Abstract
The purpose of this study was to establish whether a pulsed dose rate (PDR) treatment of 1.5 Gy given every 3 h in combination with 41°C mild hyperthermia or a continuous low dose rate (LDR) treatment with mild hyperthermia could radiosensitize two isogenic human breast carcinoma cell lines in comparison to pulsed dose rate or low dose rate irradiation alone. The radiation resistant cell line was derived from the parental cell line and was transfected to over-express DNA polymerase β. The end-points assessed were the survival of the cells using the clonogenic assay, the amount of residual DSB(s) using the comet assay and gene expression of polymerase β using RT-PCR. Results showed that the PDR and LDR treatments combined with mild hyperthermia caused significant radiosensitization when compared to PDR and LDR irradiation alone in terms of the clonogenic and comet assays with both cell lines. RT-PCR results showed that polymerase β levels of expression were not elevated in response to these treatments, implying that this polymerase may not be involved in sub-lethal damage repair or thermal radiosensitization. These results suggest a potential clinical advantage when combining LDR or PDR with hyperthermia, since they indicate that hyperthermia is an effective radiosensitizer.
Introduction
Pulsed dose rate (PDR) irradiation holds the possibility of using the radiobiological advantages of low dose rate (LDR) brachytherapy such as a high level of local control in combination with optimum sparing of normal tissues, while improving dose distributions and enhancing radiation protection. Pulsed dose rate (PDR) brachytherapy uses a single stepping radioactive source under computer control that is scanned throughout the treatment volume. This type of treatment offers the advantages of (i) computer-controlled dose distribution, (ii) convenience for nursing and for the patient in terms of visiting and mobility during the interval between doses, (iii) decreased costs resulting from a smaller inventory of radioactive sources as well as from equipment sharing by several patients and (iv) good radiation protection, since the source is returned to the safe after each pulse, eliminating exposure to staff Citation[1–5]. This technique uses a series of short high dose rate pulses separated by equally spaced time intervals such that the average dose rate is similar to that of traditional LDR brachytherapy; therefore, offering the radiobiological advantages of LDR irradiation. The theoretical basis for comparison of LDR and PDR exposure schemes was originally proposed by Brenner and Hall Citation[4]. In their article they analysed data from 36 cell lines of human origin and suggested that a pulsed regime consisting of 0.5 Gy delivered in a 10-min pulse and repeated every hour would be functionally equivalent to continuous low dose rate irradiation at 0.5 Gy h−1. A number of in vitro studies by Armour et al. Citation[6] with rat 9L gliosarcoma cells, Chen et al. Citation[2], Pomp et al. Citation[7] using human tumour cells and Fritz et al. Citation[1] (although using a larger fraction size) with V79 cells from spheroid culture provided evidence to support the suggested equivalence even when using pulses with duration shorter than 10 min. However, increased cytotoxicity was reported when using the larger fraction sizes Citation[1], Citation[2], Citation[6]. More recent studies with a range or human tumour cell lines of different radiation responses as well as some normal human cell lines showed only limited PDR and LDR equivalency Citation[8], Citation[9]. It appeared that the equivalency was dependent on pulse size and frequency, as well as there being a cell line dependency. The cell line dependency may be related to radiation resistance, capacity for sub-lethal damage repair (SLDR), as well as adaptive response differences amongst the cell lines. This study and others have shown adaptive responses to PDR treatments Citation[9], Citation[10].
Numerous studies have shown that hyperthermia is a good radiosensitizer, which is in part due to the inhibition of SLDR Citation[11–15]. More recent studies showed that mild hyperthermia given concurrently with LDR irradiation was a potent sensitizer, which was in large part due to the inhibition of repair of radiation damage Citation[6], Citation[11], Citation[13], Citation[14], Citation[16]. To date, to the best of the authors’ knowledge, only three studies have investigated the effects of mild hyperthermia combined with PDR irradiation. While all of these studies showed radiosensitization, only one showed equivalency of PDR to LDR treatments under such thermal sensitizing conditions Citation[6], Citation[16], Citation[17] and the degree of sensitization may be cell-line dependent, possibly involving radiation resistance, repair capacity and adaptive responses. Thus, based on these studies there is potential for mild hyperthermia to be an effective PDR irradiation sensitizer, which may be equivalent to LDR irradiation sensitization.
