ABSTRACT
Background: The localization and organization of language has been an ongoing research interest ever since the early findings of Paul Broca. The emergence of neuroimaging the past 20 years has given us new insights on the anatomical and structural organization of the brain. Lesion studies on patients with aphasia can provide knowledge on where and how specific language functions are organized in the brain.Aims: The primary objective of the study was to investigate the relationships between aphasia severity, aphasic symptoms, lesion location and lesion volume in patients with left hemispheric stroke in the acute phase (within one week post-stroke). Using a voxel-based lesion-symptom mapping method (VLSM), we hypothesized that lesions associated with speech comprehension deficits mainly would involve regions within the posterior superior and middle temporal lobe, and lesions associated with speech production deficits would mainly be associated to the inferior frontal areas of the left hemisphere.Methods & procedures: Findings from diffusion-weighted magnetic resonance imaging (DWI-MRI) and patients’ scores from the Norwegian Basic Aphasia Assessment (NBAA) were used to investigate our research questions. We did a whole group analysis of descriptive statistics, lesion localization and lesion volume. We thereafter divided the patients into two groups based on their median scores on the NBAA, one high comprehension group and one low comprehension group. We used VLSM to investigate the associations between the patients’ lesions and the results from the NBAA.Outcomes & Results:Lesion volume was significantly associated with all subtest from the NBAA. Our initial analysis of the whole group showed that difficulties in naming was associated with lesions within the rolandic operculum. We also found that difficulties in repetition was associated with lesions within the rolandic operculum, and in addition, the superior temporal gyrus. In the group of patients with high comprehension scores lesions within Broca’s area, insula, the superior temporal gyrus (STG) and Heschl’s gyrus were found to be associated with difficulties with overall aphasia severity, repetition, naming, and reading out loud from the NBAA. Conclusions: Lesion volume is strongly associated with aphasia severity in the acute stages of stroke. Further, lesions within Broca’s area, the insula, the STG and Heschl’s gyrus were found to be crucial areas in language comprehension and production. This confirms current views that speech and language processes depend on the integrity of the entire network comprising both cortical structures and their interconnected fibre tracts.
Introduction
The neurobiology of language
Aphasia can be defined as an acquired communication disorder, and can cause impairments in verbal comprehension and production, and in reading and writing. The most common cause of aphasia is left hemispheric stroke (Hallowell & Chapey, Citation2008). The neurobiological localization of language functions has been an ongoing research interest ever since the clinical findings of Paul Broca and Carl Wernicke in the late 19th century. The research on the neurobiological basis for language has mainly focused on lesion studies, but the past twenty years research on healthy language processing using functional magnetic resonance imaging (fMRI) has sought to understand how language is organized and processed in the brain (Friederici, Citation2011; Price, Citation2000; Price & Crinion, Citation2005). Language processing is now considered a complex system dependent of several neural sites and connections within the brain (Damasio, Citation2008).
Lesion size or lesion location?
Several studies have investigated the role of lesion size and lesion location in patients with aphasia (Cherney & Robey, Citation2008; Crinion, Holland, Copland, Thompson, & Hillis, Citation2013a; Plowman, Hentz, & Ellis, Citation2012). However, the quantification of lesion volume differs across studies depending on MRI-sequence and variability in quantification methods that makes it difficult to compare results across studies. Crinion et al. (Citation2013a) requested a gold standard for quantifying lesion volume and concluded that until the standard is clear researchers should thoroughly explain and define cut offs and quantification procedures when reporting findings from lesion quantification studies. Using diffusion tractography Forkel et al. (Citation2014) found that lesion volume was a predictor of aphasia recovery six months post-stroke. Plowman et al. (Citation2012) investigated stroke related factors in post-stroke recovery, and found that both lesion volume and lesion site was associated with aphasia severity six months post-stroke, however initial aphasia severity was found to be the most predictive factor of aphasia recovery. However, there seems to be an agreement that lesion location might be more important to consider than lesion size when investigating initial aphasia outcome post-stroke (Cherney & Robey, Citation2008; Crinion, Holland, Copland, Thompson, & Hillis, Citation2013b).
This view has been supported by recent neuroimaging studies, indicating that focal lesions of certain cortical areas or fibre tracts can have a substantial impact on recovery and therapy outcome (Abel, Weiller, Huber, Willmes, & Specht, Citation2015; Specht et al., Citation2009). In addition, there is growing evidence that lesions also have remote effects through disinhibition (Price & Crinion, Citation2005; Saur et al., Citation2006) or altered perfusion (Robson et al., Citation2017).
Towards a new model of language processing
The Classic Model of the neurobiology of language was developed by Geschwind (Citation1965) and is based on the functional view upon language that emerged after the findings of Broca and Wernicke (Geschwind, Citation1965a, Citation1965b). To simplify, the Classic Model focuses on the associations between language functions and brain structures. Within the Classic Model, language processing is considered to be dependent on activation of the posterior temporal area (referred to as Wernicke’s area) which is responsible for auditory comprehension, storage, synthesis and overall language comprehension. In the Classic Model, the location of Wernicke’s area is assumed to comprise the posterior third of the superior temporal gyrus (STG), on the border between the temporal and parietal lobe. Further, Broca’s area is postulated to be within the anterior inferior frontal area and is thought to be crucial for the production of oral and written language. According to the Classic Model, a fiber tract, the arcuate fasciculus, is essential for the flow of information from the posterior language areas to the anterior language areas making language processing possible (Geschwind, Citation1965a, Citation1965b).
The Classic Model of language has dominated the view on language processing since it emerged in the 1960s. However, newer neuroimaging studies using functional neuroimaging and voxel-based lesion symptom mapping (VLSM) have facilitated several new theories on the neurobiology of language. Tremblay and Dick (Citation2016) argue that the Classic Model of language is outdated, and more precise anatomical definitions should be used in the neurobiological descriptions of language processes. Further, the authors argue that there is no precise anatomical definition of Broca’s and Wernicke’s area; therefore, the field of the neurobiology of language should be updated with new evidence from research on language processing. Poeppel and Hickok (Citation2004) also argue that the Classic Model is empirically incorrect as it does not account for the aphasic syndromes and is underspecified in its neuroanatomical descriptions.
