Abstract
Objective
To develop a diagnostic model for predicting occult uterine sarcoma in patients with presumed uterine fibroids.
Materials and methods
We retrospectively reviewed 41631 patients with presumed uterine fibroids who presented for HIFU treatment in 13 hospitals between November 2008 and October 2023. Of these patients, 27 with occult uterine sarcoma and 54 with uterine fibroids were enrolled. Univariate analysis and multivariate logistics regression analysis were used to determine the independent risk factors for the diagnosis of occult uterine sarcoma. A prediction model was constructed based on the coefficients of the risk factors.
Results
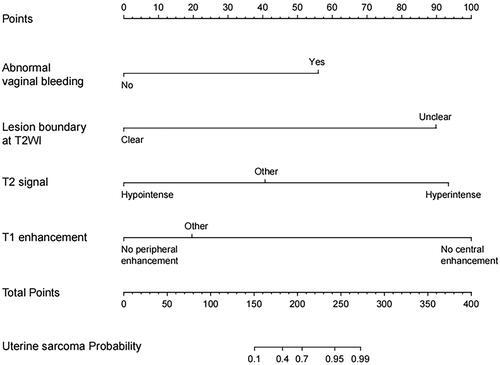
The multivariate analysis revealed abnormal vaginal bleeding, ill-defined boundary of tumor, hyperintensity on T2WI, and central unenhanced areas as independent risk factors. A scoring system was created to assess for occult uterine sarcoma risk. The score for abnormal vaginal bleeding was 56. The score for ill-defined lesion boundary was 90. The scores for lesions with hypointensity, isointensity signal/heterogeneous signal intensity, and hyperintensity on T2WI were 0, 42, and 93, respectively. The scores for lesions without enhancement on the mass margin, uniform enhancement of tumor, and no enhancement in the center of tumor were 0, 20, and 100, respectively. Patients with a higher total score implied a higher likelihood of a diagnosis of occult uterine sarcoma than that of patients with a lower score. The established model showed good predictive efficacy.
Conclusions
Our results demonstrated that the diagnostic prediction model can be used to evaluate the risk of uterine sarcoma in patients with presumed uterine fibroids.
Introduction
Uterine fibroids, also known as myomas or leiomyomas, are the most common benign tumors of the female genital tract. The prevalence varies among races, but it is generally believed that the incidence is about 25–50% in women of childbearing age [Citation1]. A study from the United States has shown that uterine fibroids were detected by ultrasound in more than 80% of African American women by the age of 50 years [Citation2]. Although most patients with uterine fibroids are asymptomatic and may be incidentally diagnosed, nearly one-third of them has symptomatic fibroids and require treatment [Citation3].
Current management strategies for uterine fibroids include surgical and non-surgical interventions. Surgical approach includes hysterectomy (via vagina, laparoscopy or abdominal surgery) and myomectomy (by hysteroscopy, laparoscopy or laparotomy), while non-surgical approach includes uterine artery embolization (UAE), other interventions performed under radiologic or ultrasound guidance and medication treatment. Due to differences in personal experience and available equipment, there are significant differences in the management of uterine fibroids using various technologies in different countries and even in different regions of the same country. Although no study breaks down the exact proportions of treatment methods in the management of uterine fibroids, the most common approach currently is hysterectomy. In the United States, over 600,000 hysterectomies are performed each year, with uterine fibroids being the main indication [Citation4]. In China, about 2.8 million patients undergo hysterectomies every year, and uterine fibroids remain the first indication for hysterectomy [Citation1,Citation5].
Over the last two decades, advances in techniques have promoted laparoscopic hysterectomy and myomectomy to the rank of a standard minimally invasive surgical procedure for uterine fibroids [Citation6–11]. However, due to lack of specific clinical manifestations in patients with uterine sarcoma, especially patients with occult uterine sarcoma, the misdiagnosis rate was high. Thus, it was not rare to see some misdiagnosed cases and accordingly inappropriate surgical treatments that led to serious consequences for patients with uterine sarcoma [Citation12]. As a result, the US Food and Drug Administration (FDA) issued a black box warning in November 2014 against use of power morcellation on presumed uterine fibroids in women undergoing myomectomy or hysterectomy [Citation13].
Recently, high intensity focused ultrasound (HIFU) has also been widely used in the treatment of uterine fibroids [Citation14–16]. Since HIFU is a noninvasive treatment and no histopathological diagnosis is available after treatment, it also raises a major concern about how to distinguish a uterine sarcoma from uterine fibroids before HIFU treatment. In addition, will treating an occult uterine sarcoma as uterine fibroids with HIFU affect the prognosis of patients? Our previous study with a small sample size has shown that HIFU treatment will not cause tumor dissemination even if the occult uterine sarcoma was treated with HIFU [Citation17]. However, obtaining a preoperative diagnosis still has great clinical significance. The best way to decrease the risks associated with misdiagnosis is to focus on early detection strategies that can reliably identify uterine fibroids and occult uterine sarcoma. Therefore, this study intended to establish a personalized clinical diagnostic model that can be used to predict uterine sarcoma.