In order to assess this potential, this study has set out to compare the responses to PDR irradiation in combination with 41°C hyperthermia to PDR irradiation alone using 1.5 Gy every 3 h fractionation scheme, for two isogenic human breast carcinoma cell lines, one a parental cell line and the other its more radioresistant variant over-expressing one of the DNA repair enzymes, polymerase β.
Methods and materials
Cell lines and culture
Two human breast carcinoma cell lines, kindly donated by Dr R. Sobol and Dr S. Wilson, were used; the parental wild type MCF7 and the variant C716. The C716 is considered to be radiation- and cisplatin-resistant compared to the parental cell line Citation[18]. These two cell lines were used because they may represent a good model of tumour tissues of similar origin, but with different intrinsic radiosensitivities. In addition, they allowed one to investigate whether the differences in response to the treatments between the two cell lines were due to the involvement of the DNA polymerase β in sub-lethal damage repair and inhibition, since both repair capacity and polymerase β have been implicated in thermal radiosensitization in some studies Citation[19], Citation[20].
The cell lines were grown in a medium combination of DMEM/F-12 supplemented with 10% foetal bovine serum, 1 mM MEM non-essential amino acids, 10 mM sodium bicarbonate and 20 mM Hepes. All cell cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Details of the cell culture have been previously described Citation[18]. For experiments, plateau phase cells were used in order to minimize cell cycle redistribution during protracted treatments. Cell cycle analysis using flow cytometry showed cells to be distributed as follows; G1 = 60%, S = 5% and G2 = 35% and no significant changes where observed during the experiments.
Irradiations
For high dose rate (HDR) and PDR irradiation experiments, 5 × 105 cells were plated in 25 cm2 flasks, while for LDR irradiation experiments, 2.5 × 105 cells were plated in 35 mM petri dishes. Medium was changed on day 7 and cells were irradiated (HDR and PDR) at plateau phase on day 10 using a 250 kVp X-ray unit at a dose rate of 142 cGy min−1. The PDR flasks were irradiated to 1.5 Gy every 3 h in a water bath at 37°C or 41°C. The LDR plates were irradiated by an array of 137Cs sources contained in a temperature-controlled irradiator, which was set to 37°C or 41°C, at a dose rate of 0.78 cGy min−1 (0.47 Gy h−1).
Clonogenic survival assay
Irradiated and untreated control cells were trypsinized (0.2% trypsin/2.5 mM EDTA, 5 min, 37°C) and counted with an Elzone electronic particle counter. They were plated to yield ∼50 colonies/60 mm dish after 14 days of incubation in a humidified atmosphere of 5% CO2 and 95% air. The dishes were stained with methylene blue and colonies of at least 50 cells were counted and survival curves were generated. The data points were then fitted with the Linear Quadratic Model Citation[21] using a non-linear least squares fit (Sigma Plot) to find the parameters α and β and their estimated standard errors. p was then calculated using the Student's t-test at the 0.05 significance level to determine the equivalence between PDR and LDR matched data points Citation[22].
Neutral comet assay
Residual DNA DSBs were determined using a neutral comet assay technique, with the lysis time and electrophoresis time optimized for the detection of DSBs in present cell lines using HDR irradiation.
Cells were trypsinized as per the clonogenic assay and diluted to ∼4 × 105 cells ml−1 and gels were prepared as described previously Citation[23], using 20 min incubation time at room temperature in 50 ml TBE buffer (90 mM Tris-Borate, 2 mM EDTA, pH 8.4; Roche Diagnostics, QC) and a 20 min electrophoresis time in 220 ml of fresh TBE buffer at 1 V cm−1 (13 V DC) in a mini-horizontal submarine gel electrophoresis chamber (Hoefer). The DNA damage parameter tail moment of 50 cells per sample was quantified using the ALKOMET v3.1 image analysis system (Richard Brancker Research Ltd., Ottawa, Ontario).
