‘Synthesis and mesomorphic properties of three-benzene-ring-containing banana-shaped liquid crystals’
published by Yuan Ming Huang and Qing-Lan Ma, Liquid Crystals 2010, 37, 1119–1126
by Wolfgang Weissflog,
Martin-Luther-Universität Halle-Wittenberg, Institut für Chemie, Physikalische Chemie, Halle, Germany
Banana-shaped liquid crystals are a topic in the field of liquid crystals; for some reviews see, for example, [Citation1–3]. Therefore, new relationships between the molecular structure and the mesophase behaviour are of high interest. Since the first report on banana-shaped liquid crystals in 1996 one of the basic questions has been ‘What is the size of the bent core?’ [Citation4]. Also ‘How many aromatic rings are necessary to form so-called banana phases?’ For a long time it seemed that at least five phenyl units were needed to form banana phases exhibiting spontaneous polarisation. In the meantime, different groups reported that bent-core four-ring compounds can also show mesophases exhibiting spontaneous or field-induced polarity [Citation5, Citation6].
1. Problem
According to the generally accepted structure–property relationships, simple bent-core three-ring compounds as reported in the paper under discussion [Citation7] should not be able to form any mesophase. This is a fundamental piece of knowledge derived from rod-like mesogens, which is correct despite the existence of banana-shaped liquid crystals.
Checking the paper with care, some other points are questionable. In Table 1 the transition temperatures are listed. All 12 homologues have the same ‘clearing temperatures’. Looking at the portion of the aliphatic chains for these molecules, which increases from 7% to 45% with the growing length of the terminal hydrocarbon chains, such constant behaviour is impossible. Furthermore, the transition temperatures and enthalpies are much too high for the target compounds. The textures shown in Figures 3 and 4 are by no means typical for any type of banana phases. It seems like a melting and crystallisation process, but nothing more. The X-ray pattern could give a hint of a kind of columnar structure but there is no evidence for a liquid crystalline phase. The broad scattering between 10° and 16° in 2θ cannot be assigned to a liquid-like lateral arrangement of the molecules (such a scattering should appear at 2θ ≈ 19.5° corresponding to d ≈ 0.45 nm) and it shows several maxima suggesting a three-dimensionally ordered arrangement with rather strong disorder, but no liquid crystal.
2. Experiments
To clarify the situation we have prepared the first and last members of the series under discussion using, however, another reaction pathway. A study of the new materials (compounds A and B) using nuclear magnetic resonance (NMR) spectroscopy (Varian Gemini 200) and elemental analysis together with calorimetric measurements (differential scanning calorimeter Pyris 1, Perkin Elmer) and polarising optical microscopy (NIKON, F-601 M equipped with a Linkam hot stage THM 600/S) gives the following results.
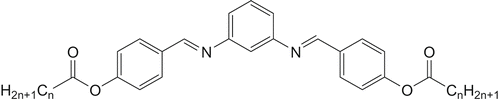
Compound A , n = 1: 0.5 mmol (540 mg) 1,3-phenylenediamine and 1.0 mmol (1.64 g) 4-acetyloxybenzaldehyde (Acros Organics) were refluxed in 30 ml methanol for 30 min without any catalyst. After cooling, the precipitate was separated and purified by several crystallisations from methanol. Yield: 1.12 g (56.0%).
Melting behaviour. Differential scanning calorimetry: heating, Cr 119.0 Is [ΔH = 38.48 kJ/mol]; cooling, formation of a glassy state near 70°C; polarising optical microscopy does not show a liquid crystalline phase on supercooling.
1H NMR (CDCl3) 200 MHz, δH 2.31 (6H, CH3, s), 7.01–7.09 (3H, Ar-H, m), 7.18–7.24 (4H, Ar-H, m), 7.39 (1H, Ar-H, t, J = 7.8 Hz), 7.92 (4H, Ar-H, d, J = 8.7 Hz), 8.47 (2H, CH=N, s).