In recent years, several functional neuroimaging and meta-analysis studies have investigated the neuroanatomical basis of language processing and proposed new models of language comprehension and language production (Ardila, Bernal, & Rosselli, Citation2016a; Dronkers, Wilkins, et al. Citation2004; Hickok & Poeppel, Citation2007; Specht, Citation2014). Based on earlier findings from lesion analysis (Dronkers, Wilkins, et al. Citation2004), fiber tractography and functional connectivity analysis, Turken and Dronkers (Citation2011) suggested a language comprehension network including the left middle temporal gyrus (MTG), the anterior superior temporal gyrus (STG/BA22), the pars orbitalis (IFGpOrb/BA47) and the superior temporal sulcus (STS/BA39). Further, Turken and Dronkers found that the inferior occipito-frontal fasciculus, the arcuate fasciculus, and the middle and inferior longitudinal fasciculi, and transcallosal projections via the tapetum were found to be the most significant white matter pathways bridging the areas crucial in language comprehension. Finally, the MTG was found to be a core region in the language comprehension network.
In a meta-analysis of the Brodmann areas (BA) involved in language by Ardila et al. (Citation2016a) the authors suggested an extended Wernickes’ area for auditory comprehension. This included the traditional core areas as the planum temporale, the posterior thirds of the STG (BA22), the posterior part of the MTG (BA21) and the auditory cortex (BA41/42), and further including corresponding areas as the inferior temporal gyrus (BA20), the fusiform gyrus (BA37), the angular gyrus (BA39) and the supramarginal gyrus (SMG/BA40) (Ardila et al., Citation2016a). Accordingly, Ardila and colleagues refer to the Brocas’ complex and suggest extending the borders of Brocas’ area, which traditionally includes the pars opercularis (IFGpOp/BA44) and the pars triangularis (IFGpTri/BA45), to also include the dorsolateral prefrontal cortex (BA46), IFGpOrb, supplementary motor area (SMA/BA6), and extending subcortically towards the basal ganglia. Finally, in their view, the insula (BA13) has a crucial coordinating role in language production and comprehension (Ardila et al., Citation2016a).
These results have been confirmed and extended by studies investigating the relationship between the patients’ lesioned areas in the brain and their language specific deficits by using voxel-based lesion-symptom mapping (VLSM) (Bates et al., Citation2003). VLSM has become a commonly used lesion mapping method in aphasia research (Baldo, Arevalo, Wilkins, & Dronkers, Citation2009; Dronkers, Wilkins, Van Valin Jr., Redfern, & Jaeger, 2004; Harvey & Schnur, Citation2015). VLSM calculates the statistical relationship between performance on a given task and the status, i.e. lesioned or not, for each voxel of the brain (Crinion et al., Citation2013b; Schwartz et al., Citation2009).
Bates et al. (Citation2003) were one of the first using VLSM. They conducted a study investigating speech fluency and auditory comprehension deficits in patients with aphasia. Bates and colleagues found that lesions within the anterior insula were an important contributor to fluency deficits in aphasia, and the middle temporal areas were associated with auditory comprehension deficits. Interestingly, they also found that lesions limited to Broca’s area was not the area that explained fluency deficits in patients with aphasia. Furthermore, their results showed that lesions within the MTG had a strong association to the patients’ auditory comprehension difficulties, especially when the contribution of Wernicke’s area was factored out. Finally, Bates et al. found that the peri-Sylvian areas contributed to both fluency and comprehension difficulties in patients with aphasia suggesting that the peri-Sylvian areas account for core language functions. Accordingly, Dronkers, Wilkins, Van Valin Jr., et al. (2004) found in their lesion study on language comprehension that the most distinct areas within the left hemisphere were the posterior MTG and underlying white matter, the anterior STG, the superior temporal sulcus (STS) and the angular gyrus. This is in line with the results by Baldo et al. (Citation2009), who investigated the associations between brain lesions and difficulties with category-specific naming. They found that lesions within the left MTG and STG were associated with naming difficulties, and that lesioned areas overlapped across naming categories (Baldo et al., Citation2009). Finally, Dronkers et al. (2004a) did not find that lesions within either Broca’s or Wernicke’s area were significant contributors to the language comprehension difficulties on the given tasks in the study.
The findings from Bates et al. and Dronkers and her colleagues raise important questions that challenge the traditional assumption about the contribution of Broca’s and Wernicke’s area in language production and comprehension. However, Bonilha and Fridriksson (Citation2009) suggest a more pragmatic approach by acknowledging that Broca’s area is crucial for speech production, but damage and disconnection from surrounding language areas might also result in fluency disorders (Bonilha & Fridriksson, Citation2009).
Based on these converging evidences from both functional neuroimaging and lesion studies, Hickok and Poeppel published in 2007 the dual-stream theory on the cortical organization of speech processing (Hickok & Poeppel, Citation2007). In short, the concept of the model is that a ventral stream processes signals for auditory comprehension, which involves structures within the superior and middle temporal lobe. The ventral stream interacts with a dorsal stream which maps acoustic speech signals to the frontal lobe articulatory networks. The dorsal stream involves structures in the posterior frontal lobe and the posterior dorsal region of the temporal lobe, and also the parietal operculum. Further, both streams share neural tissue in the left posterior STG. While the dorsal stream is left-hemisphere dominant, the ventral stream is assumed to be bilaterally organized (Hickok & Poeppel, Citation2007), or may even represent two separated streams, one for phonological and sub-lexical processing and one for prosody and voice recognition (Specht, Citation2014). The precise anatomical areas which comprise the ventral and dorsal stream, and where the two streams diverge is still a debate (Specht, Citation2014).
