?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
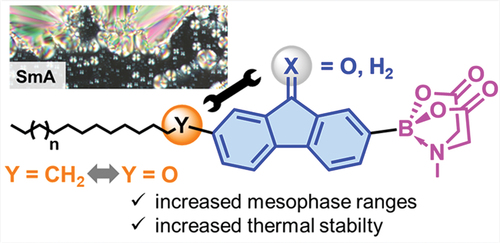
To obtain novel boron liquid crystals, we successfully combined MIDA boronates with chromophoric fluorene and fluorenone mesogenic units and studied the influence of alkoxy versus alkyl side chains by differential scanning calorimetry (DSC), polarising optical microscopy (POM) and X-ray diffraction (XRD). The fluorenes showed broader temperature ranges of the SmA phase compared to their fluorenone counterparts. By replacing the alkoxy side chains with alkyl side chains, the mesophase ranges could be significantly enlarged and the thermal stability increased.
Introduction
Fluorenes and fluorenones are important building blocks for semiconducting, chromophoric and NLO materials [Citation1,Citation2], which are relevant for OLED devices [Citation3], alignment layers [Citation4] and photonic OFET memory devices [Citation5]. Also, the incorporation of fluorenes and fluorenones into calamitic and discotic liquid crystals 1, 2, 3 is well established in the literature and the beneficial properties of both fluorene or fluorenone and mesogenic unit have been merged successfully () [Citation6–46]. Furthermore, fluorenone and fluorenol core units turned out to be strong promoters for higher order liquid crystalline phases such as SmC and SmC* in thermotropic and lyotropic systems [Citation47–49]. Moreover, electron-poor fluorenones such as 2,4,7-trinitrofluorenone (TNF) have been used to stabilise mesophases in donor-acceptor complexes [Citation50–56].
Scheme 1. Overview of preliminary work on liquid crystalline fluorenes, fluorenones and MIDA boronates.
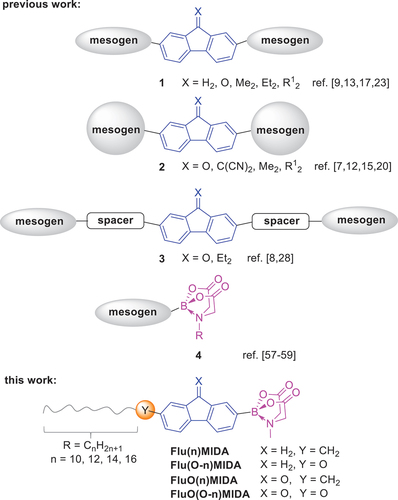
We have recently developed thermotropic liquid crystals 4, carrying the bulky and highly polar N-methyl-iminodiacetic acid (MIDA) boronate group [Citation57–59]. In contrast to non-mesomorphic precursors such as pinacol borolanes and boronic acids, it was found that the pronounced polarity of the MIDA group, indicated by the high dipole moment of 7.6 D as compared to 2.8 D for the corresponding boronic acid was essential for mesophase formation and stabilisation [Citation57]. While the previous studies mostly focused on phenyl, biphenyl and heteroaryl mesogenic units [Citation57–59], we were curious how the combination of fluorene or fluorenone moieties with the MIDA unit would affect the liquid crystalline self-assembly of the resulting fluorene or fluorenone MIDA boronates (). Our study revealed that the merging of the two molecular entities is indeed beneficial for mesophase formation. However, as detailed below, the type of side chain, i.e. alkyl vs alkoxy plays a major role with regard to mesophase stabilisation.
Results and discussion
Synthesis of the fluorene and fluorenone MIDA boronates
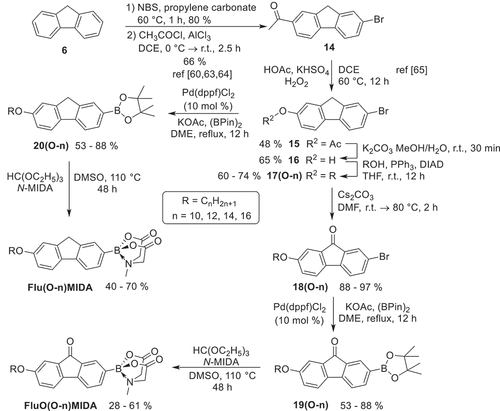
The introduction of alkyl vs alkoxy side chains at the fluorene (fluorenone) required two different synthetic strategies. The synthesis of alkyl-substituted fluorene MIDA boronates Flu(n)MIDA and fluorenone MIDA boronates FluO(n)MIDA (n = 10, 12, 14, 16) is outlined in . Starting from commercially available fluorene 6, Friedel Crafts-type acylation [Citation42] with fatty acid chlorides 7 and AlCl3 yielded the 2-acylfluorenes 8 in 57–64%. Subsequent Wolff-Kishner reduction with hydrazine hydrate and KOH provided the 2-alkylfluorenes 9 in 38–48%, which were then brominated with NBS in propylene carbonate [Citation60] to give the 2-alkyl-7-bromofluorenes 10(n) in 46–65%. Moderate yields in the Wolff-Kishner reduction were probably due to partial oxidation of the fluorene methylene unit to the corresponding carbonyl group. Even the use of an excess hydrazine hydrate (3.0 equiv.) could not completely suppress these side reactions, however any byproducts could be fortunately removed by column chromatography. 2-Alkyl-7-bromo-fluorenes 10(n) were then converted in two steps via Miyaura borylation in the presence of Pd(dppf)Cl2, KOAc and B(pin)2 [Citation61] to the pinacol borolanes 11(n), followed by treatment with triethylorthoformiate and N-MIDA to give the desired alkyl fluorene MIDA boronates Flu(n)MIDA in 59–69%. In order to get access to the fluorenone series FluO(n)MIDA, 2-alkyl-7-bromo-fluorenes 10(n) were oxidised under aerobic conditions with Triton BⓇ in pyridine following the procedure by Eakins [Citation62] to give the 2-alkyl-7-bromofluorenones 12(n) in 60–74%, which were then converted in two steps as described above to the 2-alkyl-fluorenone MIDA boronates FluO(n)MIDA in 56–71%.
Scheme 2. Synthesis of the fluorene and fluorenone MIDA boronates Flu(n)MIDA, FluO(n)MIDA with alkyl side chains.
