ABSTRACT
We have designed new chiral mesogens, OXm/n, containing an oxazoline chiral motif derived from natural L-serine amino acid. Our effort was concentrated on the evaluation of the synthetic accessibility of these new compounds and preserving their optical purity, which was determined using chiral high-performance liquid chromatography. We established materials mesomorphic properties and confirmed the phase identification by X-ray scattering studies. Depending on the ratio between the terminal alkyl chain lengths, the studied materials exhibited various smectic mesophases. The derivatives OXm/n with conformationally rigid oxazoline chiral moiety were compared with the structurally similar smectogens, ZLm/n, having a flexible (S)-lactate chiral unit, in order to investigate the effect of rigidisation of the chiral unit on the mesomorphic properties.
1. Introduction
Natural chiral compounds are very useful and cheap sources of chirality, either built in the molecular structure of liquid crystals or used as chiral dopants [Citation1,Citation2]. Compounds with intrinsic chirality are attractive for diverse applications due to their physical properties such as ferroelectricity and electro-optical switching. Additionally, the chiral functional nanomaterials based on liquid crystalline compounds can be templates for various nanostructured materials towards technological applications in holography, lasing technology, electronics and beyond.
Chiral liquid crystals with a lactic acid unit have been proven to show a rich variety of mesophases and to be an efficient pool of chirality [Citation3–9]. This fact was supported by the presence of various frustrated phases, like blue phases (BP) or twist-grain boundary phases (TGB) [Citation10–14]. Additionally, for the compounds with a lactic acid unit in the chiral terminal chain, a cholesteric phase with a very short pitch (less than 200 nm) has been found [Citation15,Citation16]. In the case of a molecular core composed of three phenyl rings, we have investigated a homologue series of compounds denoted as ZLm/n, exhibiting smectic phases including ferroelectric SmC* mesophase [Citation17–19]. By tuning the length of both terminal chains, we have observed a broad range of the SmC* phase with high values of spontaneous polarisation for selected derivatives.
The rigidisation of the chiral fragment is an effective tool to enhance the helical twisting power of chiral dopants for liquid crystals, frequently utilised for the induction of chiral mesophases. It gives better results than the introduction of the mesogen-like structural motifs to the molecular structure of chiral dopants [Citation20]. Reduction in the number of possible chiral conformers, favouring ones with higher helical twisting power, is believed to be the key effect of the rigidisation [Citation21]. Higher helical twisting power is beneficial as the chiral mesophase with required characteristics can be obtained with a lower concentration of a chiral dopant, which is usually costlier than a non-chiral LC matrix. As the dopant itself is usually non-mesogenic, it can have some adverse effects on the electro-optical properties and the stability of the mesophase.
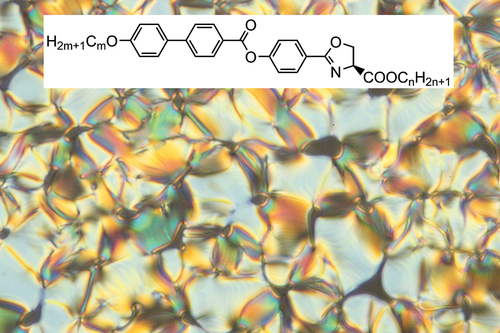
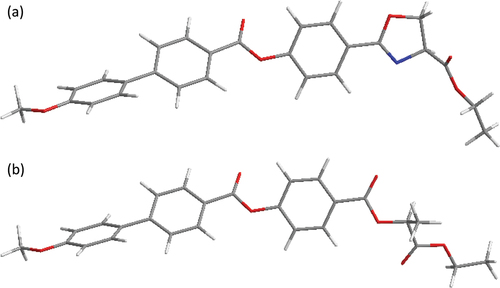
In this contribution, we designed new mesogens based on oxazoline, denoted as OXm/n (see . Although the materials are new, they comprise a structurally almost identical molecular core as the previously developed mesogens ZLm/n [Citation18,Citation19] with a lactic acid unit in the chiral moiety (see . The major difference between these two series of materials lies in the conformational rigidity of the serine derivative present in the form of a chiral five-membered oxazoline ring in comparison with the conformationally flexible lactate motif. Despite its availability from natural amino acids, the potential of oxazoline has not yet been sufficiently explored in the design of liquid crystalline derivatives. To the best of our knowledge, there are only several works on oxazolines related to the liquid crystal topic [Citation21–24], with only one focused on the obtaining of mesogenic oxazoline materials [Citation24]. Our aim is to evaluate the mesogenic properties of this type of oxazoline derivatives () and to compare their properties with the lactic acid-derived analogous mesogens (). We varied the length of the terminal chains on both the chiral and the non-chiral parts of the molecule to estimate their role on the mesogenic properties and to further evaluate the synthetic accessibility of these new compounds. The chiral purity of the materials was determined using chiral high-performance liquid chromatography (HPLC).
