ABSTRACT
The author’s work in metallomesogens stemmed from initial preparations of κ1-complexes of cyanobiphenyls of the Group 10 metals, palladium and platinum. In this personalised account, these initial studies are described, put in the context of where the field was at that time and contextualised for the way the author’s own contributions to the field then developed.
GRAPHICAL ABSTRACT
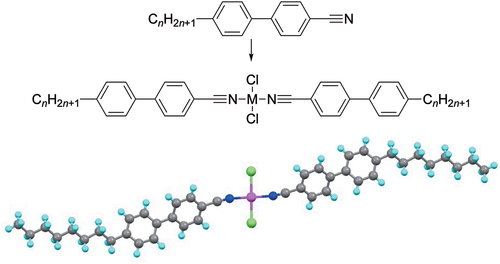
Introduction
Our story begins in Sheffield in 1984 with a conversation between the author (DWB) and the late Peter Maitlis, who had just appointed him to a temporary lectureship in Chemistry. Having come from a PhD in homogeneous photocatalysis using soluble metal-phosphine complexes with David Cole-Hamilton, DWB had been keen to pursue his interests in catalysis further and had indeed written a research proposal based on heterobinuclear metal complexes as part of his application. But Peter had seen the way the chemistry world was going, had understood that organometallics was at that time a crowded field and had therefore suggested that a visible career might be better followed in newer and less-developed areas of the subject. The conversation, held over several meetings, had ranged far and wide until Peter drew on the board a cyanobiphenyl and mentioned liquid crystals. This was a new and foreign field to both, although DWB does remember attending a lecture on the subject by George Gray while a postgraduate student at Liverpool and even being exposed to some of it even earlier on a visit to Hull as a prospective undergraduate, although ironically little of the detail had stuck from either exposure.
The cyanobiphenyl idea struck some sort of chord and while, as recalled earlier, as an organometallic chemist of many years standing, Peter saw η6-coordination as the way forward. However, as someone versed in coordination chemistry, DWB saw coordination through nitrogen and analogues of the well-known bis(benzonitrile)dichlorometal(II) complexes of palladium and platinum as the way to go [Citation1].
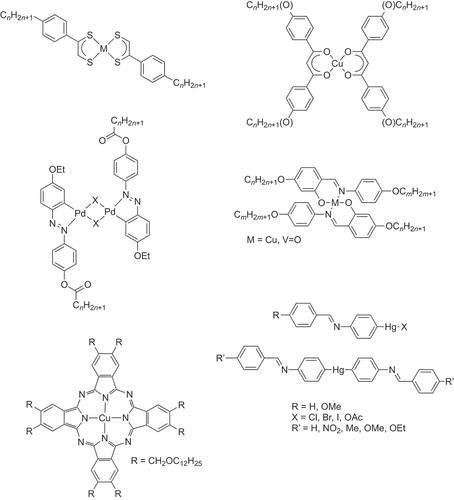
An exploration of the literature began and DWB recalls clearly that the first paper he read was from Kazu Ohta [Citation2] on some copper(II) β-diketonates and then, papers from the other names that became familiar from what was at that time a relatively limited literature – Anne-Marie Giroud with her Group 10 dithiolene complexes [Citation3], Mauro Ghedini with ortho-palladated azobenzenes [Citation4], Yury Galyametdinov with salicylaldimato complexes [Citation5], Jacques Simon with phthalocyanines [Citation6] and, of course, Daniel Vorländer with mercury complexes [Citation7,Citation8]. All were using bi- or tetra-dentate ligands with the exception of Vorländer who had prepared σ-phenyl complexes. The structures of these early complexes are collected in , although there are some other, isolated early examples which can be found in the review in reference [Citation9].
As is often the way, things began with an undergraduate project student’s work on metal complexes that turned out not to have liquid crystal properties (not his fault!), but progress was eventually made with the arrival in the summer of 1985 of Elena Lalinde from Zaragoza. Elena, now Professora Catedrática at the Universidad de la Rioja in Logroño, was then working with Pablo Espinet (In Zaragoza, before he moved to Valladolid) who in turn had at one time been a postdoctoral fellow with Peter Maitlis in Sheffield. She was to spend the summer in Sheffield and Peter suggested to me that she work with me on cyanobiphenyl complexes.
Elena is a very fine synthetic chemist, and before long she had shown how we could take trans-[PdCl2(NCPh)2] and react it with pentylcyanobiphenyl to prepare our first metallomesogen. However, this part of the story misses a key player as neither Peter nor I had a clue how to find out if we had made a liquid crystal. Enter David Dunmur.