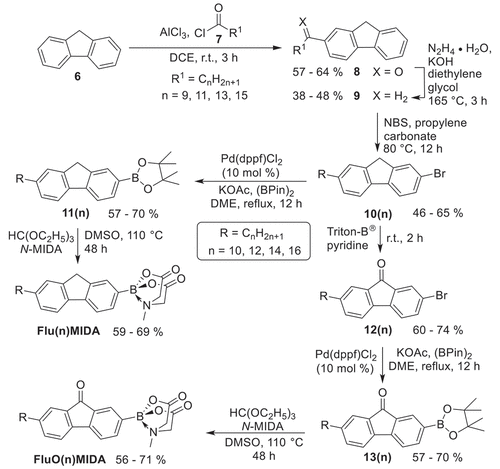
The synthesis of the alkoxy-substituted fluorene or fluorenone MIDA boronates Flu(O-n)MIDA and FluO(O-n)MIDA is outlined in . Following the known procedure fluorene 6 was brominated with NBS [Citation60,Citation63] followed by a Friedel Crafts acylation with acetyl chloride and AlCl3 [Citation64] to give 2-acetyl-7-bromo-fluorene in 53%. Subsequent Bayer-Villinger type oxidation with HOAc, KHSO4 and H2O2 [Citation65] yielded 2-O-acetyl-7-bromofluorene 15 in 48%, followed by saponification to give 2-hydroxy-7-bromo-fluorene 16 in 65%. In order to avoid oxidation of the methylene bridge in the fluorenes during Williamson etherification an alternative protocol was sought. Thus, 2-hydroxy-7-bromo-fluorene 16 was submitted to Mitsunobu etherification with alcohols, PPh3 and DIAD [Citation66] to provide the 2-alkoxy-7-bromo-fluorenes 17(O-n) in 60–74%. Compounds 17(O-n) were converted in two steps as described above via borylation and introduction of MIDA into the 7-alkoxy-fluorene MIDA boronates Flu(O-n)MIDA. The corresponding 2-alkoxy-fluorenone MIDA boronates FluO(O-n)MIDA were obtained from 2-alkoxy-7-bromo-fluorene 17(O-n) by aerobic oxidation in the presence of Cs2CO3 in DMF [Citation67], giving the fluorenones 18(O-n) in 88–97%, which were then converted in two steps to the 2-alkoxyfluorene MIDA boronates FluO(O-n)MIDA.
Mesomorphic properties of alkoxybromofluorenes, alkyl- and alkoxy-substituted fluorene and fluorenone MIDA boronates
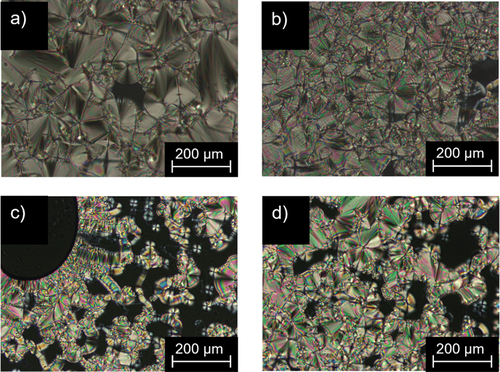
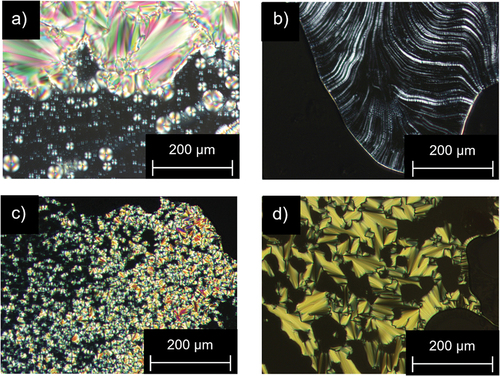
Preliminary investigations of all alkyl- and alkoxy fluorene and fluorenone derivatives by polarising optical microscopy (POM) revealed that most precursors and fluorene or fluorenone intermediates 10(n), 12(n), 18(O-n) were non-mesomorphic and showed only isotropic melting. The only exception were 2-alkoxy-7-bromofluorenes 17(O-n), which showed mosaic-like textures upon cooling (). In addition, for derivatives with side chains C12 or C14 17(O-12) and 17(O-14) respectively, at higher temperatures Maltese crosses with homeotropic alignment were visible ().
Figure 1. (Colour Online) POM images of 17(O-10) at (a) 82°C and (b) 45°C and (c) 17(O-12) at 85°C and (d)75°C (200 × magnification, taken upon cooling from the isotropic phase (5 K · min−1).
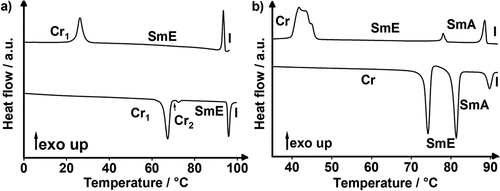
Differential scanning calorimetry (DSC) of 17(O-10) revealed upon heating an endothermal crystal to crystal transition at 67°C, followed by a hardly visible endothermal melting transition at 71°C and endothermal clearing transition at 96°C (). In the subsequent cooling cycle, the isotropic to mesophase transition appeared at 94°C and crystallisation at 27°C with a strong hysteresis due to supercooling. In case of 17(O-12) an endothermal melting transition at 74°C, followed by an endothermal mesophase to mesophase transition at 81°C and clearing transition at 90°C were visible upon heating, which appeared in the cooling cycle at 89°C, 78°C and 46°C, respectively. Similar behaviour was found for 17(O-14), while the highest homologue 17(O-16) displayed only a monotropic mesophase. The DSC results are summarised in .
Table 1. Phase transition temperatures (in °C) and phase transition enthalpies (in kJ/mol) of 17(O-n). a,b.
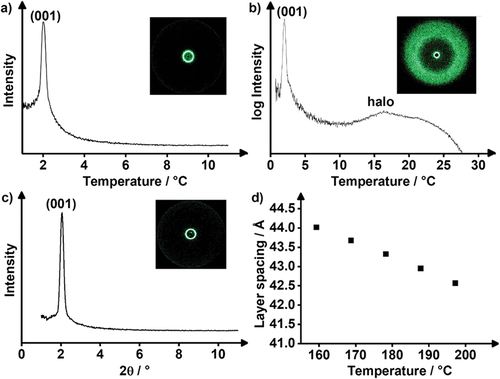
In order to assign mesophase geometries compound 17(O-12) was examined by X-ray diffraction (XRD) experiments (, ). At 85°C, a sharp reflection at 27.24 Å was detected in the small-angle section, which was assigned as the (001) layer reflection of a SmA phase (d = 28.34 Å). In the wide-angle section, a diffuse halo around 4.6 Å was observed, which was caused by the fluid alkoxy side chains (). The SAXS diffractogram at 57°C also showed the distinct (001) layer reflection at 27.24 Å and in the WAXS the halo was visible. However, the halo was superimposed by three sharp wide-angle reflections at 4.81 Å, 4.26 Å and 3.50 Å, which were indexed as (110), (200) and (210) reflection of a smectic E phase. Thus, the low-temperature phase was assigned as SmE phase with lattice parameters a = 8.52 Å, b = 5.83 Å, c = 27.24 Å in agreement with the known 4-pentyloxy-4’-bromo-benzylidene aniline [Citation68] and 2-bromo-6-dodecyloxyazulene [Citation69] displaying SmE phases. By using the formula Z = ● a ● b ● NA/M [Citation70] the number Z of molecules per unit cell was calculated to Z = 2. The observed layer distance of 27.24 Å is somewhat larger than the calculated molecular lengths of 17(O-12) in the all-trans conformation (Lcalc = 25.74 Å). In case of a smectic monolayer a smaller d-value as compared to Lcalc would be expected. In order to rationalise the experimentally observed results, a smectic bilayer with partial interdigitation of the side chains might be assumed. According to the literature in soft crystalline SmE phases, the aromatic mesogens are oriented in an antiparallel herringbone pattern [Citation71,Citation72]. Our proposed packing model with antiparallel fluorene units is shown in . For the higher temperature SmA phase of 17(O-12) also a partially interdigitated bilayer SmA was proposed in order to explain the layer distance of 28.34 Å.