Figure 1. Molecular structure of a) the studied compounds OXm/n, bearing a chiral oxazoline α-carboxylic acid as a source of chirality and b) the previously reported lactic acid derivatives ZLm/n [Citation18].
![Figure 1. Molecular structure of a) the studied compounds OXm/n, bearing a chiral oxazoline α-carboxylic acid as a source of chirality and b) the previously reported lactic acid derivatives ZLm/n [Citation18].](/cms/asset/608cdce0-07a7-450a-bf98-e353340d0e98/tlct_a_2252388_f0001_b.gif)
2. Experimental
2.1. Synthesis
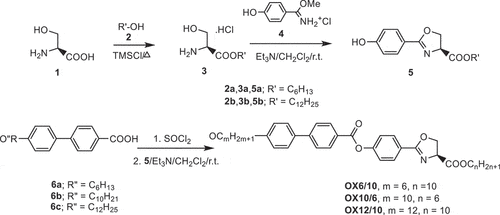
The synthetic pathway presented in led to three chiral oxazoline derivatives OXm/n. Oxazoline intermediates 5 as suitable building blocks bearing long alkyl chains were easily prepared from the commercially available L-serine 1. First, L-serine was converted into corresponding esters 3a and 3b by its esterification with alcohols 2a and 2b using Lewis acid such as bromotrimethylsilane. Slightly modified method (see Ref [Citation25]) exhibited good preparative yields of new esters. Thus, the procedure represents a good chemical tool for the introduction of long alkyl chains. Despite the fact that the presented esterification required higher temperatures for the removal of alcohol access during the purification of the products, mild temperatures (not exceeding 65–85°C) were sufficient to give the product residues without observed unfavoured by-products.
The subsequent closure of the oxazoline ring successfully proceeded by the treatment of the serine esters 3a and 3b with aryl-imidiate 4 [Citation26] in the presence of triethylamine. In contrast to the standard reaction protocol initiated by a strong inorganic base, the obtained aryl-substituted oxazolines 5a and 5b did not induce racemisation as it was observed recently in our studies [Citation27]. The condensation of a number of biphenylcarboxylic acids 6a-c with presented oxazoline intermediates 5 showed good results under the activation of the carbonyl group by its reaction with thionyl chloride. In comparison with the alternative esterification of 6, using N,N´-dicyclohexylcarbodimide (DCC), the oxazoline attachment to the biphenyl part afforded clearly the final oxazolines OXm/n in a short reaction time without any significant impurities (monitored by NMR). In contrast, the esterification of 6 in the presence of DCC results in the undesirable esterification of OX12/10 as a consequence of oxazoline ring cleavage.
2.2. Determination of the enantiomeric purity of OXm/n
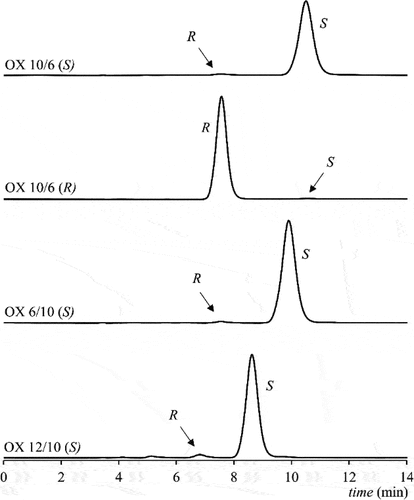
A chiral stationary phase (CSP) based on amylose was chosen as polysaccharide derivatives are the most successful CSP for the enantioseparation of this type of liquid crystals [Citation28,Citation29]. A mixture of n-hexane/dichlo-romethane 7/3 (v/v) was found to be suitable for the enantioseparation of the enantiomers of all three oxazolines. Finally, 2-propanol was added to the mobile phase, which led to a decrease in retention times and in total analysis times as well as to the significant improvement of the peak shape. A mixture of n-hexane/dichloromethane/2-propanol 70/28/2 (v/v/v) was then used for all enantioseparations and allowed us to analyse all oxazoline products in less than 12 minutes. The identification of the enantiomers in obtained chromatograms was done through overloading the column with highly concentrated samples (1 mg·mL−1) in order to obtain reliable UV spectra of smaller peaks. For the compound OX10/6, both enantiomers were synthesised, which enabled the identification of the peaks by the comparison of the retention times. The results of the enantioselective HPLC are shown in more detailed description is given in Supplemental file.