RT-PCR
Primers used in these experiments are as follows (Gibco): β2-micro-globulin 5′-GACTGGTCTTTCTATCTCTTGTACTACAC-3′ and 3′-GAAGGTTAAATGTATGAGACGAATCTTA-5′, pol β5′-GGTGAGACAAAGTTCATGGGT-3′ and 3′-GTGTTAGTTACTCATGTGGTAGG-5′. Cells were irradiated with up to 6 Gy and then incubated for various periods of time to allow for expression of RNA. Then irradiated and control cells were trypsinized as per the clonogenic assay and 1–5 × 106 cells were obtained for the RT-PCR assay. The cells were centrifuged and the RNA was extracted from the cells as per the RNeasy kit instructions (RNeasy Mini Handbook, Qiagen, 2nd ed, May 1999). Following extraction, the concentration of the RNA obtained was calculated using a spectrometer (Eppendorf). Reverse transcription (RT) was performed using the First Strand cDNA Synthesis Kit (MBI Fermentas), which allowed 1 μg of RNA to be reverse transcribed into cDNA. The PCR reaction was performed in the LightCycler (Roche) using a mixture of 2 mM MgCl2, 1Xbuffer, 0.1 U μl−1 Taq Pol (Qiagen), 0.2 mM dNTP (MBI Fermentas), 1XBSA, 1 : 40 000 SYBR Green I and 200 mM of each primer. Total volume of the reaction was 20 μl. Since the C716 cell line over-expresses polymerase β, the template was diluted to 1 : 50. The samples were analysed directly during the procedure and crossing point ratios were determined from calibrators and gene reference β2 micro-globulin to target gene ratios. The crossing points are inflection points where the signal from the products is above background levels. It was also confirmed with melting curves that there is only a single amplified cDNA sequence.
Results
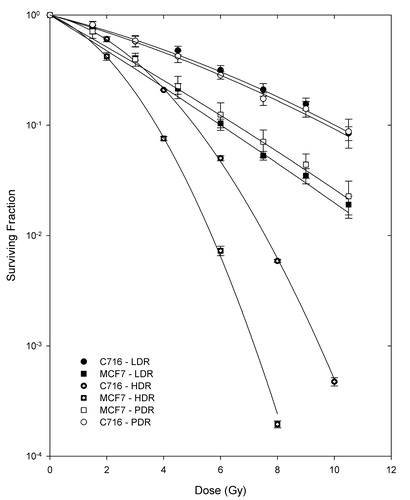
shows survival curves for the pulsed low dose rate experiments and low dose rate treatments for both cell lines. High dose rate survival curves are also included for comparison of the relative radiosensitivities of the two cell lines. From these HDR curves it can be concluded that the MCF7 cell line was more radiosensitive than the C716 cell line. In addition, it can be observed that, for both cell lines, PDR and LDR irradiations were equivalent since the results calculated from Student's t-test of matched data points all had p-values greater than 0.4 (p > 0.4).
Figure 1. Survival curves for the two cell lines after LDR, PDR and HDR irradiations. Cell lines and radiation protocols are indicated on the figure key. All figures are fitted using the LQ model and the error bars are the standard error of the mean of three experiments. Student's t-test determined that the differences between matched PDR and LDR doses are not statistically significant at the 95% confidence level for either cell line, while for HDR they were significantly different.
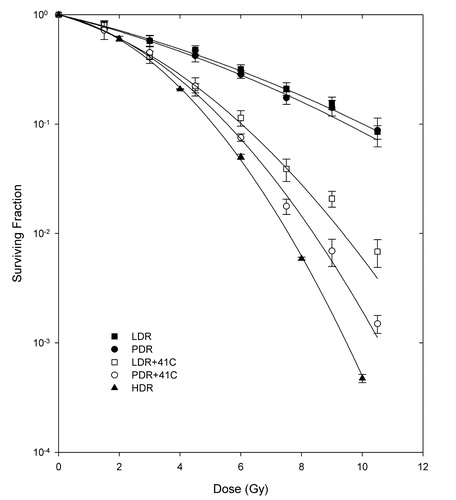
and show the response of the MCF7 and C716 cell lines to PDR and LDR with/without 41°C hyperthermia, along with HDR curves for comparison. From these curves, it can be observed that the PDR plus hyperthermia response had lower survival compared to that of the LDR plus hyperthermia treatment at higher doses in both cell lines, indicating a trend to greater radiosensitization for PDR irradiation. However, the difference in response was not significant for either cell line (p > 0.05). In addition, the equivalence between PDR with/without hyperthermia (PDRH and PDR, respectively) and LDR with/without hyperthermia (LDRH and LDR, respectively) was tested to establish the effectiveness of concurrent hyperthermia as a radiosensitizer and it was established that hyperthermia is an effective radiosensitizer at higher doses above 6 and 4.5 Gy for the MCF7 and C716 cell lines, respectively (p<0.05).