Assessment and classification of aphasia
The assessment of aphasia varies across research, languages, and theoretical traditions. One common way to define aphasia is to conceptualize aphasia dichotomously, such as fluent versus non-fluent aphasia, or Broca’s versus Wernicke’s aphasia and so forth (Hallowell & Chapey, Citation2008). In the present study we used a standardized test of aphasia severity which is based on the Boston classification of aphasia. The Boston Diagnostic Aphasia Examination (Goodglass, Citation2001), and the Norwegian Basic Aphasia Assessment (NBAA) (Reinvang & Engvik, Citation1980) are designed to classify patients into localization-based classifications of aphasia; Broca’s, Wernicke’s, anomic, conduction, transcortical motor, transcortical sensory and global aphasia syndromes. According to the Boston-classification of aphasia, the different aphasia syndromes have certain hallmark symptoms dependent on lesion location (Hallowell & Chapey, Citation2008). The Boston-classification of aphasia is based on the Classic Model of the neurobiology of language. Even though newer classifications and theories on language processing and aphasia have emerged, the Classic Model of language neurobiology is still commonly used as a theoretical framework in aphasia assessment, and the NBAA is the most frequently used aphasia assessment in Norway.
The primary objective of the present study was to investigate the relationship between symptoms of aphasia, lesion location and lesion volume in patients with left ischemic stroke in the acute phase (within one-week post-stroke). We used findings from diffusion-weighted magnetic resonance imaging (DWI-MRI) and the patients’ scores from the Norwegian Basic Aphasia Assessment (Reinvang & Engvik, Citation1980) to investigate our research questions.
Using VLSM, we hypothesized that lesions associated with speech comprehension deficits mainly would involve regions within the posterior superior and middle temporal lobe, and lesions associated with speech production deficits would mainly be associated to the inferior frontal areas of the left hemisphere.
Methods
Participants
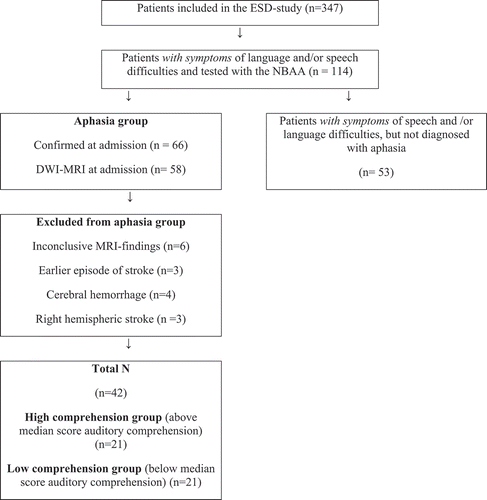
The present study is a part of two larger projects at Haukeland University Hospital (HUS); the Early Supported Discharge after stroke in Bergen-study (ESD) (Hofstad, Naess, Moe-Nilssen, & Skouen, Citation2013) and the Bergen NORSTROKE study. From January 2008 throughout December 2012 a total of 347 patients were included in the ESD-study. The patients were recruited from the Stroke Unit of the Department of Neurology at HUS. Of 347 patients included in the ESD-study a total of 114 (33%) patients with aphasia-like symptoms caused by stroke were asked to participate in the present study. The patients underwent diffusion-weighted magnetic resonance imaging within 24 hours post-onset of first symptoms. Aphasia was diagnosed based on the convergence of clinical consensus and the results from the NBAA (Reinvang & Engvik, Citation1980). The patients were tested with the NBAA within seven days post-onset of initial symptoms. Fifty-three patients were excluded from the study because they did not have aphasia when diagnosed by the speech and language pathologist. Of the remaining 66, eight were excluded because DWI-MRI was not performed. Thereafter, six patients were excluded because of inconclusive MRI findings, three patients had earlier episodes of stroke, four patients were excluded because of cerebral haemorrhage, and finally three patients were excluded because of right hemispheric lesions (see for an overview). Patients with apraxia of speech and dysarthria were not excluded from the study.
Finally, 42 patients (mean age 72.9, SD: 11.8, range: 27–89) were included in the present study. All patients had first time episode of ischemic stroke and all patients were native Norwegian speakers. Twenty-four men and 18 women were included, 36 were right-handed, one left-handed and five were uncertain. Four patients had lesions in both hemispheres, and the remaining 38 patients had lesions in the left hemisphere.
Materials and procedures
The Norwegian Basic Aphasia Assessment (NBAA). The NBAA is a standardized Norwegian test for assessment of aphasia. The test was developed by Reinvang and Engvik (Citation1980) and is based on the Boston model of aphasia. The score ranges from 0–217 where 217 is the maximum of correct responses. The NBAA consists of seven subtests measuring auditory comprehension, repetition, naming, reading comprehension, reading out loud, syntax, and writing. The overall aphasia score gives an aphasia profile indicating aphasia severity and what type of aphasia the patient has acquired. However, in the present study we did not divide the patients’ into aphasia subgroups based on the aphasia types suggested in the NBAA. This was due to the clinical routines at the hospital where subgrouping the patients based on their results from the NBAA is not part of the clinical diagnosis of aphasia. The aphasia assessment was administered to all patients as part of the existing research protocol of the ESD-Bergen project.
Diffusion-weighted magnetic resonance imaging
DWI-MRI is a magnetic resonance imaging technique increasingly used in acute stroke (Neumann-Haefelin et al., Citation1999). DWI-MRI gives in vivo images and allows a rapid characterization of stroke pathophysiology (Lee, Kidwell, Alger, Starkman, & Saver, Citation2000). By using the DWI-sequences one can easily differentiate between old and new lesions by looking at the white matter hyperintensities which are white when new, and black when old (Crinion et al., Citation2013b).
MRI specifications
All patients underwent diffusion-weighted magnetic resonance imaging within 24 hours post-onset of stroke symptoms. The DWI-MRI data were collected on a Siemens 1.5 Tesla Symphony using a DWI-sequence with TR 3200 ms, TE 94 ms, field of view 230mm, 128 × 128 matrix, in-plane voxel size 1.8 × 1.8 mm2, slice thickness 5mm, as specification parameters.
