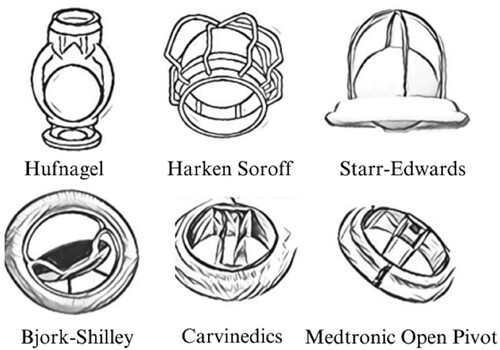
Valvular heart disease refers to any cardiovascular condition that affects one or more of the heart’s four valves: the aortic and mitral valves on the left side of the heart, and the pulmonic and tricuspid valves on the right side. While these conditions primarily develop as a result of aging, they can also be caused by congenital abnormalities, specific diseases, or physiological processes such as rheumatic heart disease and pregnancy. Surgical replacement of the faulty valve with prosthetic valves remains the preferred and most effective treatment for all types of VHD. In 2020, over 180,000 heart valve replacements were performed in the US alone [Citation1]. Charles Hufnagel is considered the pioneer in the design of prosthetic heart valves. The first Hufnagel heart valve was implanted in 1952 using a Lucite tube and methacrylate ball in the descending aorta. Over the past century, significant advancements have been made in the development of prosthetic heart valves, and continuing research is dedicated to engineering optimal designs. [Citation2] ().
The prosthetic heart valve comprises three components: the valve ring, the valve leaf, and the sewing ring (). The valve ring and leaf are typically made of titanium, 316L stainless steel (SS) or cobalt-chromium (Co-Cr) alloys, low-temperature isotropic pyrolytic carbon, or expanded polytetrafluoroethylene (ePTFE) or polyethylene terephthalate (PET) [Citation3].
Figure 2. Carbomedics valve. Valve ring and leaf made of Si-doped pyrolytic carbon [Citation4].
![Figure 2. Carbomedics valve. Valve ring and leaf made of Si-doped pyrolytic carbon [Citation4].](/cms/asset/3d37554b-a5e8-4673-9266-fa889e0585a2/ysue_a_2238971_f0002_oc.jpg)
While progressive designs of prosthetic heart valves have improved haemodynamic properties, the introduction of a foreign object into the human body comes with its own set of complications [Citation5]. The common problems include thrombosis, haemorrhage related to anticoagulant use, infections, valve failure, tissue hyperplasia, and overgrowth. Thrombogenicity or clot formation on the surfaces of the internal prosthesis is triggered by the adhesion and activation of platelets on them. This in turn is guided by the protein layer, especially human plasma fibrinogen (HPF). Inflammatory reactions such as restenosis and calcification are also caused by the release of toxic ions from the metals or alloys and the degradation of polymeric components of the artificial valves.
A promising strategy to limit thrombogenicity is to modulate HPF behaviour at the blood-material interface by altering the physicochemical properties of the valve’s (or any prosthetic device’s) surface. Surface modifications aim to optimize various aspects of blood-material interactions, including protein adsorption, thrombin generation and blood coagulation, platelet adhesion, aggregation and activation, and cellular behaviour at the prosthesis surface [Citation6].
A recent study showed the relationship between surface crystallographic structure and platelet adhesion. Valve rings are often made of titanium or pyrolytic carbon, the surface of which is often engineered to have a layer of titanium oxide [Citation7]. The rutile crystallographic structure typically has three low-index (110), (100), and (101) facets. HPF has been reported to unfold into a trinodular form on the hydrophobic (110) facet and has a globular conformation in the more hydrophilic (001) facet [Citation8]. Such conformational changes result in altered platelet adhesion in the two phases. As seen in , the hydrophilic (001) phase has a higher distribution of platelets, and therefore presents a higher risk of thrombogenicity [Citation9].
Figure 3. Platelet adhesion on the different phases after 120 minutes under static conditions. Image reproduced without modification from [Citation9].
![Figure 3. Platelet adhesion on the different phases after 120 minutes under static conditions. Image reproduced without modification from [Citation9].](/cms/asset/f82755e5-7ab3-4adc-9b6c-e51a3dd752f1/ysue_a_2238971_f0003_ob.jpg)
Two approaches have been reported for the surface modification of heart valves – the application of surface coatings, and the patterning of the valve surface.
Early approaches to surface modification involved applying a bioinert coating that acts as a physical barrier between the valve and the biomedium (blood). Various carbon coatings, including diamond-like carbon, have been utilized to enhance the biocompatibility and hemocompatibility of implants [Citation10]. Studies have shown that hydrogen-free DLC (diamond-like carbon) coatings with a higher bonding ratio of sp3/sp2 exhibit improved blood compatibility[Citation11].
