ABSTRACT
Background: Persons with aphasia (PWA) after stroke are less able or unable to communicate about their pain due to language, speech and/or cognitive impairment. Most commonly pain rating scales are used for the assessment of pain in PWA, which could not be applied to any patient aphasia because of their inability to communicate verbally their pain.
Aims: This review aims to investigate the prevalence and incidence of pain in PWA after stroke, establish which pain assessment instruments are used, and examine whether they are feasible, valid and reliable.
Methods & Procedures: A systematic literature search was made to identify studies on pain and pain assessment in PWA and persons without aphasia after stroke, or in patients with right and left hemispheric stroke. The COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN) checklist was used to evaluate the methodological quality of the studies and the properties of the measurement scales used.
Outcomes & Results: The search yielded 10 articles. The vertical, mechanical and horizontal Visual Analogue Scale, Faces Pain Scale, Verbal Rating Scale, Numeric Rating Scale, categorical site-of-pain scale, and a pictorial scale of pain intensity were used to assess pain, as were the Short-Form 36 Health Survey and the Dartmouth COOP Charts Quality of Life Scales that each have one pain item. Prevalence of pain in PWA after stroke was reported in two studies and ranged from 43.8–87.5%. Most studies described pain assessment in PWA after stroke with mild-to-moderate aphasia, while patients with severe aphasia were excluded. Various pain assessment tools were used but their feasibility, validity and reliability were generally of low methodological quality.
Conclusions: A feasible, reliable and valid instrument is not available for PWA after stroke.
Introduction
Stroke survivors experience significant pain, especially headache, shoulder pain, pain from increased muscle stiffness and central post-stroke pain. Post-stroke pain is a chronic neuropathic disorder after lesions in the central somatosensory system. It may occur not only directly after stroke but also years after (Mulla et al., Citation2015). Joint pain is equally common in patients with or without post-stroke pain (Klit, Finnerub, Overvad, Andersen, & Jensen, Citation2011). Shoulder pain and central post-stroke pain are distressing sequelae of stroke, with shoulder pain occurring in 19–74% and central post-stroke pain in 11% of patients (Kim, Citation2009; Raffaeli, Minella, Magnani, & Sarti, Citation2013). However, pain in persons with aphasia (PWA) after stroke is not well described due to difficulty with self-report assessment and (often) the inability of these patients to describe and communicate their pain (Smith, Bottemiller, Flemming, Cutrer, & Strand, Citation2013). It is unclear if this leads to under identification and under treatment of pain in PWA (Kehayia et al., Citation1997). Therefore, it is important that clinicians address the presence of pain in aphasia more appropriately. Registering the presence of pain with a self-report scale is particularly challenging in PWA. Self-report pain scales generally require respondents to understand verbal information to understand the instructions of the pain assessment instruments. Therefore, conducting self-report pain scales in patients with severe aphasia is not seldomly suitable because of the inability to understand the instructions to report whether they experience pain or able to rate their pain. For example, the Visual Analogue Scale (VAS) (Huskisson, Citation1974) requires patients to point to the position on the line to indicate how much pain they are currently feeling. Similar instructions are provided for the Numeric Rating Scale (NRS) (McCaffery & Beebe, Citation1993) and the Faces Pain Scale (FPS) (Wong & Baker, Citation1988). A description of traditionally used pain rating scales and their instructions are reported in . The combination of the inability to communicate pain because of aphasia, and the high prevalence of pain after stroke, suggets a need for adequate assessment of pain in this vulnerable population. Therefore, a systematic review was performed to evaluate the incidence and prevalence of pain among PWA and to establish which pain measurement instruments are being used. The main goal of this review was to examine whether these pain instruments are feasible, reliable and valid in PWA.
Table 1. Pain rating scales.