Data pre-processing for voxel-based lesion-symptom mapping
Lesions were traced manually slice-by-slice on patients DWI-images in MRIcron (Rorden, Karnath, & Bonilha, Citation2007). Uncertain or unclear cases were excluded because of inconclusive MRI-findings. Both the DWI images as well as the lesion maps were thereafter normalised into standard Montreal Neurological Institute (MNI) stereotactic space, using the “old normalisation” procedure of the SPM12 software. First, DWI images were normalised into the MNI space using an EPI template, as provided by SPM12. In order to achieve the most optimal normalisation, the transformation was based on the non-lesioned tissue by masking the individual DWI images with the respective lesion maps. Thereafter, the transformation was applied to the lesion map, and images were resampled to a voxel size of 2mm3.
Data analysis
For the VLSM data analysis we used the non-parametric mapping (NPM) software package in MRIcron (Rorden et al., Citation2007). In order to correct for multiple comparisons, we added the non-parametric permutation test to determine the critical t cut-off score (p < 0.05) which was based on 1,000 random permutations of the data. In order to control for false positives, Family Wise Error (FWE) control was carried out on the primary, whole group analysis, and False Discovery Rate (FDR) control was carried out on all subsequent sub-group analyses. For statistical analysis, the lesion detection threshold was set to 5% prior the analysis, thus meaning that tests were not run for voxels with less than 5% of the subjects having damage there. We used a two-sample t-test where the predictor variable was the two patient groups (if a voxel was lesioned or not). The outcome variable was the raw scores from the overall aphasia severity score and each subtest from the NBAA. The colorized maps are based on the resulting t-value of each voxel. To determine anatomical structures, the Automated Anatomical Labelled map in MRIcron was used. Lesion volume was quantified using an in-house Matlab script that extracts the number of voxels of a lesion and estimates its volume, based on the respective voxel size. Finally, we carried out independent samples t-test to investigate differences in lesion volume between the high and low comprehension group. All t-tests on lesion volume data, descriptive statistics and frequencies of the behavioral data were calculated using IBM SPSS v20.
Results
The mean score on the Norwegian Basic Aphasia Assessment was 130.7 (max: 215, min: 0, SD: 66.5 range: 0–217). A high score on the aphasia test indicates mild aphasia, and a low score indicates severe aphasia. Descriptive statistics from the patients’ scores on the subtests from the NBAA are presented in .
Table 1. Descriptive statistics of the patients scores on the Norwegian Basic Aphasia Assessment (n = 42)
Whole group lesion analysis
Our primary analysis with the whole group of patients showed, using a FWE-corrected threshold of p < 0.05, that the patients’ performance on the subtests repetition and naming were found to be associated to lesions within two specific areas. On the subtest repetition, we found that lesions within the rolandic operculum, and the STG were significantly associated with the patients’ performance on the repetition subtest. Further, on the subtest naming we also found that the patients’ results were significantly associated with lesions within the rolandic operculum (see ).
Table 2. VLSM-analysis of lesions associated with patients scores on the subtests repetition and naming, MNI-coordinates in parenthesis (n = 42)
Group differences based on auditory comprehension scores
In order to disentangle the differential effect of a lesion on comprehension and production further, the patients were divided into two groups based on the median score on the subtest auditory comprehension from the NBAA. This was done for several reasons. Firstly, impaired auditory comprehension is a key symptom of aphasia. Secondly, the division was based on the descriptive statistics of the patients, when comparing the two groups, auditory comprehension had the greatest effect size, measured with Cohen’s d (see ). Thus, indicating that the greatest difference in language performance between the groups was on their performance on the auditory comprehension subtest from the NBAA.
Table 3. Independent samples t-test of all subtests from the NBAA between the patients who scored above the median and the group who scored below the median (55.5) on the auditory comprehension subtest from the NBAA
The high comprehension group performed significantly better on all NBAA subtests than the low comprehension group. To specify, the high comprehension group consists of patients with mild aphasic symptoms, whereas the low comprehension group consists of patients with moderate to severe aphasic symptoms. An overview of the results of the independent samples t-tests are presented in .
Median score on the subtest was 55.5 (max: 70, min: 0) and the groups consisted of 21 patients in each group. Mean age was 72.9 in both groups, and each group consisted equally of 12 men and 9 women. The groups differed significantly on all subtests on the NBAA as indicated by independent samples t-tests. On the overall aphasia score the high comprehension group had a mean score of 179.1 (SD: 33.5), and the low comprehension group had a mean score of 82.2 (SD: 54.9), t = 6.9, p < .001.
Lesion volume
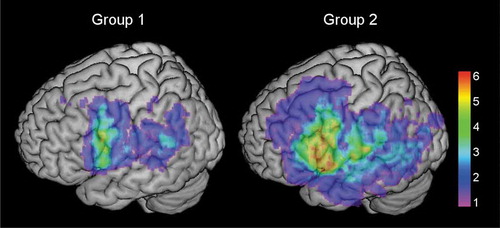
The group specific lesion localizations are illustrated in . By visual inspection of the images, the patients in the high comprehension group had lesions mostly in the inferior frontal and posterior temporal areas, while the lesions of the patients in the low comprehension group had a more heterogeneous and widely spread pattern. Independent samples t-test was performed to investigate the differences in lesion volume between the groups. Mean lesion volume in the high comprehension group was 19,019.43mm3 (SD: 22,040.46 mm3), and 43,443.81 mm3 (SD: 39,382.34 mm3) in the low comprehension group, t = 2.48, p = .017. The mean lesion volume was larger in the low comprehension group.
Figure 2. Colorized maps depicting lesion overlaps in the left hemisphere in the high comprehension group (left, group 1) and the low comprehension group (right, group 2) (n = 21 each group). Warmer areas (red) illustrate a greater lesion overlap than colder areas (purple/blue). Maximum overlap in individual voxels in the high comprehension group was 6, and 11 in the low comprehension group. Mean lesion was 19,019.43mm3 in the high comprehension group, and 43,443.81mm3 in the low comprehension group
Pearson’s product moment correlation was performed on the whole group (n = 42) to investigate the associations between lesion volume and aphasia severity and aphasic symptoms. The results yielded a significant association between the patients’ lesion volume and aphasia severity, as measured by the NBAA (r = −.59, p < .001), and the subtests auditory comprehension (r = −.40, p < .009), repetition (r = −.49, p < .001), naming (r = −.51, p < .001), reading comprehension (r = −.47, p < .002), and reading out loud (r = −.50, p < .001). The results indicate that greater lesion volume was associated with greater aphasia severity within the subtests.