The use of ultrananocrystalline diamond (UNCD) coatings avoids graphitization and film delamination in pyrolytic carbon-based mechanical heart valves. UNCD also results in minimum thrombin formation, in comparison to pyrolytic carbon alone, boron-doped UNCD, microcrystalline diamond, and silicon carbide films, represented as Pyc, BD-UNCD, MCD, and SiC, respectively, in .
Figure 4. Thrombin generation of fresh human platelets after a 1-hour exposure. Image reproduced without modification from [Citation12].
![Figure 4. Thrombin generation of fresh human platelets after a 1-hour exposure. Image reproduced without modification from [Citation12].](/cms/asset/dcc33f5f-b875-4e42-978d-8ccf1d93d6f1/ysue_a_2238971_f0004_oc.jpg)
Ceramic coatings such as TiO2 and TiN have also been studied because of their biocompatibility and the possibility of conferring high hardness, good wear resistance, and chemical stability to the valve [Citation13], [Citation14]. Studies are also continuing for the development of polymer-based coatings for heart valves. PEG-modified surfaces have been shown to have good hemocompatibility, and adhesion resistance to fibrinogen, platelets, and leukocytes [Citation15]. Despite the promise, in vivo and clinical outcomes have been disappointing for polymer-based coatings because of the poorer strengths of polymers compared to diamond or ceramic coatings.
As mentioned earlier, superhydrophobic surfaces can potentially reduce the risk of blood clotting associated with interactions between blood and materials. Ultra-Ever Dry, a commercially available superhydrophobic coating was applied to a Jude Medical™ standard pyrolytic carbon bileaflet mechanical heart valve (). The coating exhibited a remarkable ability to significantly decrease the adhesion of blood cells in a static setting compared to the uncoated pyrolytic carbon surface. This reduction in blood cell adhesion indicates a lower risk of thrombosis associated with blood-material interactions.
Figure 5. Blood droplet falling/bouncing on a pyrolytic carbon (PyC) leaflet (top) and a PyC leaflet with a hierarchical coating (bottom) from a height of 10 mm. Image reproduced without modification from [Citation16].
![Figure 5. Blood droplet falling/bouncing on a pyrolytic carbon (PyC) leaflet (top) and a PyC leaflet with a hierarchical coating (bottom) from a height of 10 mm. Image reproduced without modification from [Citation16].](/cms/asset/50628085-e816-43d3-a695-9081a4b9c196/ysue_a_2238971_f0005_ob.jpg)
As the artificial valve is a foreign object within the body, surface biofunctionalization, which involves incorporating biologically active or inactive molecules to create biomimetic surfaces, has also been widely researched [Citation17]. By biofunctionalization, the blood's defense constituents can recognize the surface as a friendly environment, leading to appropriate responses. As early as 1986, Y. Ishikawa successfully immobilized albumin onto polyethylene (PE) as a modified coating and showed that it prevented the aggregation of platelets [Citation18]. A study conducted by the University of Liege introduced a polyethylene glycol (PEG) nanogel coating for heart valves. The coating was loaded with various anti-platelet and anti-coagulant agents, and the researchers went a step further by incorporating antibiotics into the coating to provide antibacterial properties [Citation19].
Researchers from the University of California at Los Angeles and the University of Michigan reported an out-of-the-box approach to creating long-lasting implants [Citation20]. Their approach involved coating the implants with a catalyst that utilizes the patient's own blood to produce an anticoagulant called nitroxyl. The coating consists of catalysts attached to fragments of graphene, which enables the catalysts to efficiently convert glucose and an amino acid called L-arginine in the patient's blood to nitroxyl. This anticoagulant prevents clot formation around the implant.
Surface micro- and nanopatterning have also been studied to enhance the functionality of valves and other prosthetic devices [Citation21]. While the surfaces of natural heart valve cusps or leaflets may appear smooth to the naked eye, they are made of intricate nanostructures that influence blood flow properties and prevent clotting. This structure likely comes from the layered nature of the heart valve walls ((c)) [Citation17]. Researchers have developed models of these microstructures and conducted theoretical analyses to determine the optimal geometric parameters for creating rough surfaces on artificial valve cusps or leaflets ((a,b) [Citation22].
Figure 6. (a) SEM image of the micro-structured cobblestone surface structure of rabbit valve and (b) magnified image of a single cobblestone. Image reproduced without modification from [Citation22] (c) Detailed heart valve structure. Image reproduced without modification from [Citation17].