Materials and methods
The ethics committee at our institutes approved the protocol of this retrospective study (No.HF2023-015) and the requirement for an informed consent to do the research was waived.
Subjects
A total of 41631 consecutive patients with presumed uterine fibroids who presented to the Department of Gynecology of Chongqing Haifu Hospital, Department of Gynecology of the Affiliated Hospital of Zunyi Medical University, Department of Gynecology of Suining Central Hospital of Sichuan, Department of Gynecology of the Third Xiangya Hospital of Central South University, Department of Gynecology of Nanchong Central Hospital of Sichuan, Department of Gynecology of Three Gorges Central Hospital of Chongqing, Department of Gynecology of Zigong Fourth Hospital of Sichuan, Department of Gynecology of Liangshan First Hospital of Sichuan, Department of Gynecology of Southwest Hospital of Army Military Medical University, Department of Gynecology of The Second Affiliated Hospital of Chongqing Medical University, Department of Gynecology of Changsha Maternal and Child Health Care Hospital of Hunan, Department of Gynecology of the First Affiliated Hospital of Chengdu Medical College, and Department of Gynecology of Neijiang First People’s Hospital of Sichuan for HIFU treatment from November 2008 to October 2023 were retrospectively reviewed. The medical history of the patients was collected first, followed by a physical examination. All patients had routine laboratory tests, electrocardiogram (ECG), ultrasound, chest X-ray, and magnetic resonance imaging (MRI) before HIFU treatment.
Inclusion criteria were as follows: (1) patients were older than 18 years, (2) patients with pathologically diagnosed uterine sarcoma, and (3) patients with pathologically diagnosed uterine fibroids.
Exclusion criteria were as follows: (1) patients without pre-HIFU MRI, (2) the diagnosis of uterine sarcoma was made before surgery.
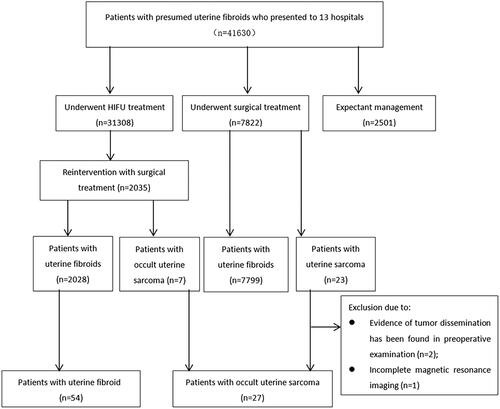
Among the 41631 patients, 31308 patients underwent HIFU treatment, 7822 patients underwent myomectomy or hysterectomy, and 2501 patients chose expectant management. Of the 7822 patients who underwent myomectomy or hysterectomy, 23 patients were pathologically diagnosed as uterine sarcoma. Among them, 3 patients were excluded due to 2 patients were suspected as uterine sarcoma by reviewing preoperative MRI, and 1 patient without enhanced MRI. In patients who underwent HIFU treatment, 2035 patients had surgical treatment during the required follow-up period, and 7 patients were pathologically diagnosed as uterine sarcoma. Thus, a total of 27 patients with occult uterine sarcoma were enrolled. Based on statistical principles of a ratio of 1 occult uterine sarcoma to 2 uterine fibroids, 54 patients who were pathologically diagnosed as uterine fibroids were finally enrolled in this study ().
MRI examination
The standard protocol for MRI examination was used in every center. MRI images were analyzed by two senior radiologists with more than 10 years of radiographic experience in obstetrics and gynecology at each center. The image features of lesions included T2WI signal intensity, T1WI signal intensity, lesion margin, lesion volume, cystic degeneration, multi-lobulated with septation, and contrast-enhancement were evaluated by two radiologists independently. If there was any disagreement with the diagnosis, their supervisor should join them and reach a consensus.
HIFU treatment
The protocol of HIFU treatment has been described previously [Citation14]. Briefly, the procedure of HIFU treatment was performed under conscious sedation (fentanyl and midazolam) using a Focused Ultrasound Tumor Therapeutic System (Model-JC or Model-JC200, Chongqing Haifu Medical Technology Co., Ltd.). This system contains an ultrasound imaging device situated at the center of the transducer, to provide real-time monitoring for the treatment. The patients were positioned prone on HIFU table, with the abdominal wall in contact with degassed water. The treatment plan was made using the sagittal ultrasound scanning mode and the targeted fibroid was divided into slices at a distance of 5 mm between the two slices. A point scan energy delivery with the treatment power of 300–400 W was used. The sonication began on the inferior side of the fibroid, moved toward the superior side, and then from the posterior area to the anterior area of the fibroid. The focus was kept at least 1.5 cm away from the endometrium and boundary of the fibroid. During the procedure, the sonication power was regulated according to the feedback from the patient and the changing grayscale on ultrasound imaging. The treatment was terminated when the entire fibroid was hyperechoic, or the enhanced ultrasound showed no blood flow in the fibroid.