Having decided to pursue liquid crystals, I had sought David out (he was a member of staff at Sheffield) and he had agreed that I could take his final-year optional lecture course on the subject, which was heavy with the physical chemistry. Nonetheless, the vocabulary and concepts started to stick and this was the beginning of a long and fruitful collaboration that lasted some 10 years and generated 22 papers. Thus, when Elena had prepared trans-[PdCl2(5CB)2] for the first time, we gave it to David to characterise and a short while later he came back with the news that we had our first liquid crystal!
The early work
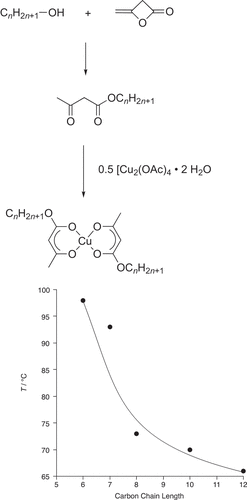
It was undergraduate student Bill Snooks who, in undertaking a 10-week, final-year project, made the first studies in the group. Motivated by the work with β-diketones mentioned above, he reacted diketene with long-chain alcohols to produce alkylacetoacetates, which were then complexed with copper(II) (). None of the complexes was mesomorphic and the observed melting points showed a steady decrease with alkyl chain length [Citation10]. There was simply insufficient anisotropy in these complexes to stabilise a mesophase and it seemed as though trying to elaborate these further would lead to what would become a crowded field.
Figure 2. Preparation and plot of the melting points of a series of bis(alkylacetoacetato)copper(II) complexes.
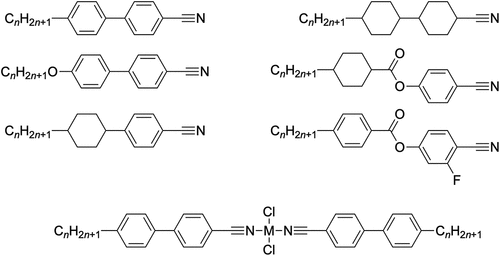
However, the summer that Elena arrived, progress became rapid. She prepared several examples of first palladium(II) complexes of cyanobiphenyls and then extended this to analogous complexes of platinum(II) – we had our foot in the door [Citation11]. As will be outlined below, Elena made other contributions, too, but having put some momentum behind the cyanobiphenyls, some of this work was then taken up by new PhD student Peter Styring (now Professor of Chemical Engineering in Sheffield) alongside undergraduate project students Mick Wragg, Lesley Green and Andy Maggs. Between them they made palladium and platinum complexes of a very wide range of organonitrile liquid crystals, and while several of them were published in Chemical Communications in 1986 [Citation11] and then in this journal in 1987 [Citation12], data for the vast majority never saw the light of day. Although a good deal of the basic data for these complexes still exists in old project reports and lab books, the Supplementary Information would be of a poor standard in trying to report them as we do now (e.g. note that in the Chemical Communications paper [Citation11], it was sufficient to say ‘All new materials have been characterised by i.r. and 1H n.m.r. spectroscopy and by C, H, N, and Cl microanalysis for which satisfactory results were obtained’, with none of those data provided for referees! [Citation11]). This is not meant to imply that the data are poor, rather that we do not have all of them readily to hand and so rather we give a flavour of what was done with the structure of some of the nitriles used given in .
Figure 3. Structures of the come of the organonitriles used to prepared metal complexes and the structure of the complexes (M = Pd or Pt).
Single crystal structures were published for one palladium and one platinum complex (), confirming the trans nature of the complexes, with the palladium complex crystallising in the triclinic P–1 space group, while the Pt complex crystallised in the monoclinic P21/a [Citation12].
As indicated, while reliable, tabulated transition temperatures do not exist for most of these complexes, Styring’s thesis [Citation13] contains phase diagrams for [PdCl2(n-CB)2] and [PdCl2(n-OCB)2], which are now reproduced in .
Figure 5. Phase diagram for (a) 4-alkyl-4-cyanobiphenyl complexes of (b) 4-alkoxy-4-cyanobiphenyl complexes of palladium. Temperatures were estimated visually from a plot in reference [Citation12].
