ABSTRACT
The liquid crystal dimers, the 1-(4-substitutedazobenzene-4′-yloxy)-4-(4-cyanobiphenyl-4′-yl)butanes (CB4OABX), are reported in which the terminal substituent is either a methyl, methoxy, butyl, butyloxy, cyano or nitro group. The butyloxy spacer endows these dimers with the required molecular curvature to exhibit the twist-bend nematic phase in addition to showing the conventional nematic phase. Their transitional properties are compared to those of the corresponding dimers with either a pentyloxy or hexyloxy spacer. As expected, the even-membered pentyloxy-based dimers show the highest nematic–isotropic transition temperature, TNI, and exhibit smectic behaviour. These observations are attributed to their linear molecular shapes. The values of both the twist-bend nematic–nematic transition temperature, TNTBN, and TNI increase on passing from the butyloxy to hexyloxy spacer, but the change in TNI is greater than that in TNTBN. Thus, the ratio TNTBN is greater for the shorter spacer reinforcing the view that molecular curvature drives the formation of the NTB phase relative to the N phase. By comparison, the melting point decreases on passing from the butyloxy to hexyloxy spacer. Thus, increasing molecular curvature simultaneously increases both the melting point and NTB phase stability and this highlights the design challenge in obtaining dimers that exhibit enantiotropic NTB–I transitions.
GRAPHICAL ABSTRACT
Introduction
The twist-bend nematic phase, NTB, is a fascinating liquid crystal phase in which achiral molecules spontaneously assemble into chiral arrangements and was the first example of spontaneous chiral symmetry breaking in a fluid with no spatial ordering [Citation1–4]. The NTB phase was predicted using symmetry arguments by Dozov [Citation5] and at the root of this prediction is the assertion that bent molecules have a strong tendency to pack into bent structures. Pure uniform bend, however, cannot fill space and must be accompanied by other local deformations of the director and specifically, either twist or splay. In the case of twist, this gives rise to the NTB phase in which the director forms a heliconical structure and is tilted with respect to the helical axis. The symmetry breaking is spontaneous such that left- and right-handed helices are degenerate and, hence, form in equal amounts. A key feature of the NTB phase is a surprisingly short pitch length, typically corresponding to just a few molecular lengths. This degeneracy is removed by molecular chirality, and the chiral twist-bend nematic phase is observed [Citation6,Citation7]. The NTB phase is normally formed on cooling a conventional nematic phase and only rarely is a direct NTB–isotropic phase transition observed [Citation8–13]. The NTB phase is not only of very significant fundamental interest but also has considerable application potential [Citation14–19]. Dozov also predicted the existence of twist-bend smectic phases and these have recently been found experimentally [Citation20–24].
A great many molecules have now been reported to show the NTB phase, and these include liquid crystal dimers (for recent examples, see references [Citation10,Citation21,Citation25–38]), higher oligomers [Citation27,Citation39–47], rigid bent core mesogens [Citation48,Citation49], hydrogen-bonded systems [Citation50–55] and polymers [Citation56]. The common structural feature to each of these structures, and in complete accord with Dozov’s prediction [Citation5], is molecular curvature. The importance of this was further underlined by predictions made using a Maier-Saupe theory for V-shaped molecules that the NTB-N transition temperature is particularly sensitive to the bend angle [Citation57]. Thus, for bend angles between 130° and 150° an NTB–N transition is observed, and for angles smaller than 130° the N phase disappears and a direct NTB–I transition observed. For angles larger than 150°, the NTB–N transition is predicted only to occur at very low temperatures.
In the vast majority of twist-bend nematogens, the required molecular curvature for the observation of the NTB phase is realised using odd-membered liquid crystal dimers [Citation58–74]. In a liquid crystal dimer, two mesogenic groups are linked through a flexible spacer, and if an odd number of atoms connects the two groups, then the molecule is, on average, bent [Citation75,Citation76]. The bend angle in such molecules depends on a number of factors including the nature of the links between the spacer and mesogenic units. The length of the spacer also governs the molecular curvature and as the spacer is increased in length, there is an increased number of conformations available to it and the liquid crystal field preferentially selects the more linear of these [Citation75]. This is apparent in the dependence of the N–I transition temperature, TNI, on spacer length for a homologous series of liquid crystal dimers. For short chain lengths, a pronounced alternation in TNI is observed in which even members exhibit the higher values, but this attenuates on increasing spacer length [Citation75]. Whereas an odd member is bent, in an even-membered dimer the two mesogenic units are essentially parallel and the molecule is linear. The linear even-members are more compatible with the nematic environment, and hence higher values of TNI are observed. As the spacer length is increased, the difference in average shape between odd and even members decreases and the values of TNI become similar.
In designing twist-bend nematogens based on odd-membered liquid crystal dimers, it would appear, therefore, that a short spacer would be the preferred choice in order to obtain a more pronounced molecular curvature. In practice, however, this has not been the case, and the overwhelming majority of twist-bend nematogens have spacer lengths of seven, nine or eleven atoms, and it is much less common to find examples containing three or five atoms (see recent review [Citation73]). The reasons for this are straightforward. In any given homologous series of dimers in which the length of the spacer is varied, the short odd-members tend to have high melting points and low liquid crystal transition temperatures, typically showing monotropic phase behaviour [Citation77,Citation78]. On increasing the spacer length, the melting points of the odd members tend to fall and the liquid crystal transition temperatures increase sharply before passing through a maximum and tending towards a limiting value [Citation75,Citation76].