Methods
Search
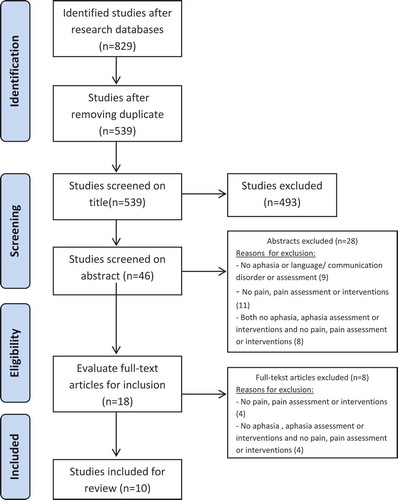
A systematic search guided by the PRISMA guidelines was conducted in June 2015 in the following databases PubMed (Medline), PsychInfo, Chinahl, Embase, Web of Science and Cochrane. Search strategies relevant to the database (using MeSH heading when appropriate) were developed to identify appropriate studies (Moher, Liberati, Tetzlaff, & Altman, Citation2009). Search terms included “stroke, cerebrovascular accident”, “aphasia, language, speech or communication disorder” and “pain, ache, pain measurement, pain assessment, pain scale”. Combined MeSH terms and text words for stroke, aphasia and pain are presented in Supplemental Material. Subsequently, relevant articles were included based on a three-step process; (1) screening based on the title, (2) screening based on the abstract and (3) screening based on the full-text of the articles. After screening the titles, all abstracts were read. Full-text was also reviewed, when it was not possible to assess eligibility based on abstract alone. Two reviewers (WA/CDV) independently selected studies based on title and abstract, and full-text papers were independently scrutinised by two reviewers (PS/CDV). The selected studies were compared and final inclusion was based on consensus between three reviewers (WA/CDV/PS).
Selection criteria
Studies meeting the following criteria:
Participants: adult stroke survivors (aged ≥ 18 years) at any stage after stroke and in any setting.
Participants: PWA or part of a cohort that included PWA and persons without aphasia.
Intervention and/or outcomes: reported outcomes of pain, pain measurement or pain assessment, or prescribed or used pain medication.
Exclusion criteria:
No aphasia.
No pain, pain assessment or interventions.
Both no aphasia, aphasia assessment or interventions and no pain, pain assessment or interventions.
No other restrictions (such as language or publication date) were utilised for the inclusion of articles.
Data extraction
A data extraction form was designed and tested before actual data extraction. Two reviewers (WA/CDV) independently extracted data on (1) characteristics of the study samples (e.g., sample size, setting, age, stroke); (2) presence of aphasia, outcome of the aphasia examination; (3) prevalence of pain and pain measurement scales used or assessment instruments and/or pain intervention; (4) findings of the included studies and (5) score of the methodological quality.
Quality assessment
The results of the review were organised to (a) describe the methodological quality of the studies and (b) summarise the measurement properties of the instruments utilised to measure pain taking into account the methodological quality.
The Consensus-based Standards for the selection of health status Measurement Instruments (COSMIN) checklist was used to critically evaluate and compare the measurement properties of the measurement instruments used and the methodological quality of the studies reporting use of those tools (Mokkink et al., Citation2010). The measurement properties contain the domains reliability, validity and responsiveness. In addition, the interpretability and feasibility was evaluated. The COSMIN checklist consists of nine boxes with 5–18 items concerning methodological standards for how each measurement property should be assessed. Each item was scored on a 4-point rating scale (i.e., “poor”, “fair”, “good” or “excellent”); this is an additional feature of the COSMIN checklist (see http://www.cosmin.nl). An overall score for the methodological quality of a study is determined for each measurement property separately, by taking the lowest rating of any items in a box. The methodological quality of pain assessment instruments was evaluated per measurement property. Assessment of the methodological quality was performed by two reviewers (CDV/PS) independently. In case of any disagreement, a third reviewer (WA) was consulted to achieve consensus.
Best evidence synthesis: levels of evidence
The results of this review were organised and presented to describe the methodological quality of the studies. Second, the results summarise all the evidence on the measurement properties of the different used instrument, taking into account the methodological quality of the studies. Similarly, the possible overall rating for a measurement property was defined as “positive”, “indeterminate” or “negative”, accompanied by levels of evidence, as proposed by the Cochrane Back Review Group (Furlan, Pennick, Bombardier, & Van Tulder, Citation2009; Van Tulder, Furlan, Bombardier, & Bouter, Citation2003). Level of evidence (LOE) “strong” indicates consistent findings in multiple studies of good methodological quality, or in one study of excellent methodological quality. LOE “moderate” indicates consistent findings in multiple studies of fair quality, or in one study of good methodological quality. LOE “Limited” corresponds with one study of fair methodological quality. The LOE “conflicting” corresponds with conflicting findings, and the level “unknown” indicates that only studies of poor methodological quality are present.
The criteria to assess the results of the measurement properties reliability, content validity, criterion validity and responsiveness were based on Terwee et al. (Citation2007) and De Vet, Terwee, Mokkink and Knol (Citation2011). The quality criteria of the measurement properties were as follow:
A positive reliability was based upon reports of intra-class correlation coefficient of weighted Kappa ≥0.70 or Pearson’s r ≥ 0.80 (Terwee et al., Citation2007).