Associations between lesions and overall aphasia severity
We investigated the patients’ overall aphasia severity scores from the NBAA and the patients’ lesions in both groups. We found a significant association between the overall aphasia severity score and lesioned areas of the brain in the high comprehension group, but not in the low comprehension group.
Our results showed that within the frontal lobe, lesions within the rolandic operculum (RO) the IFGpTri and IFGpOrb, the insula and the IFGpOp were associated with aphasia severity. Within the temporal lobe, lesions within the STG and Heschl’s gyrus were significantly associated with aphasia severity. Finally, lesions within the parietal lobe, specifically the SMG, the angular gyrus, the superior parietal lobule (SPL), the postcentral gyrus (postCG), and the inferior parietal lobule (IPL) were associated with aphasia severity.
Associations between lesions and difficulties with word, non-word and sentence repetition
We investigated the association between lesion site and the patients’ performances on the subtest repetition from the NBAA. The subtest was treated as one subtest, and not divided into word, non-word and sentence repetition because there are too few items in each category (8 non-words, and 32 words and sentences).
Within the frontal lobe, we found that lesions of the insula, the IFGpTri, the IFGpOrb, the IFGpOp, and the RO were associated with repetition difficulties. For the temporal lobe, lesions of the Heschls’ gyrus and STG showed this association, as well. For the parietal lobe lesions within the IPL, the angular gyrus, the postCG, and the SMG were associated with repetition difficulties in aphasia.
Associations between lesions and naming
Significant results were found for the associations between the patients’ lesions and the patients’ performance on the subtest naming.
In the high comprehension group, lesions within the frontal lobe that were associated with naming difficulties included the insula, the IFGpOrb, the IFGpTri, and the IFGpOp. Finally, within the temporal lobe, we found associations between the patients’ lesions in Heschl’s gyrus and the STG and their performance on the naming subtest.
Associations between lesions and difficulties reading words and sentences out loud
We found a significant association between the patients’ lesioned areas of the brain and the patients’ performance on the subtest reading out loud from the NBAA in the high comprehension group.
Lesions within the frontal lobe associated with difficulties reading out loud included the insula, the IFGpOp, the IFGpOrb, the IFGpTri, and the RO. Furthermore, in the temporal lobe, lesions within the STG, and Heschls’ gyrus were associated with the patients’ difficulties with the subtest reading out loud from the NBAA. Finally, lesions in the parietal lobe included areas within the SPL, the postCG, the SMG, the IPL, and the angular gyrus. All results are presented in .
Table 4. VLSM-analysis of lesions associated with overall aphasia severity, repetition, naming and reading out loud in the high comprehension group, MNI-coordinates in parenthesis (n = 21)
Discussion
The aim of the present study was to investigate the associations between lesion location, lesion volume, aphasia severity, and aphasic symptoms in the acute phase in a group of patients with left hemispheric stroke. We hypothesized that lesions that were associated with speech comprehension deficits would mainly affect regions within the temporal lobe, and lesions associated with speech production deficits would mainly affect the areas comprising the frontal areas of the left hemisphere.
Greater lesion volume was associated with patients’ performance on the overall aphasia score, and the subtests auditory comprehension, repetition, naming, reading comprehension and reading out loud. Thus indicating that lesion volume has an adverse effect on initial aphasia severity. This is in line with findings from several studies showing that lesion volume is an important factor in aphasia outcome and recovery (Forkel et al., Citation2014; Plowman et al., Citation2012). One important consideration in the interpretation of the results of the current study is that the results are derived from the acute stage. A more differential outcome could be expected, if the results were from the subchronic and chronic stages after the stroke, hence ruling out the possible influence of spontaneous recovery and cerebral blood flow in the acute stages of a stroke.
Our initial analysis of the whole group showed that difficulties in naming was associated with lesions within the rolandic operculum. Likewise, we also found that difficulties in repetition was associated with lesions within the rolandic operculum, and in addition, the STG. In a case study by Tonkonogy and Goodglass (Citation1981) the rolandic operculum was found to be associated with articulatory difficulties in a patient with aphasia. However, Baldo et al. (Citation2009) show that naming difficulties are a result of lesions within several areas along the left hemisphere. Friederici and Gierhan (Citation2013) emphasize that speech repetition is a complex language process. This involves several regions along the parietal, temporal and frontal cortex, which are connected by several white matter tracts such as the superior longitudinal fascile and the arcuate fasciculus. Baldo, Katseff, and Dronkers (Citation2012) also did a VLSM analysis of brain lesions related to performance on a repetition task and found that lesions along the left posterior temporo-parietal cortex were associated with repetition difficulties in aphasia. Our findings from the whole group lesion analysis are therefore not consistent with the theory of language processing as a system dependent of several neural sites and connections within the brain (Damasio, Citation2008).
In order to investigate the lesion data further, we divided the patients into two groups, based on the notion that auditory comprehension deficits is a key symptom in aphasia, and based on the patients’ descriptive results on the subtest auditory comprehension. One group consisted of patients with low auditory comprehension scores and the other of patients with high auditory comprehension scores. As one would expect, we found that in the high comprehension group the patients also scored significantly better on all subtests from the NBAA compared to the patients in the low comprehension group. Further, we found that in the high auditory comprehension group the patients’ performance on the overall aphasia severity score, the subtests repetition, naming and reading out loud were associated to lesions within specific regions within the frontal, temporal and posterior areas of the brain. Surprisingly, the group of patients with more severe auditory comprehension deficits did not have any significant associations between lesion localizations and their results on the NBAA subtests. One possible explanation of this finding is that the localization of lesions in the low auditory comprehension group is much more heterogeneous. As shown in the group differences in lesion volume it is likely that the patients in the low comprehension group have more cortical and subcortical areas that are affected.
