ABSTRACT
The optical properties of dyes dissolved in liquid crystals have led to their proposed use in a diverse range of practical applications. Such guest–host systems are typically required to fulfil a range of criteria relating to their absorption properties, degree of alignment and stability, but concurrently satisfying these requirements has proven a barrier to their widespread use. In this article, many of the proposed applications and their requirements are discussed, and an outline of some of the most prevalent classes of dye proposed in the context of guest–host systems is given, along with a summary of recent reports of dyes that exhibit thermotropic mesophases. Theoretical approaches to describing the alignment within guest–host systems are outlined, and possible strategies for the future rational design of guest–host systems are discussed.
Graphical Abstract
1. Introduction to guest–host systems
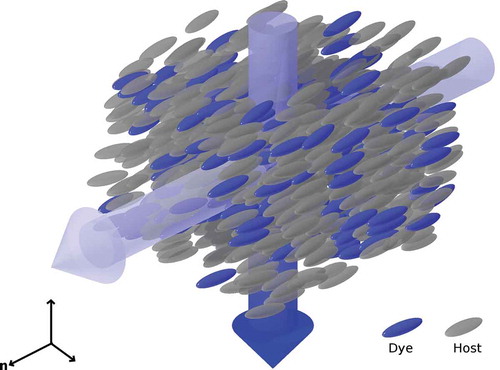
The anisotropic properties of a system comprising a guest dye dissolved in an aligned liquid-crystalline host were first reported in 1968,[Citation1] arising from the combination of the bulk liquid crystal host alignment and the anisotropic shape and absorption characteristics of the dye molecules. Such a system is shown schematically in . In this first report, the ability to switch the bulk absorption characteristics of a guest–host mixture with the application of an electric field was noted, and unsurprisingly, this ability to readily switch the bulk optical characteristics of a material led to immediate suggestions for such guest–host systems to be used in display applications.[Citation2,Citation3]
Figure 1. (colour online) Schematic diagram of an alignment guest–host system showing the absorbance of light perpendicular to the director, n, and the transmittance of light parallel to the director.
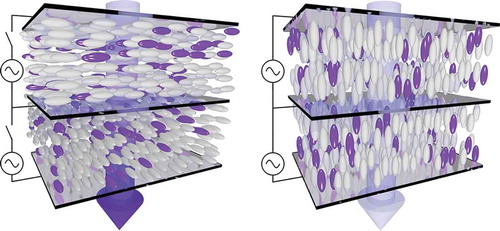
White and Taylor published the first report of the construction of such a device,[Citation4] and it was realised that these devices offer potential benefits over other modes of liquid crystal display, because the use of guest–host systems potentially negates the need for backlights and polarisers, offering bright, low-power displays.[Citation5] Subsequently, a variety of different modes of guest–host display device were proposed and tested,[Citation6,Citation7] such as the dual layer device shown in , and have been discussed in detail elsewhere.[Citation5]
Figure 2. (colour online) Schematic representation of a double layer guest–host display in the absorbing ‘off’ state (left) and in the transmitting ‘on’ state (right).
Aside from the obvious potential for use in display applications, the desirable optical properties exhibited by guest–host systems have resulted in their proposed use in a diverse range of other applications. The spontaneous ordering of guest–host systems, combined with their polarised absorption has led to suggestions for their use as precursors for high performance thin-film polarisers.[Citation8] The incorporation of dyes that undergo photochemical isomerisation into guest–host systems gives scope for their use in optical storage devices,[Citation9] as well as their use in optically controlled diffraction gratings that exhibit high efficiency and rapid response times,[Citation10] and use as active components for the photo-alignment of liquid crystals.[Citation11–Citation13] The use of multiple cells with a variety of surface alignment orientations containing guest–host mixtures can yield devices with complex polarised absorption properties, and such systems have been proposed for use in security devices.[Citation14] Electrically switched guest–host devices have also been suggested for use within applications outside of displays, such as in smart windows using polymer-dispersed liquid crystal hosts that enable the transmittance of the windows to be electrically controlled.[Citation15] Incorporating fluorescent dye molecules into these smart windows, and using the windows themselves as waveguides, potentially enables light absorbed by the windows in their absorbing state to be used for solar energy conversion.[Citation16] Combining the optical properties of chiral nematic liquid crystals with the absorption properties of guest dye molecules has also lead to electrically switched laser protection devices being developed.[Citation17]
One particular application that has been the subject of a significant amount of research in recent years is that of liquid crystal lasers.[Citation18] These systems utilise the wavelength-selective reflection observed in chiral nematic liquid crystals along with a fluorescent dye molecule as a guest, enabling lasing along the helical axis of the of the host. The very small size of these systems gives scope for arrays of lasers to be contained within a cell, providing higher outputs than individual discrete lasers,[Citation19] and the tuneable nature of the selective reflection of the chiral nematic host enables the lasing of the different individual discrete lasers to be tuned to different wavelengths.[Citation20] Such devices potentially offer the prospect of laser projection displays as well as lasers that are continuously tuneable across the full range of the visible spectrum. Incorporation of the lasing mixtures into emulsions has led to paintable lasing materials which may be applied to flexible substrates,[Citation21] and the inherent self-organising nature of the liquid crystals within these devices means such systems may be very cheap to produce, such as by inkjet printing,[Citation22] offering the prospect of disposable lasers.[Citation23]
Outside of the context of their use in devices and technologies, guest–host systems and the principles underlying their properties are of great relevance to many biological studies probing the structure, orientation and order in membrane systems through the use of polarised absorption and emission spectroscopy.[Citation24,Citation25] As well as utilising dyes to probe the nature of the orientational order in membranes, guest dyes may be used, for example, to probe properties such as electric potentials across cell membranes, which relies on a detailed understanding of the alignment of the dye molecules within these ordered systems.[Citation26,Citation27]
Despite being applied in very different contexts to the applications of guest–host liquid crystal systems described above, many of the underlying principles of molecular alignment and optical anisotropy within these systems are common to both areas of research. It is therefore important that the properties of anisotropic guest–host systems are well understood, but despite the attention they have received, there still remains a need to develop widely applicable quantitative structure–property relationships for these systems.
In this article, desirable properties of guest–host systems are outlined, before typical properties of guest dyes from different classes in liquid crystal hosts, as well phases exhibited by dyes themselves, are discussed. Current theoretical approaches concerning the alignment of dyes in liquid crystals are presented, before potential routes towards the rational design of guest–host systems are discussed.