Content validity indicates that all items of the measurement are relevant for the application of the measurement instrument. Questions about discrimination (to distinguish between persons at one point in time), evaluation (to assess change over time) or prediction (to predict future outcomes) were answered with the COSMIN checklist (Terwee et al., Citation2007).
A positive criterion validity indicates a correlation between the results of both the pain scale used and the gold standard pain measurement instrument (Terwee et al., Citation2007).
Responsiveness corresponds with a correlation with an instrument measuring the same construct ≥0.50 or at least 75% of the results are in accordance with the hypotheses or area under the curve ≥0.70, and correlation with related constructs is higher than with unrelated constructs (De Vet et al., Citation2011; Terwee et al., Citation2007).
Results
Search
The initial search strategy yielded 829 results: 224 from PubMed (Medline), 149 from PsychINFO, 125 from CINAHL, 192 from EMBASE, 62 from Web of Science and 83 from Cochrane. Of these, 493 references were excluded based on the title and 46 were excluded based on the abstract. Finally, 10 studies met all three inclusion criteria and were included in the present review ( and ).
Table 2. Characteristics of included articles.
Study characteristics
The included studies were published between 1997 and 2015. Two articles reported data from the same original research (Cruice, Worrall, & Hickson, Citation2010; Cruice, Worrall, Hickson, & Murison, Citation2005). Five studies were conducted at a stroke unit of a university or general hospital (Kehayia et al., Citation1997; Korner-Bitensky et al., Citation2006; Mazzocato, Michel-Nemitz, Anwar, & Michel, Citation2010; Price, Curless, & Rodgers, Citation1999; Smith et al., Citation2013) and one study at a rehabilitation centre (Jackson, Jackson, Kersten, & Turner-Stokes, Citation2006). Data from three studies were collected at a community setting (Cruice et al., Citation2005, Citation2010; Pomeroy et al., Citation2000). Most of the included studies used a prospective cohort design; of the 10 articles, 3 described a retrospective cohort study (Cruice et al., Citation2005; Kehayia et al., Citation1997; Mazzocato et al., Citation2010). The studies including PWA consisted of sample sizes of 33–388 participants (Pomeroy et al., Citation2000; Smith et al., Citation2013). The number of PWA varied from 13 to 138 PWA. The following types of aphasia were reported in the studies included in the review mild–moderate aphasia, receptive aphasia, severe aphasia, aphasia with severe expressive deficits and aphasia with both comprehension and expressive deficits (Kehayia et al., Citation1997; Korner-Bitensky et al., Citation2006; Pomeroy et al., Citation2000). Four of the 10 studies used a control group.
Control groups including individuals with stroke, aphasia and the presence of cognitive or psychiatric disorders or neurological disease were excluded from the review. (Benaim et al., Citation2007, Citation2010; Cruice et al., Citation2005; Korner-Bitensky et al., Citation2006). Mean age ranges from 43 to 84 years, where the youngest patient is 36 and the oldest 92 years (Jackson et al., Citation2006; Kehayia et al., Citation1997; Mazzocato et al., 2009).
Outcomes
Pain measurement
The pain assessment instruments used in the studies involving PWA were the vertical, horizontal and mechanical VAS (Benaim et al., Citation2007; Korner-Bitensky et al., Citation2006; Pomeroy et al., Citation2000; Price et al., Citation1999), the FPS (Benaim et al., Citation2007), the NRS (Price et al., Citation1999), the Verbal Rating Scale (VRS) (Benaim et al., Citation2007; Herr, Spratt, Mobily, & Richardson, Citation2004; Price et al., Citation1999), a categorical site-of-pain shoulder scale (Pomeroy et al., Citation2000) and the Scale Pain Intensity (SPIN) for patients with communication impairments (Jackson et al., Citation2006). Parallel to pain measurement instruments, the studies with pain registered as a subdomain of quality of life also used different quality of life instruments the International Quality of Life Assessment (IQOLA) SF-36 Health Survey (Ware & Gandek, Citation1998) and the Dartmouth COOP Charts (Nelson, Wasson, & Kirk, Citation1987). Studies on pain measurement in specific patients with an inability to communicate used the FPS and NRS to measure pain (Mazzocato et al., Citation2010; Smith et al., Citation2013). A summary of pain scales, the methodological quality and their accompanying LOE is presented in . The use of self-report pain scales in individuals with severe aphasia after stroke was not possible in some studies due to their inability to two studies included individuals with severe aphasia (Benaim et al., Citation2007; Cruice et al., Citation2010; Kehayia et al., Citation1997; Korner-Bitensky et al., Citation2006; Price et al., Citation1999; Smith et al., Citation2013).
Table 3. Quality of measurement properties per scale.