Figure 2. Survival curves for the MCF7 cell line after pulsed dose rate with/without hyperthermia (PDR/PDRH), low dose rate with/without hyperthermia (LDR/LDRH) and high dose rate (HDR) irradiation. Student's t-test determined that the differences between matched PDRH and LDRH doses are not statistically significant at the 95% confidence level. Differences between PDR and PDRH as well as LDR and LDRH matched doses are significant at 6 Gy and higher.
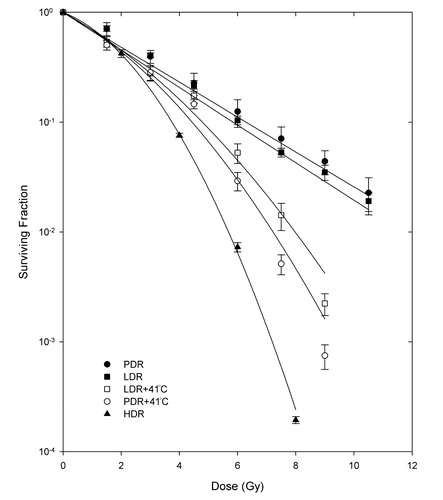
Figure 3. Survival curves for the C716 cell line after pulsed dose rate with/without hyperthermia (PDR/PDRH), low dose rate with/without hyperthermia (LDR/LDRH) and high dose rate (HDR) irradiation. Student's t-test determined that the differences between matched PDRH and LDRH doses are not statistically significant at the 95% confidence level. Differences between PDR and PDRH as well as LDR and LDRH matched doses are significant at 4.5 Gy and higher.
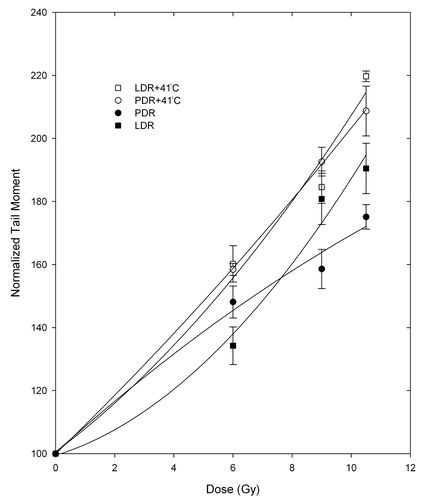
and show DNA DSB analysis through the tail moments for the MCF7 and the C716 cell lines after PDR and LDR irradiations alone or in combination with 41°C mild hyperthermia. Hyperthermia alone was not found to cause any significant increases in DSB levels in either cell line. It can be observed from that PDR plus hyperthermia treatment has marginally more DSBs than the LDR plus hyperthermia treatment for the MCF7 cell line, except for the last dose point, where the values for the two treatments overlap. , on the other hand, shows that for the C716 cell line the two treatments have very similar levels of DSBs. The equivalence between the two treatments was tested using the Student's t-test on matched data points and it was found that the two treatments are equivalent for both cell lines. The PDR with/without and LDR with/without hyperthermia treatment data were also tested and it was determined that the two treatments are significantly different for some points and not for others in both cell lines. While the trend is clear, the variation is due to the large uncertainty in the experimental data. Therefore, the data for LDR and PDR treatments were combined and tested against the combined data for LDR and PDR plus hyperthermia. This was done in order to be able to conclude whether the treatments of radiation alone and radiation with hyperthermia were significantly different from each other by adding more degrees of freedom. Combining the data in this manner showed that the treatments with no hyperthermia are statistically different from the combination treatments of radiation and hyperthermia for both cell lines.
Figure 4. Tail moments normalized to unirradiated controls following LDR and PDR irradiation with/without hyperthermia for the MCF7 cell line. Student's t-test determined that the differences between matched PDRH and LDRH doses are not statistically significant at the 95% confidence level. Differences between PDR and PDRH as well as LDR and LDRH matched doses are significant when combining the PDR and LDR data vs. PDRH and LDRH data.