Figure 3. (Colour Online) (a) SAXS profile and (b) WAXS profile of 17(O-12) at 57°C (black) and 85°C (blue).
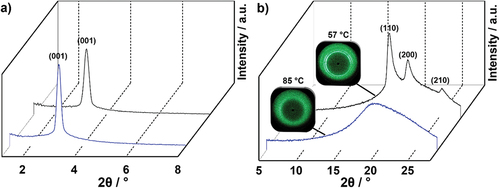
Figure 4. (Colour Online) Proposed packing model of the SmE phase of 17(O-12) in (a) top view, (b) side view and the proposed packing model of the SmA bilayer (c).
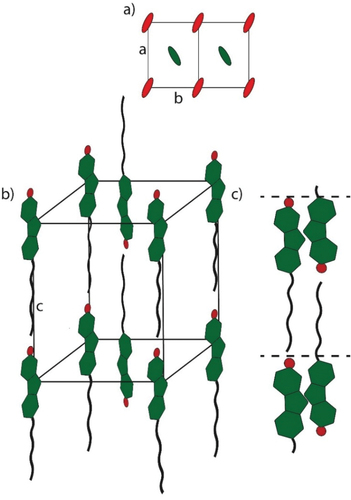
Table 2. X-ray diffraction data of bromoalkoxyfluorene 17(O-12). The measurements were performed during cooling from the isotropic liquid phase. The halo was determined from WAXS.
After having solved the mesophase assignment of 2-alkoxy-7-bromofluorenes 17(O-n), we investigated the fluorene and fluorenone MIDA boronates Flu(n)MIDA, Flu(O-n)MIDA, FluO(n)MIDA, FluO(O-n)MIDA by DSC (see and ) and POM (). A typical DSC curve is shown for alkyl fluorene MIDA boronate Flu(12)MIDA in . Upon 1st heating an endothermal melting transition at 186°C and a clearing transition at 257°C were observed. Upon 1st cooling, the mesophase reappeared at 250°C followed by a glass transition at 128°C, which was hardly visible in the DSC. Upon subsequent heating/cooling cycles, decomposition was observed. Similar results were obtained for the higher homologues Flu(n)MIDA (Figure S1–S4, ESI). In case of the alkoxy fluorene MIDA boronates, Flu(O-n)MIDA peaks were sometimes visible in the cooling cycle () and sometimes not due to thermal decomposition (Figure S1–S4, ESI). The DSC results are summarised in . For some samples, glass transitions could not be observed during the DSC measurements (). These transitions were determined via POM. This phenomenon was also observed during our previous studies on MIDA boronates [Citation57–59]. For this purpose, the corresponding sample was heated and cooled with the same rates as in the DSC. If a phase transition occurred, the heating and cooling was reiterated with a rate of 2 K/min to determine the transition temperature as accurate as possible.
Figure 5. (Colour Online) DSC traces of (a) Flu(12)MIDA, (b) Flu(O-12)MIDA, (c) FluO(12)MIDA (phase transitions (Tg) determined via POM due to the absence in DSC traces) and (d) Flu(O-12)MIDA; 1st heating/cooling of (a-c) 10 K · min−1 and 1st, 2nd, 3rd heating/cooling of d), 10 K · min−1).
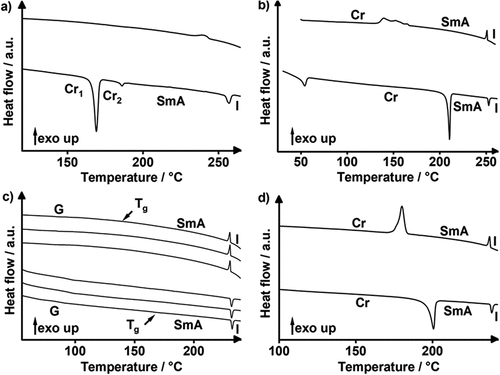
Figure 6. (Colour Online) Results from DSC measurements (rounded values, 1st heating cycle; rate 10 K·min− 1) of Flu(n)MIDA, Flu(o-n)MIDA, FluO(n)MIDA and FluO(O-n)MIDA. Gray: crystalline phase; blue: smectic a phase.
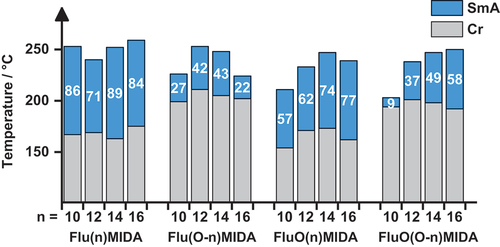
Table 3. Phase transition temperatures T (in °C) and phase transition enthalpies (in kJ/mol) of Flu(n)MIDA, FluO(n)MIDA, Flu(o-n)MIDA and FluO(O-n)MIDA.a,b
Also the series of alkoxy fluorene MIDA boronates Flu(O-n)MIDA suffered from thermal decomposition upon prolonged heating/cooling cycles. With some exceptions, the alkyl- and alkoxy-substituted fluorenone MIDA boronates displayed more reproducible DSC curves. Typical examples are shown for FluO(12)MIDA and FluO(O-12)MIDA respectively ().
The series of alkyl-fluorene MIDA boronates Flu(n)MIDA showed melting points in range of 166–174°C and clearing temperatures close to 250°C resulting in broad mesophase widths of 71–89 K irrespective of the chain lengths ().
In contrast, the corresponding alkoxy-fluorene MIDA boronates Flu(O-n)MIDA displayed higher melting and lower clearing transitions with much smaller mesophase widths of only 22–43 K as compared to the alkyl series. The maximum mesophase stability and temperature range was found for the C12 and C14 derivatives.
For the alkyl-fluorenone MIDA boronates FluO(n)MIDA melting and clearing temperatures increased with increasing chain lengths up to C14 and then decreased, resulting in phase widths of 57–77 K (). In general, alkyl-fluorenone MIDA boronates FluO(n)MIDA and their fluorene counterparts Flu(n)MIDA showed better thermal stability and reproducibility as compared to alkoxy-fluorenone MIDA boronates FluO(O-n)MIDA and alkoxy-fluorene MIDA boronates Flu(O-n)MIDA respectively.