Elemental analysis: C24H20N2O4, Mm 400.43; calc . C 71.99, H 5.03, N 7.00; found: C 71.69, H 4.87, N 6.84.
Compound B , n = 11: This homologue was synthesised by reaction of 1,3-phenylenediamine with 4-n-dodecanoyloxybenzaldehyde in the molar ratio 1:2. The substituted benzaldehyde was prepared from 4-hydroxybenzaldehyde and lauroylchloride in dichloromethane with triethylamine as catalyst according to [Citation8, Citation9]. The condensation of 0.25 mmol (270 mg) 1,3-phenylenediamine and 0.5 mmol (1.52 g) 4-n-dodecanoyloxybenzaldehyde was done in 50 ml ethanol using the same procedure as described for compound A. Ethanol was used for crystallisation. Yield: 1.08 g (63.4 %).
Melting behaviour. Differential scanning calorimetry: heating, Cr 90.7 Is [ΔH = 49.71 kJ/mol]; cooling, Is 83.6 Cr [ΔH = 49.50 kJ/mol]. On supercooling, polarising optical microscopy does not show any liquid crystalline behaviour up to 52°C.
1H NMR (CDCl3) 200 MHz δH 0.87 (6H, CH3, t, J = 6.4 Hz), 1.26–1.35 (32H, CH2, m), 1.68–1.79 (4H, OCH2CH2, m), 2.56 (4H, OCH2, t, J = 7.5 Hz), 7.02–7.09 (3H, Ar-H, m), 7.15–7.27 (4H, Ar-H, m), 7.39 (1H, Ar-H, t, J = 7.7 Hz), 7.86–7.94 (4H, Ar-H, m), 8.47 (2H, CH=N, s).
Elemental analysis: C44H60N2O4, Mm 680.97, calc . C 77.61, H 8.88, N 4.11; found C 77.47, H 8.73, N 4.07.
3. Results
The compounds reported in the paper published by Huang and Ma [Citation7] are not identical with the compounds claimed by the chemical structure in Figure 1. Looking again at the transition temperatures of the homologues n = 1–12 in Table 1, it seems to be one and the same material. Because the three-ring phenolic intermediate used by the authors exhibits a lower melting temperature at 164°C [Citation10], it cannot be excluded that dicyclohexylurea (melting point 228–232°C) was isolated in all cases which is formed during the reaction with dicyclohexylcarbodimide.
4. Conclusion
Simple bent-core molecules containing three benzene rings including a bending angle of about 120° in the central position are not able to form liquid crystalline phases. This fundamental fact which has been known for about 80 years is also true in the époque of banana-shaped liquid crystals. The situation is changed, however, if additional effects influence the molecular interactions. For example, microsegregation caused by terminal perfluoroalkyl chains [Citation11] or more than one alkyl chain at the terminal phenyl rings leading to bent-core phasmidic mesogens can result in smectic and columnar phases [Citation12–17]. The influence of hydrogen bonding should additionally be mentioned [Citation18, Citation19]. Furthermore, the fundamental conclusion is only true if the bending angle between both legs of the three-ring mesogens is near to 120°. Extending this angle to about 140° and more can also give liquid crystalline materials, as reported for bent-core three-ring molecules bearing heterocyclic five-membered fragments in the central position [Citation20–25].
References
- Pelzl , G. , Diele , S. and Weissflog , W. 1999 . Adv. Mater. , 11 : 707 – 724 .
- Amaranatha Reddy , R. and Tschierske , C. 2006 . J. Mater. Chem. , 16 : 907 – 961 .
- Takezoe , H. and Takanishi , Y. 2006 . Jap. J. Appl. Phys. , 45 : 597 – 625 .
- Niori , T. , Sekine , F. , Watanabe , J. , Furukawa , T. and Takezoe , H. 1996 . J. Mater. Chem. , 6 : 1231 – 1233 .