![Figure 5. Phase diagram for (a) 4-alkyl-4-cyanobiphenyl complexes of (b) 4-alkoxy-4-cyanobiphenyl complexes of palladium. Temperatures were estimated visually from a plot in reference [Citation12].](/cms/asset/342a8f1d-39c1-4cbd-8844-f2c7561db42e/tlct_a_2245362_f0005_b.gif)
Thus, the palladium CB complexes all show monotropic nematic phases, with clearing points in the approximate range 70–95°C, while the complexes of the OCBs all show enantiotropic phases, which are nematic for 4 ≤ n ≤ 8, while for n = 9 a SmC and a SmA phase are seen. As ever in liquid crystals, enantiotropic and monotropic behaviour are a function of both the melting point and the clearing point and, in these complexes, a comparison of each is really rather instructive. Thus, shows the melting points of the two series plotted on the same axes as a function of the total chain length, while likewise shows the clearing points plotted using Kelvin as the temperature axis (see below).
Figure 6. (Colour online) (a) Melting points (in °C) and (b) clearing points (in K) for the complexes [PdCl2(n-CB)2] and [PdCl2(n-OCB)2] plotted as function of the total terminal chain length. All of the clearing points are for N-Iso except for [PdCl2(9-OCB)2], where it is SmA-Iso. Note that the two y-axis scales are different.
![Figure 6. (Colour online) (a) Melting points (in °C) and (b) clearing points (in K) for the complexes [PdCl2(n-CB)2] and [PdCl2(n-OCB)2] plotted as function of the total terminal chain length. All of the clearing points are for N-Iso except for [PdCl2(9-OCB)2], where it is SmA-Iso. Note that the two y-axis scales are different.](/cms/asset/7ca7f291-7180-4329-b0e3-49a197a3cb2e/tlct_a_2245362_f0006_oc.jpg)
The melting points are not so dissimilar as a function of total chain length, although for the shorter-chain ligands the complexes of the OCBs have a generally higher melting point, which may be an effect of the dipole moment introduced on addition of the alkoxy oxygen to the ligand. However, for the ligands 5OCB to 7OCB, the melting points of the complexes fall below those of the complexes with ligands 6CB to 8CB, which is not expected and which may indicate that they crystallise in different space groups.
Then in considering the clearing points (), it can be seen that while not unexpectedly the temperatures found for the complexes are higher than those of the ligands, nonetheless they track one another rather well and the observed odd-even effect also tracks the total number of atoms in the terminal chain. Interestingly, if the ratio is taken between the clearing point of the complex and the clearing point of the related ligand in Kelvin, it comes out in effect as 1.2 for each pair considered. Thus, the higher clearing points for the complexes compared to the ligands would appear to have a common origin related to complexation, with those of the complexes of n-OCB being highest on account of the dipole induced from introduction of the ether oxygen.
There are many fewer data available for platinum complexes that can be relied upon and so for the purposes of this article, the two published examples will be considered, namely [PtCl2(5CB)2] and [PtCl2(8CB)2], whose melting points (189°C and 166°C, respectively) and clearing points (TN-Iso = 204 °C and 176 °C, respectively) are very significantly higher than those of the analogous palladium complexes (ΔTN-Iso = 112 °C and 87 °C, respectively) [Citation11]. Further, the ratio (in K) of the clearing point of the complexes to the ligands is between 1.5 for [PtCl2(5CB)2] and 1.4 for [PtCl2(8CB)2]. How might the differences between Pd and Pt be understood?
There is a simple, linear correlation between the length of a mesogen and its clearing point as set out in a molecular field theory of Kloczkowski and Luckhurst [Citation14] and, on this basis, it is quite reasonable to expect that the clearing points of the palladium and platinum complexes ought to be rather close (there is a difference in molecular weight of ca 89 g mol−1 (≈12%) on account of the larger value of Z for Pt, but this should not have a significant effect).
In the two crystal structures, the metal atoms sit on a crystallographic inversion centre and so both the N-M-N and Cl-M-Cl angles are formally 180°. However, as monodentate ligands and in the case of the nitriles quite extended ligands, then there is the possibility for bending about the M – N bond in particular. Further, the result of the lanthanide contraction means that while the nuclear charge at Pt is some 32 greater than at Pd, the radii of the two metal(II) centres will be all but identical, giving a much greater charge at Pt and resulting in tighter binding of ligands. On this basis, it is entirely reasonable to assume that there is greater motional flexibility in the palladium complexes compared to those of platinum and, this being the case, then there is a potential explanation for the lower mesophase stability of the palladium complexes.