In the alkoxy-fluorenone MIDA boronate series FluO(O-n)MIDA melting transitions were significantly higher as compared to the alkyl series FluO(n)MIDA, but remained independent of the chain lengths. On the other hand, clearing transitions increased significantly with increasing chain lengths. Thus, FluO(O-16)MIDA showed the broadest and most stable mesophase (58 K) within the series.
When comparing MIDA boronates with alkyl vs alkoxy side chains (), the most obvious influence of the alkyl side chains is the significant decrease of the melting points. A similar observation was reported previously for the melting into the nematic phase of calamitic alkyl-cyanobiphenyls (vs alkoxy-cyanobiphenyls) [Citation73–77] and cyanophenyl-4-alkyl-benzoates (vs cyanophenyl-4-alkoxy-benzoates) [Citation8,Citation78–84]. These experimental results might be rationalised by the lower polarisability of the alkyl chain as compared to the alkoxy chain, resulting in lower anisotropy of the connection between aryl moiety and alkyl side as compared to aryl – alkyloxy connections [Citation85]. Upon transition from the crystalline to the mesophase these dipolar interactions must be overcome in order to self-assemble into the liquid crystalline phase. The lower anisotropy of derivatives with alkyl chains thus results in reduced transition temperatures. Furthermore, according to Arakawa, the different bond geometries of aryl unit plus side chains have to be considered () [Citation85]. In case of alkyl-substituted aryl moieties an out-of-plane conformation is preferred in order to avoid unfavourable steric interactions between the CH2 unit and the ortho-H atoms. In contrast, for alkoxy-substituted aryl moieties an in-plane conformation is preferred because both lone pairs at the O atom and the C–H bonds of the neighbouring CH2 unit can adopt a gauche conformation with respect to the ortho-H atoms, thus minimising any steric interactions [Citation85]. Thus, the out-of-plane conformation (i.e. the orthogonal arrangement of the alkyl chain with respect to the plane of the aryl ring) results in a decreased tendency for crystallisation, lower crystallisation temperatures or even glass transitions and vitrification. It should be noted that Imrie recently discussed similar arguments to rationalise the influence of alkyl vs alkoxy vs thioether side chains in calamitic dimers with cyanobiphenyl [Citation86,Citation87] and/or imine unit [Citation88] showing twist-bend nematic phases. Significantly higher values of TNI were found for dimers with alkoxy or thioether chains, which was explained by the in plane conformation of the alkoxy chain and the higher shape anisotropy of these dimers [Citation86,Citation87]. DFT calculation revealed bond angles of C–O–C (119°) > C–C–C (113.5°) > C–S–C (100.5°) in agreement with the observed trend of TNI values [Citation88].
Figure 7. Out-of plane and in-plane conformations of alkyl and alkoxy chains on six-membered aromatic rings. The figure was adapted from ref [Citation85].
![Figure 7. Out-of plane and in-plane conformations of alkyl and alkoxy chains on six-membered aromatic rings. The figure was adapted from ref [Citation85].](/cms/asset/a57807ee-4fa8-41ac-829f-47ce6f276e7f/tlct_a_2319626_f0007_b.gif)
Under the POM fan-shaped textures, Maltese cross defects as well as areas with homeotropic alignment were observed for all four MIDA boronate series ().
Figure 8. (Colour Online) POM images of (a) Flu(12)MIDA at 220°C; (b) Flu(O-12)MIDA at 228°C; (c) FluO(12)MIDA at 225°C; (d) FluO(O-12)MIDA at 229°C (200 x magnification, taken upon cooling from the isotropic phase (5 K · min−1).
In order to assign the phase geometries of the fluorene and fluorenone MIDA boronates exemplarily SAXS and WAXS experiments were carried out for Flu(O-12)MIDA, FluO(O-12)MIDA, Flu(16)MIDA and FluO(14)MIDA. Unfortunately, decomposition of the samples Flu(16)MIDA and FluO(O-12)MIDA was observed during irradiation time and therefore XRD measurements were abandoned for these samples.
Flu(O-12)MIDA showed a sharp reflection in the small-angle region at 2.03, which was assigned as (001) reflection of a SmA phase with a layer distance d = 43.4 Å (, ). In the wide-angle region, the broad halo around 5.16 Å was visible, resulting from the liquid like order of the alkoxy side chains. The d value of Flu(O-12)MIDA is significantly larger than the calculated molecular lengths Lcalc = 27.6. But smaller than 2 Lcalc, which indicates the presence of a smectic bilayer (SmA) in agreement with the packing behaviour of the previously reported alkoxy-pyridine, -pyrimidine, and ester-substituted phenyl MIDA boronates [Citation58,Citation59].
Table 4. X-ray diffraction data for fluorene Flu(12)MIDA and fluorenone FluO(14)MIDA. The measurements were performed during cooling from the isotropic liquid phase. The halo was determined from WAXS.
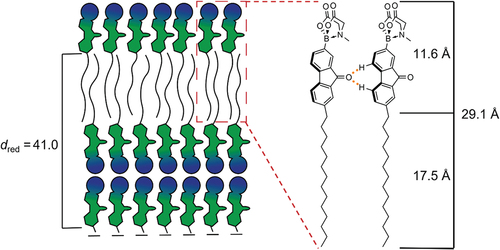
In a similar fashion FluO(14)MIDA showed the sharp (001) reflection at 43.69 Å and the broad halo around 5.23 Å, which is in good agreement with the assigned SmA phase. Temperature-dependent XRD measurements () showed a decreasing layer distance d with increasing temperature further confirming the SmA phase. Again, the reduced layer distance dred = 41.0 Å was larger than the calculated molecular lengths (Lcalc = 29.1 Å) in the all-trans conformation, but smaller than the twofold molecular length (58.2 Å). We propose a packing model of a smectic bilayer (SmA) (), consisting of a polar sublayer with MIDA headgroups stacked antiparallel with respect to each other, aromatic sublayers consisting of the fluorenone moiety and an aliphatic sublayer consisting of the fully interdigitated alkyl chains. Such a packing model would also rationalised the self-assembly of Flu(O-12)MIDA in the SmA phase. However, in contrast to the latter derivative, the fluorenone MIDA boronate FluO(14)MIDA can further stabilise the smectic layers by additional hydrogen bonds between the carbonyl group and the (C-4)-H and (C-7)-H bonds of the neighbouring fluorenone unit [Citation89].
Figure 9. (Colour Online) (a) SAXS profile and (b) WAXS profile of Flu(12)MIDA and (c) SAXS profile and (d) temperature-dependant layer spacing of FluO(14)MIDA with the corresponding 2D-pattern. (WAXS profile see ESI fig. S9).
Although we could only obtain XRD data for two derivatives, the similar behaviour of the other members in the DSC and particular in the POM investigations strongly suggested that the four series Flu(n)MIDA, Flu(O-n)MIDA, FluO(n)MIDA, FluO(O-n)MIDA behaved similarly regarding their liquid crystalline self-assembly.