2.3. Set-ups and apparatus
The synthesised materials OXm/n were studied by texture observations under a polarising microscope (POM) Nikon Eclipse E-600 (Nikon, Tokyo, Japan), equipped with Linkam LTSE350 heating stage (Linkam, Tadworth, UK) having an accuracy ±0.1 K, and with Canon EOS 700D photo camera. For these studies, we utilised commercial WAT glass 7- or 12-µm-thick cells with transparent indium tin oxide (ITO) 5 × 5 mm2 electrodes and a polymer coating, which ensured a planar geometry. The cells were filled with materials in the isotropic phase by means of capillary action. The precise phase transition temperatures, Ttr, and the corresponding enthalpy changes, ΔH, were obtained from differential scanning calorimetry (DSC, Pyris Diamond PerkinElmer 7) measurements. For these measurements, 1–3 mg of compounds was hermetically sealed into aluminium pans and inflated by nitrogen. The heating and cooling processes were conducted at 10 K/min rate.
For tilt angle, ϴopt, and spontaneous polarisation, PS, measurements, we used the same cells as for POM. ϴopt was evaluated from the angular difference between the extinction positions under the opposite values of the applied DC field ±3 V/µm. PS was measured by the integration of the current peaks recorded during polarisation switching under the application of a triangular-wave electric field of frequency 10 Hz and a magnitude 15 V/µm. Both spontaneous quantities were measured on cooling.
The pitch of the helical structure in the SmC* phase, p, was measured by analysing the light diffraction on dechiralisation lines. The measurements were performed on cooling of the sample. For this experiment, 50-µm-thick home-made planar cells were utilised to minimise the influence of the surfaces on the helical structure. The values of the pitch were evaluated from the diffraction circles, observed on a screen. This method is applicable for the pitch values 0.8 μm < p < 4 μm.
Small angle x-ray diffraction (SAXRD) technique was used to determine the structural properties of LC phases. We utilised D8 Discover diffractometer (Bruker, Karlsruhe, Germany), equipped with an X-ray ceramic tube with Cu anode (wavelength λ = 1.5418 Å), parabolic Göbel mirror monochromator and a scintillation detector. For the temperature control, Anton Paar DCS 350 chamber (Anton Paar, Graz, Austria) with an accuracy 0.1 K was used. The measurements were performed in the reflection mode on cooling of the samples prepared as thin films on a heated surface, with one free surface to assure homeotropic alignment. The layer thickness, d, was calculated from the position of the scattering angle peak using the Bragg’s law.
3. Results and discussion
3.1. Mesomorphic properties of OXm/n
For all homologues, the phase transition temperatures and the corresponding enthalpy changes were established from DSC measurements, and the results are summarised in . All phase transitions were clearly distinguishable from the DSC thermographs; for illustration, see . Liquid crystalline phases of the studied compounds were determined from the textures and their changes observed under the polarising optical microscope. For OX10/6 compound, a filament texture was observed on cooling from the isotropic phase (Iso), which is a characteristic feature for a TGBA phase growth from the isotropic phase. Deeper in the TGBA phase, oily-streak textures appeared, which are very similar to cholesteric phase ones (see . On subsequent cooling, blurred fan-shaped character of the TGBA textures dominated (see . Finally, the TGBA phase transformed into a regular SmC* phase with a typical broken fan-shaped texture and a defect structure of dechiralisation lines was clearly visible in POM (see . Dechiralisation lines are disclination defects which appear due to the frustration of a helical structure in confined geometry in a planar cell; their regular structure allows for diffraction of monochromatic light in the visible range and the measurements of the pitch length (see below).