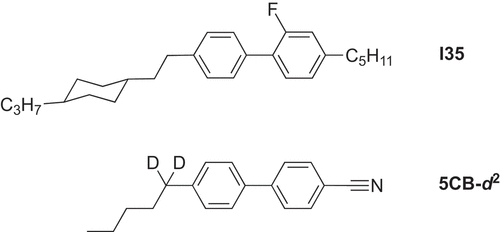
In fact, there is another interesting issue when comparing the clearing point of 5CB and its complexes, for the same molecular field theory would predict that the ratio of their clearing points (in K) should be close to 1:2 as the complex is almost exactly twice the length of the ligand, when in fact it is highest in platinum complexes where it is 1.5. In order to probe this observation, 2H NMR spectra were recorded of 5CB and its platinum complex dissolved in the liquid crystal I35 () using a d2-labelled cyanobiphenyl.Footnote1 The work was done with Geoffrey Luckhurst and Shimei Fan [Citation15]. The NMR experiment allowed values of the order parameter P2 to be obtained from the observed quadrupolar splitting and, as might have been expected, at the clearing point of the solvent, P2 was greater for the complex (0.48) compared to the ligand (0.33), although the two converged to a reasonable degree at lower reduced temperatures. An explanation was proposed in terms of what are now the fashionable cybotactic clusters, although the fact that the complexes have two flexible chains (as opposed to one for the ligand) and the model assumes rigid molecules is likely a pertinent factor.
In addition to these NMR studies, the enhanced anisotropy of the complexes was studied by optical means and solutions of [PdCl2(5CB)2] in 5CB showed slightly enhanced clearing points and birefringence () [Citation12]. This work was followed up by a more complete series of measurements for the complexes [PdCl2(n-OCB)2] (n = 5, 6, 7) and, for example, at a reduced temperature of 0.985, Δn for [PdCl2(6-OCB)2] was 0.1636 compared to 0.1433 for the free ligand [Citation16].
Figure 8. Plot of refractive indices versus temperature for a solution of [PdCI2(5CB),] in 5CB at the following compositions: o 1.27 wt per cent Pd complex; + 0.77 wt per cent Pd complex; x pure 5CB (reproduced from reference [Citation12]).
![Figure 8. Plot of refractive indices versus temperature for a solution of [PdCI2(5CB),] in 5CB at the following compositions: o 1.27 wt per cent Pd complex; + 0.77 wt per cent Pd complex; x pure 5CB (reproduced from reference [Citation12]).](/cms/asset/c92568ec-109a-4bdf-be10-8b40625e4ffd/tlct_a_2245362_f0008_b.gif)
After this, there are few reports regarding cyanobiphenyl complexes, one being the report by Hanabusa et al. of the use of PtCl2 to cross-link side-chain cyanobiphenyl polymers thus creating elastomeric materials [Citation17]. However, a particularly elegant extension was the report by Lee et al. of the palladium complexes of 3,4,5-tridodecyloxy- and 3,4,5-trioctadecyloxy-benzonitrile (), which showed a columnar mesophase between 73.3 and 92.1°C and between 58.4 and 80.4°C, respectively [Citation18]. Elegant simplicity!
Beyond cyanobiphenyls to isonitriles
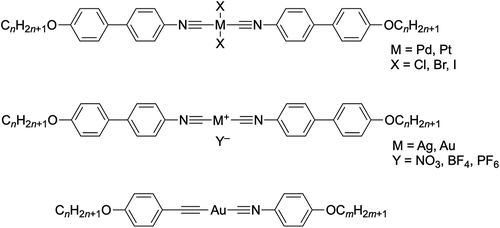
Takahashi and Espinet later mixed things up by preparing a many and interesting series of complexes of palladium(II), platinum(II) [Citation19], silver(I), gold(I) [Citation20,Citation21] and iron(0) [Citation22] based upon the isomeric isonitriles, representative examples of which are shown in . In fact, one might also regard an acetylide anion as isoelectronic with a nitrile and of course these have been used on their own and in combination with organoisonitriles to generate many more metallomesogens, with an example shown also in [Citation23,Citation24].
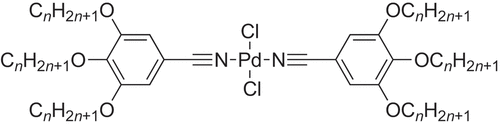
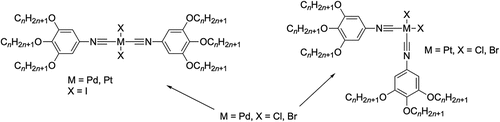
Further, Coco et al. prepared isonitrile analogues of the trialkoxybenzonitriles shown in , which they complexed to both palladium and platinum (). Interestingly, where the halide ligand was iodide, then the complexes of both metals showed a trans geometry in the solid state and (insofar as they could tell) the mesophase. However, things were not so clear cut for X = Cl and Br, so that for M = Pt, both showed exclusively a cis-geometry, whereas for M = Pd, both isomers were found at room temperature (95% trans where X = Br and 65% cis where X = Cl). Irrespective of the geometry, the complexes were mesomorphic forming columnar phases for 4 ≤ n ≤ 10 for all cis complexes and for n = 8, 10 for the trans complexes [Citation25].