2. Desirable features of guest–host systems for technological applications
From the work cited in the introductory section above, it is evident that guest–host systems have the potential for practical use in an extremely wide range of applications, but although the proposed uses are diverse, the guest–host systems themselves are typically required to exhibit very similar characteristics. Optical anisotropy is the key feature in common with all the applications outlined above, and thus a guest–host system must exhibit a strong absorbance, ideally at a wavelength of choice, as well as exhibiting a high degree of anisotropy as shown schematically in .
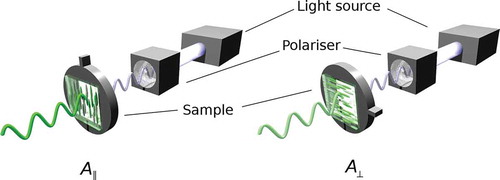
The optical characteristics of the dye(s) used are therefore critical, and it is a combination of the absorption coefficient and the concentration (often limited by the solubility) of a dye within a host that gives rise to the absorbance exhibited by a mixture. The optical anisotropy of a guest–host mixture is determined by the bulk alignment of the transition dipole moments (TDMs) of the dye molecules, itself arising from a combination of the alignment of the dye molecules within the host, and from the alignment of the TDMs within the dyes. This anisotropy is typically determined through the use of polarised UV–visible absorption spectroscopy, for which an experimental set-up for its determination is shown schematically in . In such an experiment, the absorbance of polarised light of a sample is measured with the director of the host-orientated parallel (A‖) and perpendicular (A⊥) to the electric vector of the incident polarised light, from which the dichroic ratio, R, may be determined as A‖/A⊥. The dichroic ratio may then be related to the dichroic order parameter, Sϕ, of the system according to Equation (1), which may take values between 1 for a system in which the TDMs are perfectly aligned with the director, and −0.5 for a system in which the TDMs lie perpendicular to the director. A system of randomly oriented TDMs will have a dichroic order parameter of 0.
Figure 3. (colour online) Schematic diagram of the experimental set-up for measuring the polarised absorbance of an aligned guest–host sample.
Ideally, the dichroic order parameter of a system will be as close as possible to 1, although measured order parameters are typically much lower. The minimum acceptable value of the order parameter is dependent on the specific application in question, but generally speaking, a system with a dichroic order parameter of <0.6 may be considered to have poor alignment, whereas a system with an order parameter >0.8 may be considered to be highly aligned. A threshold value of 0.75 has been proposed for certain modes of display device,[Citation28] but it is highly desirable for the optical anisotropy to be greater than this.
For many applications, it is also desirable for a guest–host mixture to exhibit a long useable lifetime in addition to the optical properties described above. Many of the proposed applications involve exposure of the systems to visible radiation (e.g. in displays, polarisers, smart windows and lasers) and thus the photochemical stability of a mixture is of paramount importance. Further, the electrical switching of guest–host systems (in displays and smart windows) and the presence of ionic dopants in some systems [Citation29,Citation30] mean that their electrochemical stability is also important. The stability of a dye molecule may be strongly dependent on its chemical environment, so the stability of a guest–host system may depend on the nature of the host mixture as well as that of the dye.
In the context of biological systems, the optimisation of properties such as dichroic alignment may not always be as critical as in other applications, but a detailed understanding of the behaviour of dye molecules within the oriented host is important for the interpretation of experimental data. Hence, the understanding of the features discussed in this section, particularly in terms of alignment, are relevant to the study of orientationally organised biological systems.
3. Dye classes in guest–host systems
3.1. Azo dyes
Much work has been reported on comparing the behaviour of different dye classes in guest–host mixtures, following the proposed use of the systems for display devices. Azo dyes containing the azo (–N=N–) linkage group are the largest class of commercial dyes, and they produce coloured compounds due to the electron delocalisation that arises from aromatic substituents appended to both ends of the azo group. Their widespread use in a range of applications is principally due to the ease and versatility of their synthesis, enabling a wide range of structures and resultant colours to be obtained, as well as due to the intense colours they exhibit.[Citation31] After having been used in the first report of the guest–host effect,[Citation1] azo dyes have subsequently been widely studied as candidates for use within guest–host systems. Their use has been largely due to the rod-like shape of the chromophore, which generally results in both good alignment of the molecules within a liquid–crystalline host and good alignment of the TDMs within the dyes.[Citation4,Citation32] The range of accessible synthetic modifications enables the optical properties of azo dyes to be tuned by using a variety of substituents. Early studies yielded azo dyes with a range of absorption maxima and colours, which exhibit order parameters of >0.7 in nematic hosts.[Citation7] Further work incorporating ester groups into the structures yielded azo dyes with order parameters of >0.8.[Citation33] As well as their good optical and alignment properties, the solubilities of azo dyes in liquid–crystalline hosts tend to be high,[Citation34,Citation35] enabling devices using azo compounds to exhibit intense colours and high-contrast ratios. However, despite the positive characteristics outlined here, azo dyes are generally considered to be impractical for applications in commercial devices due to their poor stability. The stability within the azo dye class has been shown generally to be better for red and yellow dyes than for blue and violet dyes, although there is still a tendency for all of the dyes to degrade over time.[Citation28,Citation32,Citation36,Citation37]
3.2. Anthraquinone dyes
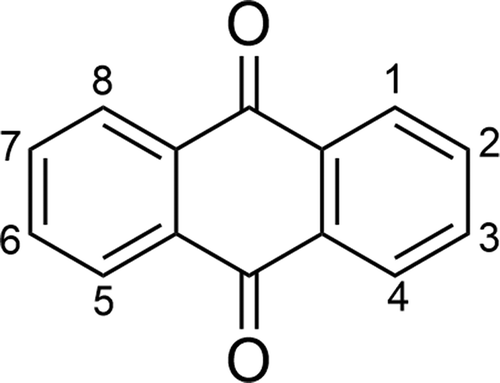
Anthraquinone dyes are another important class of commercial dyes, and they are based upon the fused ring chromophore shown in . A range of substituents may be used around the structure to obtain a full range of colours, although typically the colours of anthraquinone dyes are weaker than those of azo dyes.[Citation31] In the context of guest–host systems, anthraquinone dyes are attractive due to their stability. In particular, their light-fastness properties are generally superior to those of azo dyes,[Citation31] resulting in a significant amount of research into anthraquinone dyes in the context of liquid crystal applications.