Follow-up of patients after HIFU treatment
All patients were discharged 1 day after HIFU treatment, and vital signs were monitored throughout the hospital stay. The patients were asked to return to the hospital at 1 month, 3, 6, 12, and 24 months after HIFU for follow-up imaging evaluation. If the diagnosis of occult sarcoma was suspected, the patients were referred to gynecological surgery.
Histological examination
The histopathological examination was conducted by pathologists with more than 10 years of experience in gynecological pathology. The diagnosis of uterine sarcoma was made based on the 2014 World Health Organization (WHO) classification [Citation18].
Statistical analysis
SPSS 26 software was used for statistical analysis. Normal distribution data were expressed as mean ± standard deviation, and t-test was used for comparison the variables between the two groups. The skewed distribution data were expressed as median and interquartile range, and Wilcoxon-test was used for comparison the data between the two groups. The count data was expressed as the number of cases (%), and the Chi-square test was used for comparison the variables between the two groups. p < 0.05 was defined as a significant statistically difference. Univariate analysis and multivariate logistics regression analysis were used to establish the regression analysis model. Survival curve was calculated by Kaplan-Meier method, and the Log-rank test was used to compare the cumulative survival between the group of patients with uterine sarcoma underwent surgical treatment directly and the group of patients with uterine sarcoma underwent surgery after HIFU treatment.
Results
Baseline characteristics
The median age of patients with occult uterine sarcoma was 44.0 (interquartile range: 41.0–49.0) years, it was 43.5 (interquartile range: 33.0–48.0) years in the group of patients with uterine fibroids. The average body mass index (BMI) was 23.2 ± 2.9 in the group of patients with occult uterine sarcoma, while it was 22.2 ± 2.3 in the group of patients with uterine fibroids. The incidence of menopause was 7.4% (2/27) in the group of patients with occult uterine sarcoma, and it was 5.6% (3/54) in the group of patients with uterine fibroids. The rate of unpregnant women was 11.1% (3/27) in the group of patients with occult uterine sarcoma, while it was 29.6% (16/54) in the group of patients with uterine fibroids. The average lesion volume was 109.0 (interquartile range: 47.1–240.7) cm3 in the group of patients with occult uterine sarcoma, and it was 79.4 (interquartile range: 42.7–148.0) cm3 in the group of patients with uterine fibroids. No significant difference was observed in age, BMI, the incidence of menopause, the rate of unpregnant women, and lesion volume between the two groups (p > 0.05) ().
Table 1. Comparison of baseline characteristics between patients with uterine fibroids and occult uterine sarcoma.
Analysis of the predictive factors of occult uterine sarcoma
As shown in , there were significant differences in incidence of abnormal vaginal bleeding, incidence of large menstruation, and incidence of abdominal pain and distention between the patients with occult uterine sarcoma and patients with uterine fibroids by clinical history. We also observed a significant difference in neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), hemoglobin, and percentage of anemia between the two groups. In addition, there were significant differences in mass type, mass boundary on T2WI, signal intensity on T2WI, capsule or separation of the mass, signal intensity on T2WI, enhancement of the lesion, and diffusion restriction (defined as high b-value DWI showed high signal intensity, while the ADC image showed low signal intensity in the corresponding area in the mass) on diffusion-weighted imaging (DWI) between the patients with occult uterine sarcoma and patients with uterine fibroids (all p < 0.05).
Table 2. Comparison of clinical features between patients with uterine fibroids and patients with occult uterine sarcoma.
Table 3. Comparison of lab test results between patients with uterine fibroids and patients with occult uterine sarcoma.
Table 4. Comparison of MRI findings in patients with uterine fibroids and patients with occult uterine sarcoma.
These factors were further included for multivariate analysis, and the results revealed that abnormal vaginal bleeding (by clinical history), unclear boundary of lesions on T2WI (any portion around the mass), isointensity/heterogeneous signal intensity on T2WI (isointensity, defined as the signal intensity of the mass equal to that of myometrium/heterogeneous, defined as any combination of two signals on T2WI), hyperintensity on T2WI (the signal intensity higher than that of the myometrium on T2WI), uniform enhancement of lesions (homogenous enhancement in the mass), and no enhancement in the central area of the lesion (defined as heterogeneous, with central poor enhancement) were independent predictors of occult uterine sarcoma ().
