ABSTRACT
Background: Difficulties with word finding occasionally occur in all speakers and commonly in all aphasia types. An understanding of these difficulties and their treatment-induced improvement is of fundamental scientific and clinical importance. Phonological cueing yields immediate and treatment effects in behavioural and neural measures. Disagreement exists about the locus of the cueing effects in aphasia and whether the effects arise from the same neurocognitive mechanisms as phonological effects in picture naming by healthy speakers.
Aims: Computer simulations were conducted to expand our understanding of immediate and treatment effects on word finding in behavioural and neural measures and to examine whether a unified account of the phonological effects in health and disease is possible.
Methods & Procedure: Picture naming performance of the WEAVER computer model, which accounts for immediate phonological effects in healthy speakers, was assessed in simulations of immediate and treatment effects of phonological cueing in poststroke aphasia. The model assumes (1) a phonological encoding locus of the cueing effects, (2) a cohort mechanism in cue perception, and (3) activation of lexical and sublexical phonological levels by the cues.
Outcomes & Results: The model successfully simulated the empirically observed effects in behavioural naming performance and neural measures.
Conclusions: The simulations provide a proof of concept for the idea that immediate and treatment effects of phonological cueing in aphasia arise from the same neurocognitive mechanisms as immediate phonological effects in healthy speakers. This expands our understanding of word finding, associated difficulties, and their improvement by therapy.
Introduction
An essential component of our speaking skill is the quick finding of appropriate words for concepts that we want to express, also called word retrieval, lexical access, or word planning. Problems with word finding occasionally occur in all speakers, surfacing as pauses or errors, and frequently in all types of aphasia, often referred to as anomia (e.g. Goodglass & Wingfield, Citation1997; Laine & Martin, Citation2006). Word finding and its difficulties are typically assessed by measuring the speed and accuracy of picture naming.
In the past three decades, our understanding of word finding and associated problems has gained much from computer models and simulations of picture naming (e.g. Dell, Schwartz, Martin, Saffran, & Gagnon, Citation1997; Levelt, Roelofs, & Meyer, Citation1999; Rapp & Goldrick, Citation2000; Roelofs, Citation1992, Citation1997, Citation2004). Models have related several aspects of word-finding difficulties to damage of the language areas in the left hemisphere of the brain (e.g. Dell, Schwartz, Nozari, Faseyitan, & Coslett, Citation2013; Roelofs, Citation2014; Ueno, Saito, Rogers, & Lambon Ralph, Citation2011). However, few models have addressed treatment effects on naming performance in aphasia (e.g. Laine & Martin, Citation2006). Treatment employing semantic and phonological techniques has been successful in improving naming performance and also functional communicative effectiveness (for a meta-analysis, see Wisenburn & Mahoney, Citation2009).
In the widely used phonological cueing approach, patients are given a spoken cue (e.g. the first phoneme of the picture name) when naming fails during assessment or treatment (e.g. Best, Herbert, Hickin, Osborne, & Howard, Citation2002; Nickels, Citation2002; Raymer, Thompson, Jacobs, & Le Grand, Citation1993). For example, the phoneme /k/ is provided in naming a picture of a cat. Cueing increases naming speed and accuracy. It not only facilitates immediate naming but also leads to long-term treatment gains. Treatment-induced improvements of word finding occur in poststroke aphasia (e.g. Best et al., Citation2013) as well as in primary progressive aphasia (e.g. Meyer, Tippett, Turner, & Friedman, Citation2019), which is a type of dementia due to neurodegenerative disease with language impairment as the primary deficit. In recent years, the improvements in aphasia have not only been examined in naming speed and accuracy, but also in neuroimaging studies targeting corresponding changes in brain activations and fibre tract integrity (e.g. Nardo, Holland, Leff, Price, & Crinion, Citation2017; Van Hees et al., Citation2014). In these studies, phonological cueing has been central. However, the neuroimaging findings have not been addressed yet by computer models.
In interpreting the treatment effects in their neuroimaging study on poststroke aphasia, Nardo et al. (Citation2017) argue that “the phonemic cueing approach used in anomia treatment relies upon the same processes underlying cued-picture naming priming effects found in healthy speakers” (p. 3051), which they take to be phonological encoding processes. This is also assumed by other researchers for phonological facilitation in poststroke aphasia (Best et al., Citation2013; Hashimoto & Thompson, Citation2010; Nickels, Citation2002) and primary progressive aphasia (Mack et al., Citation2013). However, Pellet Cheneval, Glize, and Laganaro (Citation2018) note that “the mechanisms by which phonological cueing facilitates oral production remain poorly understood. Most interpretations in psycholinguistic studies favor the phonological (sub-lexical) hypothesis, whereas results from studies on aphasia report evidence suggesting a lexical locus” (p. 1468). The difference is that “The ‘lexical hypothesis’ holds that phonological facilitation is due to the reduction of the cohort of possible lexical entries for selection, while according to the ‘sublexical hypothesis’ the encoding of the word form is facilitated by pre-activating the segmental information.” (p. 1470). Pellet Cheneval et al. argue that their own evidence supports a lexical (“lemma”, p. 1471) selection locus in poststroke aphasia. Meteyard and Bose (Citation2018) report findings on poststroke aphasia that they take to “challenge the theoretical assumptions that phonological cues map to phonological processes. Instead, phonological information benefits the earliest stages of picture recognition, aiding the initial categorization of the target” (p. 658). According to them, their findings are “critically important for rehabilitation, allowing for therapy development to be more rooted in the true mechanisms through which cues are processed” (p. 658). Thus, researchers have found no agreement about the functional locus of the phonological cueing effects in aphasia and whether the effects arise from the same neurocognitive mechanisms as phonological effects in picture naming by healthy speakers.
An important question is whether the findings on phonological cueing of Pellet Cheneval et al. (Citation2018) and Meteyard and Bose (Citation2018) exclude the locus in phonological encoding that Nardo et al. (Citation2017) assume. If not, this would reconcile the empirical findings and conclusions about the locus in poststroke aphasia and healthy speakers (Meyer & Schriefers, Citation1991; Roelofs, Citation1997). To examine this, a psycholinguistic model of phonological effects in healthy speakers, the computer model WEAVER (Roelofs, Citation1997), was extended and tested on the immediate and treatment effects found in speakers with aphasia. The “cued-picture naming priming effects” (p. 3051) referred to by Nardo et al. come from picture-word interference studies with healthy participants (e.g. Damian & Martin, Citation1999; Meyer & Schriefers, Citation1991; Schriefers, Meyer, & Levelt, Citation1990). In these studies, phonological cues are presented as part of auditory or written distractor words. These distractors provide both matching and mismatching information. For example, the distractor word cap shares the phonemes /k/ and /æ/ with the picture name cat, but the phoneme /p/ differs.