Conclusion
In order to understand the liquid crystalline self-assembly of mesogens carrying the highly polar MIDA group containing alkyl- or alkoxy-substituted fluorene and fluorenone core units four series of novel calamitic liquid crystals were synthesised starting from fluorenone. Among the synthetic intermediates 2-alkoxy-7-bromofluorenes 17(O-n) were mesomorphic and showed SmE phases for chain lengths C10 – C16, while members with C12, C14 chain 17(O-12), 17(O-14) displayed additional SmA phases. The targeted alkyl- or alkoxy-fluorene and -fluorenone MIDA boronates Flu(n)MIDA, Flu(O-n)MIDA, FluO(n)MIDA, FluO(O-n)MIDA displayed SmA phases. However, due to their high clearing temperatures (close to 250°C) many derivatives suffered from thermal decomposition upon repetitive heating/cooling cycles.
Merging flat fluorene or fluorenone cores with bulky MIDA head group leads to stable SmA phases. When comparing the influence of the additional lateral dipole moment in fluorenones as compared to fluorenes on the mesophase stability and temperature range with the influence of the side chain (alkyl vs. alkoxy), the side chain has a much more pronounced impact on the mesomorphic properties. In other words, MIDA boronates with alkyl side chains displayed broader and more stable SmA phases as compared to the MIDA boronates with alkoxy side chains. On the other hand, when side chains were kept constant, the replacement of the fluorenes by fluorenones had a much smaller influence.
In general, fluorenes displayed broader temperature ranges of the SmA phase in comparison with the fluorenones. More importantly, alkyl side chains resulted in significantly decreased melting transitions as compared to derivatives with alkoxy side chains, which was rationalised by the reduced anisotropy of the connecting unit between aryl moiety and side chain and the conformational preference of an out-of-plane conformation for alkyl-substituted derivatives vs. an in-plane conformation for the alkoxy-substituted derivatives.
Our results revealed that the polar MIDA unit can be successfully combined with chromophoric mesogenic units to obtain novel liquid crystals. Moreover, the replacement of the archaetypal alkoxy side chains by alkyl side chains provides a tool to broaden the mesophase range and to overcome thermal stability problems, which might be useful for other calamitic liquid crystal classes as well.
Supplemental Material
Download PDF (6.8 MB)Acknowledgments
Generous financial support by the Deutsche Forschungsgemeinschaft (grant # LA907/20-1, HBFG and shared instrumentation grant no. INST 41/897-1 FUGG for 700 MHz-NMR and no. INST 41/1136-1 FUGG for HR LC-MS (ESI)), the DAAD PROCOPE project “WELCHYNA”# 57658198, the Ministerium für Wissenschaft, Forschung und Kunst des Landes Baden-Württemberg, Fonds der Chemischen Industrie and the Carl-Schneider-Stiftung Aalen (shared instrumentation grant) is gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02678292.2024.2319626.
Additional information
Funding
References
- Beaupré S, Boudreault PLT, Leclerc M. Solar‐Energy Production and Energy‐Efficient Lighting: Photovoltaic Devices and White‐Light‐Emitting Diodes Using Poly (2, 7‐fluorene), Poly (2, 7‐carbazole), and Poly (2, 7‐dibenzosilole) Derivatives. Adv Mater. 2010;22(8):E6–E27. doi: 10.1002/adma.200903484
- Semin S, Li X, Duan Y, et al. Nonlinear optical properties and applications of fluorenone molecular materials. Adv Opt Mater. 2021;9(23):2100327. doi: 10.1002/adom.202100327
- Tao SL, Peng ZK, Zhang XH, et al. Highly efficient non‐doped blue organic light‐emitting diodes based on fluorene derivatives with high thermal stability. Adv Funct Mater. 2005;15(10):1716–1721. doi: 10.1002/adfm.200500067
- Wei X, Zhang Z, Zhang Q, et al. Br as an alignment layer for liquid crystalline conjugated polymer chain orientation. ChemPhotoChem. 2022;6(6):e202100288. doi: 10.1002/cptc.202100288
- Mulia T, Mumtaz M, Ercan E, et al. Exploring the charge-trapping behavior of self-assembled sugar-based block copolymers with a pendent design in photoassisted memory. ACS Appl Polym Mater. 2023;5(6):3898–3911. Published online 2023. doi: 10.1021/acsapm.2c02214
- Veeraprakash B, Pratap G, Lobo NP, et al. Influence of the thiophene ring on the molecular order of structurally simple π-conjugated Smectogens: 13C NMR study. Chemphyschem. 2023;24(12):e202300074. Published online 2023. doi: 10.1002/cphc.202300074
- Zhao H, Cheng X. Fluorene thiophene α-cyanostilbene hexacatenar-generating LCs with Hexagonal columnar phases and gels with helical morphologies as well as a light-emitting LC display. Int J Mol Sci. 2023;24(11):9337. doi: 10.3390/ijms24119337
- Arakawa Y, Komatsu K, Tsuji H. 2, 7-substituted fluorenone-based liquid crystal trimers: twist-bend nematic phase induced by outer thioether linkage. Phase Transit. 2022;95(4):331–339. doi: 10.1080/01411594.2022.2044040
- Shi Y, He W, Zhang Y, et al. Mesophase properties of fluorene-core mesogens and their effects on blue phase liquid crystals. Liq Cryst. 2022;49(5):679–689. doi: 10.1080/02678292.2021.2000055
- Brey D, Popp W, Budakoti P, et al. Quantum dynamics of electron–hole separation in stacked perylene Diimide-based self-assembled nanostructures. J Phys Chem C. 2021;125(45):25030–25043. doi: 10.1021/acs.jpcc.1c06374
- Yamamoto H, Inagaki T, Park J, et al. Helical network polymers embodying high dissymmetry factors in circularly polarized luminescence: photocrosslinking polymerization of acrylate derivatives in chiral smectic liquid crystals. Macromolecules. 2021;54(19):8977–8986. doi: 10.1021/acs.macromol.1c01146