Table 5. Results of the logistics regression analysis for patients with occult uterine sarcoma.
Establish of diagnosis and prediction model of occult uterine sarcoma
Based on the regression coefficient of independent risk factors for the diagnosis of occult uterine sarcoma obtained from the regression analysis results, the clinical diagnosis and prediction model of occult uterine sarcoma was developed. The R software was used to draw a nomogram to complete the visualization of the model. According to the nomogram chart, the score for abnormal vaginal bleeding was 56. The score for ill-defined lesion boundary on T2WI was 90. The score for lesion with hypointensity on T2WI was 0, the score for lesion with isointensity signal/heterogeneous signal intensity on T2WI was 41, and the score for lesion with hyperintensity on T2WI was 93. The score for lesion without enhancement on the mass margin was 0, the score for uniform enhancement of tumor was 20, and the score for no enhancement in the central area of tumor was 100 ().
Figure 2. Nomogram for independent risk factors in predicting uterine sarcoma. The corresponding score of each independent risk factor was visualized on the nomogram. The total score was obtained by adding up the scores of independent risk factor.
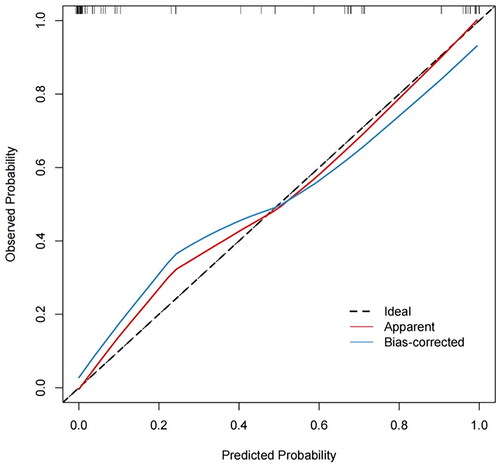
The prediction model was internally validated using the bootstrap resampling method. The number of self-sampling was B = 1000, and the area under the curve (AUC) was used to evaluated the discriminative ability of the model. The results showed that the AUC was 0.969 (95%confidence interval: 0.937–0.991, p < 0.001) (). The consistency of the model was evaluated by the calibration curve. The calibration curve of the model showed a good consistency between the predicted value of occult uterine sarcoma obtained by the nomogram and the actual observed value (). The optimal cutoff value of the total score of the nomogram was 160, the corresponding sensitivity was 96.3%, specificity was 83.3%, positive predictive value was 74.3%, and negative predictive value was 97.8%.
Figure 3. ROC Curve of the nomogram model. The AUC was 0.969 (95% confidence interval: 0.937–0.991, p < 0.001).
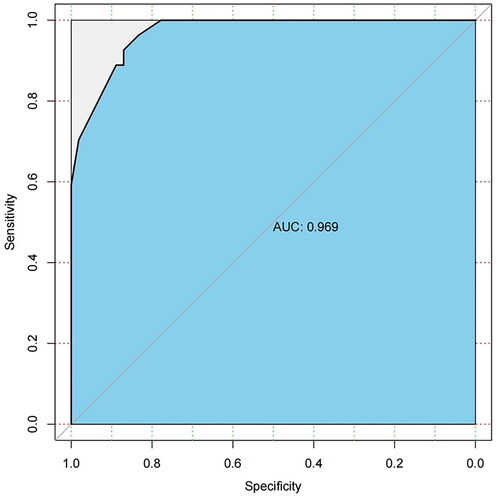
Figure 4. Calibration curve of the predictive model. The predicted value of occult uterine sarcoma obtained by the nomogram met well with the actual observed value.
Follow-up results of patients with occult uterine sarcoma treated with surgery after HIFU or treated with surgery.
All the 27 patients with occult uterine sarcoma underwent surgery after extensive physician-patient discussion. Among them, 7 patients received HIFU treatment before surgical treatment and 20 patients underwent surgery without any previous treatment. Of the 20 patients with occult uterine sarcoma who did not undergo HIFU, 5 patients chose to undergo abdominal hysterectomy, 8 patients chose to undergo laparoscopic hysterectomy, 4 patients chose to undergo abdominal myomectomy, and 3 patients chose to undergo laparoscopic myomectomy. After surgery, 6 patients were diagnosed as uterine leiomyosarcom (LMS) (1 with stage IA, 4 with stage IB and 1 with stage III), and 14 patients were diagnosed as low-grade endometrial stromal sarcoma (ESS) (5 with stage IA, 8 with stage IB and 1 with stage IIB). By the end of October 2023, after a mean follow-up of 44.9 ± 33.5 (range: 2–119) months, 15 patients were still alive, 5 patients were lost to follow-up (2 patients with stage IB of uterine LMS, 2 patients with stage IB low-grade ESS, and 1 patient with stage IIB low-grade ESS). Of the 7 patients with occult uterine sarcoma who underwent HIFU previously, 4 patients chose to undergo abdominal hysterectomy, 2 patients chose to undergo laparoscopic hysterectomy, and 1 patient chose to undergo abdominal myomectomy. Two patients were diagnosed as stage IB uterine LMS, 1 patient was diagnosed as stage IV uterine LMS, and 3 patients were diagnosed as stage IB low-grade ESS, and 1 patient with stage IIA low-grade ESS. By the end of October 2023, after a mean follow-up of 33.4 ± 23.1 (range: 3–65) months, 5 patients were alive, 1 patient with stage IB of uterine LMS died at 39 months HIFU (33 months after gynecological surgery), and 1 patient lost follow-up (uterine LMS stage IB). A significant difference in survival was observed between the two groups (p = 0.046).