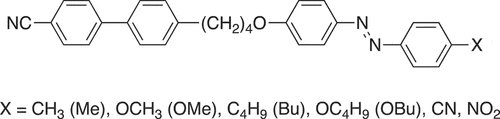
In order to obtain a better understanding of the role of short odd-membered spacers in the formation of the NTB phase, here we report a set of dimers based on an odd-membered spacer containing five atoms, namely a butyloxy spacer, the 1-(4-substitutedazobenzene-4′-yloxy)-4-(4-cyanobiphenyl-4′-yl)butanes, see . We refer to these dimers using the acronym CB4OABX in which CB denotes cyanobiphenyl, 4O the butyloxy spacer, AB azobenzene and X the terminal group. In order to establish the role played by molecular curvature, we also report the properties of the corresponding linear even-membered CB5OABX dimers based on a pentyloxy spacer and compare their properties with those of the longer odd-membered CB6OABX dimers reported previously [Citation79,Citation80].
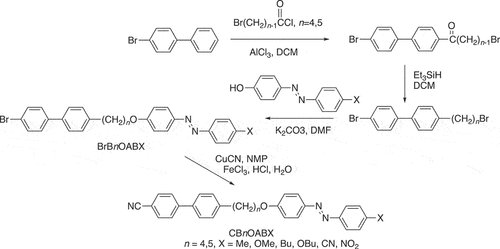
The CBnOABX dimers (n = 4,5,6) are prepared by the cyanation of the corresponding 1-(4-substitutedazobenzene-4′-yloxy)-ω-(4-bromobiphenyl-4′-yl)alkanes, see , and we also compare the transitional properties of these materials. By analogy, the acronym used to refer to these sets of dimers is BrBnOABX in which BrB denotes bromobiphenyl.
Experimental
Synthesis
The synthetic route used to obtain the CBnOABX dimers is shown in and is based upon that described for the preparation of the CB6OABX dimers [Citation79]. We note, however, that we have recently reported a convenient, one-pot synthetic method to obtain the ω-bromo-1-(4-cyanobiphenyl-4′-yl)alkanes [Citation81] that would significantly simplify the preparation of the CBnOABX dimers. The preparation of the 4-hydroxy-4′-substitutedazobenzenes has been described in detail elsewhere [Citation82–85]. The synthesis and characterisation of the CB4OABX and CB5OABX dimers and their intermediates are fully described in the ESI.
Thermal characterisation
The thermal behaviour of the materials was studied using differential scanning calorimetry (DSC) using a Mettler Toledo DSC1 equipped with a TSO 801RO sample robot and calibrated using indium and zinc standards. Heating and cooling rates were 10°C min−1, with a 3-minute isotherm between heating and cooling segments. Thermal data were extracted from the second heating trace unless otherwise stated. Samples were run in duplicate, and an average of the two measurements of temperature and change in entropy is reported. Phase characterisation was performed using polarised optical microscopy (POM), using either a Zeiss Axio Imager.A2m microscope equipped with a Linkam THMS600 heating stage or an Olympus BH2 polarising light microscope equipped with a Linkam TMS92 hot stage. Planar aligned cells with an ITO conducting layer and cell thickness of 2.9–3.5 μm were purchased from INSTEC.
Molecular modelling
The geometric parameters of the dimers studied were obtained using quantum mechanical DFT calculations with Gaussian 09 software [Citation86]. Optimisation of the molecular structures was carried out at the B3LYP/6-31 G(d) level of theory. Visualisations of the space-filling models were produced post-optimisation using the QuteMol package [Citation87].
Results and discussion
CB4OABX dimers
lists the transitional properties of the CB4OABX dimers. All six CB4OABX dimers exhibit nematic behaviour and all, but X = CN and NO2, also show the NTB phase. All nematic, N, phases were assigned on the basis of the observation of a schlieren texture containing both two and four brush point singularities and which flashed when subjected to mechanical stress, see . The values of the scaled nematic–isotropic entropy change, ∆SNI/R, listed in are consistent with this assignment [Citation88]. The transition from the nematic to the twist-bend nematic, NTB, phase was accompanied by the cessation of the flickering associated with director fluctuations and the formation of a somewhat ill-defined blocky schlieren texture, ). The monotropic nature of the NTB phases precluded their study using X-ray diffraction, and to confirm this assignment, a phase diagram was constructed using binary mixtures of CB4OABOMe and the standard twist-bend nematogen, CB7CB [Citation1], see . Complete miscibility was observed for the range of compositions studied, and all the mixtures exhibited two nematic phases, at higher temperatures the conventional N phase and at lower temperatures the NTB phase. These were identified on the basis of the observation of either a characteristic nematic schlieren texture or the blocky NTB schlieren texture, see . The value of TNTBN measured for CB4OABOMe fits perfectly the NTB–N line in the phase diagram and confirms the NTB phase assignment.
Figure 3. (Colour online) (a) The schlieren texture of the N phase for a sample sandwiched between untreated glass slides, (b) the uniform nematic texture and (c) the blocky schlieren texture of the NTB phase seen for CB4OABOMe in a cell treated for planar alignment.
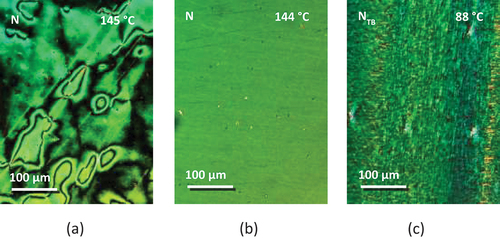
Figure 4. Phase diagram constructed for binary mixtures of CB4OABOMe and CB7CB. Triangles denote N–I transition temperatures and squares NTB–N transition temperatures. Melting points have been omitted for the sake of clarity.