Figure 3. (Colour online) DSC thermographs for compounds (a) OX10/6 and (b) OX6/10, taken on the second heating (red colour) and cooling (blue colour) runs.
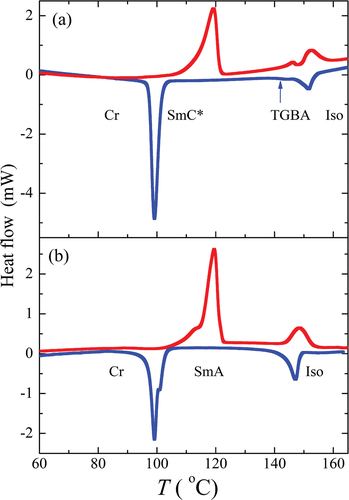
Figure 4. (Colour online) Textures of the studied compound OX10/6, obtained from POM: (a) at the isotropic-TGBA phase transition at T = 149°C, (b) in the TGBA phase at T = 147°C, (c) in the TGBA phase at T = 143°C and (d) in the SmC* phase at T = 138°C. The width of the photos corresponds to about 300 µm.
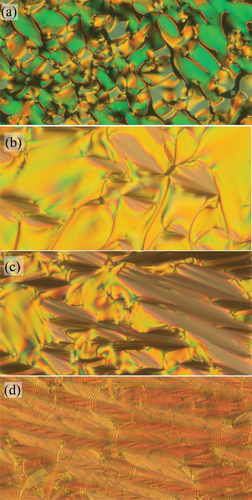
Table 1. Mesomorphic properties of the studied OXm/n compounds: melting point, m.p., the phase transition temperatures, Ttr (in oC), and enthalpy changes, ΔH (in kJ/mol), are taken from the differential scanning calorimetry (DSC) measurements, second heating and cooling runs.
For compound OX6/10, we observed a typical fan-shaped texture of the SmA phase on cooling from the isotropic liquid. The last homologue OX12/10 revealed a direct phase transition to the SmC* phase on cooling from the isotropic liquid. This material also revealed a broken fan-shaped texture with dechiralisation lines (see Figure S3 in Supplemental file). The final identification of all mesophases will be demonstrated later based on X-ray measurements.
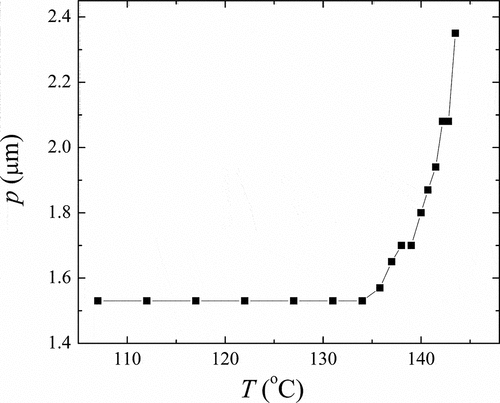
We measured spontaneous quantities and the helical pitch in the ferroelectric SmC* phase. The temperature dependence of the spontaneous polarisation, PS, and tilt angle, ϴopt, is shown in for OX10/6. Both spontaneous quantities revealed a typical behaviour: they first rapidly grew at the phase transition point and then saturated on cooling. The saturation values were about 23 deg. for ϴopt, and PS exceeded 60 nC/cm2. Both PS and ϴopt abruptly fall down at the crystallisation point. The temperature dependence of the helical pitch, measured from the positions of the diffraction circles in the light diffraction experiment, is presented in . At the transition to the SmC* phase, p first decreased and then saturated. The saturation value of the pitch was about 1.5 μm. For OX12/10 material with the SmC* phase, we were not able to measure the helical pitch by this method. We are convinced that the pitch for OX12/10 is too small and below the resolution of this method. It can be supported by the textural photos of OX12/10 (Figure S3 in Supplemental) where one can see that the dechiralisation lines are very dense and closer to each other than for OX10/6 ().