![Figure 6. (a) SEM image of the micro-structured cobblestone surface structure of rabbit valve and (b) magnified image of a single cobblestone. Image reproduced without modification from [Citation22] (c) Detailed heart valve structure. Image reproduced without modification from [Citation17].](/cms/asset/fd5b303e-5e55-4a0a-9936-10572681d50f/ysue_a_2238971_f0006_oc.jpg)
Ultrashort laser pulses have been employed to create nanotextures on pyrolytic carbon occluders to achieve superhydrophobicity. The occluders were irradiated using an ultra-short pulse laser, resulting in the creation of four distinct nanotextures, all of which exhibited superhydrophobicity (), and therefore, presumably decreased cell adherence to the occluder surfaces [Citation23].
Figure 7. The four surface textures formed on occluders subjected to ultra-short pulse laser and their water contact angles. Image adapted from [Citation23].
![Figure 7. The four surface textures formed on occluders subjected to ultra-short pulse laser and their water contact angles. Image adapted from [Citation23].](/cms/asset/6ae345bf-b383-40a0-a2a9-7f3e363e1d40/ysue_a_2238971_f0007_ob.jpg)
Apart from nanotexturing, the valve ring and leaf are often polished to achieve a mirror-like surface, which minimizes wear; wear of the metal valves can release small amounts of the metal or metal ions, which can trigger inflammatory reactions by the body. Achieving a mirror finish on the valve ring through manual polishing is time-consuming, typically taking approximately 8 to 12 hours. Chemical polishing, also referred to as ion diffusion polishing, produces a passive film on a metal surface that allows the passage of anions to the anode surface solely through diffusion. This film tends to be thicker over micro-recesses and thinner over micro-asperities, resulting in varying rates of diffusion. This creates a smooth and polished metal surface [Citation24]. Such polished surfaces have been reported to experience less wear than rough surfaces. Obviously, this is contradictory to the other methods that induce surface patterning, and therefore, more extensive studies are needed to optimize the surface properties of the valve components.
Bioprosthetic valves are made from porcine valves or bovine pericardium. Recent research has investigated ways to modify the surface of the bioprosthetic tissues to promote the regrowth of endothelial cells and prevent unwanted interactions between blood and foreign tissue. One approach involved photopolymerizing a combination of polyethylene glycol diacrylate (PEGDA) and collagen type I on the surface of the bioprosthetic valve. This was followed by the addition of an amine-rich peptide to provide reactive sites on the surface of the model [Citation25]. The mechanical properties of the surface closely resembled those of actual bioprosthetic valve tissue.
The field of synthetic heart valves has made significant progress in areas such as machining, surface design, surgical techniques, and peri-implant considerations, reaching a state of both art and science. The success of implantation depends on various factors, including surface properties at the micro or nanometer scale, hydrophilicity, biochemical bonding, and more. Surface modification plays a critical role in improving these aspects, encompassing the topographical structure, chemical composition, surface energy, hydrophilicity, and hydrophobicity of materials. However, it is essential to first comprehend the surface characteristics of natural, well-functioning valves. The ability to also use newer additive manufacturing and precisely controlled polishing techniques enhance the possibilities for designer materials and coatings. Moving forward, exploring and integrating diverse surface modification approaches, coupled with physiologically relevant testing, will prove to be crucial and invaluable. The goal is to create valves that ensure successful replacement and effectively reduce adverse inflammatory reactions. By achieving this, we can enhance patient outcomes and lay the groundwork for improved cardiac care in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Singh SK, Kachel M, Castillero E, et al. Polymeric prosthetic heart valves: a review of current technologies and future directions. Front Cardiovasc Med. Mar. 2023;10; doi:10.3389/fcvm.2023.1137827. Online.
- Gott VL, Alejo DE, Cameron DE. Mechanical heart valves: 50 years of evolution. Ann Thorac Surg. Dec. 2003;76(6):S2230–S2239. doi:10.1016/j.athoracsur.2003.09.002.
- Qi P, Maitz MF, Huang N. Surface modification of cardiovascular materials and implants. Surf Coat Technol. Oct. 2013;233:80–90. doi:10.1016/j.surfcoat.2013.02.008.
- LivaNova. Carbomedics family. https://www.vingmed.dk/wp-content/uploads/sites/3/2019/03/Carbomedic-Top-Hat.pdf. [Online].
- Mathew P, Kanmanthareddy A. Prosthetic heart valve. StatPearls: https://www.ncbi.nlm.nih.gov/books/NBK536987/#, 2022. [Online].
- Bisaria H, Bhusan Patra B, Mohanty S. Surface modification during hydroxyapatite powder mixed electric discharge machining of metallic biomaterials: a review. Surf Eng. Sep. 2022;38(7–9):680–706. doi:10.1080/02670844.2022.2155406.
- Jackson MJ, Robinson GM, Ali N, et al. Surface engineering of artificial heart valve discs using nanostructured thin films deposited by chemical vapour deposition and sol-gel methods. J Med Eng Technol. Jan. 2006;30(5):323–329. doi:10.1080/03091900500441287.