Four articles, evaluating four pain measurement scales and two quality of life scales, were assessed with the COSMIN checklist to evaluate methodological quality for each pain measurement instrument and measurement property (Benaim et al., Citation2007; Cruice et al., Citation2005; Korner-Bitensky et al., Citation2006; Pomeroy et al., Citation2000). There were no methodological studies evaluating the internal consistency, measurement error, structural validity, hypotheses testing and cross-cultural validity of the following pain measurement instruments; only the items of Reliability, Content validity, Criterion validity and Responsiveness could be rated. The methodological quality of the 10 studies is presented in for each scale and measurement property. The following section presents the results of the methodological quality per used pain measurement instrument. These results are summarised in .
Table 4. Methodological quality of each study per measurement property and pain scale.
Visual Analogue Scale
The (vertical, mechanical or horizontal) Visual Analoque Scale is an ordinal validated pain rating instrument (Kehayia et al., Citation1997). A feasibility study of the usability of the VAS in stroke patients reported that 13.5% (15/111) were excluded because of drowsiness or severe aphasia (Price et al., Citation1999). There is limited positive evidence for the reliability of the VAS vertical in LHSP and RHSP, because both inter-rater and intra-rater reliability are adequate (LHSP: ICC = 0.72 and 0.78, respectively; RHSP: ICC = 0.86 and 0.90, respectively) (Benaim et al., Citation2007). One study of excellent methodological quality presented a positive rating result in strong positive evidence for content validity (Benaim et al., Citation2007). There was limited positive evidence for criterion validity of the VAS vertical in LHSP and RHSP (r = 0.82 and 0.72, respectively) (Benaim et al., Citation2007). An examination of responsiveness across studies showed conflicting findings. Two studies of fair methodological quality confirmed a positive rating (Benaim et al., Citation2007; Pomeroy et al., Citation2000) and one study of poor quality reported a negative rating (Korner-Bitensky et al., Citation2006). Regarding generalisability of the results, no disease characteristics (e.g., severity, duration and symptoms of the stroke patients in which VAS was evaluated) were described. No floor or ceiling effects were detected (Benaim et al., Citation2007; Korner-Bitensky et al., Citation2006; Pomeroy et al., Citation2000).
Faces Pain Scale
The FPS is designed to measure pain and disability (Wong & Baker, Citation1988). Scores on the FPS were highly correlated with scores on the VAS and VRS in both left and right hemisphere stroke patients (Benaim et al., Citation2007). Patients who suffer from a left hemisphere stroke, 60% of PWA, preferred the FPS to the VAS and VRS with a significant difference compared to RHSP. A second study found that, when patients were unable to self-report, nurses rely on their own observations to assess pain and that of the research population. Of the participants, 13.4% (52/388) were unable to fill out the FPS and NRS and 30.1% of 388 patients suffered from left hemisphere stroke (Smith et al., Citation2013). The study utilised the FPS by LHSP and RHSP found that 7.9% (5/63) of the left hemisphere stroke patients could not participate (fill out the FPS) due to severe language disorders (Benaim et al., Citation2007).
There was limited evidence that the reliability of the FPS in RHSP is inadequate (inter-rater reliability: K = 0.44; intra-rater reliability: K = 0.53). Inter-rater reliability of the FPS in LHSP was inadequate (K = 0.64), while only intra-rater reliability of the FPS in LHSP was adequate (K = 0.74) (Benaim et al., Citation2007). One study of excellent methodological quality reported positive ratings results in strong positive evidence (Benaim et al., Citation2007). There was moderate positive evidence for criterion validity, because one study of good methodological quality described a positive result. One study of fair methodological quality reported positive evidence for responsiveness (Benaim et al., Citation2007). No floor or ceiling effects were detected; no information was available on other aspects of generalisability.
Verbal Rating Scale
The VRS is a pain rating scale in rank order of pain intensity and assigns each one a score as a function of its rank. The 4 points consist of: “no pain”, “mild pain”, “moderate pain” and “severe pain” (Seymour, Citation1982). Results on the VRS showed limited evidence for both inter-rater and intra-rater reliability of the VRS in LHSP (K = 0.46 and K = 0.39, respectively) and both inter-rater and intra-rater reliability are inadequate in RHSP (K = 0.52 and K = 0.57) (Benaim et al., Citation2007). There was strong positive evidence for content validity: one study of excellent methodological quality reported positive results (Benaim et al., Citation2007). For responsiveness, there were conflicting findings: two studies of fair methodological quality and one study with poor methodological quality (Benaim et al., Citation2007; Korner-Bitensky et al., Citation2006; Pomeroy et al., Citation2000).