The WEAVER model provides a functional account of the word-form encoding component of word finding and has been applied to phonological effects of auditory distractor words in healthy speakers (Roelofs, Citation1997, Citation2002, Citation2004). Its key assumptions are (1) a locus of the effects in phonological encoding, (2) a cohort mechanism in perception, and (3) activation of lexical and sublexical phonological levels by the distractors. WEAVER is part of the neurocognitive model WEAVER++/ARC (Roelofs, Citation2014), which relates functional processes in word finding to brain areas and synthesizes behavioural psycholinguistic, functional neuroimaging, tractography, and aphasic evidence. Developed within the theory on word finding of Levelt et al. (Citation1999), the model holds that the naming of a picture of an object involves conceptual identification of the object based on its perceived form (e.g. a cat), retrieval of the corresponding lemma, also called lexical selection (e.g. the lemma of the word cat), the encoding of the word form (involving morphological, phonological, and phonetic encoding), and finally articulation. Lemma retrieval and word-form encoding make up word finding.
The remainder of the article is organized as follows. I start by briefly reviewing the relevant behavioural and neuroimaging findings on auditory phonological cueing and outline the WEAVER++/ARC and WEAVER models. Next, the computer simulation methods are explained. Then, I report the results of simulations targeting the immediate and long-term treatment effects of auditory phonological cueing in behavioural and neural measures observed by Nardo et al. (Citation2017) as well as the behavioural findings on immediate effects of Pellet Cheneval et al. (Citation2018) and Meteyard and Bose (Citation2018).
Phonological cueing effects
Phonological cueing in aphasia involves the use of auditory cues that provide phonological information on the target word. For example, if a patient is unable to name a picture (e.g. of cat), spoken cues are given about the first sound (e.g. /k/) or the rhyme (e.g. /æt/) of the picture name, or the name itself is provided (e.g. /kæt/). Cueing is used to help retrieve the picture name during tests of word-finding ability (immediate) and during training to obtain long-term improvements of the ability (treatment). Improvements in naming speed and accuracy are obtained for treated words, but there is commonly little or no generalization to untreated words (for a review, see Best et al., Citation2013).
Nardo et al. (Citation2017) assessed immediate and treatment effects of auditory phonological cueing on behavioural naming performance and brain activation in a group of eighteen participants with aphasia. Brain activation concerned the blood-oxygenation-level-dependent (BOLD) signal measured by functional magnetic resonance imaging (fMRI). The participants were right-handed native speakers of English, who had anomia as determined by the Boston Naming Test (Kaplan, Goodglass, & Weintraub, Citation1983). All patients had a left-hemisphere stroke with spared or partially spared left inferior frontal cortex.
The same phonological cues were used during treatment and testing of naming with (to be) treated words and untreated words. Participants were asked to name the pictures as quickly and accurately as possible. Naming reaction time (RT) and accuracy were assessed. Picture naming performance was tested before and after treatment outside the MRI scanner (free naming) and inside the scanner measuring the BOLD response (cued naming). In the free naming condition (Experiment 1), participants named the pictures without phonological cues, just before (T1) and after a six-week treatment (T2), and three months later (T3). In the cued naming condition (Experiment 2), participants named pictures in the scanner just before (T1) and after the treatment (T2). During the cued naming tests, the stimulus onset asynchrony (SOA) between auditory cue and picture was 0 ms. The cue was the name of the picture, the whole word condition (e.g. cue /kæt/ for a picture of cat); its initial phonemes consisting of the onset phoneme plus vowel, the initial condition (e.g. /kæ/); its final phonemes consisting of the vowel plus offset phoneme, the final condition (e.g. /æt/); or noise was presented, the control condition. During treatment, the pictures were named after a whole word cue, an initial cue, and again after a whole word cue.
Picture naming RTs were shorter and accuracy was higher at T3 and T2 than at T1 for free naming and at T2 than at T1 for cued naming. This was observed for treated words but not for the untreated words. Naming RTs were also shorter and accuracy was higher for the whole, initial, and final cues relative to noise, at both T1 and T2, and for both the (to be) treated words and the untreated words.
Immediate and treatment effects of phonological cueing occurred in the right anterior insular cortex (AI), the left and right supplementary motor area (SMA), the left and right anterior cingulate cortex (ACC), the left (for immediate cueing) and right inferior frontal gyrus (IFG), and the left precentral gyrus. The literature shows that the left AI, SMA, and precentral gyrus are involved in phonetic encoding and the control of articulation, and the left IFG in phonological encoding (e.g. Indefrey, Citation2011; Indefrey & Levelt, Citation2004; for reviews, de Zubicaray & Piai, Citation2019; Kemmerer, Citation2015, Citation2019), and the left and right AI, right IFG, and ACC in top-down executive control (e.g. Duncan, Citation2010; Posner, Citation2012). This suggests that the activation of the right AI and IFG reflects right hemisphere recruitment for word-form encoding, top-down control, or both.
Activations were lower for the easy (treated, phonological cue) than for the hard (untreated, noise) conditions, which suggests less effort. However, there was an immediate but not a treatment effect of phonological cueing in left IFG. As Thompson and Woollams (Citation2017) remark, “it is striking that they did not observe any therapy-related increases in left perilesional regions, despite being careful to select patients with at least partially spared left inferior frontal cortex” (p. 2774). The activation reduction for treated relative to untreated words in the right AI and right IFG correlated with the reduction in free naming RT.
The findings of Nardo et al. (Citation2017) favour a locus in phonological encoding. A meta-analysis of neuroimaging studies by Indefrey (Citation2011) and Indefrey and Levelt (Citation2004) associated phonological encoding with the left IFG, where the immediate cueing effect occurred. The meta-analysis localized lemma retrieval (lexical selection) to the middle section of the left middle temporal gyrus (MTG), but immediate and treatment effects did not happen in this area. Also, these effects did not occur in occipital-temporal areas, where the picture is visually processed and recognized. This suggests that the locus of the phonological cueing effect is phonological encoding and not lemma retrieval (Pellet Cheneval et al., Citation2018) or picture recognition (Meteyard & Bose, Citation2018).