What happened next?
During that eventful stay that Lalinde made to Sheffield in the summer of 1985, I recall having been away for 2 weeks on holiday and coming back to find a new ligand system, which had been proposed to her by Maitlis. These were the alkoxystilbazoles (), which were made at that time by the reaction of an alkoxybenzaldehyde with 4-picoline in acetic anhydride giving a recovered yield of <5% [Citation26]. Despite the awful yields (not that long after we began to prepare them in much greater quantity and in much higher yields using a Heck coupling between 4-alkoxyiodobenzene and 4-vinylpyridine) these were attractive. For, while organonitriles are in general rather poor ligands, pyridines are rather better and so there was room to hope for more stable/inert complexes that might result. The simple 4-alkoxystilbazoles never gave anything terribly interesting with palladium and platinum as the complexes were very high melting/clearing and rather difficult to study, but complexes of related dialkoxystilbazoles did open up quite a field of work [Citation27,Citation28]. However, while nitrile complexes of rhodium(I) had been really thermally labile, the stilbazoles formed a whole series of complexes () with both rhodium and iridium of the formula cis-[MCl(CO)2(stilbazole)] [Citation29], and developing these ideas led us to work in both Langmuir-Blodgett films (with the late Tim Richardson) [Citation30] and second-order non-linear optics [Citation31].
Figure 12. 4-alkoxystilbazoles. Complexes with Rh, Ir and Ag and both hydrogen- and halogen-bonded mesogens.
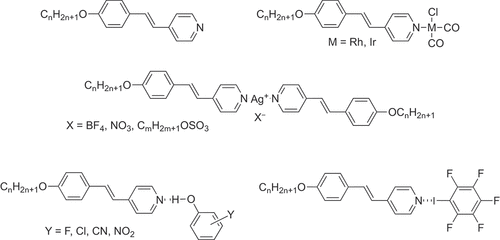
Stilbazoles [Citation32] also opened up our work on hydrogen-bonded liquid crystals [Citation33] following inspiration from Takashi Kato and Jean Fréchet [Citation34] and, in turn, this led us to halogen-bonded liquid crystals () where we reported the first examples [Citation35] – an area we have reviewed recently [Citation36].
However, stilbazoles also got us into silver chemistry [Citation37] and while we published our first paper on silver in 1986 [Citation38], the most recent one was published (albeit using related dialkoxyphenylpyridine ligands) in 2022 [Citation39]. Through silver () and with major contributions from Peter Styring, Sarah Hudson and Bertrand Donnio, we discovered ionic nematic phases [Citation37,Citation40] (now more common through some lovely work by former postdoc Viorel Cîrcu and co-workers [Citation41]), in 1986/1987 we doubled the number of materials showing thermotropic cubic phases (from around 6 to 12) [Citation42], we studied the cubic-columnar transition in detail [Citation43] and obtained freeze-fracture electron microscopy images [Citation44], we developed a material with a direct N-Cub transition which, working with Anne-Marie Levelut (sadly no longer with us), Michéle Veber and Bertrand Donnio gave us a monodomain X-ray determination of a cubic phase (Ia3d) [Citation45] and, having been able to stabilise the S4 phase that often occurred alongside the cubic phase, once more with Anne-Marie and Bertrand we were able to use X-ray to determine that it has tetragonal symmetry [Citation46]. Furthermore, using a wider range of stilbazole complexes, we were able to propose some very strong structure-activity correlations on the formation of cubic phases in calamitic mesogens [Citation28,Citation43,Citation44,Citation47,Citation48], which then informed studies with Antonina Smirnova on solvent-modified mesomorphism [Citation49].