The molecular structure of the anthraquinone chromophore does not lend itself as readily to creating rod-like structures as the azo chromophore does. This possible drawback is evident from order parameters in the region of 0.6 in nematic hosts obtained for anthraquinone dyes with a range of colours, obtained from using various hydroxyl and amine substituents in the 1-, 4-, 5- and 8-positions.[Citation28] Synthesis of anthraquinones with a wider range of amine-based substituent groups resulted in order parameters of up to 0.7 being achieved for compounds with a range of colours in nematic hosts.[Citation38] It was subsequently found that the use of sulphide substituents yielded materials exhibiting order parameters higher than 0.8 in nematic hosts in some cases.[Citation39] More rod-like anthraquinones have been investigated by adding substituents in the 2-, 3-, 6- and 7-positions, providing relatively high order parameters in nematic hosts, but the high degree of alignment displayed by these compounds is still not obtained as readily with as wide a range of colours as that obtained with azo dyes.[Citation39,Citation40]
The solubility of anthraquinone dyes in liquid crystal hosts has generally been found to be lower than that of azo dyes,[Citation34] especially in the case of sulphide-substituted anthraquinones.[Citation41] Greater solubility has been observed for asymmetric dyes compared with symmetrically substituted dyes, but at the cost of ease of synthesis.[Citation39,Citation42–Citation44]
3.3. Other dyes
Although studies of azo dyes and anthraquinone dyes dominate the literature relating to guest–host systems, particularly in the context of display applications, a significant body of work has also been carried out on the behaviour of other chromophores in liquid crystal hosts, albeit to a lesser extent. Examples of the typical properties of some of these different dye structures alongside those of azo and anthraquinone dyes are summarised in .
Table 1. Examples of structures from different dye classes proposed for use in guest–host systems, along with typical reported properties in nematic hosts.
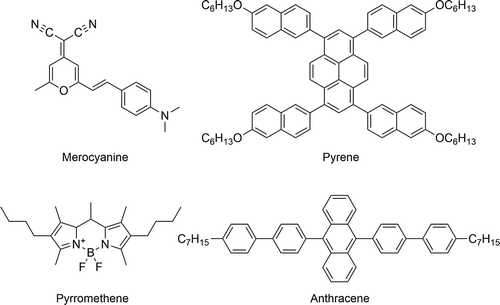
More recent work in the context of liquid crystal lasers has prompted further comparisons of dyes in the context of guest–host systems, but with slightly different requirements than those in displays. For laser applications, the order parameter of a dye is not as crucial to its practical use as it is for display applications, although the orientation of the TDM of a dye can affect the lasing wavelength,[Citation55] and the order parameter can determine the efficiency of the laser.[Citation56] For lasing to occur in a liquid crystal laser, a high molar absorption coefficient, a high quantum yield of emission and a high degree of stability and solubility are all important characteristics,[Citation55] and as such, studies have focused on different dye classes to those above, such as merocyanines,[Citation57] pyrenes,[Citation58] anthracenes [Citation55] and pyrromethenes.[Citation57] Some examples of structures from these dye classes are shown in .
4. Dyes as thermotropic liquid crystals
The formation of lyotropic liquid crystal phases from certain dye structures is well known and covered extensively in the field of chromonic liquid crystals,[Citation59,Citation60] but the formation of thermotropic liquid crystal phases from dye molecules has, until recently, been virtually unreported. Early reports on studies of tetrazine dyes identified liquid-crystalline phases exhibited by the dyes themselves,[Citation61,Citation62] and although devices tend not to utilise dyes alone as the anisotropic medium, the solubility of these compounds was found to be particularly high (10–20 mol%) in other nematic hosts.[Citation45]
Much more recently, there have been reports of dyes from other classes exhibiting mesophases themselves, including compounds based on the typically much more stable benzothiadiazole,[Citation63] anthraquinone [Citation64] and indigo [Citation65] chromophores, and examples of these are shown in . Such studies demonstrate the potential for a high degree of compatibility between guests and hosts, potentially providing a route to overcoming solubility difficulties such as those outlined for anthraquinones above, enabling mixtures exhibiting intense colours to be formulated, and enabling chromophores previously discounted for use in guest–host systems on solubility grounds to be considered.
5. Dye alignment models and theoretical approaches
As described above, optimisation of the dichroic order parameter of a guest–host system is critical when choosing guest and host species for use in many guest–host applications, and both the molecular alignment of the dye within the host and TDM alignment within the dye need to be optimised for a system to exhibit a high degree of optical anisotropy.
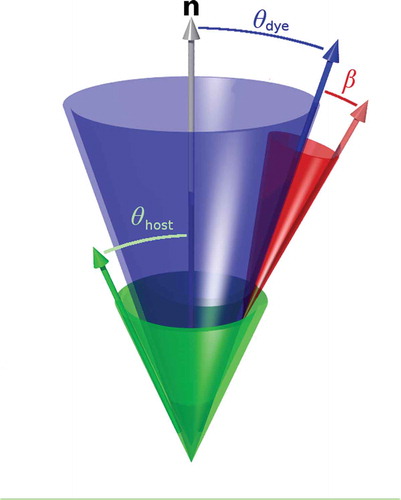
Quantitatively, these two contributions to the dichroic order parameter, Sϕ, of a guest–host system may be expressed by Equation (2), where Sθ is the order parameter of the long molecular axis of the dye within the host, and Sβ is the order parameter of the TDM within the dye.[Citation66] The angular brackets denote an ensemble average value of P2 (cos θ) values from which Sθ is derived, arising from a range of angles, θ, between the long molecular axes of the dyes and the host director. In contrast, whereas the TDM is typically assumed to be fixed at a single angle, β, against the long molecular axis of the dye.[Citation28] This relationship between the contributions to the order parameters arises from the closure relation of Wigner functions used to describe the translation of the molecular frame into the laboratory frame,[Citation67–Citation69] and Equation (2) only strictly holds for uniaxial molecules in a uniaxial phase; as such, any contributions from molecular and phase biaxiality are assumed to be zero. The relative orientations relating to this model are shown in . This model is valuable in terms of rationalising observed order parameters, for example, enabling the negative dichroism of some azo and anthraquinone dyes to be rationalised and providing estimates for values of β.[Citation70–Citation72]
Figure 7. (colour online) Schematic visualisation of the orientations of the host molecules (green) the dye molecules (blue) and the transition dipole moments of the dyes (red) in a guest–host system relative to the director, n.