Categorical site-of-pain scale (shoulder)
The categorical site-of-pain scale (shoulder) contains the four categories no pain, pain easy to pinpoint in one localised spot of the shoulder, pain generalised all around the shoulder area and diffuse pain radiating away from the shoulder joint area (Pomeroy et al., Citation2000). One study of fair methodological quality evaluated the content validity and responsiveness of the categorical site-of-pain scale. For both measurement properties, there was limited negative evidence. Results on inter-rater reliability and intra-rater reliability were poor (K = 0.156–0.385 and K = 0.300–0.559, respectively) (Pomeroy et al., Citation2000).
IQOLA SF-36
The SF-36 is a multi-purpose, short-form health survey which contains 36 questions. It yields an 8-scale profile of scores as well as summary physical and mental measures. The IQOLA Project was established in 1991 to translate the SF-36 Health Survey and to validate, norm and document the translations as required for their use internationally (Ware & Gandek, Citation1998). There is limited negative evidence for criterion validity of the Australian version of the SF-36 completed by proxy respondents. One study of poor methodological quality described positive correlations between aphasic and proxy respondent on the item Body Pain of the IQOLA SF-36 (ICC = 0.75) (Cruice et al., Citation2005). Proxy respondents of PWA rated their partners’ pain with the IQOLA SF-36 significantly lower than the PWA’s score (ICC = 0.75). Depending on the item, exact agreement ranged from 25% to 91% (Cruice et al., Citation2005). In addition, PWA who could self-report at interview and had moderate comprehension ability at the time of interviewing were included. However, the number of excluded participants is unknown (Cruice et al., Citation2010).
Dartmouth COOP charts
The Dartmouth COOP Charts is a measurement system of individual scales for each measure which are displayed on a chart which is a direct indicator of function in the domain. COOP charts for adults contains the domains physical function, emotional function, daily activities, social activities, social support, change in health, overall health, pain and quality of life. A 5-point scale with descriptors and cartoon illustrations of levels 1–5, rating of “1” = no impairment and “5” = most impaired, was used (Nelson et al., Citation1987). The study on the use of the Dartmouth COOP Charts to measure pain describes that proxy respondents showed a significant negative bias in rating their aphasic partners’ pain. There was limited negative evidence for criterion validity as it was reported by only one study of fair methodological quality (ICC = 0.54) (Cruice et al., Citation2005).
SPIN for patients with communication impairments
The SPIN of patients with communicaton disorders is based on a total communication approach which was established and serial pain ratings made by the patient were found to be consistent with independent clinical records. The SPIN appears to have potential as a method for quantifying pain severity in people with limited communication (Jackson et al., Citation2006). The aim of the study on a pictorial scale of pain intensity was to develop and characterise a step-by-step process for introducing this new scale. Because the article describes a single case study, the COSMIN checklist could not be completed. No specified examination of aphasia was used. The patient was able to indicate yes or no and understand pictures easier than words and used hand gestures to respond (Jackson et al., Citation2006). Results concerning validity yield outcomes of good validity (SPIN-VAS r = 0.79; SPIN-NRS r = 0.92; NRS-VAS r = 0.87). Self-reported pain ratings showed daily fluctuations, but the overall pattern reflected an increase in medication and was consistent with the documented reports (Jackson et al., Citation2006).
Prevalence of pain
Two studies reported prevalence of pain in PWA after stroke, ranging from 43.8% to 87.5% (Mazzocato et al., Citation2010; Pomeroy et al., Citation2000). A prospective study reported higher prevalence of pain in stroke patients without aphasia (83.3–87.5%) compared with PWA after stroke (60–75%) (Pomeroy et al., Citation2000). Another study reported a prevalence of pain in 69% of the 42 stroke patients. Out of the total population, 38.1% (16/42) had difficulties communicating; for 15 of these participants, this was due to aphasia or altered level of consciousness. Of these 38.1%, the prevalence of one-location pain was 43.8% (7/16) and of two or more pain locations was 56.2% (9/16), as measured with the National Institutional Health Stroke Scale (Mazzocato et al., Citation2010).
Pain intervention
A study on pain intervention reported a significant difference in prescribed dosages and actually used pain medication in PWA and persons without aphasia after stroke; 88% of patients without aphasia were prescribed pain medication and 56% actually used this medication. Of the PWA with mild-to-moderate aphasia, 51% were prescribed medication and 29% actually used this medication; for PWA with severe aphasia, the percentage was 55% and 27%, respectively (Kehayia et al., Citation1997). Patients with severe cognitive and/or psychological problems, as indicated in the neuropsychology report (percentage not mentioned), were excluded (Kehayia et al., Citation1997). A retrospective cohort study including 42 stroke patients reported that 69% of their study population were treated with opioids (Mazzocato et al., Citation2010).