Discussion
Currently, there is still a lack of diagnostic tools for predicting occult uterine sarcoma in clinical practice. Predicting the risk of uterine sarcoma from the existing clinical information of patients and developing individualized monitoring and treatment plans are the hot topics in gynecology. Over the last decade, multiple studies showed that the following MRI features of isointense to hyperintense mass at T2WI, ill-defined margin of the uterine mass, no enhancement in the central area of the uterine mass, and high signal intensity at high–b value DWI and corresponding low signal intensity on ADC maps are associated with LMS [Citation17,Citation19–22]. Wang et al. compared the MRI features of ESS and LMS lesions and didn’t find any significant difference in mass volume, the proportion of irregular margins, ill-defined margins, high signal intensity on T1WI and T2WI, and pocket-like degeneration between the ESS and LMS lesions. However, lobulated with internal septation was seen in 75% of ESS lesions, which was not seen in uterine LMS [Citation17]. Several studies also found that DWI might play a role in the differential diagnosis of uterine sarcoma and uterine fibroids [Citation23–25]. Although multiple studies have tried to establish ADC values for differentiating LMS from different types of uterine fibroids, there is a significant overlap between them [Citation26,Citation27]. Therefore, ADC values should be interpreted with caution, in combination with other MRI features. Meanwhile, Cho et al. found that a NLR> 2.1 (p = 0.041) was an independent predictor of uterine sarcoma [Citation28]. In present study, we found that there were significant differences in incidence rate of abnormal vaginal bleeding, rate of increased menstrual volume, rate of abdominal pain, NLR, PLR, hemoglobin, percentage of anemia, type of mass, mass boundary, signal intensity on T2WI, lesion with or without cystic cavity/septation, signal intensity on T1WI, features of contrast enhancement and DWI between the two groups (all p < 0.05). The results of multivariate logistics regression analysis showed that the rate of abnormal vaginal bleeding, mass boundary, signal intensity of the mass, contrast enhancement of the lesion are independent predictors of occult uterine sarcoma.
In recent years, the nomogram model has been widely used to establish models in the diagnosis and prognosis prediction of tumors. The nomogram model incorporates multiple clinical risk factors for prediction, and the prediction accuracy is higher than the traditional single factor model [Citation29–32]. In this study, we analyzed the clinically relevant risk factors, screened the relevant variables to predict the diagnosis of occult uterine sarcoma, and constructed a clinical prediction model. Based on the regression coefficient of four independent risk factors, a diagnostic prediction model of occult uterine sarcoma was successfully developed, and a nomogram was drawn.
Lakhman et al. revealed that combination of 3 or more MRI imaging features such as irregular borders, hyperintense at T1WI (hemorrhage), central unenhanced areas (coagulative necrosis), increases the sensitivity and specificity of diagnosis up to 95–100% [Citation19]. In a consensus statement, Hindman et al. summarized that using combined T2-weighted imaging, DWI with a b value of 1000s/mm2, and ADC mapping, the accuracy for detecting uterine LMS was 88%–94.6%, with sensitivity of 83%–100% and specificity of 88–100% [Citation33]. According to the corresponding value on the nomogram, we obtained the corresponding score of each independent risk factor (). The score for abnormal vaginal bleeding was 56. The score for ill-defined margin was 90. The score for lesion with isointensity/heterogeneous signal intensity on T2WI was 41, and the score for hyperintensity on T2WI was 93. The score for uniform enhancement of tumor was 20, and the score for central non-enhancement was 100. The scores for all variables were added and the total score ranged from 0 to 339 points. The optimal cutoff value of the nomogram total score for uterine sarcoma was 160, with a corresponding sensitivity of 96.3%, specificity of 83.3%, positive predictive value of 74.3%, and negative predictive value of 97.8%. The AUC of this model was 0.969, showing good predictive efficacy (). We further verified this model using the calibration curve, and the results showed a good consistency between the predicted value of occult uterine sarcoma obtained by the nomogram and the actual observed value (). Our results, along with other studies, have found that ill-defined margin and central non-enhancement are important features in distinguishing uterine sarcoma from uterine fibroids [Citation19,Citation33]. However, we didn’t find imaging features of intratumoral hemorrhage in any of the masses in this study, which may be explained by that occult uterine sarcomas have a lower probability of intratumoral bleeding because of the small size. Although DWI is not an independent influencing factor and was not included in this model in conjunction with other MRI features, the predictive accuracy, sensitivity, and specificity of this model are consistent with other studies [Citation33]. Therefore, by quantifying the corresponding predictive factors through nomogram, a more accurate prediction model can be created.