Pellet Cheneval et al. (Citation2018) examined immediate effects of auditory phonological cueing on behavioural naming performance in a group of fourteen participants with aphasia. The participants were right-handed native speakers of French, who had moderate anomia as determined by the French version of the Boston Naming Test. All patients had a left-hemisphere stroke. Seven patients made predominantly semantic errors, referred to as the lexical-semantic profile, and seven patients made predominantly phonological errors, called the phonological profile.
To assess the functional locus of phonological cueing, first-phoneme cues were used that were compatible with many words (e.g. cue /k/) or with only a few words (e.g. cue /g/) in the language, called the lexical cohort size. The phonological cues were presented before picture onset with an SOA of 1,100 ms. Given that cohort size involves a lexical rather than sublexical manipulation, its effect would imply a lexical locus. Participants with a lexical-semantic profile showed a cohort size effect in naming accuracy and participants with a phonological profile showed a cohort size effect in naming RT. Pellet Cheneval et al. (Citation2018) take the cohort size effects to provide evidence that the phonological cueing influences lemma retrieval rather than phonological encoding.
Meteyard and Bose (Citation2018) examined immediate effects of auditory phonological cueing on behavioural naming performance in a group of ten participants with aphasia. The participants were right-handed native speakers of English, who showed a “wide range of picture naming abilities” (p. 660) and “variable impairments for both input and output phonology but with better preserved conceptual and lexical semantics” (p. 662). All patients had a left-hemisphere stroke.
The pictures were presented after the offset of the phonological cues, with an interstimulus interval of 750 ms (the SOA was not reported). The phonological cue was the first phoneme of the picture name (e.g. the cue /k/ for a picture of a cat). In the control condition, a 1-kHz pure tone was presented. Naming accuracy (but not RT) was assessed.
Naming accuracy was higher with the phonological cues than in the control condition. Moreover, when a phonological cue was presented, naming accuracy was higher for pictures with less visual information, that is, when “visual input is sparse and provides less information for easy identification” (Meteyard & Bose, Citation2018, p. 667). There was no interaction with lexical frequency or length in phonemes. On the basis of these findings, Meteyard and Bose conclude that phonological cueing helps the earliest stages of picture recognition rather than lexical selection (Pellet Cheneval et al., Citation2018) or phonological encoding (Nardo et al., Citation2017), an insight that they take to be critically important for treatment.
Outline of the WEAVER++/ARC model
To help elucidate immediate and treatment effects of phonological cueing, the WEAVER psycholinguistic model (Roelofs, Citation1997), which accounts for phonological effects of distractor words in healthy speakers, was applied to the immediate and treatment effects found in speakers with anomia. The model is part of an incremental modelling approach that extends a model to new empirical domains outside its original scope, as is done in the present article. The WEAVER model was designed to account for word-form encoding, specifically addressing effects in picture naming RT. WEAVER++ (Levelt et al., Citation1999) is an extended version encompassing both lemma retrieval and word-form encoding, embedding WEAVER as a proper part. The WEAVER++/ARC model (Roelofs, Citation2014) maps the functional processes assumed by WEAVER++ to grey matter areas and white-matter fibre tracts in the brain and addresses evidence from lesion-deficits analyses in aphasia.
The WEAVER++/ARC model assumes that picture naming engages an associate network in declarative memory and condition-action rules in procedural memory. The associative network is accessed by spreading activation while condition-action (if-then) rules select nodes among the activated ones depending on the goal specified in working memory (here, to name a picture). illustrates the associative network mapped onto areas of the brain based on the meta-analysis of neuroimaging studies by Indefrey (Citation2011) and Indefrey and Levelt (Citation2004). Shown is the network representation of the word cat, which consists of a lexical concept (CAT), a lemma (cat), a lexical output form or morpheme (<cat>), output phonemes (/k/, /æ/, and /t/), and a syllable motor programme ([kæt]). Lemmas specify the grammatical properties of words (e.g. word category: noun, N), crucial for the production of phrases and sentences. The network also contains input phonemes (/k/, /æ/, and /t/) and lexical input forms (<cat>), which serve speech recognition.
Figure 1. Illustration of the WEAVER++/ARC model: An associative network and condition-action rules mapped onto areas of the brain. The word-form encoding component of word finding is highlighted in red. N = noun
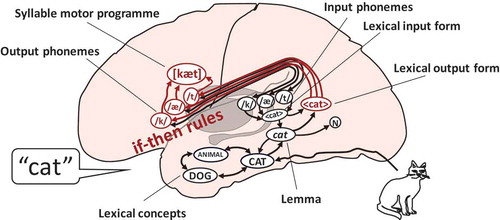
In picture naming, activation spreads continuously from lexical concepts to lemmas, lexical output forms, output phonemes, and syllable motor programmes (Roelofs, Citation2008). Condition-action rules select a concept for a selected percept (conceptual identification), a lemma for the selected concept (lemma retrieval), one or more morphemes (lexical output forms) for the selected lemma (morphological encoding), and phonemes for the selected morphemes, which are then syllabified to create a phonological word representation (phonological encoding). Finally, condition-action rules select motor programmes for the syllables in the phonological word (phonetic encoding), followed by articulation.
The condition-action rules are thought to be realized by the basal ganglia, thalamus, frontal cortex (including Broca’s area), and cerebellum and the declarative associative network by temporal and inferior frontal areas of the brain. Lexical concepts are thought to be represented in anterior-ventral temporal cortex, lemmas in left middle MTG, lexical output forms in left posterior superior temporal gyrus (STG) and left posterior MTG (henceforth STG/MTG, Wernicke’s area), output phonemes in left posterior IFG (Broca’s area), and syllable motor programmes in left ventral precentral gyrus. Converging evidence for these function-to-brain mappings from functional neuroimaging comes from large-scale lesion-deficit analyses showing that semantic errors due to incorrect lemma retrieval are most strongly correlated with damage to left middle MTG (Schwartz et al., Citation2009), and phoneme selection errors with damage to frontal areas, including the left IFG (Schwartz, Faseyitan, Kim, & Coslett, Citation2012). Schwartz et al. restricted their analysis of phoneme errors to nonword responses in picture naming, which provides evidence that the phoneme errors had an origin in phonological encoding rather than lexical form selection (see also Schwartz, Wilshire, Gagnon, & Polansky, Citation2004). This was further corroborated by an absence of a correlation between phoneme error rate and damage to left posterior STG/MTG, where lexical output forms are thought to be stored (and damage should have resulted in malapropisms, such as the word cap for cat). The assumption of the model that lexical output forms are stored in left posterior STG/MTG is also supported by evidence from specific targeted fMRI studies with healthy participants (e.g. Graves, Grabowski, Mehta, & Gupta, Citation2008).