- Hang JF, Lin H, Zhao KQ, et al. Butterfly mesogens based on carbazole, fluorene or fluorenone: mesomorphous, gelling, photophysical, and photoconductive properties. Eur J Org Chem. 2021;2021(13):1989–2002. doi: 10.1002/ejoc.202100108
- Liu X, Zuo R, Li N, et al. First biscyanovinyl diphenylfluorenone-based hexacatenars: synthesis, liquid crystal and photophysical properties. Phase Transit. 2021;94(3–4):245–255. doi: 10.1080/01411594.2021.1931201
- Bhowmik PK, Jo TS, Koh JJ, et al. Poly (pyridinium salt) s containing 2, 7-diamino-9, 9′-dioctylfluorene moieties with various organic counterions exhibiting both lyotropic liquid-crystalline and light-emitting properties. Molecules. 2021;26(6):1560. doi: 10.3390/molecules26061560
- Li B, Liu Y, Tao Y, et al. Concentration-dependent conformational isomerization of fluorenone-based polycatenar in 2D polymorphic self-assembly. J Phys Chem C. 2020;124(46):25396–25402. doi: 10.1021/acs.jpcc.0c07996
- Bader K, Müller C, Molard Y, et al. Fluorenone imidazolium salts as novel de Vries materials. RSC Adv. 2020;10(40):23999–24016. doi: 10.1039/d0ra04650g
- Dmochowska E, Bombalska A, Kula P. Synthesis and mesomorphic properties of four ring, rod-like fluorene derivatives–the influence of the lateral substitution on mesomorphic properties of 2, 7-bis (4-alkylphenyl)-fluorenes. Liq Cryst. 2020;47(1):17–27. doi: 10.1080/02678292.2019.1622047
- Jasiurkowska-Delaporte M, Rozwadowski T, Juszyńska-Gałązka E, et al. Relaxation dynamics and crystallization study of glass-forming chiral-nematic liquid crystal S, S-2, 7-bis (4-pentylphenyl)-9, 9-dimethylbutyl 9H-fluorene (5P-Am* FLAm*-P5). Eur Phys J E. 2019;42(9):1–11. doi: 10.1140/epje/i2019-11887-6
- Boopathi AA, Janani K, Lobo NP, et al. 13C NMR investigations of hairy-rod-like π-conjugated mesogens. J Phys Chem B. 2019;123(26):5651–5664. doi: 10.1021/acs.jpcb.9b04282
- Zhao K, Xiao Y, Guo C, et al. Control the self-assembly of fluorenone-based polycatenars by tuning chain length. Tetrahedron. 2019;75(3):409–415. doi: 10.1016/j.tet.2018.12.009
- Pratap G, Narasimhaswamy T, Shanmugam P. Palladium (II) catalyzed arylation and methylene oxidation of 2, 7‐Dibromo fluorenes with Heteroaryl Esters: synthesis of Mesogenic2‐Heteroaryl and 2, 7‐Diheteroaryl‐9‐fluorenones. ChemistrySelect. 2019;4(5):1795–1799. doi: 10.1002/slct.201803612
- Kim SW, Lee DM, Kim JH, et al. Highly polarised electroluminescence in low aspect ratio mesogenic molecules. Liq Cryst. 2019;46(7):1136–1144. doi: 10.1080/02678292.2018.1558465
- Gupta S, Choudhury T, Dmochowska E, et al. A new mesogenic fluorene derivative: 2, 7-bis (4-pentylphenyl)-9, 9-diethyl-9H-fluorene. Liq Cryst. 2019;46(4):543–549. doi: 10.1080/02678292.2018.1512169
- Yang Y, Wang H, Wang HF, et al. Molecular engineering of mesomorphic fluorene-bridged triphenylene triads: thermotropic nematic/columnar mesophases, and p-type semiconducting behavior. Cryst Growth Des. 2018;18(8):4296–4305. doi: 10.1021/acs.cgd.8b00083
- Zhao KQ, Gao Y, Yu WH, et al. Discogens possessing aryl side groups synthesized by Suzuki Coupling of Triphenylene Triflates and their Self‐Organization behavior. Eur J Org Chem. 2016;2016(16):2802–2814. doi: 10.1002/ejoc.201600270
- Thiery S, Heinrich B, Donnio B, et al. Luminescence modulation in liquid crystalline phases containing a dispiro [fluorene-9, 11′-indeno [1, 2-b] fluorene-12′, 9′′-fluorene] core. J Mater Chem C. 2014;2(21):4265–4275. doi: 10.1039/C4TC00038B
- Zabulica A, Perju E, Bruma M, et al. Novel luminescent liquid crystalline polyazomethines. Synthesis and study of thermotropic and photoluminescent properties. Liq Cryst. 2014;41(2):252–262. doi: 10.1080/02678292.2013.852258
- Kim H, Kim J, Ka JW, et al. Synthesis and thermal transition behaviour of new reactive mesogens with propiolate (-C≡ C-COO-) linkages. Liq Cryst. 2012;39(7):803–811. doi: 10.1080/02678292.2012.681810
- Marin L, Perju E, Damaceanu MD. Designing thermotropic liquid crystalline polyazomethines based on fluorene and/or oxadiazole chromophores. Eur Polym J. 2011;47(6):1284–1299. doi: 10.1016/j.eurpolymj.2011.03.004
- Lehmann M, Köhn C, Figueirinhas JL, et al. Biaxial nematic mesophases from shape‐persistent mesogens with a fluorenone bending unit. Chem: Eur J. 2010;16(28):8275–8279. doi: 10.1002/chem.201001214
- Sović T, Kappaun S, Koppitz A, et al. Main‐Chain Liquid Crystalline Polymers Based on Bis‐Etherified 9, 9‐Dihexyl‐2, 7‐bis (4′‐hydroxy‐1, 1′‐biphen‐4‐yl) fluorenes. Macromol Chem Phys. 2007;208(13):1458–1468. doi: 10.1002/macp.200600657
- Güntner R, Farrell T, Scherf U, et al. Novel rod-like fluorene-trimers exhibiting smectic LC mesophases. J Mater Chem. 2004;14(17):2622–2626. doi: 10.1039/B406339M
- Lehmann M, Levin J. Rigid phenylene ethynylene units linked by a V-shaped centre. An approach to biaxial nematogens? Mol Cryst Liq Cryst. 2004;411(1):273–281. doi: 10.1080/15421400490435260
- Reddy KP, Brown TL. Synthesis and thermal behaviour of mesomorphic cu II Schiff’s base complexes. J Mater Chem. 1991;1(5):757–764. doi: 10.1039/JM9910100757
- Hoatson GL, Goetz JM, Palffy-Muhoray P, et al. 2H-NMR study of orientational order in binary mixtures: a nematic phase of biaxial molecules. Mol Cryst Liq Cryst. 1991;203(1):45–59. doi: 10.1080/00268949108046045
- Hoatson GL, Goetz JM. Experimental deuteron magnetic resonance study of orientational order in the nematic phase of a binary mixture of biaxial molecules. J Chem Phys. 1991;94(5):3885–3895. doi: 10.1063/1.460664 Article history.