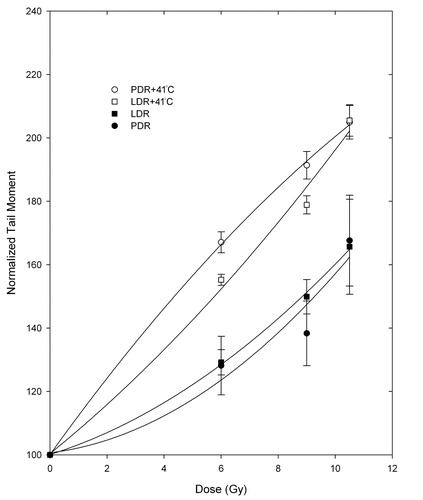
Figure 5. Tail moments normalized to unirradiated controls following LDR and PDR irradiation with/without hyperthermia for the C716 cell line. Student's t-test determined that the differences between matched PDRH and LDRH doses are not statistically significant at the 95% confidence level. Differences between PDR and PDRH as well as LDR and LDRH matched doses are significant when combining the PDR and LDR data vs. PDRH and LDRH data.
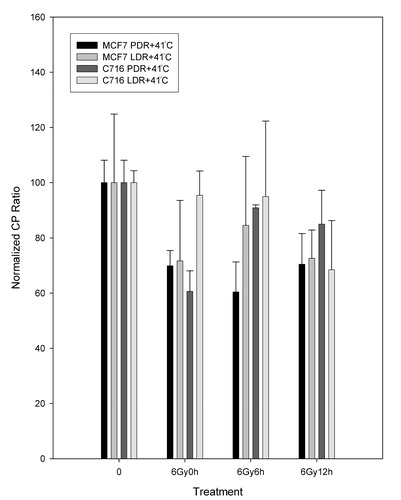
For polymerase β analysis, the RTPCR normalized crossing point ratios are shown for the MCF7 and the C716 cell lines after PDR and LDR irradiations alone () and in combination with 41°C mild hyperthermia (). From it can be observed that there are no significant differences in levels of expression between the PDR and LDR treatments as time progresses in either cell line. Therefore, the two treatments are equivalent for both cell lines since the results calculated from Student's t-test of matched data points all had p-values greater than 0.08 (p > 0.08). shows that for the MCF7 cell line the polymerase β levels of expression follow a similar trend for the two hyperthermia plus radiation combination treatments. Results from the Student's t-test confirmed that the two types of treatment are not statistically different from each other. The polymerase is down-regulated compared to untreated controls and that is significant for the PDR plus hyperthermia treatment for the measurements taken immediately after treatment and following 6 h after treatment. A similar trend is observed with the C716 cell line. The two radiation and hyperthermia combination treatments are not significantly different from each other and only the down-regulation observed immediately after the PDR plus hyperthermia treatment is statistically significant from the untreated levels. Data for hyperthermia alone was also taken and analysed. In general, it can be observed that, for both cell lines, the levels of expression for hyperthermia alone were not statistically different from the untreated levels (p > 0.3). Note that the comparison of the expression levels for LDR and hyperthermia vs hyperthermia alone and PDR and hyperthermia vs hyperthermia alone were also not significantly different.
Figure 6. Normalized CP ratios for the β polymerase levels of expression following LDR and PDR irradiation for the MCF7 and C716 cell lines. Student's t-test determined that the differences between matched PDR and LDR doses, as well as LDR and PDR untreated vs. treated dose points are not statistically significant at the 95% confidence level for either cell line.
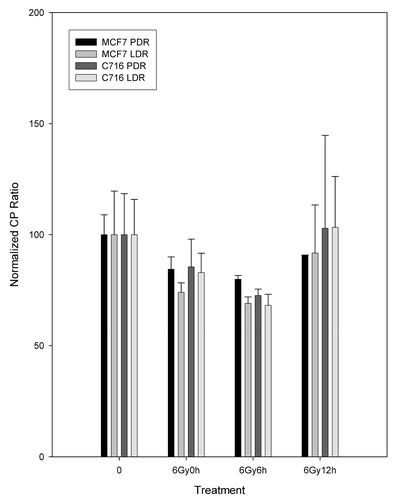
Figure 7. Normalized CP ratios for the β polymerase levels of expression following LDR and PDR irradiation with hyperthermia (LDRH and PDRH) for the MCF7 and C716 cell lines. Student's t-test determined that the differences between matched PDRH and LDRH doses, as well as LDRH untreated vs treated dose points are not statistically significant at the 95% confidence level for either cell line. Significant differences were found for the PDRH untreated vs. 6 Gy at 0 h and 6 Gy after 6 h data points for the MCF7 cell line and PDRH untreated vs. 6 h at 0 h data points for the C716 cell line.