Discussion
This is the first systematic review to document the incidence and prevalence of pain, and the measurement properties of pain assessment instruments, in PWA after stroke. The broad search strategy resulted in only 10 relevant publications that actually described pain or pain assessment or pain medication in PWA or patients with inability to communicate after stroke. There were no studies that reported the incidence of pain. Five studies explicitly excluded PWA with severe aphasia after stroke because of the inability to complete pain measurement instruments (Benaim et al., Citation2007; Cruice et al., Citation2010; Kehayia et al., Citation1997; Korner-Bitensky et al., Citation2006; Price et al., Citation1999). One article reported a significant difference in prescribed proportions and actually used pain medication in PWA and persons without aphasia after stroke (Kehayia et al., Citation1997). These findings underline the difficulty of identifying pain in PWA after stroke. None of the 10 studies reported incidence rates of pain in this specific population. The few studies that described prevalence of pain in PWA after stroke with mild-to-moderate aphasia or difficulty to communicate reported a prevalence of 43.8–87.5%. There is strong positive evidence for content validity, moderate positive evidence for criterion validity and limited positive evidence for responsiveness of the FPS in LHSP and RHSP. Regarding reliability, there are conflicting findings in LHSP and limited negative results in RHSP. In addition, patients with a left hemispheric stroke prefer the FPS to the VAS or VRS (Benaim et al., Citation2007). The VAS vertical showed limited positive evidence for reliability and criterion validity and strong positive evidence for content validity (Benaim et al., Citation2007). In contrast to the conflicting findings reported for the responsiveness of the VAS vertical (Benaim et al., Citation2007; Korner-Bitensky et al., Citation2006; Pomeroy et al., Citation2000), there is strong positive evidence for content validity of the VRS in contrast to limited negative evidence for reliability and responsiveness (Benaim et al., Citation2007). Regarding the feasibility, reliability and validity, four studies were evaluated on their methodological quality. Reliability, content validity and responsiveness rates were judged to be fair (Benaim et al., Citation2007; Pomeroy et al., Citation2000), and poor ratings were observed on criterion validity and responsiveness (Cruice et al., Citation2005; Korner-Bitensky et al., Citation2006; Price et al., Citation1999). The study utilising the FPS in LHSP and RHSP scored excellent rating on content validity and good rating on criterion validity (Benaim et al., Citation2007). Additionally, quality assessment revealed that studies with good or fair methodological quality reported poor methodological quality of the measurement properties of the pain assessment tools VRS, Categorical site-of-pain scale, IQOLA SF-36 and the Dartmouth COOP Charts. Poor quality was reported because of missing items (or no report for reasons for missing items), no adequate sample size or the lack of a gold standard.
A strength of the present study is the sensitive search string and the various databases used. In addition, the PRISMA guidelines provide a transparent methodology. Another strength is that, by using the COSMIN method, a meta-analysis could be performed on the quality of the measurement properties of the pain instruments in the different studies. A limitation of the study is that, due to the scarcity of the number of studies on pain in aphasia and their heterogeneity, a meta-analysis of the results was not possible. Our findings stress that more research is required on how to effectively measure pain in aphasia. For example, instead of (or in addition to) a self-report pain scale, the use of an observational instrument might be helpful to reliably assess symptoms of pain in PWA after stroke. Although several such instruments have been developed for people with dementia (Corbett et al., Citation2014), they have not been tested in PWA. Therefore, assessment of psychometric properties of these observational instruments in PWA is warranted. Based on our findings, the vertical VAS and FPS are recommended for pain assessment in PWA. When it is impossible to use a self-report pain scale because of total inability to communicate, an observation scale for pain used in patients with dementia (e.g., the PAINAD Pain Assessment IN Advanced Dementia or the PACSLAC-D: Pain Assessment Checklist for Seniors with Limited Ability to Communicate – Dementia) might be considered (Zwakhalen, Hamers, Abu-Saad, & Berger, Citation2006). This study confirms that most of the studies on pain assessment in PWA after stroke focus on mild-to-moderate aphasia. Of the various pain assessment tools used, the feasibility, validity and reliability generally show low quality. The pain scales VAS vertical and FPS provide the best results on methodological quality. Patients with a left hemispheric stroke prefer the use of FPS rather the VAS and VRS.
