ABSTRACT
Background
We require high-quality information on the current burden, the types of therapy and resources available, methods of delivery, care pathways and long-term outcomes for people with aphasia.
Aim
To document and inform international delivery of post-stroke aphasia treatment, to optimise recovery and reintegration of people with aphasia.
Methods & Procedures
Multi-centre, prospective, non-randomised, open study, employing blinded outcome assessment, where appropriate, including people with post-stroke aphasia, able to attend for 30 minutes during the initial language assessment, at first contact with a speech and language therapist for assessment of aphasia at participating sites. There is no study-mandated intervention. Assessments will occur at baseline (first contact with a speech and language therapist for aphasia assessment), discharge from Speech and Language Therapy (SLT), 6 and 12-months post-stroke. Our primary outcome is changed from baseline in the Amsterdam Nijmegen Everyday Language Test (ANELT/Scenario Test for participants with severe verbal impairments) at 12-months post-stroke. Secondary outcomes at 6 and 12 months include the Therapy Outcome Measure (TOMS), Subjective Index of Physical and Social Outcome (SIPSO), Aphasia Severity Rating Scale (ASRS), Western Aphasia Battery Aphasia Quotient (WAB-AQ), stroke and aphasia quality of life scale (SAQoL-39), European Quality of Life Scale (EQ-5D), lesion description, General Health Questionnaire (GHQ-12), resource use, and satisfaction with therapy provision and success. We will collect demography, clinical data, and therapy content. Routine neuroimaging and medication administration records will be accessed where possible; imaging will be pseudonymised and transferred to a central reading centre. Data will be collected in a central registry. We will describe demography, stroke and aphasia profiles and therapies available. International individual participant data (IPD) meta-analyses will examine treatment responder rates based on minimal detectable change & clinically important changes from baseline for primary and secondary outcomes at 6 and 12 months. Multivariable meta-analyses will examine associations between demography, therapy, medication use and outcomes, considering service characteristics. Where feasible, costs associated with treatment will be reported. Where available, we will detail brain lesion size and site, and examine correlations with SLT and language outcome at 12 months.
Conclusion
International differences in care, resource utilisation and outcomes will highlight avenues for further aphasia research, promote knowledge sharing and optimise aphasia rehabilitation delivery. IPD meta-analyses will enhance and expand understanding, identifying cost-effective and promising approaches to optimise rehabilitation to benefit people with aphasia.
Introduction
People with aphasia are a highly heterogeneous group. Our insight into the relationships between recovery patterns and individual participant characteristics (e.g., age, education, mood, cognitive abilities and socioeconomic status), stroke (type, severity and location) and aphasia profiles are limited. While some evidence suggests that the above factors are likely to influence initial aphasia profile, language recovery and performance on assessment tools, the sample sizes are often small and data collection remains inadequate to guide best practice.
Delivery, quality of care, level of support and definitions of standard aphasia care vary within and across regions and countries, limiting our ability to build an overall picture of the status of aphasia care within a clinically relevant population. Long-term recovery, service needs, patient pathways following rehabilitation, referral rates to community support groups, adherence to rehabilitation, home-based therapy, access to and the effect of emerging community support groups each remain unclear. This has resulted in a poor understanding of how these factors may contribute to aphasia outcomes in the clinical population.
Aphasia research and clinical practice would benefit from high-quality information on the burden of aphasia, the regional differences in existing practice, which approaches work well within the constraints of current capacity, long-term outcomes after post-stroke aphasia and what happens to those with aphasia after discharge from speech and language therapy (SLT). Implementation of standardised and consistent assessment procedures across international clinical settings would facilitate the rapid generation of high-quality aphasia data that is comparable across settings, aphasia services, regions and countries. Such comparable data could contribute to the establishment of clinical registries and international stroke trials for post-stroke aphasia outcome and rehabilitation.
Prospective data collection with a standardised protocol that adheres to aphasia Core Outcome Set recommendations (Wallace et al., Citation2019) would accurately and robustly capture information on participants, delivery and type of care available within service delivery constraints, and third-sector involvement. Collation of these data at individual, therapeutic and services levels would identify gaps in the evidence for treatment of aphasia populations, current practice for aphasia treatment, geographic differences in care pathways, quality and access to care and outcomes. Whilst data from RCTs form the gold standard for efficacy of aphasia interventions, these data may exhibit selection bias in the people represented in the trials. Prospective data collection in the wider clinical population would supplement and expand on evidence from RCTs to provide a broader picture of the aphasia population, the state of aphasia care regionally and internationally, and permit analyses of rich, international data using predictive models to identify and inform best practice.