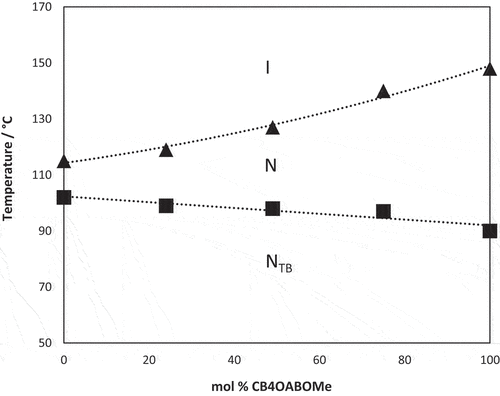
Figure 5. (Colour online) (a) The schlieren nematic texture and (b) the blocky schlieren texture of the NTB phase seen for a mixture of CB4OABOMe/CB7CB containing 75 mol % CB4OABOMe sandwiched between untreated glass slides.
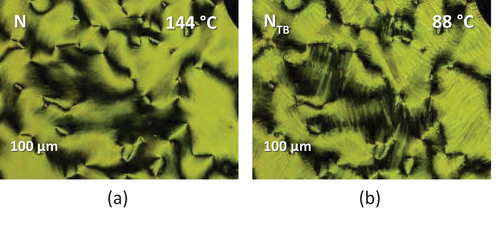
Table 1. The transition temperatures and associated scaled entropy changes of the CB4OABX dimers.
CB5OABX dimers
The transitional properties of the CB5OABX dimers are listed in . All six dimers are enantiotropic nematogens, and the nematic phase was identified using polarised light microscopy as described earlier; a representative texture is shown as . On cooling the nematic phase of CB5OABMe and CB5OABOBu, a focal conic fan texture developed, see , indicating the formation of a smectic phase. The monotropic nature of these smectic phases and their tendency to crystallise precluded their study using X-ray diffraction.
Figure 6. (Colour online) (a) The nematic texture and (b) the focal conic fan texture seen for CB5OABMe sandwiched between untreated glass slides.
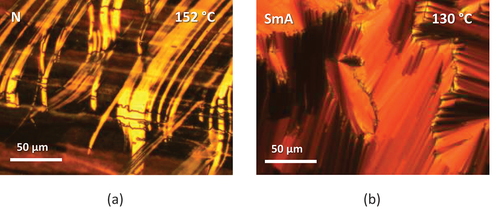
Table 2. The transitional properties of the CB5OABX dimers.
Comparison of the CBnOABX dimers
The melting points of the CBnOABX dimers are compared in . For any given X, the melting point is the lowest for the CB6OABX dimer with the exception of X = OMe for which CB6OABOMe melts 5°C higher than CB4OABOMe. This exception to the general trend may be attributed to the favourable mixed mesogenic unit that is facilitated by the longer spacer. It is clear that the even-membered dimers tend to have the highest melting points reflecting the greater ability of the more linear even-membered dimers to pack into a crystalline structure, see . The notable exception to this trend is the much higher melting point seen for CB4OABCN compared to CB5OABCN, but the physical significance of this observation is not clear. The different trends in the melting points for a given value of n on varying X may reflect, in part, the role played by the spacer in the packing of the molecules in order to maximise the interaction between the unlike mesogenic units and the ability to form intercalated arrangements [Citation90,Citation91]. It is noteworthy, however, that the dimer containing the butyl terminal group has the lowest melting point for each value of n, and this reflects both the flexibility of the butyl chain, and that it protrudes out of the plane of the phenyl ring to which it is attached [Citation92].
Figure 7. (Colour online) A comparison of the melting points of the CBnOABX dimers. The value of n is indicated on each bar, and the terminal substituents, X, are arranged in order of increasing van der Waals volume [Citation89].
![Figure 7. (Colour online) A comparison of the melting points of the CBnOABX dimers. The value of n is indicated on each bar, and the terminal substituents, X, are arranged in order of increasing van der Waals volume [Citation89].](/cms/asset/7e2175ec-58a5-42f0-a3dc-bd03800f1ae8/tlct_a_2198505_f0007_oc.jpg)
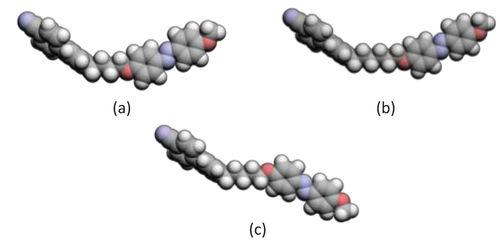
The values of TNI for the CBnOABX dimers show a more regular dependence on X () than do their melting points (). Specifically, the values of TNI shown by the CB5OABX dimers are higher than those of the corresponding odd-membered dimers, and this may be understood in terms of the linear shape adopted by even-membered dimers as described earlier and is shown in . The values of TNI shown by the dimers with n = 6 are greater than those of the corresponding dimers with n = 4. This reflects the rather general observation that within a homologous series of dimers in which the length of the spacer is varied, the clearing temperature of the odd members tends to pass through the maximum value on increasing spacer length, whereas those of the even members simply fall [Citation75]. This behaviour may be accounted for in terms of the average shapes of the dimers that may be visualised, to a first approximation, in terms of the all-trans conformation, and it is apparent that the shorter odd-membered spacer gives rise to a more pronounced molecular curvature, see . On increasing the length of the odd-membered spacer from n = 4 to n = 6, the enhanced flexibility allows the dimer to adopt more linear conformers, and this increases TNI. For long odd-membered spacers, the dilution of the mesogenic units offsets this effect and TNI falls. For a given value of n, the values of TNI on varying X may be understood in terms of how the terminal substituent changes the shape and polarisability of the mesogenic unit to which it is attached. As with the melting points, the lowest values of TNI are observed for the butyl substituent and again, this reflects that the butyl chain protrudes at an angle from the plane of the phenyl ring to which it is attached [Citation93–95].