In summary, a feasible, reliable and valid pain assessment instrument is not yet available for PWA after stroke. Therefore, future research is needed to facilitate a valid, feasible and reliable pain assessment tool in PWA after stroke.
Supplementary_material.docx
Download MS Word (12.3 KB)Acknowledgments
The authors declare that there are no conflicts of interest in relation to this article.
The authors thank Claudia Pees, and the Walaeus Library of Leiden University Medical Center, for their help in developing the search strategies. This work was supported by Topaz Leiden and Leiden University Medical Center.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Benaim, C., Froger, J., Cazottes, C., Gueben, D., Porte, M., Desnuelle, C., & Pelissier, J. Y. (2007). Use of the faces pain scale by left and right hemispheric stroke patients. Pain, 128, 52–58. doi:10.1016/j.pain.2006.08.029
- Corbett, A., Achterberg, W., Husebo, B., Lobbezoo, F., De Vet, H., Kunz, M., … Lautenbacher, S., on behalf of EU-COST action td 1005 Pain Assessment in Patients with Impaired Cognition, especially Dementia’ Collaborators. (2014). An international road map to improve pain assessment in people with impaired cognition: The development of the Pain Assessment in Impaired Cognition (PAIC) meta-tool. BMC Neurology, 14, 229. doi:10.1186/s12883-014-0229-5
- Cruice, M., Worrall, L., & Hickson, L. (2010). Health-related quality of life in people with aphasia: Implications for fluency disorders quality of life research. Journal of Fluency Disorders, 35, 173–189. doi:10.1016/j.jfludis.2010.05.008
- Cruice, M., Worrall, L., Hickson, L., & Murison, R. (2005). Measuring quality of life: Comparing family members’ and friends’ ratings with those of their aphasic partners. Aphasiology, 19, 111–129. doi:10.1080/02687030444000651
- De Vet, H. C. W., Terwee, C. B., Mokkink, L. B., & Knol, D. L. (2011). Measurement in medicine. Cambridge: Cambridge University Press.
- Furlan, A. D., Pennick, V., Bombardier, C., & Van Tulder, M.; Editorial Board CBRG. (2009). Updated method guidelines for systematic reviews in de Cochrane back review group. Spine, 34, 1929–1941. doi:10.1097/BRS.0b013e3181b1c99f
- Goodglass, H., Kaplan, E., & Barresi, B. (2001). Boston Diagnostic Aphasia Examination (3rd ed.). Austin, TX: Pro-Ed. (BDAE-3).
- Herr, K. A., Spratt, K., Mobily, P. R., & Richardson, G. (2004). Pain intensity assessment in older adults: Use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. The Clinical Journal of Pain, 20, 207–219. doi:10.1097/00002508-200407000-00002
- Huskisson, E. C. (1974). Measurement of pain. The Lancet, 304, 1127–1131. doi:10.1016/S0140-6736(74)90884-8
- Jackson, D., Jackson, S., Kersten, P., & Turner-Stokes, L. (2006). A pictorial scale of pain intensity (SPIN) for patients with communication impairments. International Journal of Therapy and Rehabilitation, 13, 457–463. doi:10.12968/ijtr.2006.13.10.22193
- Jensen, M. P., Karoly, P., & Braver, S. (1986). The measurement of clinical pain intensity: A comparison of six methods. Pain, 27, 117–126. doi:10.1016/0304-3959(86)90228-9
- Kehayia, E., Korner-Bitensky, N., Singer, F., Becker, R., Lamarche, M., Georges, P., & Retik, S. (1997). Differences in pain medication use in stroke patients with aphasia and without aphasia. Stroke, 28, 1867–1870. doi:10.1161/01.STR.28.10.1867
- Kim, J. S. (2009). Post-stroke pain. Expert Review of Neurotherapeutics, 9, 711–721. doi:10.1586/ern.09.19
- Klit, H., Finnerup, N. B., Overvad, K., Andersen, G., Jensen, T. S., & Kiechl, S. (2011). Pain following stroke: A population-based follow-up study. PLoS ONE, 6, e27607. doi:10.1371/journal.pone.0027607
- Korner-Bitensky, N., Kehayia, E., Tremblay, N., Mazer, B., Singer, F., & Tarasuk, J. (2006). Eliciting information on differential sensation of heat in those with and without poststroke aphasia using a visual analogue scale. Stroke, 37, 471–475. doi:10.1161/01.STR.0000198872.75377.34
- Mazzocato, C., Michel-Nemitz, J., Anwar, D., & Michel, P. (2010). The last days of dying stroke patients referred to a palliative care consult team in an acute hospital. European Journal of Neurology, 17, 73–77. doi:10.1111/j.1468-1331.2009.02744.x
- McCaffery, M., & Beebe, A. (1993). Pain: Clinical manual for nursing practice. Baltimore: V.V.Mosby Company.