Discussion
The results in this study confirmed the effectiveness of mild 41°C hyperthermia administered concomitantly with LDR irradiation, supporting data from several studies that have demonstrated the effectiveness of mild hyperthermia as a radiosensitizer Citation[6], Citation[24–26].
In addition, survival data for the MCF7 and C716 cell lines show that PDR as well as LDR irradiation combined with 41°C hyperthermia both have a significantly lower survival than either treatment alone. This is especially true for the C716 where the difference between the two treatments is significant already at 4.5 Gy compared to 6 Gy for the MCF7. This radiation and hyperthermia combination effectiveness in both cell lines is most likely due to SLD repair inhibition by the added hyperthermia treatment. In addition, since both cell lines are thermally radiosensitized to a similar degree, it indicates that polymerase β, which is over-expressed in the radioresistant cell line, may not play a role in repair of this type of radiation damage, supporting earlier findings Citation[27].
The combination hyperthermia treatment appears on the graphs to be more effective for the PDR treatment than for the LDR for both cell lines; however, the two combination treatments are not statistically different from each other (except for one data point for the C716). Therefore, the LDR-PDR equivalence is maintained with or without hyperthermia for this cell line and indicates that hyperthermia is effective with PDR irradiation. These results support those in one study that used rat cells treated with PDR irradiation (0.25 Gy/0.5 h, 1 Gy/2 h, 3 Gy/6 h) with and without 41°C hyperthermia, as well as 0.5 Gy h−1 LDR irradiation. This study also found that the hyperthermia treatment sensitized the PDR and LDR treatments to the same degree Citation[6]. Another study using human cell lines found that the 1.5 Gy/3 h treatment combined with hyperthermia was only slightly effective for two tumour cell lines and not at all for the normal cells, although the hyperthermia used was 40°C, which may account for the reduced effect Citation[17].
Results from the comet assay show that, for both cell lines, PDR plus hyperthermia and LDR plus hyperthermia treatments are equivalent to each other. In addition, although PDR/LDR and PDR/LDR plus hyperthermia are statistically different from each other for most of the doses investigated in both cell lines (p<0.05), there are some dose points that do not show this difference. In order to be able to draw definite conclusions about these experiments, the data from treatments without hyperthermia were combined together as were the data for the combined treatments with hyperthermia. This was done to increase the number of degrees of freedom and also it was justified since the equivalence between LDR and PDR with/without hyperthermia has already been established. This resulted in significant differences between the treatments with and without hyperthermia. Thus, radiosensitization by concomitant hyperthermia with PDR and LDR irradiation is correlated for the survival end-point and the DNA DSB end-point for each cell line. However, the correlation does not hold for the differences between the two cell lines for hyperthermia plus radiation, since the amounts of DSBs are not different between the two cell lines, meaning the more radioresistant cell line does not have less DSBs than the parental cell line. However, this may be explained as a result of the way in which cells identify or define a DSB. Radiosensitive cells could view both opposed and more widely spaced opposing lesions as DSBs while, in resistant cells, a DSB could be identified as two closely opposed SSBs (although it would still be viewed as a DSB by the assay). Hence, resistant cells could repair this break as independent SSBs, while the sensitive cells may use other, more error-prone repair pathways Citation[28]. This agrees with a previous study on isogenic human ovarian carcinoma cell lines, in which a radiosensitive and resistant cell line showed differences in clonogenic survival, no differences in DNA DSBs and more error prone repair in the sensitive cell line Citation[29]. The comet assay does not distinguish DSBs as seen by the sensitive cell line from two closely spaced SSBs as seen by the radioresistant cell line; hence, recording a similar response in the two cell lines. There have been no studies to date using the comet assay to compare PDR or LDR for these treatments combined with hyperthermia to compare survival results to DNA damage. There is, however, one study comparing LDR to LDR combined with camptothecin, which found strong correlations between the levels of residual DNA DSBs and surviving fraction after concurrent treatment with 25 μM camptothecin and low dose rate irradiation Citation[23].