Epilogue
To pull this article together has been a great deal of fun, not least because it made me dig out so many old reports and in so doing recall so many previous students and co-workers. I have named many, but by no means all, of the key players and I think those not named are otherwise recognised as co-authors in the references. But I did want to reflect once more on how it all started. From sage advice to change direction and find something ‘new and different’ to do, from eschewing the organometallic route and following an instinct for κ1 rather than η6 coordination, from the support of being given the chance to work over two summers with a hugely talented visitor (Elena did come back in summer 1986, but that is another story) who not only got the whole thing moving but also opened up the stilbazoles, to the fortune in having a colleague in the department to show that the ideas actually had something to them. Cyanobiphenyls are 50-years old, and this story has almost a 40-year history attached to it. George Gray was also a very early supporter of the work we started to do and that feels apposite given that, after all, it was he and his research group that showed what the cyanobiphenyls could do. It’s a privilege to have been part of that story.
Acknowledgments
It has been a real pleasure to be able to recall all of those with whom I was able to work in this enterprise over many years and who are named either explicitly in the text or through the references cited. We were funded generously by the EPSRC (and its predecessor the SERC), the EU, the Royal Society, the British Council and by the Universities of Sheffield, Exeter and now York, while palladium and platinum came from the wonderful loans scheme run by Johnson Matthey. All are thanked warmly.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
Notes
1. DWB went to Southampton to prepare the complex with Geoffrey Luckhurst, staying on a day after giving a seminar there to the inorganic chemists. Whereas previously the platinum complexes had been made under reflux in toluene leading to only quite moderate yields [Citation12], the route had recently been modified to carry it out in a small excess of molten ligand above 100°C to which PtCl2 was added [Citation50]. These regioselectively deuteriated CBs take a bit of making and are, therefore, valuable materials. DWB remembers well the look of discomfit he encountered on his collaborator’s face when this beautiful, pure and colourless material has heated to the fluid state only to have ‘dirty’ PtCl2 powder dumped into it. Nonetheless, coming back after coffee, a slightly dirty yellow product was recovered that cleaned up just fine.
References
- This conversation has been referred to previously inLiu X-H, Abser MN, Bruce DW. Synthesis and characterization of rod-like metallomesogens of Mn(I) based on Schiff base ligands. J Organomet Chem. 1999;551:271–280 doi: 10.1016/S0022-328X(97)00437-3.
- Ohta K, Ishii A, Yamamoto I, et al. Discotic liquid crystals of organocopper complexes: the substituent effects. J Chem Soc Chem Commun. 1984;16:1099–1101. doi: 10.1039/c39840001099
- Giroud AM, Mueller-Westerhoff UT. Mesomorphic transition metal complexes. Mol Cryst Liq Cryst Lett. 1977;41(1):11–13. doi: 10.1080/01406567708071945
- Ghedini M, Longeri M, Bartolino R. Transition metals complexes to ordered mesophases – palladium Azo complexes. Mol Cryst Liq Cryst. 1982;84(1):207–211. doi: 10.1080/00268948208072141
- Ovchinnikov IV, Galyametdinov Y, Ivanova GI, et al. Liquid-crystal complexes of Schiff bases with copper. Dokl Akad Nauk SSSR. 1984;276:126–128.
- Piechocki C, Simon J, Skoulios A, et al. Discotic mesophases obtained from substituted metallophthalocyanines – towards liquid-crystalline one-dimensional conductors. J Am Chem Soc. 1982;104(19):5245–5247. doi: 10.1021/ja00383a050
- Vorländer D. Die Erforschung der molekularen Gestalt mit Hilfe der kristallinischen Flüssigkeiten. Z Phys Chem. 1923;105(1):211. doi: 10.1515/zpch-1923-10514
- Bruce DW, Heyns K, Vill V. Vorländer’s wheel. Liq Cryst. 1997;23(6):813–819. doi: 10.1080/026782997207740
- Donnio B, Guillon D, Deschenaux R, et al. Metallomesogens. In: McCleverty J, and Meyer T, editors. Comprehensive coordination chemistry II. Vol. 7, Oxford (UK): Elsevier; 2003. pp. 357–627. doi: 10.1016/B0-08-043748-6/06150-8
- Snooks WA. The synthesis of transition metal diketonate complexes as potential coloured liquid crystals [ BSc Thesis]. University of Sheffield; 1985. ( available from the author).
- Bruce DW, Lalinde E, Styring P, et al. Novel transition metal-containing nematic and smectic liquid crystals. J Chem Soc Chem Commun. 1986;1986(8):581–582. doi: 10.1039/c39860000581
- Adams H, Bailey NA, Bruce DW, et al. Mesogenic transition metal complexes. Liquid crystal phase behaviour and crystal and molecular structure of some nitrile complexes of the platinum metals. Liq Cryst. 1987;2(3):381–393. doi: 10.1080/02678298708086683
- Styring P. [ PhD Thesis]. University of Sheffield; 1988.