Figure 5. (Colour online) The temperature dependence of the spontaneous polarisation (■) and tilt angle (●) for OX10/6 material in the ferroelectric SmC* phase.
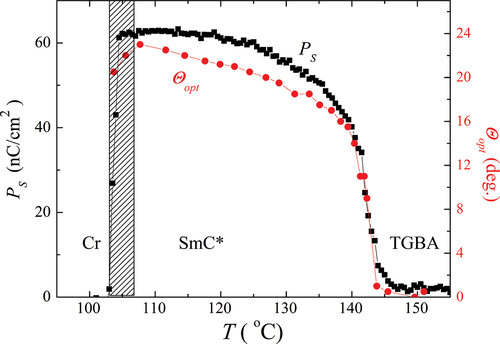
Figure 6. The temperature dependence of the helical pitch, p, for OX10/6 material in the ferroelectric SmC* phase.
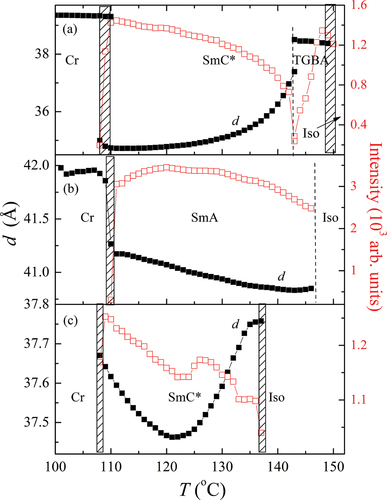
Small-angle X-ray diffraction technique was applied to establish the layer spacing values. A sharp peak corresponding to smectic layers arrangement was detected. The temperature dependence of d(T) together with the intensity of the measured signal, obtained from SAXRD measurements, is presented in for all the materials under study. For OX10/6 compound (), there was a clear anomaly in the intensity at the TGBA-SmC* transition temperature, which can be explained by fluctuations in the vicinity of the phase transition. In the TGBA phase, d(T) slightly grew on cooling (negative thermal expansion coefficient), which is usually explained by the increase in the orientational order of the molecules. At the TGBA-SmC* phase transition, d(T) abruptly fell down and then saturated. Such a layer spacing behaviour is related to the temperature dependence of the tilt angle in the SmC* phase. At the SmC*-crystal (Cr) phase transition, a coexistence region was observed. For OX6/10, almost linear increase in d(T) was found () on cooling in the SmA phase. The intensity of the signal dropped down at the transition to the crystalline phase. For OX12/10 homologue (), the coexistence of phases was observed at the isotropic–SmC* and SmC*–Cr transitions. In the SmC* phase, a pronounced dropdown of d(T) was observed at the Iso-SmC* phase transition, which is connected with the molecular tilt increase. Deeper in the SmC* phase, d(T) started growing. Such a behaviour is usually explained by the interplay of the tilt angle increase, the molecular chains stretching, the changes in the molecular conformations and an increase in the orientational order during the cooling process.
Figure 7. (Colour online) The temperature dependence of the layer thickness (■) and the intensity of the scattered signal or the full width at half maximum values (□), obtained from the X-ray diffraction measurements for a) OX10/6; b) OX 6/10 and c) OX12/10 compounds. Dashed areas between the phases mark the coexistence regions.
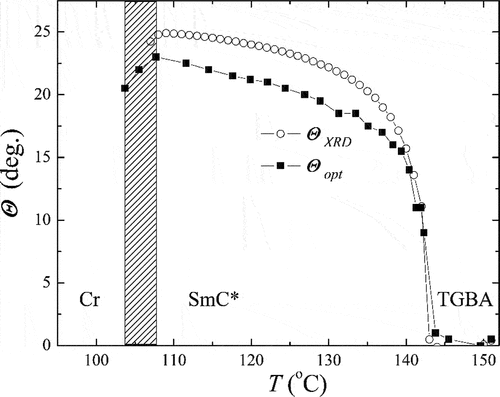
For the material OX10/6 with both the orthogonal SmA (or TGBA) phase and the tilted SmC* phase, we were able to calculate the molecular tilt, ΘXRD, from d(T) data. We calculated ΘXRD from d(T) considering a simple relation ΘXRD = acos (dC /dA), where dC is the layer spacing values measured in the SmC* phase and dA is the layer spacing value linearly extrapolated from the d(T) dependence in the SmA phase. We compared the values of the molecular tilt calculated from the layer spacing measurements, ϴXRD, with the molecular tilt measured optically, ϴopt (see . On cooling the sample in the SmC* phase, the tilt angle grew and the optical tilt, which achieved 23 degrees, was found to be slightly smaller than ϴXRD, which approached 25 degrees. All presented data were taken during the cooling of the sample from the isotropic phase.