Aphasia severity, repetition and reading out loud
The overall aphasia severity score and the patients’ performance on both the repetition and reading out loud subtests were all associated with the same lesion patterns within the inferior frontal (RO, insula, IFGpTri, IFGpOrb and the IFGpOp), temporal (STG), and parietal areas (SMG, SPL, IPL, angular gyrus, postCG) of the left hemisphere (see ).
The results indicate that patients with extending lesions within the frontal, temporal and parietal areas of the left hemisphere have difficulties with more than repetition and reading out loud as indicated by the association to the overall aphasia score. This is in line with the notion that language is a complex process involving the activity of several brain regions, that all contribute to the processing of language (Damasio, Citation2008; Turken & Dronkers, Citation2011). As Turken and Dronkers (Citation2011) point out in their study on the structural and functional connectivity of regions associated with auditory sentence comprehension, the language comprehension network consists of several regions and pathways extending beyond the traditionally recognized language areas. It involves a large-scale network including several white-matter pathways such as the arcuate fasciculus, the inferior occipito-frontal fasciculus, the middle and inferior longitudinal fasciculi, the uncinate fasciculus and the tapetum. However, Turken and Dronkers (Citation2011) emphasize that the left MTG is a significant area in language comprehension. Our results do not confirm the left MTG to be significant, but the STG and Heschl’s gyrus were found to be associated with several subtests and the overall aphasia score.
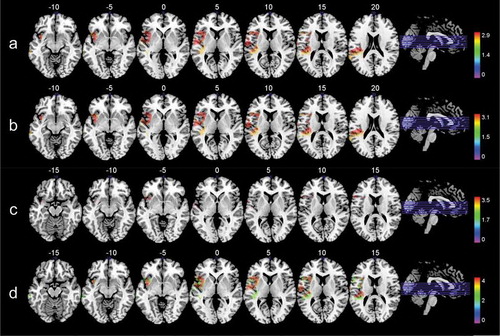
As seen in and illustrated in the test statistic values share the same numbers across brain regions. By visual inspection in , one can see that the lesion patterns in all analysis are similar, except for the naming subtest. This indicates that the same subgroup of patients share the same lesion patterns across the analysis.
Figure 3. Colorized multislice maps of lesions associated with A: aphasia severity, B: repetition, C: naming, D: reading out loud in the high comprehension group (n = 21). All maps include FDR-corrections with permutations, p < .05. Warmer areas (red) indicate a greater lesion overlap than colder areas (purple/blue). Color bars indicate Z scores
Our results show that several regions within the frontal, temporal and parietal areas are related to aphasia severity in the acute stage, but some regions overlap to a greater extent indicating that certain areas are more crucial in aphasia in the acute stages post-stroke. Furthermore, our findings are consistent with previous VLSM studies investigating aphasia and lesion location suggesting that certain areas are more involved in speech production, and certain areas are more involved in speech comprehension (Buchsbaum, Hickok, & Humphries, Citation2001).
Naming difficulties associated with a different lesion pattern
We found that focal lesions within the IFGpTri, IFGpOp, the insula, the STG and Heschl’s gyrus were associated with naming difficulties in the high comprehension group. These findings are to a certain degree consistent with Baldo et al. (Citation2009) who investigated the effect of lesion site on category-specific naming in patients with aphasia. Their results showed that lesions within the left MTG, STG and the insula were associated with difficulties in naming. Further, Baldo and colleagues found that smaller regions, such as the IFGpTri, the IFGpOrb, the SMG and the angular gyrus were also significantly associated with difficulties in naming tools and animals. However, the latter results could only be partly replicated by our study, since we did not find lesions within the parietal lobe to be associated with naming difficulties. One possible explanation might be the assessment of naming difficulties. While Baldo and colleagues used category-specific tests and investigated differences in lesion sites based on the categories, we did not divide the subtest naming based on categorical properties.
General discussion
Five lesioned areas appeared to be more significant in aphasia severity post-stroke, since they all were consistently associated with several subtests (see ). These areas were the IFGpTri, IFGpOp, insula, STG and Heschl’s gyrus.
The IFGpOp and IFGpTri correspond to the classical Broca’s area. These areas have been related to a range of language functions; from language production, grammar, and verbal fluency (Ardila, Bernal, & Rosselli, Citation2016b), to motor sequencing in speech and morphosyntax (Ardila & Bernal, Citation2007), and to a broader role in the unification of semantic, syntactic and phonological language processing (Hagoort, Citation2005). Our results confirm the traditional assumption that Broca’s area is a crucial region in speech production; however, it does not confirm that lesions within Broca’s area necessarily lead to Broca’s aphasia.
The insula has also been suggested to be important for the coordination of both language comprehension and production (Ardila et al., Citation2016a). Oh, Duerden, and Pang (Citation2014) did a meta-analysis of fMRI-studies investigating the insula in both speech and language tasks. They found that speech perception activated the left dorsal mid-insula, and expressive language tasks activated the more ventral parts of the mid-insula. Their findings suggest that the mid-insula is crucial in both speech and language processing. Further, they suggest that the insula has a role in the coordination of higher-order cognitive functions in speech and language processing (Oh et al., Citation2014). Further, a lesion within the insula may also affect nearby language-related fiber tracts, like the arcuate fasciculus or the tracts through the extreme capsule (Weiller, Bormann, Saur, Musso, & Rijntjes, Citation2011).
The STG was suggested to have a central role in the dual-stream model of speech processing, since it is active both during speech perception tasks, and speech recognition tasks (Hickok and Poeppel (Citation2007). Further, Buchsbaum et al. (Citation2001) suggested that the dorsal stream involves the posterior STG and that it projects to Broca’s area. The authors suggest that the function of these areas is to connect speech sounds with speech motor functions. In a study by Butler, Lambon Ralph, and Woollams (Citation2014) it was found that phonological processing involved the left posterior perisylvian region, including Heschl’s gyrus, the posterior middle STG and STS, and also white matter underlying the posterior STG. In accordance with Butler et al. (Citation2014) our results confirm that lesions of the STG and Heschl’s gyrus and the perisylvian region may contribute to deficits in both language comprehension and language production.