In hearing speech, input phonemes activate lexical input forms, lemmas, and lexical concepts. Also, activation spreads from input to output phonemes, both directly and via lexical output forms and lemmas. In the activation of lexical input forms, a cohort principle applies (e.g. Marslen-Wilson, Citation1987; Norris, Citation1994). For example, in processing the /k/ of the cue /kæ/, all lexical input forms that are compatible with the /k/ receive activation, including <cat>. Next, in processing the /æ/, only lexical input forms that are compatible with the /æ/ continue receiving activation. Assuming that the amount of activation that spreads to lexical output forms and lemmas depends on the competition among lexical input forms, less activation will be received by the target lemma and lexical output form when the lexical cohort is large than when it is small. Thus, in WEAVER, cohort effects are expected in naming RT (Roelofs, Citation1997) and error patterns (Roelofs, Citation2004), in both word-form encoding and lemma retrieval (cf. Roelofs, Meyer, & Levelt, Citation1996).
To associate processes in the model to the BOLD response in the experiment of Nardo et al. (Citation2017), a linking hypothesis is needed. In discussing such hypothesis for reading, Taylor, Rastle, and Davis (Citation2013) distinguish between engagement and effort, which reflect “the extent to which a model component or brain region is engaged by a stimulus” and “once a model component or brain region is engaged, how much effort is exerted in processing that stimulus” (p. 769). For example, words but not nonwords have a lexical orthographic form representation, thus reading words should engage lexical form processing more than reading nonwords, leading to higher BOLD response for words in areas underpinning lexical form processing. Lexical form processing is engaged by both high- and low-frequency words, with high-frequency words being read quicker and more accurately than low-frequency ones, suggesting less effort for high-frequency words, as reflected in a lower BOLD response. Similarly, following earlier WEAVER++ simulations concerning the BOLD response (Roelofs, Citation2018; Roelofs & Hagoort, Citation2002; Roelofs, Van Turennout, & Coles, Citation2006), I assume that the duration of a process in the model relates to the magnitude of the BOLD response. That is, the shorter the encoding process is engaged, the less energy is consumed, which maps onto a lower BOLD response.
Treatment may reduce effort and increase engagement, however. For example, if treatment affects phonological encoding by strengthening the connections between lexical output form and output phoneme nodes, encoding will take less time and the BOLD response will be lower. However, treatment may also have a parallel opposite effect. If treatment leads to renewed recruitment and thus greater engagement of a brain region, such as the left IFG for phonological encoding, this may lead to an increased BOLD response.
Method
In the simulations, the encoding of a word form consisted of spreading of activation through the form network and selection of a lexical output form for a selected lemma (morphological encoding), selection of phonemes for the selected lexical output form and their syllabification to create a phonological word representation (phonological encoding), and selection of motor programmes for the syllables in the phonological word (phonetic encoding). The network structure and parameter values were the same as in earlier simulations (Roelofs, Citation1997, Citation2004). The network included words similar to those in simulations by Dell and colleagues (Dell et al., Citation1997, Citation2013), among others. The target was cat and the other words were dog, fish, fog, and mat. This small network consisted of five lexical output form nodes, ten output phoneme nodes, five syllable programme nodes, and corresponding connections. To examine the effect of varying the size of the lexicon, the simulations were also run with the small network embedded within a larger network. This larger network contained all animal names of the Boston Naming Test (i.e. octopus, camel, snail, seahorse, beaver, rhinoceros, pelican, and unicorn). The simulations with the larger network yielded the same outcomes as those with the smaller network, which suggests that the size of the lexicon does not affect the simulation outcomes.
Activation spread through the network according to a linear function with spreading rate r and a decay factor d: a(m, t + Δt) = a(m, t)(1 – d) + Σn r a(n, t). The rate r indicates the strength of the connections between node m and the other nodes n, and a(m, t) and a(n, t) their levels of activation at time t. In the present simulations, damage was simulated by decreasing the strength of the connections between the lexical output node and output phoneme nodes (set at 0.5 × r), corresponding to a locus in phonological encoding (cf. Dell et al., Citation2013). Treatment was simulated by restoring the strength to its normal value (cf. Van Hees et al., Citation2014). The simulations started by providing external activation to the lexical output node, followed by a spread of activation through the network. Condition-action rules selected the corresponding nodes for the lexical output form, output phonemes followed by their syllabification, and syllable motor programme. The phonological cues were simulated by activating the corresponding output phonemes and compatible lexical output forms over time. For example, for the cue /kæ/, first the output phoneme node /k/ and the lexical output form <cat> were activated for 125 ms, and then /æ/ and <cat> for another 125 ms (the same parameter value as in Roelofs, Citation1997). Mean word-form encoding times were computed as in Roelofs (Citation1997, Citation2002, Citation2004).
The simulations were coded and run using the C programming language and the programming environment of Microsoft Visual Studio 2017. The source codes of the simulations are available from the archive of the Open Science Framework (osf.io/ue4bn) and the author.
Results
Immediate and treatment effects in behavioural and neural measures
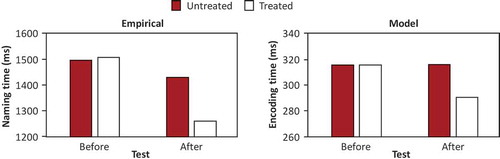
shows the mean RTs obtained by Nardo et al. (Citation2017, Experiment 1) for free picture naming in tests conducted before and after the six-week treatment, and the corresponding simulation results concerning word-form encoding times in the model. Nardo et al. observed that naming RT was shorter after (T2) than before treatment (T1) for treated words but not for untreated words. The model captures these findings.