- Kloczkowski I, Luckhurst GR. On the relationship of the nematic-isotropic transition temperature of an oligomer to that of its constituent units. Liq Cryst. 1988;3(1):95–99. doi: 10.1080/02678298808086352
- Fan SM, Bruce DW, Luckhurst GR. On the transitional properties of rigid oligomers. The orientational order of the monomer 4- n -pentyl-4′-cyanobiphenyl and its platinum dichloride linked dimer. Liq Cryst. 1994;16(6):1093–1099. doi: 10.1080/02678299408027878
- Bruce DW, Dunmur DA, Manterfield MR, et al. High birefringence materials using metal-containing liquid crystals. J Mater Chem. 1991;1(2):255–258. doi: 10.1039/jm9910100255
- Hanabusa K, Suzuki T, Koyama T, et al. Effect of metal complexation on the behavior of liquid-crystalline polymer. J Macromol Sci Chem. 1990;A27(9):1379–1387. doi: 10.1080/10601329008544845
- Lee M, Yoo Y-S, Choi M-G. Synthesis and mesomorphic properties of palladium(II) complexes based on 3,4,5-trialkoxy benzonitrile ligands. Bull Korean Chem Soc. 1997;18:1067–1070.
- Kaharu T, Tanaka T, Sawada M, et al. Liquid-crystalline palladium– and platinum–isonitrile complexes: synthesis, mesomorphic properties and molecular structure. J Mater Chem. 1994;4(6):859–865. doi: 10.1039/JM9940400859
- Kaharu T, Ishii R, Takahashi S. Liquid-crystalline gold–isonitrile complexes. J Chem Soc Chem Commun. 1994;11:1349–1350. doi: 10.1039/C39940001349
- Benouazzane M, Coco S, Espinet P, et al. Liquid crystalline behaviour in gold(I) and silver(I) ionic isocyanide complexes: smectic and columnar phases. J Mater Chem. 2002;12(3):691–696. doi: 10.1039/b109061e
- Coco S, Espinet P, Marcos E. Mesomorphic trigonal bipyramidal iron(0) isocyanide complexes. J Mater Chem. 2000;10(6):1297–1302. doi: 10.1039/a910347n
- Alejos P, Coco S, Espinet P. Liquid-crystals based on alkynylgold(I) isonitrile complexes. New J Chem. 1995;19:799–805.
- Kaharu T, Ishii R, Adachi T, et al. Liquid-crystalline (lsonitrile)gold(I) acetylide complexes. J Mater Chem. 1995;5(4):687–692. doi: 10.1039/jm9950500687
- Coco S, Díez-Expósito F, Espinet P, et al. Columnar organization in mesogenic cis- and trans-[MX2(C≡NR)2] complexes (M = Pd, Pt). Chem Mater. 1998;10:3666–3671 doi: 10.1021/cm980400+.
- Bruce DW, Dunmur DA, Lalinde E, et al. 4-alkyloxy-4’-stilbazoles: new heterocyclic mesogens. Liq Cryst. 1988;3(3):385–395. doi: 10.1080/02678298808086385
- Donnio B, Bruce DW. Liquid-crystalline complexes of palladium(II) and platinum(II) with Di- and Tri-alkoxystilbazoles: ligand control of mesomorphism. J Chem Soc Dalton Trans. 1997;16:2745–2755. doi: 10.1039/a702756g
- Fazio D, Mongin C, Donnio B, et al. Bending and shaping: cubics, calamitics and columnars. J Mater Chem. 2001;11(11):2852–2863. doi: 10.1039/b104724h
- Bruce DW, Dunmur DA, Esteruelas MA, et al. The synthesis and mesomorphism of stilbazole complexes of rhodium and iridium(I). J Mater Chem. 1991;1(2):251–254. doi: 10.1039/jm9910100251
- Richardson T, Topaçli A, Abd Majid WH, et al. Langmuir-Blodgett films of stilbazole complexes of iridium(I) and rhodium(I). Adv Mater Opt Electron. 1994;4(4):243–251. doi: 10.1002/amo.860040403
- Bruce DW, Thornton A. Electronic hyperpolarisabilities of some stilbazole complexes of Rh(I) and Ir(I). Mol Cryst Liq Cryst. 1993;231(1):253–256. doi: 10.1080/10587259308032510
- Bruce DW. The materials chemistry of alkoxystilbazoles and their metal complexes. Adv Inorg Chem. 2001;52:151–204 doi: 10.1016/S0898-8838(05)52003-8.