- Takatoh K, Sakamoto M. Liquid crystalline derivatives containing fluorene and fluorenone structures. Mol Cryst Liq Cryst Inc Nonlinear Opt. 1990;182(1):339–349. doi: 10.1080/00268949008035764
- Takatoh K, Sunohara K, Sakamoto M. Mesophase transition of series materials containing fluorene, fluorenone and biphenyl structures with chiral end groups. Mol Cryst Liq Cryst Inc Nonlinear Opt. 1988;164(1):167–178. doi: 10.1080/00268948808072121
- Ziemnicka B, Doane JW. Nematic mesophases in 9-methyl and 9-bromo-2-fluorenyl 4-alkoxybenzoates. Mol Cryst Liq Cryst. 1987;150(1):361–373. doi: 10.1080/00268948708074809
- Sakashita H, Komori S, Kamon KK, et al. X-ray diffraction study on the reentrant smectic a phase in a binary liquid crystal system. Mol Cryst Liq Cryst. 1987;149(1):49–60. doi: 10.1080/00268948708082970
- Shimizu Y, Kusabayashi S. Occurrence of the smectic C phase for 3-(2-hydroxy-4-alkoxybenzylidene-amino) dibenzofurans. Mol Cryst Liq Cryst. 1986;132(3–4):221–233. doi: 10.1080/00268948608079543
- Davison IR, Hall DM, Sage I. Fluorene analogues of biphenyls: comparison of mesogenic behaviour. Mol Cryst Liq Cryst. 1985;129(1–3):17–35. doi: 10.1080/15421408408084163
- Arora SL, Ziemnicka B, Doane JW. Synthesis of some new fluorene esters with mesomorphic behavior. Mol Cryst Liq Cryst. 1985;127(1):341–351. doi: 10.1080/00268948508080850
- Arora SL, Vaz MAP, Doane JW. Mesomorphic behavior of some new fluorene compounds. Mol Cryst Liq Cryst. 1984;110(1–4):161–174. doi: 10.1080/00268948408074504
- Zhang D, Liu Y, Gao H, et al. α-cyanostilbene and fluorene based bolaamphiphiles: synthesis, self-assembly, and AIEE properties with potential as white-light emissive materials and light-emitting liquid crystal displays. J Mater Chem C. 2020;8(48):17474–17481. doi: 10.1039/D0TC04571C
- Lincker F, Attias AJ, Mathevet F, et al. Influence of polymorphism on charge transport properties in isomers of fluorenone-based liquid crystalline semiconductors. Chem Commun. 2012;48(26):3209–3211. doi: 10.1039/C2CC17276C
- Harjung MD, Schubert CPJ, Knecht F, et al. New amphiphilic materials showing the lyotropic analogue to the thermotropic smectic C* liquid crystal phase. J Mater Chem C. 2017;5(30):7452–7457. doi: 10.1039/C7TC02030A
- Roberts JC, Hudson ZM, Lemieux RP. The influence of alkoxy chain length on the ferroelectric properties of chiral fluorenol liquid crystals. J Mater Chem. 2008;18(28):3361. doi: 10.1039/b804673e
- McCubbin JA, Tong X, Zhao Y, et al. Directed metalation-cross coupling route to ferroelectric liquid crystals with a chiral fluorenol core: the effect of intermolecular hydrogen bonding on polar order. Chem Mater. 2005;17(10):2574–2581. doi: 10.1021/cm047860p
- Kouwer PH, van den Berg O, Jager WF, et al. Induced liquid crystalline diversity in molecular and polymeric charge-transfer complexes of discotic mesogens. Macromolecules. 2002;35(7):2576–2582. doi: 10.1021/ma011628c
- Usoltseva N, Bykova V, Kudrik E, et al. Induction of mesomorphic properties in non-mesogenic octa (decyloxy) phthalocyanines. Mol Cryst Liq Cryst Sci Technol. 2001;367(1):509–516. doi: 10.1080/10587250108028671
- Goldmann D, Mahlstedt S, Janietz D. Mesomorphic donor-acceptor twin molecules with covalently linked sheet-like pentaalkyne and nitrofluorenone sub-units. Liq Cryst. 1998;24(6):881–890. doi: 10.1080/026782998206713
- Fletcher ID, Omenat A, Serrano JL. The effects of steric interactions on the stability of smectic a Phases in mixtures of Calamitic, Organometallic complexes with 2, 4, 7-trinitro-9-fluor Enone. Anales de Química. Vol. 94. Springer; 1998. pp. 226–230.
- Boden N, Bushby RJ, Hubbard JF. Modulating the phase behaviour of lyotropic discotic liquid crystals by incorporation of an electron acceptor. Mol Cryst Liq Cryst Sci Technol. 1997;304(1):195–200. doi: 10.1080/10587259708046961
- Zamir S, Singer D, Spielberg N, et al. Columnar mesophases of octa-alkyloxydibenzopyrenes and their charge transfer complexes: synthesis, X-ray and NMR. Liq Cryst. 1996;21(1):39–50. doi: 10.1080/02678299608033794
- Möller M, VV T, Wendling J, et al. Observation of a nematic phase displayed by a polysiloxane with trinitrofluorenones as side groups. Makromol Chem Macromol Chem Phys. 1992;193(10):2659–2668. doi: 10.1002/macp.1992.021931014
- Wöhrle T, Gündemir R, Frey W, et al. Thermotropic MIDA boronates as a case study for the role of Dipolar interactions in liquid crystalline self‐assembly. Chem Eur J. 2017;23(17):4149–4159. doi: 10.1002/chem.201605648
- Schilling C, Schulz F, Köhn A, et al. Liquid crystalline benzoic acid Ester MIDA Boronates: synthesis and mesomorphic properties. Org Mater. 2020;2(4):288–299. doi: 10.1055/s-0040-1715900
- Schilling C, Bauer A, Knöller JA, et al. Tailoring boron liquid crystals: mesomorphic properties of iminodiacetic acid boronates. J Mol Liq. 2022;367:120519. doi: 10.1016/j.molliq.2022.120519
- Chavez IR, Cai M, Tlach B, et al. Benzobisoxazole cruciforms: a tunable, cross-conjugated platform for the generation of deep blue OLED materials. J Mater Chem C. 2016;4(17):3765–3773. doi: 10.1039/C5TC03622D
- Sun X, Qiu J, Strong SA, et al. Discovery of potent and novel S-nitrosoglutathione reductase inhibitors devoid of cytochrome P450 activities. Bioorg Med Chem Lett. 2011;21(19):5849–5853. doi: 10.1016/j.bmcl.2011.07.103
- Eakins GL, Alford JS, Tiegs BJ, et al. Tuning HOMO–LUMO levels: trends leading to the design of 9‐fluorenone scaffolds with predictable electronic and optoelectronic properties. J Phys Org Chem. 2011;24(11):1119–1128. doi: 10.1002/poc.1864
- Kang QK, Li Y, Chen K, et al. Rhodium‐catalyzed stereoselective deuteration of benzylic C–H bonds via reversible η6‐coordination. Angew Chem Int Ed. 2022;61(11):e202117381. doi: 10.1002/anie.202117381
- Kovalev AI, Shapovalov AV, Sukhorukova EV, et al. Synthesis and luminescent properties of branched oligophenylenefluorenes. Mendeleev Commun. 2011;21(1):9–11. doi: 10.1016/j.mencom.2011.01.004
- Urata Y, Hashimoto K, Mitani M, et al. Published online 2016.