Figure 2. Mean naming times before and after phonological cueing treatment for treated and untreated words obtained by Nardo et al. (Citation2017, Experiment 1) and corresponding word-form encoding times in the model. Note that “treated” before treatment refers to the words that were part of the treatment later. ms = milliseconds
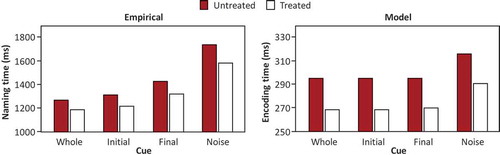
shows the mean RTs obtained by Nardo et al. (Citation2017, Experiment 2) for picture naming with whole, initial, and final cues and in the noise condition after the six-week treatment for treated and untreated words, and the corresponding simulation results concerning word-form encoding times in the model. Nardo et al. observed shorter naming RTs for the whole, initial, and final cues relative to the noise condition, for both the treated and untreated words. The model captures these findings.
Figure 3. Mean naming times with whole, initial, and final cues and in the noise condition for treated and untreated words obtained after six-week treatment by Nardo et al. (Citation2017, Experiment 2) and corresponding word-form encoding times in the model. ms = milliseconds
In the model, selection of lexical form, phoneme, and syllable programme nodes occurs when the activation of the nodes exceeds a threshold. Immediate cueing increases activation levels of nodes, which leads to shorter selection times. To test whether cueing speeds up only phonological encoding (i.e. selection of phoneme nodes and syllabification) or also morphological encoding (i.e. selection of the lexical form node) and phonetic encoding (i.e. selection of the syllable programme node), the simulations were run with the selection threshold of the lexical form node or the syllable programme node set to zero, which precludes speeding up of morphological and phonetic encoding. The simulations showed that this did not affect the magnitude of the facilitation effect of cueing on word-form encoding, indicating that the cueing effects arise in phonological encoding and not in the other word-form encoding stages.
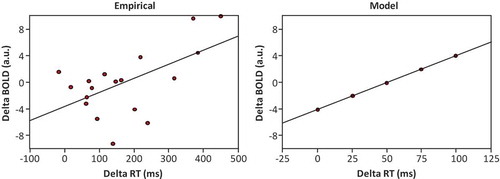
Nardo et al. (Citation2017) observed faster naming and lower right IFG activation for the whole and partial cues relative to the noise condition, and after treatment (T2) than before treatment (T1). This suggests less effort for top-down control or phonological encoding due to immediate cueing and treatment. shows the relationship between the treatment effect (delta RT: treated vs. untreated words) for free naming (Experiment 1) and the treatment effect in the BOLD response (delta BOLD: treated vs. untreated words) in the right IFG (Experiment 2) obtained by Nardo et al. and the corresponding relationship in the model. The BOLD response was assumed to be linearly related to the encoding duration in the simulation, with the intercept and slope estimated from the empirical data (cf. Roelofs, Citation2018). Moreover, it was assumed that the right IFG has taken over functionality of the left IFG (in reaction to its damage, before treatment). The model captures the empirical findings.
Figure 4. Relationship between the treatment effect (treated vs. untreated words) in free naming time (delta RT) and in BOLD response (delta BOLD) in the right IFG after six-week treatment obtained by Nardo et al. (Citation2017) and in the model. a.u. = arbitrary unit; RT = reaction time; ms = milliseconds
Nardo et al. (Citation2017) observed an immediate but not a treatment effect of phonological cueing in left IFG. According to the model, the immediate effect of phonological cueing reflects the online facilitation of phonological encoding, simulated by temporarily increasing the activation of nodes that match the information in the phonological cues. The immediate conditions differ only in effort (cf. Taylor et al., Citation2013). The model assumes that the BOLD response reflects the encoding duration, so the response should be lower in the whole and partial cueing conditions than in the noise condition, as empirically observed. However, if treatment restores functionality in the left IFG through a structural change (i.e. an increase in connection strengths) leading to a greater engagement of this area, then this should increase the BOLD response (cf. Taylor et al., Citation2013), contrary to what Nardo et al. observed. However, if the restored functionality of the left IFG also decreases the encoding duration (because of the increased capacity to do the encoding, thereby reducing effort, as reflected in the RT), then this should reduce the BOLD response, counteracting the increase due to greater engagement of the area. The net result may be no change in BOLD response as a function of treatment, as empirically observed. Clearly, this account is speculative and needs to be tested in future research. For the right IFG, there is no such structural change from treatment, but only less effort because of the increased capacity of left IFG. Consequently, treatment should cause a reduction of the BOLD response in right IFG, as empirically observed.
To conclude, Nardo et al. (Citation2017) provide evidence that phonological cueing induces immediate and treatment effects in phonological encoding. Assuming a locus in phonological encoding, WEAVER successfully simulates the empirical findings.
Cohort size effects
Based on lexical cohort size effects in immediate phonological cueing, Pellet Cheneval et al. (Citation2018) argue, however, that phonological cueing influences lemma retrieval rather than phonological encoding. They observed that participants with a lexical-semantic profile show a cohort size effect in naming accuracy and a main effect of cueing in naming RT, and participants with a phonological profile show a cohort size effect in naming RT.
In WEAVER, a phonological cue not only activates corresponding output phonemes but also lemmas and lexical output forms, due to a cohort mechanism in perception. Under the assumption that the amount of activation received by lexical output forms depends on the competition among lexical input forms, less activation will be received by a target output form when the lexical cohort is large than when it is small. To simulate the cohort size manipulation, the activation received by the lexical output node was (arbitrarily) halved for the large cohort condition. Phonological encoding was assumed to be spared for the lexical-semantic profile and impaired for the phonological profile. Pellet Cheneval et al. (Citation2018) presented the auditory cues more than a second before the picture. At such large SOAs, no phonological effects are found in healthy speakers (Damian & Martin, Citation1999; Meyer & Schriefers, Citation1991). Therefore, it was assumed that when phonological cues are presented well in advance, patients covertly repeated the cues until picture presentation.
shows the mean naming times for cues with small and large cohorts obtained by Pellet Cheneval et al. (Citation2018) and corresponding word-form encoding times in the model. Lexical cohort size was simulated by reducing the perceptual activation input to the lexical output nodes for the large cohort condition relative to the small cohort condition. Consequently, the corresponding output phoneme nodes were activated less by their lexical output node. This had an impact on the encoding time when the activation of these output phoneme nodes was already low because of the phonological impairment assumed for the phonological profile (i.e. the reduced connection strength between the lexical output node and the output phoneme nodes).