- Price DJ, Willis K, Richardson T, et al. Hydrogen-bonded liquid crystals from nitrophenols and alkoxystilbazoles. J Mater Chem. 1997;7(6):883–891. doi: 10.1039/a700575j
- Kato T, Fréchet JMJ. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J Am Chem Soc. 1989;111(22):8533–8534. doi: 10.1021/ja00204a044
- Nguyen HL, Horton PN, Hursthouse MB, et al. Halogen bonding: a new interaction for liquid crystal formation. J Am Chem Soc. 2004;126(1):16–17. doi: 10.1021/ja036994l
- Präsang C, Bruce DW. Halogen-bonded liquid crystals. Helv Chim Acta. 2023;106(5):e202300008. doi: 10.1002/hlca.202300008
- Bruce DW, Calamitics, cubics and columnars - liquid-crystalline complexes of silver(I). Acc Chem Res. 2000;33(12):831–840. doi: 10.1021/ar990159k
- Bruce DW, Dunmur DA, Lalinde E, et al. Novel thermotropic ionic mesogens. Nature. 1986;323(6091):791–792. doi: 10.1038/323791a0
- Herod JD, Cowling SJ, Bruce DW. Synthesis and mesomorphism of 3,4-dialkoxyphenylpyridine complexes of silver(I). J Mol Liq. 2022;362:119707. doi: 10.1016/j.molliq.2022.119707
- Bruce DW, Dunmur DA, Maitlis PM, et al. Nematic phases in ionic melts: mesogenic ionic complexes of silver(I). Chem Mater. 1989;1:479–481. doi: 10.1021/cm00005a001
- Pană A, Pasuk I, Micutz M, et al. Nematic ionic liquid crystals based on pyridinium salts derived from 4-hydroxypyridine. Cryst Eng Comm. 2016;18(27):5066–5069. doi: 10.1039/C6CE00618C
- Bruce DW, Dunmur DA, Hudson SA, et al. Polymorphic ionic mesogens of silver(I): ionic materials exhibiting a thermotropic cubic mesophase. Mol Cryst Liq Cryst. 1991;206(1):79–92. doi: 10.1080/00268949108037720
- Donnio B, Bruce DW, Heinrich B, et al. The synthesis, mesomorphism and characterisation by X-Ray diffraction and freeze-fracture electron microscopy, of polycatenar liquid crystals of silver(I) Showing columnar and cubic mesophases. Chem Mater. 1997;9(12):2951–2961. doi: 10.1021/cm970300o
- Donnio B, Bruce DW, Delacroix H, et al. Freeze-fracture electron microscopy of thermotropic cubic and hexagonal mesophases. Liq Cryst. 1997;23(1):147–153. doi: 10.1080/026782997208758
- Bruce DW, Donnio B, Hudson SA, et al. X-Ray diffraction from mesophases of some stilbazole complexes of silver(I); monodomain determination of a thermotropic cubic phase. J Phys II France. 1995;5(2):289–302. doi: 10.1051/jp2:1995129
- Levelut AM, Donnio B, Bruce DW. Preliminary communication characterisation by X-ray diffraction of the S4 phase of some silver(I) complexes of alkoxystilbazoles. Liq Cryst. 1997;22(6):753–756. doi: 10.1080/026782997208884
- Donnio B, Rowe K, Roll CP, et al. On the formation of cubic phases. Mol Cryst Liq Cryst. 1999;332(1):2893–2900. doi: 10.1080/10587259908023782
- Mongin C, Donnio B, Bruce DW. On the formation of the thermotropic cubic phase: insights from monoacetylide complexes of Pt(II). J Am Chem Soc. 2001;123(34):8426–8427. doi: 10.1021/ja015944i
- Smirnova AI, Bruce DW. Solvent-dependent mesomorphism of polycatenar silver(I) complexes in organic solvents. J Mater Chem. 2006;16(44):4299–4306. doi: 10.1039/b612528j
- Bruce DW, Donnio B, Maggs AA, et al. Melt syntheses of [PtCl2L2]. Inorg Chim Acta. 1991;188(1):41–43. doi: 10.1016/S0020-1693(00)80914-7

![Figure 4. (Colour online) Molecular structure of (a) [PdCl2(5CB)2] and (b) [PtCl2(8CB)2].](/cms/asset/930d1c7e-63ef-4fe9-a295-c304c7a857fb/tlct_a_2245362_f0004_oc.jpg)