Patients with a higher score implied a higher likelihood of an occult uterine sarcoma diagnosis than that of patients with a lower score. As shown in , MR images obtained from a 32-year-old patient showed a hypointense lesion with clear margins, and contrast-enhanced MRI showed mild enhancement. The patient did not report abnormal vaginal bleeding. According to the nomogram chart, the total score was only 20 points. Thus, the diagnosis of uterine sarcoma was very unlikely. The pathological results confirmed the diagnosis of uterine fibroids after surgery. In contrast, MR images obtained from a 46-year-old patient showed a hyperintense mass with an irregular margin. Contrast-enhanced MRI showed central non-enhancement of the lesion (). According to the nomogram chart, this patient scored 0 points for no abnormal vaginal bleeding, 90 points for unclear boundaries, 93 points for high signal intensity on T2WI, 100 points for no enhancement in the central area, and a total score of 283 points. This high score indicated that the risk of diagnosis of uterine sarcoma in this patient was greater than 99%. The pathological results confirmed the diagnosis of uterine sarcoma after surgery. Therefore, this model is reliable and can be used to evaluate and select patients before HIFU treatment.
Figure 5. MR images obtained from a 32-year-old patient who reported no abnormal vaginal bleeding. A. T2WI showed a hypointense tumor with clear boundary. B. Contrast-enhanced MRI showed mild enhancement of tumor. According to the nomogram chart, the scores for no abnormal vaginal bleeding clear margin of tumor, low signal intensity on T2WI, and weak enhancement were 0, 0, 0, and 20 points. The total score was 20 points. The final diagnosis was uterine fibroids after surgery.
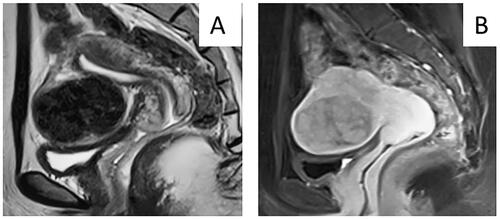
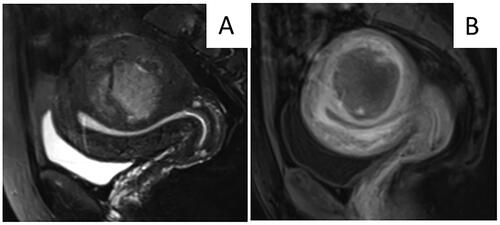
Figure 6. MRI obtained from a 46-year-old patient who did not report abnormal vaginal bleeding. A. T2WI of MRI showed a hyperintense mass with an irregular margin. B. Contrast enhanced MRI showed no enhancement in the center of the lesion. According to the nomogram chart, the patient scored 0 points for no abnormal vaginal bleeding, 90 points for unclear margin of the lesion, 93 points for hyperintensity on T2WI, 100 points for no enhancement in the central area. The total score for this patient was 283 points. The diagnosis of uterine sarcoma was confirmed after surgery.
Compared with laparotomy, laparoscopic myomectomy increased the risk of peritoneal dissemination of occult uterine sarcoma [Citation34,Citation35]. Our pilot study with a small size of subjects showed that there was no significant difference in overall survival rate between the patients with uterine sarcoma treated with abdominal surgery and patients with uterine sarcoma treated with HIFU for presumed uterine fibroids followed by open surgery [Citation17]. However, as larger studies were performed, there was a significant discrepancy between patients treated with direct surgery versus those treated with HIFU for persumed fibroids followed by surgery. The discrepancy may be related to the underlying sarcoma pathology. Uterine sarcoma subtypes include ESS and uterine LMS. Past studies have shown that patients with ESS have a better prognosis than that of patients with uterine LMS [Citation36]. The 5-year survival rate of patients with stage I uterine LMS was estimated to be 76%, and stage II was estimated to be 60%, stage III was estimated to be 45%, and stage IV was estimated to be 29% [Citation37]. While the 5-year survival rate for patients with stage I and stage II ESS was 90%, for patients with stage III and stage IV ESS the survival rate was 50% [Citation38]. In our study, although the survival rate of patients treated with surgery was a little bit higher than that of patients treated with HIFU followed by surgery during the follow up period, the incidence of uterine LMS in patients who underwent HIFU followed by surgery during the follow-up period was higher than that in the group treated with surgery. Therefore, this difference is more likely to be related to the type of sarcomas, rather than treatment.