Figure 5. Mean naming times for cues with small and large cohorts for patients with a phonological and a lexical-semantic profile obtained by Pellet Cheneval et al. (Citation2018) and corresponding word-form encoding times in the model. ms = milliseconds
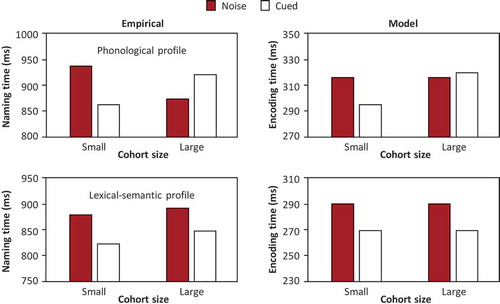
The patients with a phonological profile showed a cohort size effect in naming RT (upper left panel of ) and the patients with a lexical-semantic profile showed a main effect of cueing but not an interaction with cohort size in RT (lower left panel of ). Cohort size affected accuracy in this group. Thus, effects tend to be present in either accuracy or RT. This suggests that different speed-accuracy trade-offs were adopted by the groups. Importantly, the model captures the main effect of cueing and the effect of cohort size. To conclude, the simulation shows that a cohort size effect may happen in phonological encoding, reconciling the findings of Pellet Cheneval et al. (Citation2018) and Nardo et al. (Citation2017).
Visual information effect
Meteyard and Bose (Citation2018) observed that when a phonological cue was presented, naming accuracy was higher for pictures with less visual information. However, no interaction with lexical frequency or length in phonemes was observed. Based on these findings, Meteyard and Bose conclude that immediate phonological cueing helps picture recognition rather than lexical selection or phonological encoding.
However, an alternative interpretation of this visual effect is that the picture name is activated less in the lexical network when pictures provide less visual information. If less activation reaches the form nodes, there is more room for facilitation by phonological cueing. To examine this by simulation, the amount of activation input to the lexical output form node was (arbitrarily) reduced by 20 percent. This doubled the magnitude of the phonological cueing effect in the simulation, in line with the finding of Meteyard and Bose (Citation2018). In the model, frequency effects arise from the speed of condition-action rule application rather than network activation (Roelofs, Citation1996, Citation1997; Roelofs, Piai, & Schriefers, Citation2011). Immediate effects of cueing concern network activation, so a frequency effect is not expected. Moreover, although phonological encoding time increases with length in phonemes, leading to more room for facilitation, first-phoneme cues provide proportionally less information about the word as length increases, counteracting the potential beneficiary effect of the expanded room for facilitation (Roelofs, Citation1997). To conclude, the simulation shows that an effect of visual information may happen in word-form encoding, reconciling the findings of Meteyard and Bose and Nardo et al. (Citation2017).
Discussion
The aim of the present study was to obtain greater clarity about the locus of immediate and treatment effects of phonological cues during picture naming in aphasia and whether the effects arise from the same neurocognitive mechanisms as effects of phonological distractor words in healthy speakers. To this end, a psycholinguistic model of phonological distractor effects in healthy speakers, WEAVER, was applied through simulations to the immediate and treatment effects of phonological cues found in aphasia. The results show that the model successfully simulates the empirically observed effects in behavioural and neural measures. This suggests that the effects of phonological cues in aphasia may arise from the same neurocognitive mechanisms as effects of phonological distractor words in healthy speakers, namely from phonological encoding mechanisms.
The argument of Nardo et al. (Citation2017) for a phonological encoding locus is based on their fMRI finding that immediate cueing effects occur in left IFG. There were also activations in other areas, but not in left middle MTG or in occipital-temporal cortex. Meta-analyses, lesion-deficit analyses, and targeted neuroimaging studies have associated picture perception, lemma retrieval, and phonological encoding with, respectively, occipital-temporal cortex, left middle MTG, and left IFG, supporting the claim of Nardo et al. that cueing effects occur in phonological encoding and not in picture perception or lemma retrieval. Clearly, fMRI does not have the temporal resolution to separate lexical form selection and sublexical phonological encoding in time. However, the temporal sequence plays no role in the argumentation for a phonological locus based on the fMRI evidence. Instead, the argument is that lexical form selection and sublexical phonological encoding are underpinned by different areas of the brain, namely left posterior STG/MTG and left IFG respectively, for which there is converging evidence from meta-analyses of neuroimaging studies, large scale lesion-deficit analyses, and targeted neuroimaging. Thus, finding phonological cueing effects in left IFG but not in posterior STG/MTG, as Nardo et al. did, supports a locus in phonological encoding and not in lexical form selection.
The purpose of the simulations was to examine the possibility of a unified computational account of the phonological effects in picture naming by persons with aphasia and healthy individuals. To this end, simulations of the behavioural and neural findings of Nardo et al. (Citation2017) were conducted. Moreover, the simulations examined whether the behavioural findings of Pellet Cheneval et al. (Citation2018) and Meteyard and Bose (Citation2018) can be reconciled with the locus in phonological encoding assumed by Nardo et al. The reason why the WEAVER model was used for the simulations is that it is, to my knowledge, the only model in the literature that (1) addresses phonological effects in picture naming RTs, (2) assumes a locus in phonological encoding for these effects, and (3) assumes a particular mapping of functional stages in picture naming to areas of the brain. The models of Dell et al. (Citation1997, Citation2013) address naming errors but not RTs and have not been applied to phonological effects of cues or distractor words. This also holds for other models addressing patient data (e.g. Rapp & Goldrick, Citation2000; Ueno et al., Citation2011). The model of Starreveld and La Heij (Citation1996) addresses phonological effects of written distractors in RTs, but does not assume a sublexical phonological level in picture naming and makes no claims about the mapping of processing levels onto the brain. Activation of the IFG remains unexplained in this model without sublexical phonological level.
The central thesis in the simulations was that to explain the cueing data, one needs to assume (1) a locus in phonological encoding of the cueing (and distractor) effects, (2) a cohort principle in spoken word perception, and (3) activation of lexical and sublexical phonological levels in production by the cues (and distractors). To provide a proof of concept for the sufficiency of these assumptions, I have used a model, WEAVER, that addresses phonological effects of distractor words in RTs, assumes a locus in phonological encoding for these effects, and assumes a particular mapping of functional stages in picture naming to areas of the brain. Using WEAVER, the simulations demonstrate that assuming an influence of phonological cueing on phonological encoding is sufficient to account for the immediate and treatment effects observed in poststroke aphasia. Moreover, the simulations demonstrate that there is no need to assume that immediate phonological cueing effects in poststroke aphasia are located in lemma retrieval or in early stages of picture recognition, as assumed by Pellet Cheneval et al. (Citation2018) and Meteyard and Bose (Citation2018). This formal demonstration of the sufficiency and necessity of theoretical assumptions is of clinical and fundamental importance.