Limitations
Drawing lesions and determining lesions in VLSM is a time consuming, and subjective procedure. MRI in the acute stage might also show distortion due to gliosis, atrophy and ventricular changes which may be misinterpreted as the core lesion (Geva, Baron, Jones, Price, & Warburton, Citation2012). However, the inter-rater reliability in the present study was enhanced by a close cooperation between the authors by discussing and excluding uncertain or unclear cases. Furthermore, distinguishing whether aphasic difficulties arise from the lesioned area or from disconnection of undamaged areas of the brain might be impossible to infer (Price, Citation2000). One cannot draw a causal relationship between lesions and language functions based on the results of lesion studies. Lesion studies merely tells us that the neuronal systems and the connections within a specific lesioned area of the brain are associated or necessary for the lost function (Price, Citation2000).
Conclusions
To summarize, initial aphasia severity is clearly associated with lesion volume in the acute stage. A further investigation to see if lesion volume still is associated with aphasia severity in the more subchronic and chronic stages of aphasia would be interesting. Furthermore, our results show that lesions within Broca’s area, insula, the STG and Heschl’s gyrus seem to be part of a network that all are associated with difficulties with overall aphasia severity, repetition, naming and reading out loud. These areas therefore seem to be crucial in both language comprehension and production. This confirms current views that speech and language processes depend on the integrity of the entire network comprising both cortical structures and their interconnecting fiber tracts in the left hemisphere.
The results further confirm that a much cleaner picture can be obtained when patients are categorized by specific deficits and not by their “classical” subtypes. One should further emphasize that the patient group with low comprehension scores represented a very heterogonous group in terms of lesion patterns. This is an interesting aspect in its own, as it highlights that severely disturbed language comprehension can occur from lesions at various places within the network.
APH-PA_19-169-File001.docx
Download MS Word (22.1 KB)Acknowledgments
We are sincerely thankful for the participation of the patients in the study, and the collaboration of the speech and language pathologists, the Department of radiology and to our collaborators in the ESD-study and the Bergen NORSTROKE study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Abel, S., Weiller, C., Huber, W., Willmes, K., & Specht, K. (2015). Therapy-induced brain reorganization patterns in aphasia. Brain, 138, 1097–1112. doi:10.1093/brain/awv022
- Ardila, A., & Bernal, B. (2007). What can be localized in the brain? Toward a “factor” theory on brain organization of cognition. International Journal of Neuroscience, 117, 935–969. doi:10.1080/00207450600912222
- Ardila, A., Bernal, B., & Rosselli, M. (2016a). How localized are language brain areas? A review of Brodmann areas involvement in oral language. Archives of Clinical Neuropsychology : The Official Journal of the National Academy of Neuropsychologists, 31, 112–122. doi:10.1093/arclin/acv081
- Ardila, A., Bernal, B., & Rosselli, M. (2016b). Why Broca’s area damage does not result in classical Broca’s aphasia. Frontiers in Human Neuroscience, 10, 249. doi:10.3389/fnhum.2016.00249
- Baldo, J. V., Arevalo, A., Wilkins, D., & Dronkers, N. F. (2009). Voxel-based lesion analysis of category-specific naming on the Boston Naming Test. CRL Technical Report, 21, 3–12. 10.1.1.173.6265
- Baldo, J. V., Katseff, S., & Dronkers, N. F. (2012). Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: Evidence from voxel-based lesion symptom mapping. Aphasiology, 26, 338–354. doi:10.1080/02687038.2011.602391
- Bates, E., Wilson, S. M., Sayin, A. P., Dick, F., Sereno, M. I., Knight, R. T., & Dronkers, N. F. (2003). Voxel-based lesion-symptom mapping. Nature Neuroscience, 6, 448–450. doi:10.1038/nn1050
- Bonilha, L., & Fridriksson, J. (2009). Subcortical damage and white matter disconnection associated with non-fluent speech. Brain, 132, e108–e108. doi:10.1093/brain/awn200
- Buchsbaum, B. R., Hickok, G., & Humphries, C. (2001). Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science, 25, 663–678. doi:10.1207/s15516709cog2505_2
- Butler, R. A., Lambon Ralph, M. A., & Woollams, A. M. (2014). Capturing multidimensionality in stroke aphasia: Mapping principal behavioural components to neural structures. Brain, 137, 3248–3266. doi:10.1093/brain/awu286
- Cherney, L. R., & Robey, R. R. (2008). Aphasia Treatment: Recovery, prognosis and clinical effectiveness. In R. Chapey (Ed.), Language intervention strategies in aphasia and related neurogenic communication disorders (5 ed., pp. 186-202). Baltimore, PA: Lippincott Williams & Wilkins.
- Crinion, J., Holland, A., Copland, D., Thompson, C. K., & Hillis, A. E. (2013a). Quantifying brain lesions in neuroimaging research examining language recovery after stroke. NeuroImage, 73, 208–214. doi:10.1016/j.neuroimage.2012.07.044
- Crinion, J., Holland, A. L., Copland, D. A., Thompson, C. K., & Hillis, A. E. (2013b). Neuroimaging in aphasia treatment research: Quantifying brain lesions after stroke. NeuroImage, 73, 208–214. doi:10.1016/j.neuroimage.2012.07.044
- Damasio, H. (2008). Neural basis of language disorders. In R. Chapey (Ed.), Language Intervention Strategies in Aphasia and Related Neurogenic Communication Disorders (5 ed., pp. 20-41). Baltimore, PA: Lippincott Williams & Wilkins.