- Weissflog , W. , Dunemann , U. , Findeisen-Tandel , S. , Tamba , M.-G. , Kresse , H. , Pelzl , G. , Diele , S. , Baumeister , U. , Eremin , A. , Stern , S. and Stannarius , R. 2009 . Soft matter , 5 : 1840 – 1847 . and references therein
- Dep , R. , Nath , R.K. , Paul , M.K. , Rao , N.V.S. , Tuluri , F. , Shen , Y. , Shao , R. , Chen , D. , Zhu , C. , Smalyukh , I.I. and Clark , N.A. 2010 . J. Mater. Chem. , 20 : 7332 – 7336 .
- Huang , Y.M. and Ma , Q.L. 2010 . Liq. Cryst. , 37 : 1119 – 1126 .
- Koßmehl , G. and Bahr , J. 1991 . Z. Naturforsch. , 46b : 245 – 254 .
- Neubert , M.E. , Colby , C. , Ezenylilimba , M.C. , Jirousek , M.R. , Leonhardt , D. and Leung , K. 1988 . Mol. Cryst. Liq. Cryst. , 154 : 127 – 150 .
- Oswal , S.B. , Malek , N.I. and Pandya , A.K. 2009 . Int . J. Polym. Mat. , 58 : 202 – 216 .
- Kovalenko , L. and Weissflog , W. Unpublished results
- Kishikawa , K. , Furusawa , S. , Yamaki , T. , Kohmoto , S. , Yamamoto , M. and Yamaguchi , K. 2002 . J. Am. Chem. Soc. , 124 : 1597 – 1605 .
- Judele , R. , Laschat , S. , Baro , A. and Nimtz , M. 2006 . Tetrahedron , 62 : 9681 – 9687 .
- Kishikawa , K. , Oda , K. , Aikyo , S. and Kohmoto , S. 2007 . Angew. Chem. Int. Ed. , 46 : 764 – 768 .
- Shimura , H. , Yoshio , M. , Hamasaki , H. , Mukai , T. , Ohno , H. and Kato , T. 2009 . Adv. Mater. , 21 : 1591 – 1594 .
- Su , C. , Lee , L.X. , Yu , S.H. , Shih , Y.K. , Su , J.C. , Li , F.J. and Lai , C.K. 2004 . Liq. Cryst. , 31 : 745 – 749 .
- Huck , D.M. , Nguyen , H.L. , Donnio , B. and Bruce , D.W. 2004 . Liq. Cryst. , 31 : 503 – 507 .
- Kishikawa , K. , Nakahara , S. , Nishikawa , Y. , Kohmoto , S. and Yamamoto , M. 2005 . J. Am. Chem. Soc. , 127 : 2565 – 2571 .
- Malthete , J. , Levelut , A.M. and Liebert , L. 1992 . Adv. Mater. , 4 : 37 – 41 .
- Torgova , S.I. , Geivandova , G.A. , Francescangeli , O. and Strigazzi , A. 2003 . Pramana , 61 : 239 – 248 .
- Kang , S. , Saito , Y. , Watanabe , N. , Tokita , M. , Takanishi , Y. , Takezoe , H. and Watanabe , J. 2006 . J. Phys. Chem. B , 110 : 5205 – 5214 .
- Galardo , H. , Bortoluzzi , A.J. and de Oleveira Santos , D.M.P. 2008 . Liq. Cryst. , 35 : 719 – 725 .
- Wild , J.H. , Bartle , K. , Kirkman , N.T. , Kelly , S.M. , O´Neill , M. , Stirner , T. and Tuffin , R.P. 2005 . Chem. Mater. , 17 : 6354 – 6360 .
- Han , J. , Chang , X.Y. , Zhu , L.R. , Wang , Y.M. , Meng , J.B. , Lai , S.W. and Chui , S.Y. 2008 . Liq. Cryst. , 35 : 1379 – 1394 .
- Lee , C.H. and Yamamoto , T. 2001 . Mol. Cryst. Liq. Cryst. , 369 : 95 – 102 .