The contributions from the molecular and TDM alignments may be related directly to the absorbance, A, of an aligned sample, discussed above, typically using Equation (3),[Citation28,Citation73–Citation75] where ψ is the angle between the host director and the electric vector of the polarised light, ε is the molar absorption coefficient of the dye and c is the concentration of dye in the sample of path length, l. However, this expression may be given in the much simpler and more intuitive form shown in Equation (4).
Equations (3) and (4) appear to provide an approach to determining the order parameter, Sϕ (=SθSβ), based on the cos2 ψ dependence of A, by measuring the absorbance of an aligned sample at many values of ψ and by fitting the resultant absorbances to a cos2 ψ function. This approach theoretically results in a more accurate order parameter than that obtained from Equation (1), for which just two absorbance values, measured at ψ = 0° and ψ = 90°, are used. Such an approach may be appropriate for a non-birefringent sample, but most liquid crystal hosts exhibit birefringence, and consequently the absorbance of an aligned sample may not simply vary as a function of cos2 ψ.[Citation76–Citation78] However, at ψ = 0° and ψ = 90°, the electric vector of the polarised light is aligned with one of the two optical axes of a birefringent sample, such that Equation (1) holds under these two conditions.
For a more thorough theoretical description of the contributions to the dichroic order parameter, a logical step is to incorporate terms arising from molecular biaxiality, as it is well established that some degree of molecular biaxiality is usually present even in uniaxial phases,[Citation79] and chromophores commonly comprise planar structures that may be expected to exhibit a greater degree of biaxiality than typically more rod-like host molecules. In the context of the cone model shown in , the additional terms result in an asymmetric distribution of the TDM (red) about the long molecular axes (blue).
If the Wigner functions arising from molecular biaxiality are considered, Equation (5) is obtained, where Sxx − Syy is the biaxial order parameter of the dye molecules,[Citation69,Citation79,Citation80] and α is the Euler angle describing the orientation of the TDM in the xy plane of the molecular frame. For rod-like dye structures that exhibit relatively high order parameters, it is immediately apparent that the term arising from molecular biaxiality need not be considered as the sin2 β term will be very small. However, in the case of dyes that exhibit negative dichroism, this term will be larger, and may be significant in terms of the overall order parameter.
Regardless of the specific assumptions imposed, splitting the dichroic order parameter into contributions from the molecular and TDM alignments is appealing from a theoretical standpoint as the two independent contributions may be considered separately.
6. Rational design of guest–host systems
The real barrier to the widespread practical application of dyes in liquid crystals appears to be the combination of the different properties that must be optimised concurrently. For example, some azo dyes may be considered to fulfil the desired absorption and optical anisotropy characteristics, but lack the stability required, whereas some anthraquinone dyes may fulfil the stability requirements, while falling short of the alignment or optical requirements.
In the context of alignment, an intuitive approach to dye design is simply to consider molecules of higher aspect ratio when improvements in alignment are required. Indeed, this is a common theme in the guest–host literature,[Citation75,Citation81,Citation82] and unsurprisingly guest–host systems comprising more rod-like dye structures do tend to exhibit higher order parameters than those using less rod-like dyes. However, elongation of dye structures can come at a cost, for example, in terms of switching times and solubility.[Citation82] Relatively recently, it has been shown experimentally that increasing the rod-like nature of a dye is not necessarily the only way to increase the order parameter. The incorporation of triptycene end groups on to an anthraquinone dye has been demonstrated to result in an increase in its dichroic order parameter,[Citation83] as shown in , and this effect is thought to be due to the ‘internal free volume’ of the substituent triptycene groups.
Figure 8. Two 1,5-diamine substituted anthraquinone dye structures and their order parameters in a nematic host.[Citation78].
![Figure 8. Two 1,5-diamine substituted anthraquinone dye structures and their order parameters in a nematic host.[Citation78].](/cms/asset/987a7d9d-81e6-4b86-b583-82b26d1f86c6/tlct_a_1189613_f0008_b.gif)
This example of the complex behaviour observed for just one of the many properties of dyes which must be optimised, suggests that rational-design approaches that enable ‘screening’ of candidate dyes, and/or guest–host systems as a whole, are highly desirable and may be preferable to more trial-and-error-based synthetic approaches.
The simulation of molecular alignment in anisotropic phases using molecular dynamics (MD) simulations has become increasingly accessible with ever increasing computational resources and the development and assessment of force fields for liquid crystal simulations.[Citation84,Citation85] There are now multiple reports of simulations of guest molecules within liquid crystal hosts, providing favourable comparisons with results of electron paramagnetic resonance [Citation86–Citation88] and nuclear magnetic resonance [Citation89,Citation90] experiments, as well as providing assessments of the validity of mean field models of molecular alignment.[Citation91]
The increase in computational resources has also made electronic structure calculations more accessible with time. Density functional theory (DFT) calculations on relatively large molecules are now routine, and time-dependent density functional theory (TD-DFT) calculations have been shown to predict the absorption properties of many dyes well. Some studies have reported calculations of dye TDM orientations in the context of liquid crystal applications,[Citation92–Citation95] and more recent studies in more diverse fields have also focussed on the orientations of electronic TDMs. For example, in the contexts of chromophores in organic light-emitting diodes,[Citation96] protein fluorescence,[Citation97] and energy transfer in chlorophyll,[Citation98] time-resolved spectroscopy has been combined with electronic structure calculations enabling assessment of the validity of the calculations.
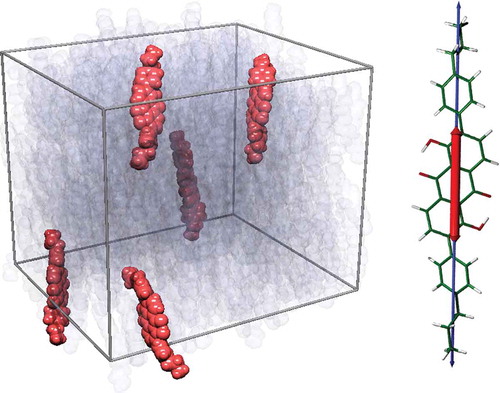
Combining the results of an MD simulation of anthraquinone dye molecules in a nematic host with those of TD-DFT calculations of the dye has enabled the calculation of a dichroic order parameter for a dye in a liquid crystal host without any direct input of experimental data, using Equation (2).[Citation99] A snapshot of the MD simulation is shown in . In this study, the high experimental dichroic order parameter was primarily attributed to the high degree of alignment between the TDM and the long axis of the dye, also shown in . Some equivalent studies have also been carried out for biological systems, combining MD simulations and electronic structure calculations to determine the orientation of probes in a phospholipid membrane,[Citation100,Citation101] and in the context of designing fluorescent probes to mimic the alignment behaviour of biological molecules.[Citation102]
Figure 9. (colour online) A snapshot of a liquid crystal guest–host MD simulation (left) showing the dye molecules in orange and the host molecules in translucent grey. The visible TDM (red) and long molecular axis (blue) are also shown overlaid on the DFT-optimised structure of the dye (right).