- Struczyńska M, Firkowska-Boden I, Scheuer K, et al. Rutile facet-dependent fibrinogen conformation: why crystallographic orientation matters. Colloids Surf B Biointerfaces. Jul. 2022;215:112506. doi:10.1016/j.colsurfb.2022.112506. Online.
- Struczyńska M, Firkowska-Boden I, Levandovsky N, et al. How crystallographic orientation-induced fibrinogen conformation affects platelet adhesion and activation on TiO 2. Adv Healthc Mater. May 2023;12(13), doi:10.1002/adhm.202202508. [Online]
- Jozwik K, Karczemska A. The new generation Ti6Al4V artificial heart valve with nanocrystalline diamond coating on the ring and with Derlin disc after long-term mechanical fatigue examination. Diam Relat Mater. Apr. 2007;16(4–7):1004–1009. doi:10.1016/j.diamond.2006.12.051.
- Huang N, Yang P, Leng YX, et al. Surface modification for controlling the blood-materials interface. Key Eng Mater. Jun. 2005;288–289:295–298. doi:10.4028/www.scientific.net/KEM.288-289.295.
- Zeng H, Yin W, Catausan G, et al. Reprint of ‘ultrananocrystalline diamond integration with pyrolytic carbon components of mechanical heart valves,’. Diam Relat Mater. Mar. 2016;63:227–231. doi:10.1016/j.diamond.2016.01.018.
- Vera ML, Schuster J, Rosenberger MR, et al. Evaluation of the haemocompatibility of TiO2 coatings obtained by anodic oxidation of Ti-6Al-4 V. Proc Mater Sci. 2015;8:366–374. doi:10.1016/j.mspro.2015.04.086.
- Leng YX, Chen JY, Yang P, et al. The microstructure and mechanical properties of TiN and TiO2/TiN duplex films synthesized by plasma immersion ion implantation and deposition on artificial heart valve. Surf Coat Technol. Oct. 2006;201(3–4):1012–1016. doi:10.1016/j.surfcoat.2006.01.024.
- Hansson KM, Tosatti S, Isaksson J, et al. Whole blood coagulation on protein adsorption-resistant PEG and peptide functionalised PEG-coated titanium surfaces. Biomaterials. Mar. 2005;26(8):861–872. doi:10.1016/j.biomaterials.2004.03.036.
- Bark DL, Vahabi H, Bui H, et al. Hemodynamic performance and thrombogenic properties of a superhydrophobic bileaflet mechanical heart Valve. Ann Biomed Eng. Feb. 2017;45(2):452–463. doi:10.1007/s10439-016-1618-2.
- Yu W, Jiang Y, Lin F, et al. Surface biofunctionalization of tissue engineered for the development of biological heart valves: a review. Coatings. Sep. 2022;12(9):1322. doi:10.3390/coatings12091322. Online.
- Ishikawa Y, Sasakawa S, Takase M, et al. Effect of albumin immobilization by plasma polymerization on platelet reactivity. Thromb Res. Jul. 1984;35(2):193–202. doi:10.1016/0049-3848(84)90214-7.
- Liege Univeriste. “PV-COAT: A prosthetic valve with a bioactive surface coating to reduce the prevalence of thrombosis,” ERC CONSOLIDATOR GRANT: https://www.news.uliege.be/cms/c_10166645/en/pv-coat-a-prosthetic-valve-with-a-bioactive-surface-coating-to-reduce-the-prevalence-of-thrombosis, 2015. [Online].
- Xue T, Peng B, Xue M, et al. Integration of molecular and enzymatic catalysts on graphene for biomimetic generation of antithrombotic species. Nat Commun. Feb. 2014;5(1):3200. doi:10.1038/ncomms4200. Online.
- Jain A, Bajpai V. Alteration in Ti6Al4V implant surface properties with micro textures density. Surface Engineering. Feb. 2022;38(2):174–182. doi:10.1080/02670844.2022.2058163.
- Ye X, Bhushan B, Zhou M, et al. The surface microstructure of cusps and leaflets in rabbit and mouse heart valves. Beilstein J Nanotechnol. May 2014;5:622–629. doi:10.3762/bjnano.5.73.
- Vigano G, ten Brink GH, Groenendijk M, et al. Laser texturing of a St. Jude Medical RegentTM mechanical heart valve prosthesis: the proof of concept. Interact Cardiovasc Thorac Surg. Nov. 2021;33(6):986–991. doi:10.1093/icvts/ivab185.
- Liu H, Huang L, Leng Y, et al.. 2010. Surface finishing on the pivot orifice of an artificial heart valve ring. Plat Surf Finishing. 97(8):33–37.
- Fahrenholtz MM, Wen S, Grande-Allen KJ. Development of a heart valve model surface for optimization of surface modifications. Acta Biomater. Oct. 2015;26:64–71. doi:10.1016/j.actbio.2015.08.021.