- Purohit A, Radeke H, Azure M, et al. Synthesis and biological evaluation of pyridazinone analogues as potential cardiac positron emission tomography tracers. J Med Chem. 2008;51(10):2954–2970. doi: 10.1021/jm701443n
- Park KK, Tsou LK, Hamilton AD. Facile and selective aerobic oxidation of arylalkanes to aryl ketones using cesium carbonate. Synthesis. 2006;2006(21):3617–3620. doi: 10.1055/s-2006-950189
- Osiecka N, Massalska-Arodź M, Galewski Z, et al. Dynamics and phase transitions of 4-bromobenzylidene-4’-pentyloxyaniline and 4-bromobenzylidene-4’-hexyloxyaniline as studied by dielectric spectroscopy. Acta Phys Pol A. 2013;124(6):913–916. doi: 10.12693/APhysPolA.124.913
- Schulz F, Ehni P, Wank B, et al. Alkoxy-bromo-azulenes displaying ambient temperature smectic E-phases. Liq Cryst. 2021;48(6):832–843. doi: 10.1080/02678292.2020.1821919
- Lehmann M, Köhn C, Meier H, et al. Supramolecular order of stilbenoid dendrons: importance of weak interactions. J Mater Chem. 2006;16(5):441–451. doi: 10.1039/B510713J
- Iino H, Usui T, Ichi HJ. Liquid crystals for organic thin-film transistors. Nat Commun. 2015;6(1):6828. doi: 10.1038/ncomms7828
- Diele S, Tosch S, Mahnke S, et al. Structure and packing in smectic E and smectic a phases in the series of 4‐n‐Alkyloxy‐4′‐alkanoylbiphenyls. Cryst Res Technol. 1991;26(6):809–817. doi: 10.1002/crat.2170260625
- Gray GW, Mosley A. Trends in the nematic–isotropic liquid transition temperatures for the homologous series of 4-n-alkoxy-and 4-n-alkyl-4′-cyanobiphenyls. J Chem Soc Perkin Trans. 1976;1:97–102. doi: 10.1039/P29760000097
- Sarkar P, Sarkar P, Mandal P, et al. Molecular structure and packing in the crystalline state of 4-n-ethyl-4′-cyanobiphenyl (2CB) by single crystal X-ray diffractometry. Mol Cryst Liq Cryst Sci Technol. 1998;325(1):91–97. doi: 10.1080/10587259808025386
- Gray GW, Harrison KJ, Nash JA. New family of nematic liquid crystals for displays. Electron Lett. 1973;6(9):130–131. doi: 10.1049/el:19730096
- Oweimreen GA, Morsy MA. DSC studies on p-(n-alkyl)-p′-cyanobiphenyl (RCB’s) and p-(n-alkoxy)-p′-cyanobiphenyl (ROCB’s) liquid crystals. Thermochim Acta. 2000;346(1–2):37–47. doi: 10.1016/S0040-6031(99)00411-6
- Hulme DS, Raynes EP, Harrison KJ. Eutectic mixtures of nematic 4′-substituted 4-cyanobiphenyls. J Chem Soc, Chem Commun. 1974;(3): 98–99. 10.1039/C39740000098
- Neuvonen H, Neuvonen K, Pasanen P. Evidence of substituent-induced electronic interplay. Effect of the remote aromatic ring substituent of phenyl benzoates on the sensitivity of the carbonyl unit to electronic effects of phenyl or benzoyl ring substituents. J Org Chem. 2004;69(11):3794–3800. doi: 10.1021/jo035521u
- Oweimreen GA, Al-Tawfiq AM. Thermodynamics of solution of nonmesomorphic solutes at infinite dilution in the Isotropic and nematic phases of p-cyanophenyl p-n-alkylbenzoates. J Chem Eng Data. 1997;42(5):996–1003. doi: 10.1021/je970075r
- Balakrishnan NS, Bayle JP, Ho MS, et al. Determination of the orientational ordering of 4′-cyanophenyl-4-alkylbenzoates by 13C NMR. Liq Cryst. 1993;14(2):591–601. doi: 10.1080/02678299308027675
- Das MK, Sarkar G, Das B, et al. Determination of the orientational order parameter of the homologous series of 4-cyanophenyl 4-alkylbenzoate (n. CN) by different methods. J Phys Condens Matter. 2012;24(11):115101. doi: 10.1088/0953-8984/24/11/115101
- Zhu DL, Li HX, Xu ZM, et al. Visible light driven, nickel-catalyzed aryl esterification using a triplet photosensitiser thioxanthen-9-one. Org Chem Front. 2019;6(14):2353–2359. doi: 10.1039/C9QO00536F
- Mori A, Hashimoto M, Ujiie S. Self‐ordered states derived from 2‐benzoyloxy‐and 2‐benzoylamino‐5‐cyanotroponoids and their corresponding benzenoids: formation of hexagonal self‐assembly of liquid crystalline 2‐trialkoxybenzoylamino‐5‐cyanotroponoids in gel states through intramolecular hydrogen bonding and π–π interaction. Liq Cryst. 2008;35(9):1109–1128. doi: 10.1080/02678290802389571
- Gray GW, Hird M, Lacey D, et al. The synthesis and transition temperatures of some fluoro-substituted 4-cyanophenyl and 4-cyanobiphenyl-4′-yl 4-pentyl-and 4-butoxy-benzoates. Mol Cryst Liq Cryst. 1989;172(1):165–189. doi: 10.1080/00268948908042160
- Arakawa Y, Ishida Y, Shiba T, et al. Effects of alkylthio groups on phase transitions of organic molecules and liquid crystals: a comparative study with alkyl and alkoxy groups. Cryst Eng Comm. 2022;24(10):1877–1890. doi: 10.1039/D1CE01470F
- Paterson DA, Crawford CA, Pociecha D, et al. The role of a terminal chain in promoting the twist-bend nematic phase: the synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkyloxyanilinebenzylidene-4′-oxy) hexanes. Liq Cryst. 2018;45(13–15):2341–2351. doi: 10.1080/02678292.2018.1525503
- Walker R, Pociecha D, Strachan GJ, et al. Molecular curvature, specific intermolecular interactions and the twist-bend nematic phase: the synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkylanilinebenzylidene-4′-oxy) hexanes (CB6O. m). Soft Matter. 2019;15(15):3188–3197. doi: 10.1039/C9SM00026G
- Cruickshank E, Strachan GJ, Majewska MM, et al. The effects of alkylthio chains on the properties of symmetric liquid crystal dimers. New J Chem. 2023;47(15):7356–7368. doi: 10.1039/D2NJ06252F
- Miao K, Hu Y, Zha B, et al. Polymorphic self-assemblies of 2, 7-bis (decyloxy)-9-fluorenone at the Solid/Gas interface: role of C–H··· O☐ C Hydrogen Bond. J Phys Chem C. 2017;121(7):3947–3957. doi: 10.1021/acs.jpcc.7b00040