Of clinical relevance, the simulation results suggest that phonological cueing influences the phonological encoding component of word finding. This implies that patients with impaired phonological encoding are treated with a technique that targets their underlying impairment, explaining why phonological cueing yields improvements (cf. Best et al., Citation2013). Of fundamental scientific relevance, the simulation outcomes provide a proof of concept for the proposition that the immediate and treatment effects of phonological cues in aphasia arise from the same neurocognitive mechanisms as effects of phonological distractor words in healthy speakers.
Improvements of word finding by phonological cueing treatment in poststroke aphasia have been examined in naming speed and accuracy, and recently also in neuroimaging studies targeting changes in brain activations and fibre tract integrity (Nardo et al., Citation2017; Van Hees et al., Citation2014). These neuroimaging findings had not been addressed yet by computer models. The present simulations filled this gap by showing that the WEAVER model successfully captures the empirically observed effects in naming performance and neural measures, thereby providing a computationally explicit account.
Conclusion
The reported simulation outcomes provide a proof of concept for the idea that the effects of phonological cueing in aphasia arise from the same neurocognitive mechanism as phonological effects in healthy speakers, namely from the phonological encoding mechanism. The simulation outcomes suggest that phonological cueing treatment targets the underlying deficit in patients with impaired phonological encoding, which explains why the treatment yields improvements.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Best, W., Greenwood, A., Grassly, J., Herbert, R., Hickin, J., & Howard, D. (2013). Aphasia rehabilitation: Does generalisation from anomia therapy occur and is it predictable? A case series study. Cortex, 49, 2345–2357. doi:10.1016/j.cortex.2013.01.005
- Best, W., Herbert, R., Hickin, J., Osborne, F., & Howard, D. (2002). Phonological and orthographic facilitation of word-retrieval in aphasia: Immediate and delayed effects. Aphasiology, 16, 151–568. doi:10.1080/02687040143000483
- Damian, M. K., & Martin, R. C. (1999). Semantic and phonological codes interact in single word production. Journal of Experimental Psychology: Learning, Memory, and Cognition, 25, 345–361. doi:10.1037//0278-7393.25.2.345
- de Zubicaray, G. I., & Piai, V. (2019). Investigating the spatial and temporal components of speech production. In G. I. de Zubicaray & N. O. Schiller (Eds.), The Oxford handbook of neurolinguistics (pp. 471–497). Oxford: Oxford University Press.
- Dell, G. S., Schwartz, M. F., Martin, N., Saffran, E. M., & Gagnon, D. A. (1997). Lexical access in aphasic and nonaphasic speakers. Psychological Review, 104, 801–838. doi:10.1037/0033-295X.104.4.801
- Dell, G. S., Schwartz, M. F., Nozari, N., Faseyitan, O., & Coslett, H. B. (2013). Voxel-based lesion-parameter mapping: Identifying the neural correlates of a computational model of word production. Cognition, 128, 380–396. doi:10.1016/j.cognition.2013.05.007
- Duncan, J. (2010). How intelligence happens. Yale: Yale University Press.
- Goodglass, H., & Wingfield, A. (Eds.). (1997). Anomia: Neuroanatomical and cognitive correlates. San Diego: Academic Press.
- Graves, W. W., Grabowski, T. J., Mehta, S., & Gupta, P. (2008). The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of Cognitive Neuroscience, 20, 1698–1710. doi:10.1162/jocn.2008.20113
- Hashimoto, N., & Thompson, C. K. (2010). The use of the picture–word interference paradigm to examine naming abilities in aphasic individuals. Aphasiology, 24, 580–611. doi:10.1080/02687030902777567
- Indefrey, P. (2011). The spatial and temporal signatures of word production components: A critical update. Frontiers in Psychology, 2, Article 255. doi:10.3389/fpsyg.2011.00255.
- Indefrey, P., & Levelt, W. J. M. (2004). The spatial and temporal signatures of word production components. Cognition, 92, 101–144. doi:10.1016/j.cognition.2002.06.001
- Kaplan, E., Goodglass, H., & Weintraub, S. (1983). Boston naming test. Philadelphia: Lea & Febiger.
- Kemmerer, D. (2015). Cognitive neuroscience of language. New York: Psychology Press.
- Kemmerer, D. (2019). From blueprints to brain maps: The status of the Lemma Model in cognitive neuroscience. Language, Cognition and Neuroscience, 34, 1085–1116. doi:10.1080/23273798.2018.1537498
- Laine, M., & Martin, N. (2006). Anomia: Theoretical and clinical aspects. New York, NY: Psychology Press.
- Levelt, W. J. M., Roelofs, A., & Meyer, A. S. (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22, 1–38. doi:10.1017/S0140525X99001776
- Mack, J. E., Cho-Reyes, S., Kloet, J. D., Weintraub, S., Mesulam, M. M., & Thompson, C. K. (2013). Phonological facilitation of object naming in agrammatic and logopenic primary progressive aphasia (PPA). Cognitive Neuropsychology, 30, 172–193. doi:10.1080/02643294.2013.835717
- Marslen-Wilson, W. (1987). Functional parallelism in spoken word-recognition. Cognition, 25, 71–102. doi:10.1016/0010-0277(87)90005-9
- Meteyard, L., & Bose, A. (2018). What does a cue do? Comparing phonological and semantic cues for picture naming in aphasia. Journal of Speech, Language, and Hearing Research, 61, 658–674. doi:10.1044/2017_JSLHR-L-17-0214
- Meyer, A. M., Tippett, D., Turner, R. S., & Friedman, R. B. (2019). Long-term maintenance of anomia treatment effects in primary progressive aphasia. Neuropsychological Rehabilitation, 29, 1439–1463. doi:10.1080/09602011.2018.1425146
- Meyer, A. S., & Schriefers, H. (1991). Phonological facilitation in picture-word interference experiments: Effects of stimulus onset asynchrony and types of interfering stimuli. Journal of Experimental Psychology: Learning, Memory, and Cognition, 17, 1146–1160. doi:10.1037/0278-7393.17.6.1146
- Nardo, D., Holland, R., Leff, A. P., Price, C. J., & Crinion, J. T. (2017). Less is more: Neural mechanisms underlying anomia treatment in chronic aphasic patients. Brain, 140, 3039–3054. doi:10.1093/brain/awx234
- Nickels, L. (2002). Therapy for naming disorders: Revisiting, revising and reviewing. Aphasiology, 16, 935–979. doi:10.1080/02687030244000563
- Norris, D. (1994). Shortlist: A connectionist model of continuous speech recognition. Cognition, 52, 189–234. doi:10.1016/0010-0277(94)90043-4
- Pellet Cheneval, P., Glize, B., & Laganaro, M. (2018). The lexical or sub-lexical locus of facilitation by phonemic cueing in aphasic speakers: The effect of onset cohort size. Aphasiology, 32, 1468–1489. doi:10.1080/02687038.2017.1423273
- Posner, M. I. (2012). Attention in a social world. Oxford: Oxford University Press.