- Dronkers, N. F., Wilkins, D. P., Van Valin, R. D., Jr., Redfern, B. B., & Jaeger, J. J. (2004). Lesion analysis of the brain areas involved in language comprehension. Cognition, 92, 145–177. doi: 10.1016/j.cognition.2003.11.002
- Forkel, S. J., Thiebaut de Schotten, M., Dell’Acqua, F., Kalra, L., Murphy, D. G., Williams, S. C., & Catani, M. (2014). Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain, 137, 2027–2039. doi:10.1093/brain/awu113
- Friederici, A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91, 1357–1392. doi:10.1152/physrev.00006.2011
- Friederici, A. D., & Gierhan, S. M. E. (2013). The language network. Current Opinion in Neurobiology, 23, 250–254. doi:10.1016/j.conb.2012.10.002
- Geschwind, N. (1965a). Disconnexion syndromes in animals and man. I. Brain, 88, 237–294. doi:10.1093/brain/88.2.237
- Geschwind, N. (1965b). Disconnexion syndromes in animals and man. II. Brain, 88, 585–644. doi:10.1093/brain/88.3.585
- Geva, S., Baron, J.-C., Jones, P. S., Price, C. J., & Warburton, E. A. (2012). A comparison of VLSM and VBM in a cohort of patients with post-stroke aphasia. NeuroImage: Clinical, 1, 37–47. doi:10.1016/j.nicl.2012.08.003
- Goodglass, H., Kaplan, E., & Barresi, B. (2001). The Assessment of Aphasia and Related Disorders (Third ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
- Hagoort, P. (2005). On Broca, brain, and binding: A new framework. Trends in Cognitive Sciences, 9, 416–423. doi:10.1016/j.tics.2005.07.004
- Hallowell, B., & Chapey, R. (2008). Introduction to language intervention strategies in adult aphasia. In R. Chapey (Ed.), Language intervention strategies in aphasia and related neurogenic communication disorders (5 ed., pp. 3-19). Baltimore, PA: Lippincott Williams & Wilkins.
- Harvey, D. Y., & Schnur, T. T. (2015). Distinct loci of lexical and semantic access deficits in aphasia: Evidence from voxel-based lesion-symptom mapping and diffusion tensor imaging. Cortex, 67, 37–58. doi:10.1016/j.cortex.2015.03.004
- Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews. Neuroscience, 8, 393–402. doi:10.1038/nrn2113
- Hofstad, H., Naess, H., Moe-Nilssen, R., & Skouen, J. S. (2013). Early supported discharge after stroke in Bergen (ESD Stroke Bergen): A randomized controlled trial comparing rehabilitation in a day unit or in the patients’ homes with conventional treatment. International Journal of Stroke, 8, 582–587. doi:10.1111/j.1747-4949.2012.00825.x
- Lee, L. J., Kidwell, C. S., Alger, J., Starkman, S., & Saver, J. L. (2000). Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke, 31(5), 1081-1089. doi:10.1161/01.STR.31.5.1081
- Neumann-Haefelin, T., Wittsack, H. J., Wenserski, F., Siebler, M., Seitz, R. J., Modder, U., & Freund, H. J. (1999). Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke, 30, 1591–1597. doi:10.1161/01.STR.30.8.1591
- Oh, A., Duerden, E. G., & Pang, E. W. (2014). The role of the insula in speech and language processing. Brain and Language, 135, 96–103. doi:10.1016/j.bandl.2014.06.003
- Plowman, E., Hentz, B., & Ellis, C., Jr. (2012). Post-stroke aphasia prognosis: A review of patient-related and stroke-related factors. Journal of Evaluation in Clinical Practice, 18, 689–694. doi:10.1111/j.1365-2753.2011.01650.x
- Poeppel, D., & Hickok, G. (2004). Towards a new functional anatomy of language. Cognition, 92, 1–12. doi:10.1016/j.cognition.2003.11.001
- Price, C. J. (2000). The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy, 197, 335–359. doi:10.1046/j.1469-7580.2000.19730335.x
- Price, C. J., & Crinion, J. (2005). The latest on functional imaging studies of aphasic stroke. Current Opinion in Neurology, 18, 429–434. doi:10.1097/01.wco.0000168081.76859.c1
- Reinvang, I., & Engvik, H. (1980). Håndbok. Norsk Grunntest for Afasi. Oslo: Universitetsforlaget.
- Robson, H., Specht, K., Beaumont, H., Parkes, L. M., Sage, K., Lambon Ralph, M. A., & Zahn, R. (2017). Arterial spin labelling shows functional depression of non-lesion tissue in chronic Wernicke’s aphasia. Cortex, 92, 249–260. doi:10.1016/j.cortex.2016.11.002
- Rorden, C., Karnath, H. O., & Bonilha, L. (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19, 1081–1088. doi:10.1162/jocn.2007.19.7.1081
- Saur, D., Lange, R., Baumgaertner, A., Schraknepper, V., Willmes, K., Rijntjes, M., & Weiller, C. (2006). Dynamics of language reorganization after stroke. Brain, 129, 1371–1384. doi:10.1093/brain/awl090
- Schwartz, M. F., Kimberg, D. Y., Walker, G. M., Faseyitan, O., Brecher, A., Dell, G. S., & Coslett, H. B. (2009). Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain, 132, 3411–3427. doi:10.1093/brain/awp284
- Specht, K. (2014). Neuronal basis of speech comprehension. Hearing Research, 307, 121–135. doi:10.1016/j.heares.2013.09.011
- Specht, K., Zahn, R., Willmes, K., Weis, S., Holtel, C., Krause, B. J., … Huber, W. (2009). Joint independent component analysis of structural and functional images reveals complex patterns of functional reorganisation in stroke aphasia. NeuroImage, 47, 2057–2063. doi:10.1016/j.neuroimage.2009.06.011
- Tonkonogy, J., & Goodglass, H. (1981). Language function, foot of the third frontal gyrus, and rolandic operculum. Archives of Neurology, 38, 486–490. doi:10.1001/archneur.1981.00510080048005
- Tremblay, P., & Dick, A. S. (2016). Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain and Language, 162, 60–71. doi:10.1016/j.bandl.2016.08.004
- Turken, A., & Dronkers, N. (2011). The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. [Original Research]. Frontiers in Systems Neuroscience, 5. doi:10.3389/fnsys.2011.00001
- Weiller, C., Bormann, T., Saur, D., Musso, M., & Rijntjes, M. (2011). How the ventral pathway got lost – And what its recovery might mean. Brain and Language, 118, 29–39. doi:10.1016/j.bandl.2011.01.005