A further comparative study of five anthraquinone dyes proposed for use within guest–host systems has provided insights into structure–property relationships of the dyes.[Citation103] Variations in dye colour have been rationalised in terms of the electronic properties of the dye substituents, and reported trends in absorption anisotropy have been rationalised in terms of TDM orientations and molecular aspect ratios, determined from the results of DFT and TD-DFT calculations. Electronic structure calculations of oxidised and reduced forms of the dyes studied also enabled redox potentials of the dyes to be calculated, providing a good match with experimental values.[Citation103] Such calculations may be used to provide indications of the stability of compounds proposed for use within guest–host systems, again without direct input of experimental data.
In principle, this range of computational techniques enables such screening to be carried out, and may provide a method of targeting synthetic approaches towards likely candidate dye molecules for use within guest–host systems.
7. Conclusions
In the decades following the discovery of the optical anisotropy of systems comprising dyes dissolved in aligned liquid crystal hosts, their potential applications and relevance have been shown to extend well beyond the display applications with which they have typically been associated. Although the scope for these systems appears wide, the applications typically require similar characteristics to be displayed by the guest–host mixtures, suggesting that if current barriers may be overcome, a wide range of proposed devices may be realised.
Advances in synthetic chemistry have enabled increasingly exotic host and dye structures to become accessible, providing advances, for example, in synthesising multiple classes of thermotropic liquid crystal dyes. However, the range of currently accessible structures also highlights the need for quantitative design parameters and structure–property relationships to be developed in order for efficient synthetic studies to be carried out.
In principle, the selection of computational techniques outlined in the previous section enables a range of properties of guest–host systems including colour, alignment and stability to be predicted. The ever advancing capabilities of classical and quantum chemical calculations allied with synthetic advances provides an extremely promising combination of techniques to be used in the molecular and material design of guest–host systems for a wide range of applications. Although research into guest–host systems appears less prevalent in recent years than it was following the initial report on their properties, the recent proposals of so many diverse applications for these systems combined with this combination of current computational and synthetic methods provide an attractive area for further research.
Acknowledgements
I am grateful to Dr John Moore, Prof. John Goodby, Dr Laurence Abbott and Dr Stephen Cowling for their support, insight and useful discussions.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Heilmeier GH, Zanoni LA. Guest-host interactions in nematic liquid crystals. A new electro-optic effect. Appl Phys Lett. 1968;13:91–92. doi:10.1063/1.1652529.
- Sussman A. Electrooptic liquid crystal devices: principles and applications. IEEE Trans Parts Hybrids Packag. 1972;8:24–37. doi:10.1109/TPHP.1972.1136587.
- Gordon E, Anderson LK. New display technologies—an editorial viewpoint. Proc IEEE. 1973;61:807–813. doi:10.1109/PROC.1973.9169.
- White DL, Taylor GN. New absorptive mode reflective liquid-crystal display device. J Appl Phys. 1974;45:4718–4723. doi:10.1063/1.1663124.
- Raj D. Dichroic display technology potentials and limitations. Mater Chem Phys. 1996;43:204–211. doi:10.1016/0254-0584(95)01639-C.
- Cole HS, Kashnow RA. A new reflective dichroic liquid-crystal display device. Appl Phys Lett. 1977;30:619–621. doi:10.1063/1.89282.
- Uchida T, Seki H, Shishido C, et al. Bright dichroic guest-host LCDs without a polarizer. Proc SID. 1981;22:41–46.
- Peeters E, Lub J, Steenbakkers JAM, et al. High-contrast thin-film polarizers by photo-crosslinking of smectic guest-host systems. Adv Mater. 2006;18:2412–2417. doi:10.1002/adma.200600355.
- Lutfor MR, Hegde G, Kumar S, et al. Synthesis and characterization of bent-shaped azobenzene monomers: guest-host effects in liquid crystals with Azo dyes for optical image storage devices. Opt Mater. 2009;32:176–183. doi:10.1016/j.optmat.2009.07.006.
- De Sio L, Ricciardi L, Serak S, et al. Photo-sensitive liquid crystals for optically controlled diffraction gratings. J Mater Chem. 2012;22:6669–6673. doi:10.1039/c2jm16077c.
- Yaroshchuk O, Reznikov Y. Photoalignment of liquid crystals: basics and current trends. J Mater Chem. 2012;22:286–300. doi:10.1039/C1JM13485J.
- Guo Q, Srivastava AK, Chigrinov VG, et al. Polymer and Azo-dye composite: a photo-alignment layer for liquid crystals. Liq Cryst. 2014;41:1465–1472. doi:10.1080/02678292.2014.926404.
- Weng S-C, Fuh AY-G, Tang F-C, et al. Effect of surface condition on liquid crystal photoalignment by light-induced Azo dye adsorption phenomena. Liq Cryst20161–9. doi:10.1080/02678292.2016.1163740
- Carrasco-Vela C, Quintana X, Oton E, et al. Security devices based on liquid crystals doped with a colour dye. Opto-Electron Rev. 2011;19:496–500. doi:10.2478/s11772-011-0049-8.
- Kim M, Park KJ, Seok S, et al. Fabrication of microcapsules for dye-doped polymer-dispersed liquid crystal-based smart windows. ACS Appl Mater Interfaces. 2015;7:17904–17909. doi:10.1021/acsami.5b04496.
- Debije MG. Solar energy collectors with tunable transmission. Adv Funct Mater. 2010;20:1498–1502. doi:10.1002/adfm.200902403.
- Xie H, Wang L, Wang H, et al. Electrically tunable properties of wideband-absorptive and reflection-selective films based on multi-dichroic dye-doped cholesteric liquid crystals. Liq Cryst. 2015;42:1698–1705. doi:10.1080/02678292.2015.1055600.
- Coles HJ, Morris SM. Liquid-crystal lasers. Nat Photon. 2010;4:676–685. doi:10.1038/nphoton.2010.184.