Figure 9. (Colour online) A comparison of the nematic–isotropic transition temperatures of the CBnOABX dimers. The value of n is indicated on each bar, and the terminal substituents, X, are arranged in order of increasing van der Waals volume [Citation89].
![Figure 9. (Colour online) A comparison of the nematic–isotropic transition temperatures of the CBnOABX dimers. The value of n is indicated on each bar, and the terminal substituents, X, are arranged in order of increasing van der Waals volume [Citation89].](/cms/asset/402b6120-ef88-4f43-b968-ef14aac72914/tlct_a_2198505_f0009_oc.jpg)
The values of the scaled entropy change associated with the N–I transition, ∆SNI/R, are several times larger for the CB5OABX dimers than for the corresponding odd-membered dimers. This may be understood in terms of the conformational and orientational contributions to the total entropy change. Although in the previous discussion we noted that, to a first approximation, the alternation in the values of TNI on increasing n may be understood in terms of the change in shape as represented by the all-trans conformation, such an explanation does not account for the very much larger values of ∆SNI/R seen for even-membered dimers. Instead, we must remember that the spacer is flexible and that even-membered dimers have a greater number of conformations in which the two mesogenic units are more or less parallel, and these conformers are preferentially selected by the nematic environment. This gives rise to a greater conformational change at TNI for even-membered than odd-membered dimers. It has been estimated that this accounts for around 20% of the total entropy change seen for an even-membered dimer, whereas this conformational contribution is vanishingly small for an odd-membered dimer [Citation77]. The major contribution to the large difference in ∆SNI/R between odd- and even-membered dimers, however, arises from the alternation in the long-range orientational order [Citation77,Citation96]. The value of ∆SNI/R increases on passing from n = 4 to n = 6 for any given terminal substituent X, and this reflects the decrease in molecular biaxiality on increasing spacer length [Citation97,Citation98]. This reinforces the view that the shorter odd-membered dimers exhibit a more pronounced molecular curvature.
All six members of the CB6OABX dimers exhibit the NTB phase, whereas, as we have seen, just four members of the CB4OABX dimers do, with no NTB phase seen for X = CN and NO2, see . The CB4OABX dimers show lower values of TNTBN than their CB6OABX counterparts by around 12°C, a smaller reduction than seen for TNI of around 17°C. It should be noted that, if this average reduction in TNTBN is applied to the X = CN and NO2 dimers with n = 4, then the expected values of TNTBN are considerably lower than the lowest temperature to which their nematic phases could be cooled prior to crystallisation, 122°C and 111°C, respectively. It may appear counter-intuitive that the values of TNTBN are lower for the CB4OABX dimers than their more linear CB6OABX counterparts, . This indicates that the stability of the NTB phase is not simply associated with molecular curvature. Instead, the increase in molecular flexibility on increasing n facilitates a better interaction between mesogenic groups and this compensates for the loss of entropy due to the additional polar order in the NTB phase [Citation57], counteracting the reduction in molecular curvature, and the stability of the NTB phase increases. This also accounts for the observed increase in the value of TNI. It is noteworthy, however, that although the absolute values of TNTBN increase on moving from n = 4–6, the scaled temperature TNTBN/TNI decreases (), indicating that the stability of the NTB phase increases relative to that of the N phase as molecular curvature increases. As expected, the linear even-membered CB5OABX dimers do not exhibit the NTB phase but instead show smectic behaviour for X = Me and OBu, see Table 7. This reflects the greater ease of packing linear molecules into a lamellar phase.
BrBnOABX dimers
The BrB4OABX dimers did not show liquid crystallinity, and their melting points are listed in . These are higher than those of the corresponding BrB6OABX dimers [Citation79], and the differences range from 13°C for X = OMe to 35°C for X = CN. The BrB6OABX dimers with X = Me and NO2 also did not exhibit liquid crystalline behaviour. The remaining four members showed conventional nematic phases and their values of TNI were lower than those of the corresponding CB6OABX dimers by, on average, 32°C. This was attributed to the decrease in structural anisotropy on replacing a cyano group by a bromine atom [Citation99] and the increased tendency for cyanobiphenyl-based materials to associate in an antiparallel fashion further enhancing structural anisotropy [Citation100]. As we saw earlier, reducing n from 6 to 4 resulted in a decrease in TNI, and it would be reasonable to assume that the BrB4OABX dimers would show lower values of TNI than their BrB6OABX counterparts. This coupled with the higher melting points shown by the BrB4OABX dimers accounts for the absence of liquid crystallinity.
Table 3. The melting points and associated scaled entropy changes of the BrB4OABX dimers.
The transitional properties of the BrB5OABX series are listed in . All six dimers exhibited an enantiotropic nematic phase identified on the basis of the observation of a characteristic schlieren texture when viewed through the polarised light microscope and a representative texture is shown as . In addition, on cooling the nematic phase of BrB5OABCN, a fan-like texture developed, indicating the formation of a smectic phase, see . The rapid crystallisation of this phase precluded its study using X-ray diffraction, although the value of the entropy change associated with the transition strongly suggests a liquid-like smectic phase. The values of TNI are on average 22°C lower for the BrB5OABX dimers compared to those of the corresponding CB5OABX dimers, and this may again be attributed to the change in structural anisotropy and reduced tendency to self-organise in an anti-parallel fashion. A smectic phase is observed for BrB5OABCN, whereas only an N phase is seen for CB5OABCN and this may be cooled to much lower temperatures than the value of TSmN seen for BrB5OABCN. The physical significance of this observation is unclear. The values of TNI shown by the BrB5OABCN dimers are, on average, 85°C higher than those of the corresponding Br6OABCN dimers, and this may be attributed to the difference in shape between even and odd-membered dimers as discussed earlier.