Results from RT-PCR show that the levels of expression of the polymerase β change very little in both cell lines following both LDR and PDR treatments. Polymerase β expression was not affected in a significant way by hyperthermia alone, which is consistent with the published data that this polymerase is not sensitive to mild hyperthermia Citation[30]. Hyperthermia combined with the LDR and PDR treatments did not have a significant effect on polymerase β expression in either cell line, except for immediately after PDR treatment in both cell lines. Most studies in the literature propose that this polymerase is involved in ionizing radiation damage repair, particularly in base excision repair Citation[18], Citation[31], Citation[32]. However, one study showed that cells over-expressing polymerase β were more sensitive to irradiation treatments by increasing apoptosis Citation[33], while another showed that two cell lines, a wild type and the other a polymerase β knockout, had identical radiation survival clonogenic assay responses, as well as the same levels of apoptosis Citation[34]. This latter study by Miura et al. Citation[34] proposes two pathways for base excision repair (BER), one dependent on polymerase β, the other (concluded as the predominant one in the study) on proliferating cell nuclear antigen (PCNA). It may be that in the present study the PCNA-dependent pathway is the main route in the repair of radiation-induced DNA damage, as well. Another possibility that cannot be ruled out is that post-transcriptional processes are affected by hyperthermia and radiation. These could be further investigated.
In summary, the data shows that mild hyperthermia given concomitantly with PDR irradiation is at least as effective as when given with LDR irradiation. In addition, the thermal radiosensitization is also observed at the DNA DSB level supporting the concept of the inhibition DNA damage repair mechanisms. The lack of differences between the parental cell line and the polymerase β-over-expressing resistant cell line also supports the previous finding that polymerase β may not be involved in thermal radiosensitization Citation[27].
References
- Fritz P, Weber KJ, Frank C, Flentje M. Differential effects of dose rate and superfractionation on survival and cell cycle of V79 cells from spheroid and monolayer culture. Radiation Oncology 1996; 39: 73–79
- Chen C-Z, Huang Y, Hall EJ, Brenner DJ. Pulsed brachytherapy as a substitute for continuous low dose rate: An in vitro study with human carcinoma cells. International Journal of Radiation Oncology, Biology & Physics 1997; 37: 137–143
- White JR, Armour EP, Armin A-R, Dewitt C, Corry PM, Martinez A. Reproducible rat model for comparing late rectal toxicity from different brachytherapy techniques. International Journal of Radiation Oncology, Biology & Physics 1997; 37: 1155–1161
- Brenner DJ, Hall EJ. Conditions for the equivalence of continuous to pulsed low dose rate brachytherapy. International Journal of Radiation Oncology, Biology & Physics 1991; 20: 181–190
- Visser AG, Van Den Aardweg GJMJ, Levendag PC. Pulsed dose rate and fractionated high dose rate brachytherapy: Choice of brachytherapy schedules to replace low dose rate treatments. International Journal of Radiation Oncology, Biology & Physics 1996; 34: 497–505
- Armour EP, Wang Z, Corry P, Martinez A. Equivalence of continuous and pulse simulated low dose rate irradiation in 9L gliosarcoma cells at 37° and 41°C. International Journal of Radiation Oncology, Biology & Physics 1991; 22: 109–114
- Pomp J, Woudstra EC, Kampinga HH. Pulsed-dose-rate and low-dose-rate brachytherapy: Comparison of sparing effects in cells of a radiosensitive and a radioresistant cell line. Radiation Research 1999; 151: 449–453
- Niedbala M, Alsbeih G, Ng CE, Raaphorst GP. Equivalence of pulsed-dose-rate to low-dose-rate irradiation in tumor and normal cell lines. Radiation Research 2001; 155: 297–303
- Raaphorst GP, Ng CE, Smith D, Niedbala M. Evidence for adaptive response and implications in pulse-simulated low-dose-rate radiotherapy. International Journal of Radiation Oncology, Biology & Physics 2000; 48: 1139–1144
- Kreder NC, Van Bree C, Peters GJ, Love WJ, Haveman J. Enhanced levels of deoxycytidine kinase and thymidine kinase 1 and 2 after pulsed low dose rate irradiation as an adaptive response to radiation. Oncology Reports 2002; 9: 141–144