Although the nomogram prediction model in this study demonstrated good predictive ability, it has several limitations. Firstly, bias may have occurred because this model was established based on retrospective analysis. Secondly, the small sample size of this study may result in incomplete inclusion of variables and omission of some important variables. Thirdly, external data validation can better prove the practicability and credibility of the model, while the validation of this model only used internal data validation. Therefore, future studies with larger subject sizes are needed, and external validation should be considered.
Conclusions
In summary, our results indicated that abnormal vaginal bleeding, ill-defined uterine tumor margin, signal intensity of lesion on T2WI, and enhancement feature of the lesion are independent predictors of occult uterine sarcoma. The established nomogram prediction model showed good predictive efficacy. Based on these preoperative predictions, the nomogram can help evaluate and select patients before surgery. Given its limitations, more clinical data is required for external validation of the model to improve the prediction model’s clinical applicability.
Disclosure statement
Lian Zhang is a senior consultant to Chongqing Haifu. The other authors have no conflict of interests to declare.
Data availability statement
The data that support the findings of this study are available upon request from the corresponding author, LZ. The data are not publicly available because they contain information that can compromise the privacy of the research participants.
Additional information
Funding
References
- Consensus for diagnosis and treatment of uterine myoma. Zhonghua Fu Chan Ke Za Zhi. 2017;52(12):793–800. doi: 10.3760/cma.j.issn.0529-567x.2017.12.001.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99.
- Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149(1):3–9. doi: 10.1002/ijgo.13102.
- Flynn M, Jamison M, Datta S, et al. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006;195(4):955–964. doi: 10.1016/j.ajog.2006.02.020.
- Chen BP, Clymer JW, Turner AP, et al. Global hospital and operative costs associated with various ventral cavity procedures: a comprehensive literature review and analysis across regions[J]. J Med Econ. 2019;22(11):1210–1220. doi: 10.1080/13696998.2019.1661680.
- Song T, Kim TJ, Lee SH, et al. Laparoendoscopic single-site myomectomy compared with conventional laparoscopic myomectomy: a multicenter, randomized, controlled trial. Fertil Steril. 2015;104(5):1325–1331. doi: 10.1016/j.fertnstert.2015.07.1137.
- Wu PC, Sheu BC, Huang KJ, et al. Laparoendoscopic two-site myomectomy (LETS-M) using conventional laparoscopic instruments and the glove-port technique. J Formos Med Assoc. 2022;121(11):2248–2256. doi: 10.1016/j.jfma.2022.04.013.
- Munro MG. Hysteroscopic myomectomy of FIGO type 2 leiomyomas under local anesthesia: bipolar radiofrequency needle-based release followed by electromechanical morcellation. J Minim Invasive Gynecol. 2016;23(1):12–13. doi: 10.1016/j.jmig.2015.08.002.
- Moawad NS, Palin H. Hysteroscopic myomectomy. Obstet Gynecol Clin North Am. 2022;49(2):329–353. doi: 10.1016/j.ogc.2022.02.012.
- Loddo A, Djokovic D, Drizi A, et al. Hysteroscopic myomectomy: the guidelines of the International Society for Gynecologic Endoscopy (ISGE). Eur J Obstet Gynecol Reprod Biol. 2022;268:121–128. doi: 10.1016/j.ejogrb.2021.11.434.
- Dumitraşcu MC, Nenciu C-G, Nenciu A-E, et al. Laparoscopic myomectomy – the importance of surgical techniques. Front Med. 2023;10:1251421. doi: 10.3389/fmed.2023.1158264.
- Ricci S, Stone RL, Fader AN. Uterine LMS: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145(1):208–216. doi: 10.1016/j.ygyno.2017.02.019.
- Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. Published 2014; [cited 2020 Aug 5]. Available from: https://wayback.archive-it. Org/7993/20170404182209/https:/www.fda.gov/MedicalDevices/ Safety/AlertsandNotices/ucm424443.htm.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125(3):354–364. doi: 10.1111/1471-0528.14689.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018;35(1):56–61. doi: 10.1080/02656736.2018.1473894.
- Liu X, Tang J, Luo Y, et al. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: a retrospective study. BJOG. 2020;127(11):1422–1428. doi: 10.1111/1471-0528.16262.