3.2. Comparison of OXm/n with ZLm/n compounds
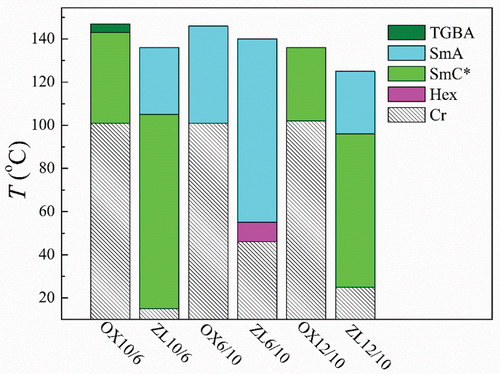
We compared the conformationally rigid oxazoline derivatives OXm/n with the structurally almost identical smectogens ZLm/n having a flexible (S)-lactate chiral unit. The synthesis and the general mesomorphic properties of the lactate derivatives ZLm/n were published recently [Citation18]. For general chemical formula of OXm/n and ZLm/n, see , respectively. shows a schematic bar diagram presenting the phase sequences for OXm/n in comparison with the analogous ZLm/n compounds. From the comparison, it is evident that the presented oxazoline derivatives reveal higher temperature scales and much higher values of the melting points than analogous homologues ZLm/n. It is probably the cost of lower flexibility of OXm/n molecular structure. Additionally, it is also apparent that the derivatives with longer achiral chains show the preference for the tilted SmC* phase than for the non-tilted SmA (TGBA) phases.
Figure 9. (Colour online) Schematic bar diagram showing the phase sequence for OXm/n in comparison with the analogous ZLm/n compounds. Phases are distinguished by different colours and/or a pattern.
To further explore the molecular structures of these two types of mesogens, we carried out a molecular modelling. The results of DFT calculations obtained using Firefly QC package [Citation30,Citation31] revealed big differences in the molecular structures of the global minimum potential energy conformers of OXm/n and ZLm/n series (). While the lactate materials are of the zig-zag shape, quite ordinary for rod-like smectics, the rigid oxazoline ring introduces much larger bend to the molecular structure. Specifically, the angle between the principle axis of the mesogenic core and the terminal chain attached to the chiral centre is much lower (116° for OX vs. 142° for ZL). We can theorise that such a bent shape of the global potential energy minimum might be disturbing if such a conformer is still abundant in the mesophase temperature interval leading to the observed reduction of the mesophase range. As a consequence of higher bend angle, OXm/n materials are slightly shorter than ZLm/n. This is supported by the experimental data as the layer spacing of OXm/n is a bit smaller than that of ZLm/n, although the tilt angle values obtained by the optical method are similar. We measured the values of the polarisation for OX10/6 () and can compare them with the analogous ZL10/6, which was published in Podoliak et al. [Citation18]). For ZL10/6, the polarisation values grow up to 110 nC/cm2 at the room temperature, reaching higher values than which are detected for the homologue OX10/6 (see ). Nevertheless, the temperature range is substantially shifted towards higher temperatures for oxazoline derivatives in comparison with ZLm/n homologues (). From this aspect, any comparison of the polarisation values or other parameters is rather problematic.