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G.; the PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
- Mokkink, L. B., Terwee, C. B., Patrick, D. L., Alonso, J., Stratford, P. W., Knol, D. L., … De Vet, H. C. W.. (2010). The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Quality of Life Research, 19, 539–549. doi:10.1007/s11136-010-9606-8
- Mulla, S. M., Wang, L., Khokhar, R., Izhar, Z., Agarwal, A., Couban, R., … Busse, J. W. (2015). Management of central poststroke pain: Systematic review of randomized controlled trials. Stroke, 46, 2853–2860. doi:10.1161/STROKEAHA.115.010259
- Nelson, E. C., Wasson, J., Kirk, J., Keller, A., Clark, D., Dietrich, A., … Zubkoff, M. (1987). Assessment of function in routine clinical practice: Description of the COOP chart method and preliminary findings. Journal of Chronic Diseases, 40, 55S–63S. doi:10.1016/S0021-9681(87)80033-4
- Nespoulous, J. L., Lecours, A. R., Lafond, D., Lemay, A., Puel, M., Joannette, Y., … Rascol, A. (1992). Protocole Montréal-Toulouse d’examen linguistique de l’aphasie (MT86). Isbergues, France: L’Ortho-Edition.
- Pomeroy, V. M., Frames, C., Faragher, E. B., Hesketh, A., Hill, E., Watson, P., & Main Chris, J. (2000). Reliability of a measure of post-stroke shoulder pain in patients with and without aphasia and/or unilateral spatial neglect. Clinical Rehabilitation, 14, 584–591. doi:10.1191/0269215500cr365oa
- Price, C. I. M., Curless, R. H., & Rodgers, H. (1999). Can stroke patients use visual analogue scales? Stroke, 30, 1357–1361. doi:10.1161/01.STR.30.7.1357
- Price, D. D., Bush, F. M., Long, S., & Harkins, S. W. (1994). A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain, 56, 217–226. doi:10.1016/0304-3959(94)90097-3
- Raffaeli, W., Minella, C. E., Magnani, F., & Sarti, D. (2013). Population-based study of central post-stroke pain in Rimini district, Italy. Journal of Pain Research, 17, 705–711.
- Schewan, C. M., & Kertesz, A. (1980). Reliability and validity characteristics of the Western Aphasia Battery (WAB). Journal of Speech and Hear Disorders, 45, 308–324.
- Scott, J., & Huskisson, E. C. (1979). Vertical or horizontal visual analogue scales. Annals of the Rheumatic Diseases, 38, 560. doi:10.1136/ard.38.6.560
- Seymour, R. A. (1982). The use of pain scales in assessing the efficacy of analgesics in post-operative dental pain. European Journal of Clinical Pharmacology, 23, 441–444. doi:10.1007/BF00605995
- Smith, J. H., Bottemiller, K. L., Flemming, K. D., Cutrer, M. F., & Strand, E. A. (2013). Inability to self-report pain after a stroke: A population-based study. Pain, 154, 1281–1286. doi:10.1016/j.pain.2013.04.006
- Syder, D., Body, R., Parker, M., & Boddy, M. (1993). Sheffield Screening Test for acquired language disorders (SST) [Manual]. Windsor: Nfer- Nelson.
- Terwee, C. B., Bot, S. D. M., De Boer, M. R., Van Der Windt, D. A. W. M., Knol, D. L., Dekker, J., … De Vet, H. C. W. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology, 60, 34–42. doi:10.1016/j.jclinepi.2006.03.012
- Van Tulder, M., Furlan, A., Bombardier, C., & Bouter, L.; Editorial Board CBRG. (2003). Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine, 28, 1290–1299. doi:10.1097/01.BRS.0000065484.95996.AF
- Ware, J. E., & Gandek, B. (1998). Overview of the SF-36 health survey and the International Quality of Life Assessment (IQOLA) Project. Journal of Clinical Epidemiology, 51, 903–912. doi:10.1016/S0895-4356(98)00081-X
- Wong, D. L., & Baker, C. M. (1988). Pain in children: Comparison of assessment scales. Pediatric Nursing, 14, 9–17.
- Zwakhalen, S. M., Hamers, J. P., Abu-Saad, H. H., & Berger, M. P. (2006). Pain in elderly people with severe dementia: A systematic review of behavioural pain assessment tools. BMC Geriatrics, 6, 3. doi:10.1186/1471-2318-6-3