- Hands PJW, Morris SM, Wilkinson TD, et al. Two-dimensional liquid crystal laser array. Opt Lett. 2008;33:515–517. doi:10.1364/OL.33.000515.
- Morris SM, Hands PJW, Findeisen-Tandel S, et al. Polychromatic liquid crystal laser arrays towards display applications. Opt Express. 2008;16:18827–18837. doi:10.1364/OE.16.018827.
- Hands PJW, Gardiner DJ, Morris SM, et al. Band-edge and random lasing in paintable liquid crystal emulsions. Appl Phys Lett. 2011;98:141102. doi:10.1063/1.3574915.
- Gardiner DJ, Hsiao WK, Morris SM, et al. Printed photonic arrays from self-organized chiral nematic liquid crystals. Soft Matter. 2012;8:9977–9980. doi:10.1039/c2sm26479j.
- Gardiner DJ, Morris SM, Hands PJW, et al. Paintable band-edge liquid crystal lasers. Opt Express. 2011;19:2432–2439. doi:10.1364/OE.19.002432.
- Klymchenko AS, Oncul S, Didier P, et al. Visualization of lipid domains in giant unilamellar vesicles using an environment-sensitive membrane probe based on 3-hydroxyflavone. Biochim Biophys Acta Biomembr. 2009;1788:495–499. doi:10.1016/j.bbamem.2008.10.019.
- Benninger RKP, Vanherberghen B, Young S, et al. Live cell linear dichroism imaging reveals extensive membrane ruffling within the docking structure of natural killer cell immune synapses. Biophys J. 2009;96:L13–L15. doi:10.1016/j.bpj.2008.10.005.
- Matson M, Carlsson N, Beke-Somfai T, et al. Spectral properties and orientation of voltage-sensitive dyes in lipid membranes. Langmuir. 2012;28:10808–10817. doi:10.1021/la301726w.
- Lambacher A, Fromherz P. Orientation of hemicyanine dye in lipid membrane measured by fluorescence interferometry on a silicon chip. J Phys Chem B. 2001;105:343–346. doi:10.1021/jp002843i.
- Bahadur B. In: Bahadur B, editor. Liquid crystals: applications and uses. Chapter 11, 3. Singapore: World Scientific Publishing Co. Pte. Ltd; 1992. p. 65–208.
- Gardiner DJ, Davenport CJ, Newton J, et al. Electro-optic bistability in organosiloxane bimesogenic liquid crystals. J Appl Phys. 2006;99:113517. doi:10.1063/1.2203391.
- Khosla S, Raina KK, Coles HJ. Electrically induced storage effects in smectic a phase of dyed low molar mass siloxane liquid crystals. Curr Appl Phys. 2003;3:135–140. doi:10.1016/S1567-1739(02)00191-8.
- Christie RM. Colour Chemistry. Cambridge: Royal Society of Chemistry; 2001.
- Seki H, Shishido C, Yasui S, et al. Dichroic azo dyes for guest-host liquid-crystal cell. Jpn J Appl Phys Part 1. 1982;21:191–192. doi:10.1143/JJAP.21.191.
- Matsui M, Okada S, Kadowaki M, et al. Synthesis of perfluorobutyl-substituted Ester-Disazo dyes and their application to guest-host liquid crystal displays. Liq Cryst. 2002;29:707–712. doi:10.1080/02678290210129920.
- Griffiths J, Feng K-C. The influence of intramolecular hydrogen bonding on the order parameter and photostability properties of dichroic azo dyes in a nematic liquid crystal host. J Mater Chem. 1999;9:2333–2338. doi:10.1039/a901936g.
- Palsson LO, Szablewski M, Roberts A, et al. Orientation and solvatochromism of dyes in liquid crystals. Mol Cryst Liq Cryst. 2003;402:279–289. doi:10.1080/744816685.
- Seki H, Uchida T, Shishido C. Light-stability of dichroic guest-host cells. Jpn J Appl Phys. 1980;19:L501–L503. doi:10.1143/JJAP.19.L501.
- Gray GW. Dyes and liquid crystals. Dyes Pigm. 1982;3:203–209. doi:10.1016/0143-7208(82)80023-5.
- Pellatt MG, Roe IHC, Constant J. Photostable anthraquinone pleochroic dyes. Mol Cryst Liq Cryst. 1980;59:299–316. doi:10.1080/00268948008071430.
- Saunders FC, Harrison KJ, Raynes EP, et al. New photostable anthraquinone dyes with high-order parameters. IEEE Trans Electron Devices. 1983;30:499–503. doi:10.1109/T-ED.1983.21156.
- Heppke G, Knippenberg B, Moller A, et al. Colored and black liquid-crystalline mixtures with new anthraquinone dyes. Mol Cryst Liq Cryst. 1983;94:191–204. doi:10.1080/00268948308084256.
- Naito K, Iwanaga H. Relation between molecular structures of dichroic dyes and their solubilities in fluorinated liquid crystals. Jpn J Appl Phys Part 1. 1998;37:3422–3427. doi:10.1143/JJAP.37.3422.
- Iwanaga H, Naito K, Nakai Y. The molecular structures and properties of anthraquinone-type dichroic dyes. Mol Cryst Liq Cryst. 2001;364:211–218. doi:10.1080/10587250108024989.
- Iwanaga H. Development of highly soluble anthraquinone dichroic dyes and their application to three-layer guest-host liquid crystal displays. Materials. 2009;2:1636–1661. doi:10.3390/ma2041636.
- Iwanaga H, Naito K. Highly soluble anthraquinone dyes with CF3-groups for guest-host liquid crystal displays. Jpn J Appl Phys Part 2. 1998;37:L356–L358. doi:10.1143/JJAP.37.L356.
- Pelzl G, Zaschke H, Demus D. Liquid-crystalline tetrazine compounds as dyes for guest-host displays with positive contrast. Displays. 1985;6:141–147. doi:10.1016/0141-9382(85)90081-2.
- Isenberg A, Krücke B, Pelzl G, et al. New liquid crystalline tetrazine derivatives for guest-host displays. Cryst Res Technol. 1983;18:1059–1068. doi:10.1002/crat.2170180814.
- Wolarz E, Moryson H, Bauman D. Dichroic fluorescent dyes for guest-host liquid-crystal displays. Displays. 1992;13:171–178. doi:10.1016/0141-9382(92)90027-O.
- Bauman D, Kuball HG. Uv-visible linear dichroism of naphthalene bicarboxylic acid-derivatives dissolved in nematic liquid-crystal. Chem Phys. 1993;176:221–231. doi:10.1016/0301-0104(93)85019-5.