4. Conclusions
We have found a feasible synthetic route leading to chiral mesogenic oxazoline derivatives and developed a chiral HPLC method, which confirmed the enantio-selectivity of the used synthetic protocol. The synthesised materials exhibited various lamellar phases depending on the lengths of the terminal chains, including ferroelectric SmC* phase with a tilt angle up to 23° and a spontaneous polarisation reaching 60 nC/cm2. As the first attempt, we concentrated on the selected homologues which would be comparable with the analogous lactic acid derivatives [Citation18]. We have not followed in a systematic study when we discovered that oxazoline derivatives do not have ideal mesomorphic properties. Despite our expectation to develop materials of strong chirality in a broad range of temperatures, only a narrow TGBA phase was present in one homologue OX10/6 (which is not present in the analogous ZL10/6). Anyway, the presence of the TGBA phase suggests for strong enough chirality of oxazoline derivatives. The comparison of OXm/n with the analogous materials ZLm/n revealed the effects of the chirality rigidisation, which leads to higher melting points and reduced mesophase temperature intervals for the studied materials. The reduced mesomorphism is, most probably, caused by a highly bent geometry of the oxazoline materials and less effective tendencies for packing into layers.
We can conclude that oxazoline can serve as an alternative source of chirality for liquid crystalline materials although with limitations. Nevertheless, the new compounds can be useful and effective as chiral dopants in non-chiral smectic matrices.
Supplemental Material
Download PDF (545.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02678292.2023.2252388.
Additional information
Funding
References
- Goodby JWG, editor. Ferroelectric liquid crystals: principles, properties, and applications. Philadelphia: Gordon and Breach Science Publishers; 1991.
- Kitzerow H-S, Bahr C, editors. Chirality in liquid crystals. New York: Springer; 2001. doi: 10.1007/b97374
- Wu S, Hsu H. Synthesis and mesomorphic properties of fluoro‐substituted chiral liquid crystals derived from (S)‐lactic acid with alkoxyethanols. Liq Cryst. 2007;34:1159–1165. doi: 10.1080/02678290701663845
- Wu S‐L, Lai F‐S. Synthesis and ferroelectric properties of new chiral liquid crystals derived from (S)‐lactic acid with alkoxyethanols. Liq Cryst. 2005;32:1243–1249. doi: 10.1080/02678290500139799
- Brombach F, Neudörfl JM, Blunk D. The chiral pool as valuable natural source: new chiral mesogens made from lactic acid. Mol Cryst Liq Cryst. 2011;542(1):62/[584]–74/[596]. doi: 10.1080/15421406.2011.569689
- Ocak H, Canli NY, Eran BB. Synthesis, mesomorphic and dielectric properties of new bent-core liquid crystal with a terminal lactate group. J Mol Liquids. 2021;1223:128975. doi: 10.1016/j.molstruc.2020.128975
- Wang F, Liu W, Li Y, et al. A “core determination” phenomenon in four dichiral liquid crystals containing two lactate groups. Mol Cryst Liq Cryst. 2023;756(1):1–10. doi: 10.1080/15421406.2022.2112494
- Wu X, Wu L, Guo Y, et al. Chirality driven mesomorphic behaviour difference: dichiral compounds containing two lactate groups. Liq Cryst. 2020;47(4):471–476. doi: 10.1080/02678292.2019.1672820
- Senthil S, Srividhya D, Manjunathan S, et al. Synthesis and mesomorphic properties of chiral liquid crystal dimers containing lactate units. J Mol Struct. 2008;877(1–3):50–55. doi: 10.1016/j.molstruc.2007.07.022
- Kašpar M, Bílková P, Bubnov A, et al. New chlorine‐substituted liquid crystals possessing frustrated TGB a and SmQ phases. Liq Cryst. 2008;35(5):641–651. doi: 10.1080/02678290802056212
- Novotná V, Kašpar M, Hamplová V, et al. Synthesis and mesomorphic properties of new compounds exhibiting TGBA and TGBC liquid crystalline phases. Liq Cryst. 2008;35(3):287–298. doi: 10.1080/02678290701862215
- Novotná V, Kašpar M, Hamplová V, et al. Ferroelectric, antiferroelectric and TGB phases in lactic acid derivatives. Liq Cryst. 2012;39(4):477–486. doi: 10.1080/02678292.2011.653411