- Wang Q, Wu X, Zhu X, et al. MRI features and clinical outcomes of unexpected uterine sarcoma in patients who underwent high-intensity focused ultrasound ablation for presumed uterine fibroids. Int J Hyperthermia. 2021;38(2):39–45. doi: 10.1080/02656736.2021.1921288.
- Kurman RJ, Carcangiu ML, Herrington CS, et al. 2014 WHO Classification of Tumours of Female Reproductive Organs.
- Lakhman Y, Veeraraghavan H, Chaim J, et al. Differentiation of uterine LMS from atypical leiomyoma: diagnostic accuracy of qualitative MR imaging features and feasibility of texture analysis. Eur Radiol. 2017;27(7):2903–2915. doi: 10.1007/s00330-016-4623-9.
- Smith J, Zawaideh JP, Sahin H, et al. Differentiating uterine sarcoma from leiomyoma: BET1T2ER Check!. Br J Radiol. 2021;0:20201332.
- Kaganov H, Ades A, Fraser DS. Preoperative magnetic resonance imaging diagnostic features of uterine LMSs: a systematic review. Int J Technol Assess Health Care. 2018;34(2):172–179. doi: 10.1017/S0266462318000168.
- Rio G, Lima M, Gil R, et al. T2 hyperintense myometrial tumors: can MRI features differentiate leiomyomas from LMSs? Abdom Radiol. 2019;44(10):3388–3397. doi: 10.1007/s00261-019-02097-x.
- Barral M, Placé V, Dautry R, et al. Magnetic resonance imaging features of uterine sarcoma and mimickers. Abdom Radiol. 2017;42(6):1762–1772. doi: 10.1007/s00261-017-1076-9.
- Li HM, Liu J, Qiang JW, et al. Diffusion-weighted imaging for differentiating uterine LMS from degenerated leiomyoma. J Comput Assist Tomogr. 2017;41(4):599–606. doi: 10.1097/rct.0000000000000565.
- DeMulder D, Ascher SM. Uterine LMS: can MRI differentiate LMS from Benign leiomyoma before treatment? AJR Am J Roentgenol. 2018;211(6):1405–1415. doi: 10.2214/ajr.17.19234.
- Sun S, Bonaffini PA, Nougaret S, et al. How to differentiate uterine LMS from leiomyoma with imaging. Diagn Interv Imaging. 2019;100(10):619–634. doi: 10.1016/j.diii.2019.07.007.
- Bura V, Pintican RM, David RE, et al. MRI findings in-between leiomyoma and LMS: a Rad-Path correlation of degenerated leiomyomas and variants. Br J Radiol. 2021;94(1125):20210283.
- Cho HY, Kim K, Kim YB, et al. Differential diagnosis between uterine sarcoma and leiomyoma using preoperative clinical characteristics. J Obstet Gynaecol Res. 2016;42(3):313–318. doi: 10.1111/jog.12915.
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi: 10.1016/s1470-2045(14)71116-7.
- Pan YX, Chen JC, Fang AP, et al. A nomogram predicting the recurrence of hepatocellular carcinoma in patients after laparoscopic hepatectomy. Cancer Commun. 2019;39(1):55. doi: 10.1186/s40880-019-0404-6.
- Lv J, Liu YY, Jia YT, et al. A nomogram model for predicting prognosis of obstructive colorectal cancer. World J Surg Oncol. 2021;19(1):337. doi: 10.1186/s12957-021-02445-6.
- Liu H, Li Z, Zhang Q, et al. Multi‑institutional development and validation of a nomogram to predict prognosis of early-onset gastric cancer patients. Front Immunol. 2022;13:1007176. doi: 10.3389/fimmu.2022.1007176.
- Hindman N, Kang S, Fournier L, et al. MRI evaluation of uterine masses for risk of LMS: a consensus statement. Radiology. 2023;306(2):e211658. doi: 10.1148/radiol.211658.
- Takamizawa S, Minakami H, Usui R, et al. Risk of complications and uterine malignancies in women undergoing hysterectomy for presumed benign leiomyomas. Gynecol Obstet Invest. 1999;48(3):193–196. doi: 10.1159/000010172.
- George S, Barysauskas C, Serrano C, et al. Retrospective cohort study evaluating the impact of intraperitoneal morcellation on outcomes of localized uterine LMS. Cancer. 2014;120(20):3154–3158. doi: 10.1002/cncr.28844.
- Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine LMS: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112(4):820–830. doi: 10.1002/cncr.23245.
- Byar KL, Fredericks T, Uterine LMS. Uterine Leiomyosarcoma. J Adv Pract Oncol. 2022;13(1):70–76. doi: 10.6004/jadpro.2022.13.1.6.
- Chan JK, Kawar NM, Shin JY, et al. ESS: a population-based analysis. Br J Cancer. 2008;99(8):1210–1215. doi: 10.1038/sj.bjc.6604527.