Figure 10. (Colour online) (a) The schlieren nematic and (b) fan-like optical textures observed for a sample of BrB5OABCN sandwiched between untreated glass slides.
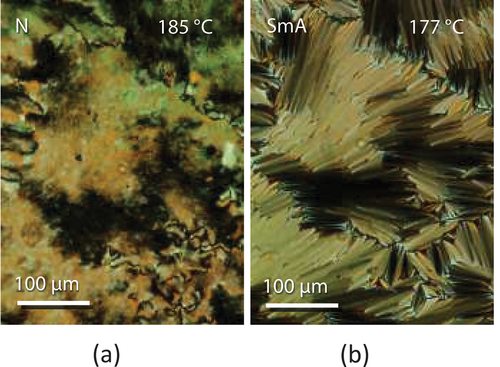
Table 4. The transition temperatures and associated scaled entropy changes for the BrB5OABX dimers.
Conclusions
The higher melting points seen for the CB4OABX dimers compared to those of the corresponding CB6OABX materials support the emerging observation that short odd-membered spacers, although promoting molecular bend, paradoxically appear to enhance packing efficiency in the crystal phase. This suggests that the reduction in the melting point on increasing spacer length is associated with the increase in molecular flexibility and, therefore, entropically driven. The values of both TNI and TNTBN are lower for the CB4OABX dimers than for the corresponding CB6OABX materials but the reduction is greater for TNI. Thus, where applicable, the scaled transition temperature, TNTBNI/TNI, is in fact higher for the dimer with the shorter spacer, indicating that direct NTB–isotropic transitions are more likely to be observed for short odd-membered spacers, as appears to be the case in the very limited number of examples observed to date [Citation8–11]. The challenge is now to design short, odd-membered dimers having low melting points in order to realise an enantiotropic NTB–I transition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cestari M, Diez-Berart S, Dunmur DA, et al. Phase behavior and properties of the liquid-crystal dimer 1′′,7′′-bis(4-cyanobiphenyl-4′-yl) heptane: a twist-bend nematic liquid crystal. Phys Rev E. 2011;84(3):031704.
- Borshch V, Kim YK, Xiang J, et al. Nematic twist-bend phase with nanoscale modulation of molecular orientation. Nature Commun. 2013;4(1):2635.
- Zhu CH, Tuchband MR, Young A, et al. Resonant carbon K-edge soft X-ray scattering from lattice-free heliconical molecular ordering: soft dilative elasticity of the twist-bend liquid crystal phase. Phys Rev Lett. 2016;116(14):147803.
- Dunmur D. Anatomy of a discovery: the twist–bend nematic phase. Crystals. 2022;12(3):309.
- Dozov I. On the spontaneous symmetry breaking in the mesophases of achiral banana-shaped molecules. Europhys Lett. 2001;56(2):247–253.
- Walker R, Pociecha D, Storey JMD, et al. The chiral twist-bend nematic phase (N*TB). Chem Eur J. 2019;25(58):13329–13335.
- Walker R, Pociecha D, Salamonczyk M, et al. Intrinsically chiral twist-bend nematogens: interplay of molecular and structural chirality in the N-TB phase. Chemphyschem. DOI:10.1002/cphc.202200807
- Dawood AA, Grossel MC, Luckhurst GR, et al. Twist-bend nematics, liquid crystal dimers, structure-property relations. Liq Cryst. 2017;44:106–126.
- Dawood AA, Grossel MC, Luckhurst GR, et al. On the twist-bend nematic phase formed directly from the isotropic phase. Liq Cryst. 2016;43(1):2–12.
- Arakawa Y, Komatsu K, Shiba T, et al. Methylene- and thioether-linked cyanobiphenyl-based liquid crystal dimers CBnSCB exhibiting room temperature twist-bend nematic phases and glasses. Mater Adv. 2021;2(5):1760–1773.
- Arakawa Y, Arai Y, Horita K, et al. Twist–bend nematic phase behavior of cyanobiphenyl-based dimers with propane, ethoxy, and ethylthio spacers. Crystals. 2022;12(12):1734.
- Wang DL, Liu J, Zhao WG, et al. Facile synthesis of liquid crystal dimers bridged with a phosphonic group. Chem Eur J. 2022;28(70). DOI:10.1002/chem.202202146
- Archbold CT, Davis EJ, Mandle RJ, et al. Chiral dopants and the twist-bend nematic phase – induction of novel mesomorphic behaviour in an apolar bimesogen. Soft Matter. 2015;11(38):7547–7557.
- Xiang J, Varanytsia A, Minkowski F, et al. Electrically tunable laser based on oblique heliconical cholesteric liquid crystal. Proc Nat Acad Sci USA. 2016;113(46):12925–12928.
- Xiang J, Li YN, Li Q, et al. Electrically tunable selective reflection of light from ultraviolet to visible and infrared by heliconical cholesterics. Adv Mater. 2015;27(19):3014–3018.
- Salili SM, Xiang J, Wang H, et al. Magnetically tunable selective reflection of light by heliconical cholesterics. Phys Rev E. 2016;94(4):042705.
- Aya S, Salamon P, Paterson DA, et al. Fast-and-giant photorheological effect in a liquid crystal dimer. Adv Mater Interfaces. 2019;6(9):1802032.
- Wang Y, Zheng ZG, Bisoyi HK, et al. Thermally reversible full color selective reflection in a self-organized helical superstructure enabled by a bent-core oligomesogen exhibiting a twist-bend nematic phase. Mater Horiz. 2016;3(5):442–446.