Aim
To document and inform international delivery of post-stroke aphasia treatment, to optimise recovery and reintegration of people with aphasia into society.
Objectives
To establish national clinical registries for aphasia rehabilitation across the world; individual participant data (IPD) meta analyses of which will inform research and clinical care of people with aphasia.
Research questions
Which aphasia treatments are used across participating sites (e.g., type, frequency, duration) and what are the associated outcomes (e.g., communication and language outcomes, discharge destination, referrals, resource use and support service access)?
What is the association between baseline demographics, site of enrolment, components of care, duration and frequency of any treatments and outcomes at 6 and 12 months post-stroke?
What is the relationship between lesion size, site and pattern with patho-linguistic variables including:
aphasia severity and profile prior to therapy
communication outcome at 6 and 12 months?
What is the relationship between medication administration and therapy outcome at 6 months and 12 months post-stroke?
What are participant and carer perceptions of care and intervention success at 12 months post-stroke?
What are the costs associated with different pathways of care (including staff involved, clinical, support services and participant provided services used)?
Methods
Ethics
This project currently involves investigators across 11 countries, with an open invitation for others to join. Ethics Committee approval requirements vary between participating countries. The study will be carried out in compliance with a shared protocol, and the principles laid down in the Declaration of Helsinki, in accordance with the International Conference on Harmonisation (ICH) Harmonised Tripartite Guideline for Good Clinical Practice (GCP), and in accordance with applicable regulatory requirements. The protocol will be submitted for approval to the appropriate Ethics Committee in each participating country, centre or regions, as appropriate, and written approval obtained, before participants are enrolled from that country.
Preparatory & pilot work
Work is underway (Malta IPD = 42, Sweden IPD = 6 & Cyprus IPD = 25) to pilot assessment and data collection procedures using a truncated version of the data collection form (baseline demographics, stroke severity stroke and aphasia severity, therapy description, post-therapy/discharge from SLT, 6 and 12-month aphasia severity and independence). Preparatory work is also underway in Chile and Norway to generate language-specific adaptations of key assessment tools prior to data collection.
Experience from this work has highlighted the need to take a pragmatic approach to recruitment and data acquisition, including permitting data collection not only in a population-based (registry) context, but also from research study contexts. Pilot work has highlighted recruitment issues within an acute setting, as well as problems recruiting participants to a registry-based study whilst offering only standard care. Recommendations include aligning data collection items with planned research studies and engaging with routine data collection from stroke registries to maximise data collection across different countries. These recommendations have been taken forward.
Design
This is a multicentre, prospective, non-randomised study, enrolling a cohort of stroke survivors with aphasia across participating international sites.
Data acquisition
Data for I-PRAISE will be acquired using three designs:
Prospective Data Collection in a Registry
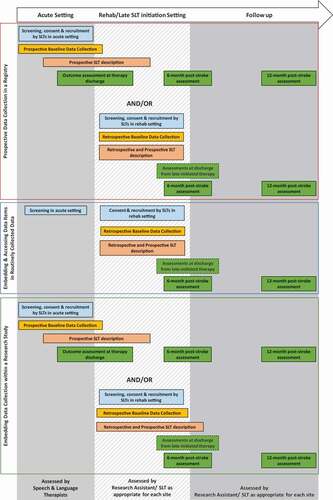
Data on demography, therapy and outcomes will be prospectively collected for participants who are recruited in the acute/early subacute phase after stroke. Baseline assessments will take place at (or as close to) first contact with the speech and language therapist. Outcomes will be assessed at discharge, 6 and 12 months post-stroke (: Description of assessment tools; : Schedule of measurements; : Study overview and data acquisition sources). For participants who’s first contact with SLT is in the chronic setting, we will retrospectively access and document data including demography, time since stroke and SLT received since stroke onset; outcomes will then be prospectively assessed.