- Rapp, B., & Goldrick, M. (2000). Discreteness and interactivity in spoken word production. Psychological Review, 107, 460–499. doi:10.1037/0033-295X.107.3.460
- Raymer, A. M., Thompson, C. K., Jacobs, B., & Le Grand, H. R. (1993). Phonological treatment of naming deficits in aphasia: Model-based generalization analysis. Aphasiology, 7, 27–53. doi:10.1080/02687039308249498
- Roelofs, A. (1992). A spreading-activation theory of lemma retrieval in speaking. Cognition, 42, 107–142. doi:10.1016/0010-0277(92)90041-F
- Roelofs, A. (1996). Morpheme frequency in speech production: Testing WEAVER. In G. E. Booij & J. van Marle (Eds.), Yearbook of Morphology 1996 (pp. 135–154). Dordrecht: Kluwer Academic Publishers.
- Roelofs, A. (1997). The WEAVER model of word-form encoding in speech production. Cognition, 64, 249–284. doi:10.1016/S0010-0277(97)00027-9
- Roelofs, A. (2002). Spoken language planning and the initiation of articulation. Quarterly Journal of Experimental Psychology, Section A: Human Experimental Psychology, 55, 465–483. doi:10.1080/02724980143000488
- Roelofs, A. (2004). Error biases in spoken word planning and monitoring by aphasic and nonaphasic speakers: Comment on Rapp and Goldrick (2000). Psychological Review, 111, 561–572. doi:10.1037/0033-295X.111.2.561
- Roelofs, A. (2008). Tracing attention and the activation flow in spoken word planning using eye movements. Journal of Experimental Psychology: Learning, Memory, and Cognition, 34, 353–368. doi:10.1037/0278-7393.34.2.353
- Roelofs, A. (2014). A dorsal-pathway account of aphasic language production: The WEAVER++/ARC model. Cortex, 59, 33–48. doi:10.1016/j.cortex.2014.07.001
- Roelofs, A. (2018). A unified computational account of cumulative semantic, semantic blocking, and semantic distractor effects in picture naming. Cognition, 172, 59–72. doi:10.1016/j.cognition.2017.12.007
- Roelofs, A., & Hagoort, P. (2002). Control of language use: Cognitive modeling of the hemodynamics of Stroop task performance. Cognitive Brain Research, 15, 85–97. doi:10.1016/S0926-6410(02)00218-5
- Roelofs, A., Meyer, A. S., & Levelt, W. J. M. (1996). Interaction between semantic and orthographic factors in conceptually driven naming: Comment on Starreveld and La Heij (1995). Journal of Experimental Psychology: Learning, Memory, and Cognition, 22, 246–251. doi:10.1037/0278-7393.22.1.246
- Roelofs, A., Piai, V., & Schriefers, H. (2011). Selective attention and distractor frequency in naming performance: Comment on Dhooge and Hartsuiker (2010). Journal of Experimental Psychology: Learning, Memory, and Cognition, 37, 1032–1038. doi:10.1037/a0023328
- Roelofs, A., Van Turennout, M., & Coles, M. G. H. (2006). Anterior cingulate cortex activity can be independent of response conflict in Stroop-like tasks. Proceedings of the National Academy of Sciences USA, 103, 13884–13889. doi:10.1073/pnas.0606265103
- Schriefers, H., Meyer, A., & Levelt, W. J. M. (1990). Exploring the time-course of lexical access in language production: Picture-word interference studies. Journal of Memory and Language, 29, 86–102. doi:10.1016/0749-596X(90)90011-N
- Schwartz, M. F., Faseyitan, O., Kim, J., & Coslett, H. B. (2012). The dorsal stream contribution to phonological retrieval in object naming. Brain, 135, 3799–3814. doi:10.1093/brain/aws300
- Schwartz, M. F., Kimberg, D. Y., Walker, G. M., Faseyitan, O., Brecher, A., Dell, G. S., & Coslett, H. B. (2009). Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain, 132, 3411–3427. doi:10.1093/brain/awp284
- Schwartz, M. F., Wilshire, C. E., Gagnon, D. A., & Polansky, M. (2004). Origins of nonword phonological errors in aphasic picture naming. Cognitive Neuropsychology, 21, 159–186. doi:10.1080/02643290342000519
- Starreveld, P. A., & La Heij, W. (1996). Time course analysis of semantic and orthographic context effects in picture naming. Journal of Experimental Psychology: Learning, Memory, and Cognition, 22, 896–918. doi:10.1037/0278-7393.22.4.896
- Taylor, J. S. H., Rastle, K., & Davis, M. H. (2013). Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin, 139, 766–791. doi:10.1037/a0030266
- Thompson, H. E., & Woollams, A. M. (2017). Reduced neural ‘effort’ after naming treatment in anomia. Brain, 140, 2773–2775. doi:10.1093/brain/awx264
- Ueno, T., Saito, S., Rogers, T. T., & Lambon Ralph, M. A. (2011). Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron, 72, 385–396. doi:10.1016/j.neuron.2011.09.013
- Van Hees, S., McMahon, K., Angwin, A., de Zubicaray, G., Read, S., & Copland, D. A. (2014). Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabilitation and Neural Repair, 28, 325–334. doi:10.1177/1545968313508654
- Wisenburn, B., & Mahoney, K. (2009). A meta-analysis of word-finding treatments for aphasia. Aphasiology, 23, 1338–1352. doi:10.1080/02687030902732745