- Yuan CL, Huang WB, Zheng ZG, et al. Stimulated transformation of soft helix among helicoidal, heliconical, and their inverse helices. Sci Adv. 2019;5(10):eaax9501.
- Imrie CT, Walker R, Storey JMD, et al. Liquid crystal dimers and smectic phases from the intercalated to the twist-bend. Crystals. 2022;12(9):1245.
- Alshammari AF, Pociecha D, Walker R, et al. New patterns of twist-bend liquid crystal phase behaviour: the synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-10-(4-alkylaniline-benzylidene-4′-oxy)decanes (CB10O· m). Soft Matter. 2022;18(25):4679–4688.
- Pociecha D, Vaupotic N, Majewska M, et al. Photonic bandgap in achiral liquid crystals—a twist on a twist. Adv Mater. 2021;33(39):2103288.
- Salamonczyk M, Vaupotic N, Pociecha D, et al. Multi-level chirality in liquid crystals formed by achiral molecules. Nature Commun. 2019;10(1):1922.
- Abberley JP, Killah R, Walker R, et al. Heliconical smectic phases formed by achiral molecules. Nature Commun. 2018;9(1):228.
- Arakawa Y, Ishida Y, Komatsu K, et al. Thioether-linked benzylideneaniline-based twist-bend nematic liquid crystal dimers: insights into spacer lengths, mesogenic arm structures, and linkage types. Tetrahedron. 2021;95:132351.
- Cruickshank E, Anderson K, Storey JMD, et al. Helical phases assembled from achiral molecules: twist-bend nematic and helical filamentary B-4 phases formed by mesogenic dimers. J Molec Liq. 2022;346:118180.
- Arakawa Y, Komatsu K, Inui S, et al. Thioether-linked liquid crystal dimers and trimers: the twist-bend nematic phase. J Molec Struct. 2020;1199:126913.
- Arakawa Y, Komatsu K, Ishida Y, et al. Thioether-linked azobenzene-based liquid crystal dimers exhibiting the twist-bend nematic phase over a wide temperature range. Liq Cryst. 2021;48(5):641–652.
- Forsyth E, Paterson DA, Cruickshank E, et al. Liquid crystal dimers and the twist-bend nematic phase: on the role of spacers and terminal alkyl chains. J Molec Liq. 2020;320:114391.
- Strachan GJ, Harrison WTA, Storey JMD, et al. Understanding the remarkable difference in liquid crystal behaviour between secondary and tertiary amides: the synthesis and characterisation of new benzanilide-based liquid crystal dimers. Phys Chem Chem Phys. 2021;23(22):12600–12611.
- Arakawa Y, Komatsu K, Ishida Y, et al. Carbonyl- and thioether-linked cyanobiphenyl-based liquid crystal dimers exhibiting twist-bend nematic phases. Tetrahedron. 2021;81:131870.
- Walker R, Majewska M, Pociecha D, et al. Twist-bend nematic glasses: the synthesis and characterisation of pyrene-based nonsymmetric dimers. Chemphyschem. 2021;22(5):461–470.
- Pocock EE, Mandle RJ, Goodby JW. Experimental and computational study of a liquid crystalline dimesogen exhibiting nematic, twist-bend nematic, intercalated smectic, and soft crystalline mesophases. Molecules. 2021;26(3):532.
- Arakawa Y, Komatsu K, Feng J, et al. Distinct twist-bend nematic phase behaviors associated with the ester-linkage direction of thioether-linked liquid crystal dimers. Mater Adv. 2021;2(1):261–272.
- Knezevic A, Dokli I, Novak J, et al. Fluorinated twist-bend nematogens: the role of intermolecular interaction. Liq Cryst. 2021;48(5):756–766.
- Abberley JP, Walker R, Storey JMD, et al. Molecular structure and the twist-bend nematic phase: the role of terminal chains. Liq Cryst. 2020;47(8):1232–1245.
- Al-Janabi A, Mandle RJ. Utilising saturated hydrocarbon isosteres of para benzene in the design of twist-bend nematic liquid crystals. Chemphyschem. 2020;21(8):697–701.
- Arakawa Y, Ishida Y, Tsuji H. Ether- and thioether-linked naphthalene-based liquid-crystal dimers: influence of chalcogen linkage and mesogenic-arm symmetry on the incidence and stability of the twist–bend nematic phase. Chem Eur J. 2020;26(17):3767–3775.
- Majewska MM, Forsyth E, Pociecha D, et al. Controlling spontaneous chirality in achiral materials: liquid crystal oligomers and the heliconical twist-bend nematic phase. Chem Commun. 2022;58(34):5285–5288.
- Tuchband MR, Paterson DA, Salamonczykc M, et al. Distinct differences in the nanoscale behaviors of the twist–bend liquid crystal phase of a flexible linear trimer and homologous dimer. Proc Nat Acad Sci USA. 2019;116(22):10698–10704.
- Arakawa Y, Komatsu K, Ishida Y, et al. Thioether-linked liquid crystal trimers: odd–even effects of spacers and the influence of thioether bonds on phase behavior. Materials. 2022;15(5):1709.
- Arakawa Y, Komatsu K, Shiba T, et al. Phase behaviors of classic liquid crystal dimers and trimers: alternate induction of smectic and twist-bend nematic phases depending on spacer parity for liquid crystal trimers. J Molec Liq. 2021;326:115319.
- Al-Janabi A, Mandle RJ, Goodby JW. Isomeric trimesogens exhibiting modulated nematic mesophases. RSC Adv. 2017;7(75):47235–47242.