- Podoliak N, Novotná V, Kašpar M, et al. Anomalous phase sequence in new chiral liquid crystalline materials. Liq Cryst. 2014;41(2):176–183. doi: 10.1080/02678292.2013.846424
- Novotná V, Stulov S, Hamplová V, et al. The cholesteric and TGB phases under the applied electric field. Liq Cryst. 2021;48(9):1283–1294. doi: 10.1080/02678292.2020.1858513
- Novotná V, Hamplová V, Glogarová M, et al. Effect of the applied electric field on new cholesterics with extremely short pitch. Liq Cryst. 2018;45(4):634–640. doi: 10.1080/02678292.2017.1376126
- Smekhova A, Novotná V, Fekete L, et al. Ultra-short helix pitch and spiral ordering in cholesteric liquid crystal revealed by resonant soft X-ray scattering. Soft Matter. 2022;18(1):89–96. doi: 10.1039/D1SM01543E
- Hamplová V, Novotná V, Kašpar M. Lactic acid derivatives with three-phenyl ring molecular core: design and mesomorphic properties. Ferroelectrics. 2014;468(1):18–27. doi: 10.1080/00150193.2014.932655
- Podoliak N, Novotná V, Hamplová V, et al. Structural optimisation of lactic acid derivatives to obtain enhanced ferroelectric properties in smectic phases. Liq Cryst. 2023;50(1):149–156. doi: 10.1080/02678292.2022.2131917
- Vojtylová T, Kašpar M, Hamplová V, et al. Chiral HPLC for a study of the optical purity of new liquid crystalline materials derived from lactic acid. Phase Transit. 2014;87(8):758–769. doi: 10.1080/01411594.2014.893344
- Kuball H-G, Brüning H, Müller T, et al. Helical twisting power of chiral mono- and bis-aminoanthraquinones. Intramolecular and intermolecular chirality transfer in liquid-crystal phases. J Mater Chem. 1995;5(12):2167–2174. doi: 10.1039/JM9950502167
- Kuball H-G, Brüning H. Helical twisting power and circular dichroism as chirality observations: The intramolecular and intermolecular chirality transfer. Chirality. 1997;9:407–423. doi: 10.1002/(SICI)1520-636X(1997)9:5/6<407:AID-CHIR3>3.0.CO;2-2
- Kelly S, Buchecker R, inventors; F. Hoffmann-La Roche AG, assignee. Chiral oxazolines as dopants for liquid crystals mixtures. European Patent EP0534258. 1993 Mar 31.
- Shoshi M, inventor; Ricoh Company, Ltd., assignee. Optically active oxazoline compounds, liquid crystal composition containing the same and optical switching method using the same. United States patent US-5159084-A. 1992 Oct 27.
- Serrano JL, Sierra T, Gonzalez Y, et al. Improving FLC properties. Simplicity, planarity, and rigidity in new chiral oxazoline derivatives. J Am Chem Soc. 1995;117(32):8312–8321. doi: 10.1021/ja00137a003
- Maiti M, Gao L-J, Huang C, et al. Bifunctional aryloxyphosphoramidate prodrugs of 2′-C-Me uridine: synthesis and anti-HCV activity. Org Biomol Chem. 2016;14:8743–8757. doi: 10.1039/C6OB01189F
- Li L-B, Dan W-J, Tan F-F, et al. Synthesis and antibacterial activities of yanglingmycin analogues. Chem Pharm Bull. 2015;63(1):33–37. doi: 10.1248/cpb.c14-00578
- Pomeisl K, Vaňkátová P, Hamplová V. Enantioselective high‐performance liquid chromatography of aryl‐substituted oxazolines as an efficient tool for determination of chiral purity of serine medicinal components. J Sep Sci. 2022;45(13):2217–2227. doi: 10.1002/jssc.202100958
- Vaňkátová P, Folprechtová D, Kalíková K, et al. Enantiorecognition ability of different chiral selectors for separation of liquid crystals in supercritical fluid chromatography; critical evaluation. J Chromatogr A. 2020;1622:461138. doi: 10.1016/j.chroma.2020.461138
- Vaňkátová P, Kubíčková A, Kalíková K. Enantioseparation of liquid crystals and their utilization as enantiodiscrimination materials. J Chromatogr A. 2022;1673:463074. doi: 10.1016/j.chroma.2022.463074
- Firefly version 8 [Internet]. Moscow: Granovsky AA; [cited 2023 May 17]. Available from: http://classic.chem.msu.su/gran/firefly/index.html
- Schmidt MW, Baldridge KK, Boatz JA, et al. General atomic and molecular electronic structure system. J Comput Chem. 1993;14(11):1347–1363. doi: 10.1002/jcc.540141112