- Spiro JI, McPherson S, Cook JA, Ling CC, DeGraff W, Mitchell JB. Sensitization of low-dose rate irradiation by nonlethal hyperthermia. Radiation Research 1991; 127: 111–114
- Nevaldine B, Longo JA, Hahn PJ. Hyperthermia inhibits the repair of DNA double-strand breaks induced by ionizing radiation as determined by pulsed-field gel electrophoresis. International Journal of Hyperthermia 1994; 10: 381–388
- Raaphorst GP. Recovery of sublethal radiation damage and its inhibition by hyperthermia in normal and transformed mouse cells. International Journal of Radiation Oncology, Biology &. Physics 1992; 22: 1035–1041
- Heller DP, Raaphorst GP. Low dose-rate irradiation of human glioma cells and thermoradiosensitization by mild hyperthermia. Radiation Oncology Investigations 1993; 1: 218–226
- Raaphorst GP, Mao JP, Ng CE. Sublethal radiation damage repair and its inhibition by hyperthermia in two human melanoma cell lines of different radiosensitivites. Melanoma Research 1994; 4: 157–161
- Raaphorst GP, Ng CE, Shahine B. Comparison of radiosensitization by 41°C hyperthermia during low dose rate irradiation and during pulsed simulated low dose rate irradiation in human glioma cells. International Journal of Radiation Oncology, Biology & Physics 1999; 44: 185–188
- Niedbala M, Ng CE, Raaphorst GP. Response to pulsed dose rate irradiation with and without mild hyperthermia using tumour and normal cell lines. International Journal of Hyperthermia 2001; 17: 539–544
- Raaphorst GP, Cybulski SE, Sobol R, Ng CE. The response of human breast tumour cell lines with altered polymerase β levels to cisplatin and radiation. Anticancer Research 2001; 21: 2079–2084
- Dikomey E, Jung H. Correlation between thermal radiosensitization and heat-induced loss of DNA polymerase beta activity in CHO cells. International Journal of Radiation Biology 1996; 63: 215–221
- Dikomey E, Jung H. Correlation between polymerase beta activity and thermal radiosensitization in Chinese hamster ovary cells. Recent Results in Cancer Research 1988; 109: 35–41
- Hall EJ. Radiobiology for the radiologist 4th. J. B. Lippincott Company, Philadelphia, PA 1994
- Mendenhall W, Beaver RJ. Introduction to probability and statistics 9th. Duxbury Press, Belmont, CA 1994
- Owen DG, McNamee JP, Raaphorst GP, Ng CE. Potentiation of cell killing by low dose rate radiation by camptothecin is related to an increase in the level of DNA double strand breaks. Radiation Research 2002; 157: 149–157
- Sugahara T, Saito M. Hyperthermic oncology. Taylor & Francis, London 1988; 1 and 2
- Wang Z, Armour EP, Corry PM, Martinez A. Elimination of dose-rate effects by mild hyperthermia. International Journal of Radiation Oncology 1992; 24: 965–973
- Armour EP, McEachern D, Wang Z, Corry PM, Martinez A. Sensitivity of human cells to mild hyperthermia. Cancer Research 1993; 53: 2740–2744
- Raaphorst GP, Yang DP, Niedbala G. Is DNA polymerase β important in thermal radiosensitization?. International Journal of Hyperthermia 2004; 20: 140–143
- Olive PL. The role of DNA single- and double-strand breaks in cell killing by ionizing radiation. Radiation Research 1998; 150: S42–S51
- Abbott Howley HE, Raaphorst GP. Comparison of repair and rejoining fidelity between two isogenic human ovarian carcinoma cell lines. International Journal of Radiation Biology 2002; 78: 1095–1102
- Raaphorst GP, Yang DP, Ng CE. Effect of protracted mild hyperthermia on polymerase activity in a human melanoma cell line. International Journal of Hyperthermia 1994; 10: 827–834
- Winters TA, Russell PS, Kohli M, Dar ME, Neumann RD, Jorgensen TJ. Determination of human DNA polymerase utilization for the repair of a model ionizing radiation-induced DNA strand break lesion in a defined vector substrate. Nucleic Acids Research 1999; 27: 2423–2433
- Vens C, Dahmen-Mooren E, Verwijs-Janssen M, Blyweert W, Graversen L, Bartelink H, Begg AC. The role of DNA polymerase β in determining sensitivity to ionizing radiation in human tumor cells. Nucleic Acids Research 2002; 30: 2995–3004
- Frechet M, Canitrot Y, Bieth A, Dogliotti E, Cazaux C, Hoffmann J-S. Deregulated DNA polymerase β strengthens ionizing radiation-induced nucleotidic and chromosomal instabilities. Oncogene 2002; 21: 2320–2327
- Miura M, Watanabe H, Okochi K, Sasaki Tl, Shibuya H. Biological response to ionizing radiation in mouse embryo fibroblasts with a targeted disruption of the DNA polymerase β gene. Radiation Research 2000; 153: 773–780