- Mandle RJ, Goodby JW. Progression from nano to macro science in soft matter systems: dimers to trimers and oligomers in twist-bend liquid crystals. RSC Adv. 2016;6(41):34885–34893.
- Mandle RJ, Goodby JW. A liquid crystalline oligomer exhibiting nematic and twist-bend nematic mesophases. Chemphyschem. 2016;17(7):967–970.
- Mandle RJ, Goodby JW. A nanohelicoidal nematic liquid crystal formed by a non-linear duplexed hexamer. Angew Chem-Int Ed. 2018;57(24):7096–7100.
- Simpson FP, Mandle RJ, Moore JN, et al. Investigating the Cusp between the nano-and macro-sciences in supermolecular liquid-crystalline twist-bend nematogens. J Mater Chem C. 2017;5(21):5102–5110.
- Chen D, Nakata M, Shao R, et al. Twist-bend heliconical chiral nematic liquid crystal phase of an achiral rigid bent-core mesogen. Phys Rev E. 2014;89(2):022506.
- Sreenilayam SP, Panov VP, Vij JK, et al. The N-TB phase in an achiral asymmetrical bent-core liquid crystal terminated with symmetric alkyl chains. Liq Cryst. 2017;44:244–253.
- Jansze SM, Martinez-Felipe A, Storey JMD, et al. A twist-bend nematic phase driven by hydrogen bonding. Angew Chem-Int Ed. 2015;54:643–646.
- Walker R, Pociecha D, Salamonczyk M, et al. Supramolecular liquid crystals exhibiting a chiral twist-bend nematic phase. Mater Adv. 2020;1(6):1622–1630.
- Walker R, Pociecha D, Crawford CA, et al. Hydrogen bonding and the design of twist-bend nematogens. J Molec Liq. 2020;303:112630.
- Walker R, Pociecha D, Martinez-Felipe A, et al. Twist-bend nematogenic supramolecular dimers and trimers formed by hydrogen bonding. Crystals. 2020;10(3):175.
- Walker R, Pociecha D, Abberley JP, et al. Spontaneous chirality through mixing achiral components: a twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem Commun. 2018;54(27):3383–3386.
- Walker R. The twist-bend phases: structure–property relationships, chirality and hydrogen-bonding. Liq Cryst Today. 2020;29(1):2–14.
- Stevenson WD, An JG, Zeng XB, et al. Twist-bend nematic phase in biphenylethane-based copolyethers. Soft Matter. 2018;14(16):3003–3011.
- Greco C, Luckhurst GR, Ferrarini A. Molecular geometry, twist-bend nematic phase and unconventional elasticity: a generalised Maier–Saupe theory. Soft Matter. 2014;10(46):9318–9323.
- Cruickshank E, Salamonczyk M, Pociecha D, et al. Sulfur-linked cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq Cryst. 2019;46(10):1595–1609.
- Paterson DA, Gao M, Kim YK, et al. Understanding the twist-bend nematic phase: the characterisation of 1-(4-cyanobiphenyl-4′-yloxy)-6-(4-cyanobiphenyl-4′-yl)hexane (CB6OCB) and comparison with CB7CB. Soft Matter. 2016;12(32):6827–6840.
- Arakawa Y, Tsuji H. Selenium-linked liquid crystal dimers for twist-bend nematogens. J Molec Liq. 2019;289:111097.
- Archbold CT, Andrews JL, Mandle RJ, et al. Effect of the linking unit on the twist-bend nematic phase in liquid crystal dimers: a comparative study of two homologous series of methylene- and ether-linked dimers. Liq Cryst. 2017;44:84–92.
- Mandle RJ, Archbold CT, Sarju JP, et al. The dependency of nematic and twist-bend mesophase formation on bend angle. Sci Rep. 2016;6(1):36682.
- Mandle RJ, Davis EJ, Voll CCA, et al. The relationship between molecular structure and the incidence of the N-TB phase. Liq Cryst. 2015;42:688–703.
- Mandle RJ, Goodby JW. Dependence of mesomorphic behaviour of methylene-linked dimers and the stability of the N-TB/N-X phase upon choice of mesogenic units and terminal chain length. Chem: Eur J. 2016;22(27):9366–9374.
- Pocock EE, Mandle RJ, Goodby JW. Molecular shape as a means to control the incidence of the nanostructured twist bend phase. Soft Matter. 2018;14(13):2508–2514.
- Ivsic T, Baumeister U, Dokli I, et al. Sensitivity of the N-TB phase formation to the molecular structure of imino-linked dimers. Liq Cryst. 2017;44:93–105.
- Lesac A, Baumeister U, Dokli I, et al. Geometric aspects influencing N-N-TB transition – implication of intramolecular torsion. Liq Cryst. 2018;45(7):1101–1110.
- Ahmed Z, Welch C, Mehl GH. The design and investigation of the self-assembly of dimers with two nematic phases. RSC Adv. 2015;5(113):93513–93521.
- Panov VP, Nagaraj M, Vij JK, et al. Spontaneous periodic deformations in nonchiral planar-aligned bimesogens with a nematic-nematic transition and a negative elastic constant. Phys Rev Lett. 2010;105(16):167801.
- Sebastian N, Tamba MG, Stannarius R, et al. Mesophase structure and behaviour in bulk and restricted geometry of a dimeric compound exhibiting a nematic–nematic transition. Phys Chem Chem Phys. 2016;18(28):19299–19308.
- Stevenson WD, Zou HX, Zeng XB, et al. Dynamic calorimetry and XRD studies of the nematic and twist-bend nematic phase transitions in a series of dimers with increasing spacer length. Phys Chem Chem Phys. 2018;20(39):25268–25274.