- Martyński T, Mykowska E, Stolarski R, et al. Derivatives of 4-amino-n-ethylnaphthalimide for use in nematic liquid-crystals. Dyes Pigm. 1994;25:115–129. doi:10.1016/0143-7208(94)85043-7.
- Kumar S. Self-organization of disc-like molecules: chemical aspects. Chem Soc Rev. 2006;35:83–109. doi:10.1039/B506619K.
- Bauman D, Mykowska E, Wolarz E. Molecular orientation of perylene-like dyes in liquid crystal 8OCB. Mol Cryst Liq Cryst Sci Technol Sect. 1998;321:333–347. doi:10.1080/10587259808025100.
- Adamski A, Chrzumnicka E, Paluszkiewicz J, et al. Orientational order of tetra N-hexylesters of perylene and tetrachloroperylene tetracarboxylic acids in low-molar-mass liquid crystal investigated using absorption and fluorescence methods. Liq Cryst. 2014;41:768–775. doi:10.1080/02678292.2014.889232.
- Chen ZH, Swager TM. Synthesis and characterization of fluorescent acenequinones as dyes for guest-host liquid crystal displays. Org Lett. 2007;9:997–1000. doi:10.1021/ol062999m.
- Zhang X, Gorohmaru H, Kadowaki M, et al. Benzo-2,1,3-thiadiazole-based, highly dichroic fluorescent dyes for fluorescent host-guest liquid crystal displays. J Mater Chem. 2004;14:1901–1904. doi:10.1039/B402645D.
- Uchimura M, Watanabe Y, Araoka F, et al. Development of laser dyes to realize low threshold in dye-doped cholesteric liquid crystal lasers. Adv Mater. 2010;22:4473–4478. doi:10.1002/adma.201001046.
- Morris SM, Ford AD, Pivnenko MN, et al. Correlations between the performance characteristics of a liquid crystal laser and the macroscopic material properties. Phys Rev E. 2006;74:061709. doi:10.1103/PhysRevE.74.061709.
- Mowatt C, Morris SM, Song MH, et al. Comparison of the performance of photonic band-edge liquid crystal lasers using different dyes as the gain medium. J Appl Phys. 2010;107:043101. doi:10.1063/1.3284939.
- Yo W, Makoto U, Fumito A, et al. Extremely low threshold in a pyrene-doped distributed feedback cholesteric liquid crystal laser. Applied Physics Express. 2009;2:102501. doi:10.1143/APEX.2.102501.
- Lydon J. Chromonic review. J Mater Chem. 2010;20(10071–10099). doi:10.1039/b926374h.
- Lydon J. Chromonic liquid crystalline phases. Liq Cryst. 2011;38:1663–1681. doi:10.1080/02678292.2011.614720.
- Pelzl G, Schubert H, Zaschke H, et al. Field-induced colour change of liquid crystalline dyes. Krist Tech. 1979;14:817–823. doi:10.1002/crat.19790140708.
- Süsse M, Skubatz R, Demus D, et al. Synthese Und Kristallin-Flüssiges Verhalten Substituierter 1-Phenylpiperazine. J Prakt Chem. 1986;328:349–358. doi:10.1002/prac.19863280309.
- Gallardo H, Conte G, Tuzimoto PA, et al. New luminescent liquid crystals based on 2,1,3-benzothiadiazole and bent five-membered N-heterocyclic cores. Liq Cryst. 2012;39:1099–1111. doi:10.1080/02678292.2012.698313.
- Sj C, Ellis C, Jw G. Anthraquinone liquid crystal dichroic dyes – a new form of chromonic dye? Liq Cryst. 2011;38:1683–1698. doi:10.1080/02678292.2011.620181.
- Porada JH, Neudorfl J-M, Blunk D. Planar and distorted indigo as the core motif in novel chromophoric liquid crystals. New J Chem. 2015;39:8291–8301. doi:10.1039/C5NJ01594D.
- Rothschild KJ, Clark NA. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979;25:473–487. doi:10.1016/S0006-3495(79)85317-5.
- Van Gurp M. The use of rotation matrices in the mathematical-description of molecular orientations in polymers. Colloid Polym Sci. 1995;273:607–625. doi:10.1007/BF00652253.
- Zannoni C. In: Emsley JW, editor. Nuclear magnetic resonance of liquid crystals. Chapter 1. Dordrecht: D. Reidel Publishing Company; 1985. p. 1–34.
- Van Gurp M, Van Langen H, Van Ginkel G, Samori B, Thulstrup EW, editors. Polarized spectroscopy of ordered systems. Chapter 19. Dordrecht: Kluwer; 1987. p. 455–490.
- Matsui M, Sumiya Y, Shibata K, et al. Fluorine-containing negative dichroic 1,4-bis(acylamino) anthraquinone dyes. Liq Cryst. 1997;23:821–832. doi:10.1080/026782997207759.
- Ivashchenko AV, Petrova OS, Titov VV. Heteroaromatic azo dyes exhibiting negative dichroism in liquid-crystals. Mol Cryst Liq Cryst. 1984;108:51–60. doi:10.1080/00268948408072097.
- Imazeki S, Kaneko M, Ozawa T, et al. Anthraquinone dyes with negative dichroism. Mol Cryst Liq Cryst. 1988;159:219–231.
- Uchida T, Wada M. Guest-host type liquid-crystal displays. Mol Cryst Liq Cryst. 1981;63:19–43. doi:10.1080/00268948108071985.
- Toda K, Nagaura S, Watanabe T, et al. Polarization spectra of azo dyes in oriented nematic liquid-crystal. Nippon Kagaku Kaishi1975459–462. doi:10.1246/nikkashi.1975.459
- Seki H, Uchida T, Shibata Y. Dichroic dyes for guest-host liquid-crystal cells. Mol Cryst Liq Cryst. 1986;138:349–365. doi:10.1080/00268948608071769.
- Gregoriou VG, Tzavalas S, Bollas ST. Angular dependence in infrared linear dichroism: a reevaluation of the theory. Appl Spectrosc. 2004;58:655–661. doi:10.1366/000370204873024.
- Libowitzky E, Rossman GR. Principles of quantitative absorbance measurements in anisotropic crystals. Phys Chem Miner. 1996;23:319–327. doi:10.1007/BF00199497.