- Henderson PA, Imrie CT. Methylene-linked liquid crystal dimers and the twist-bend nematic phase. Liq Cryst. 2011;38(11–12):1407–1414.
- Mandle RJ. A Ten-year perspective on twist-bend nematic materials. Molecules. 2022;27(9):2689.
- Mandle RJ. Designing liquid-crystalline oligomers to exhibit twist-bend modulated nematic phases. Chem Rec. 2018;18(9):1341–1349.
- Imrie CT, Henderson PA. Liquid crystal dimers and higher oligomers: between monomers and polymers. Chem Soc Rev. 2007;36(12):2096–2124.
- Imrie CT, Henderson PA, Yeap GY. Liquid crystal oligomers: going beyond dimers. Liq Cryst. 2009;36(6–7):755–777.
- Date RW, Imrie CT, Luckhurst GR, et al. Smectogenic dimeric liquid-crystals – the preparation and properties of the alpha,omega-bis(4-normal-alkylanilinebenzylidine-4′-oxy)alkanes. Liq Cryst. 1992;12(2):203–238.
- Attard GS, Date RW, Imrie CT, et al. Nonsymmetrical dimeric liquid-crystals - the preparation and properties of the alpha-(4-cyanobiphenyl-4′-yloxy)-omega-(4-n-alkylanilinebenzylidene-4′-o xy)alkanes. Liq Cryst. 1994;16(4):529–581.
- Paterson DA, Walker R, Abberley JP, et al. Azobenzene-based liquid crystal dimers and the twist-bend nematic phase. Liq Cryst. 2017;44:2060–2078.
- Paterson DA, Xiang J, Singh G, et al. Reversible isothermal twist–bend nematic–nematic phase transition driven by the photoisomerization of an azobenzene-based nonsymmetric liquid crystal dimer. J Am Chem Soc. 2016;138(16):5283–5289.
- Gibb CJ, Storey JM, Imrie CT. A convenient one-pot synthesis, and characterisation of the ω-bromo-1-(4-cyanobiphenyl-4′-yl) alkanes (CBnBr). Liq Cryst. 2022;49(12):1706–1716.
- Blatch AE, Luckhurst GR. The liquid crystal properties of symmetric and non-symmetric dimers based on the azobenzene mesogenic group. Liq Cryst. 2000;27(6):775–787.
- Imrie CT, Karasz FE, Attard GS. Side-chain liquid-crystalline copolymers. 2. Polystyrene-based side-chain polymers containing nitroazobenzene. Macromolecules. 1994;27(6):1578–1581.
- Imrie CT, Schleeh T, Karasz FE, et al. Dependence of the transitional properties of polystyrene-based side-chain liquid-crystalline polymers on the chemical nature of the mesogenic group. Macromolecules. 1993;26(3):539–544.
- Imrie CT, Karasz FE, Attard GS. Side-chain liquid-crystalline copolymers containing charge-transfer groups. Liq Cryst. 1991;9(1):47–57.
- Frisch MJ, Trucks, GW, Schlegel, HB, et al. Gaussian 09 (revision B.01). Wallingford (CT): Gaussian Inc.; 2010.
- Tarini M, Cignoni P, Montani C. Ambient occlusion and edge cueing for enhancing real time molecular visualization. IEEE Trans Visualization Comput Graphics. 2006;12(5):1237–1244.
- Attard GS, Garnett S, Hickman CG, et al. Asymmetric dimeric liquid-crystals with charge-transfer groups. Liq Cryst. 1990;7(4):495–508.
- Bondi A. Van der Waals volumes + radii. J Phys Chem. 1964;68(3):441–451.
- Imrie CT. Non-symmetric liquid crystal dimers: how to make molecules intercalate. Liq Cryst. 2006;33(11–12):1449–1454.
- Walker R, Pociecha D, Faidutti C, et al. Remarkable stabilisation of the intercalated smectic phases of nonsymmetric dimers by tert-butyl groups. Liq Cryst. 2022;49(7–9):969–981.
- Abberley JP, Storey JMD, Imrie CT. Structure-property relationships in azobenzene-based twist-bend nematogens. Liq Cryst. 2019;46(13–14):2102–2114.
- Walker R, Pociecha D, Strachan GJ, et al. Molecular curvature, specific intermolecular interactions and the twist-bend nematic phase: the synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkylanilinebenzylidene-4′-oxy)hexanes (CB6O.M). Soft Matter. 2019;15(15):3188–3197.
- Paterson DA, Crawford CA, Pociecha D, et al. The role of a terminal chain in promoting the twist-bend nematic phase: the synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkyloxyanilinebenzylidene-4′-oxy)hexanes. Liq Cryst. 2018;45(13–15):2341–2351.
- Abberley JP, Jansze SM, Walker R, et al. Structure-property relationships in twist-bend nematogens: the influence of terminal groups. Liq Cryst. 2017;44:68–83.
- Emsley JW, Luckhurst GR, Shilstone GN. The orientational order of nematogenic molecules with a flexible core – a dramatic odd even effect. Molec Phys. 1984;53(4):1023–1028.
- Lee HC, Lu ZB, Henderson PA, et al. Cholesteryl-based liquid crystal dimers containing a sulfur–sulfur link in the flexible spacer. Liq Cryst. 2012;39(2):259–268.
- Donaldson T, Staesche H, Lu ZB, et al. Symmetric and non-symmetric chiral liquid crystal dimers. Liq Cryst. 2010;37(8):1097–1110.
- Paterson DA, Abberley JP, Harrison WT, et al. Cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq Cryst. 2017;44:127–146.
- Dunmur DA. The magic of cyanobiphenyls: celebrity molecules. Liq Cryst. 2015;42:678–687.