- Benedict JB, Freudenthal JH, Hollis E, et al. Orientational dependence of linear dichroism exemplified by dyed spherulites. J Am Chem Soc. 2008;130:10714–10719. doi:10.1021/ja802322t.
- Kiefer R, Baur G. Molecular biaxiality in nematic liquid crystals as studied by infrared dichroism. Mol Cryst Liq Cryst. 1989;174:101–126.
- Clark MG, Saunders FC. Asymmetric orientational distribution of an anthraquinone dye in nematic liquid-crystal hosts. Mol Cryst Liq Cryst. 1982;82:267–276. doi:10.1080/01406568208247016.
- Jones F, Reeve TJ. Orientation of dyes in liquid crystalline media. J Soc Dyers Colour. 1979;95:352–359. doi:10.1111/j.1478-4408.1979.tb03433.x.
- Bahadur B, Sarna RK, Bhide VG. Guest-host interaction of pleochroic dyes in liquid-crystal mixtures E8 and Pch-1132. Mol Cryst Liq Cryst. 1981;75:121–132. doi:10.1080/00268948108073608.
- Long TM, Swager TM. Using “internal free volume” to increase chromophore alignment. J Am Chem Soc. 2002;124:3826–3827. doi:10.1021/ja017499x.
- Tiberio G, Muccioli L, Berardi R, et al. Towards in silico liquid crystals realistic transition temperatures and physical properties for N-cyanobiphenyls via molecular dynamics simulations. Chem Phys Chem. 2009;10:125–136.
- Boyd NJ, Wilson MR. Optimization of the gaff force field to describe liquid crystal molecules: the path to a dramatic improvement in transition temperature predictions. Phys Chem Chem Phys. 2015;17:24851–24865. doi:10.1039/C5CP03702F.
- Oganesyan VS, Kuprusevicius E, Gopee H, et al. Electron paramagnetic resonance spectra simulation directly from molecular dynamics trajectories of a liquid crystal with a doped paramagnetic spin probe. Phys Rev Lett. 2009;102:013005. doi:10.1103/PhysRevLett.102.013005.
- Kuprusevicius E, Edge R, Gopee H, et al. Prediction of EPR spectra of liquid crystals with doped spin probes from fully atomistic molecular dynamics simulations: exploring molecular order and dynamics at the phase transition. Chem Eur J. 2010;16:11558–11562. doi:10.1002/chem.201001439.
- Chami F, Wilson MR, Oganesyan VS. Molecular dynamics and EPR spectroscopic studies of 8CB liquid crystal. Soft Matter. 2012;8:6823–6833. doi:10.1039/c2sm25429h.
- Weber ACJ, Pizzirusso A, Muccioli L, et al. Efficient analysis of highly complex nuclear magnetic resonance spectra of flexible solutes in ordered liquids by using molecular dynamics. J Chem Phys. 2012;136:174506. doi:10.1063/1.4705271.
- Pizzirusso A, Di Pietro ME, De Luca G, et al. Order and conformation of biphenyl in cyanobiphenyl liquid crystals: a combined atomistic molecular dynamics and 1H NMR study. Chem Phys Chem. 2014;15:1356–1367.
- Pizzirusso A, Di Cicco MB, Tiberio G, et al. Alignment of small organic solutes in a nematic solvent: the effect of electrostatic interactions. J Phys Chem B. 2012;116:3760–3771. doi:10.1021/jp3003799.
- Matsui M, Shirai K, Tanaka N, et al. Synthesis of tris-, tetrakis-, and pentakisazo dyes and their application to guest-host liquid crystal displays. J Mater Chem. 1999;9:2755–2763. doi:10.1039/a905852d.
- Matsui M, Tanaka N, Andoh N, et al. Synthesis and properties of novel dichroic Disazo dyes containing the tetrafluoro-P-phenylene moiety for guest-host liquid crystal displays. Chem Mater. 1998;10:1921–1930. doi:10.1021/cm980075q.
- Song DH, Kim JP. Effect of transition moments and orientational behavior of dichroic dyes on the optical anisotropy of poly(vinyl alcohol) polarizing films. Dyes Pigm. 2009;80:219–225. doi:10.1016/j.dyepig.2008.07.007.
- Matsui M, Suzuki M, Mizuno K, et al. Application of polyfluorophenazines to dichroic fluorescent dyes in guest-host liquid crystal displays. Liq Cryst. 2004;31:1463–1467. doi:10.1080/02678290412331293404.
- Steiner F, Bange S, Vogelsang J, et al. Spontaneous fluctuations of transition dipole moment orientation in oled triplet emitters. J Phys Chem Lett. 2015;6:999–1004. doi:10.1021/acs.jpclett.5b00180.
- Schmitt M, Krugler D, Bohm M, et al. A genetic algorithm based determination of the ground and excited state structure and the orientation of the transition dipole moment of benzimidazole. Phys Chem Chem Phys. 2006;8:228–235. doi:10.1039/B512686J.
- Linke M, Lauer A, Von Haimberger T, et al. Three-dimensional orientation of the qy electronic transition dipole moment within the chlorophyll a molecule determined by femtosecond polarization resolved vis pump−Ir probe spectroscopy. J Am Chem Soc. 2008;130:14904–14905. doi:10.1021/ja804096s.
- Mt S, Lc A, Sj C, et al. Dyes in liquid crystals: experimental and computational studies of a guest–host system based on a combined DFT and MD approach. Chem Eur J. 2015;21:10123–10130. doi:10.1002/chem.201406372.
- Timr Š, Bondar A, Cwiklik L, et al. Accurate determination of the orientational distribution of a fluorescent molecule in a phospholipid membrane. J Phys Chem B. 2013;118:855–863. doi:10.1021/jp4067026.
- Timr Š, Brabec J, Bondar A, et al. Nonlinear optical properties of fluorescent dyes allow for accurate determination of their molecular orientations in phospholipid membranes. J Phys Chem B. 2015;119:9706–9716. doi:10.1021/acs.jpcb.5b05123.
- Nåbo LJ, List NH, Witzke S, et al. Design of new fluorescent cholesterol and ergosterol analogs: insights from theory. Biochim Biophys Acta Biomembr. 2015;1848:2188–2199. doi:10.1016/j.bbamem.2015.04.018.
- Sims MT, Abbott LC, Cowling SJ, et al. Molecular design parameters of anthraquinone dyes for guest-host liquid-crystal applications: experimental and computational studies of spectroscopy, structure and stability. J Phys Chem C. Forthcoming 2016. doi:10.1021/acs.jpcc.6b03607