Embedding and Accessing Data Items in Routinely Collected Data
Table 1. Description of assessments
Table 2. Schedule of measurements
The UK, Sweden and Australia have established registries that routinely collect data on stroke admissions for clinical audit. Certain baseline demographic data are already routinely collected within these audits, and the addition of assessments in keeping with the I-PRAISE protocol is feasible. Data from these audits will be used to screen for eligible participants, with patients consented in the chronic phase. Baseline demographic data and pre-recruitment SLT therapies will be retrospectively described; ongoing SLT therapies and outcomes will be prospectively assessed (see ).
Embedding and Accessing Data Items in Routinely Collected Data
Studies that offer an experimental SLT intervention are appealing for funders and participants alike; collaborators will embed I-PRAISE demography and clinical assessments along with study-specific data collection within such planned studies. Where recruitment is feasible in an acute stroke setting, demographic, clinical and outcome data will be prospectively collected. Where recruitment occurs in a chronic setting, baseline demography and pre-recruitment SLT regimens will be retrospectively collected from therapy and medical notes, and outcomes will be prospectively assessed (see ).
All 3 data acquisition designs will follow the procedures outlined below.
Setting
This study will be conducted in participating hospitals, outpatient clinics, primary care centres, rehabilitation centres and nursing homes with available SLT services, across all participating countries. We welcome expressions of interest in the participation of additional sites and countries.
Sites
Individual sites within each country will be identified as those having the capacity to provide SLT services following stroke and have agreed to collect data for I-PRAISE according to the standardised protocol and data collection form.
Participants
Whilst we aim to be as inclusive as possible, we acknowledge that some severely impaired participants may lack the capacity to consent to inclusion in I-PRAISE, or their comorbidities may present an obstacle to participation. People will be considered for inclusion in I-PRAISE if they meet the following criteria:
Clinical diagnosis of stroke resulting in aphasia, confirmed by assessment using standard practice at participating sites.
Informed consent provided by the patient (facilitated by accessible materials, where appropriate) and, if required, by their legal advisor, according to the standard procedures in operation at the participating site.
Able to participate in the assessment protocol for at least 30 minutes during the first assessment.
Accurate documentation of time between stroke and initial assessment.
Exclusion criteria:
Language impairment due to an aetiology other than stroke.
Dysarthria alone.
Uncorrected severe vision/hearing impairments.
Pre-stroke clinical diagnosis of dementia.
Severe neurological or cognitive conditions that preclude assessment or engagement with standard therapy.
Known life-threatening illness that is likely to lead to death in the next 6 months.
Involvement in concurrent aphasia/stroke intervention studies will not impact on screening for feasibility of inclusion in I-PRAISE.
Details of intervention and control
I-PRAISE will not investigate a study-mandated experimental SLT intervention. Conventional/Usual therapy will be documented via a standardised protocol.
Recruitment
All stroke survivors at participating sites will be screened for eligibility for inclusion in I-PRAISE on first contact with SLT services, in an acute setting or in a chronic setting. We will impose no restrictions on the time frame from index stroke to study inclusion in order to capture information on usual practice for the time to contact with SLT following stroke. Each country’s coordinator will engage with SLTs who will be responsible for screening and therapy delivery. Coordinators will establish study teams who will share information and conduct training with clinicians on eligibility criteria and use of the assessment tools where necessary. Screening for eligibility will take place at first contact with the therapist for assessment of aphasia after stroke, or as near as possible to this date. The therapist will then provide every potential participant or their representative with the Participant Information Sheet (PIS), and a “Notification of Interest” form. Participants will be given a minimum of 24 hours to consider participation. Those who would like to participate in the study will be asked to complete the Notification of Interest form and return it to the screening clinician. The number of and reasons for non-inclusions will be recorded on a recruitment log.
Capacity to consent
Prior to inclusion in the study, written or oral informed consent will be obtained from each participant (or the participant’s legally accepted representative) according to the ethics, regulatory and legal requirements of the participating country and site. Those participants who agree to be included in the study will be consented by a member of the clinical team who has been assigned the responsibility of obtaining consent from participants at that centre and will involve the use of accessible information sheets and forms. The participant will be informed that their medical records may be examined by authorised monitors or by the appropriate Independent Ethics Committee (IEC)/Institutional Review Board (IRB) members.
Those participants who are able to be screened and consented in acute settings will be recruited. If consent and recruitment are not practical in an acute setting, then patients can be approached in a chronic setting, with retrospective collection of baseline demographic data, where appropriate.
Assessments
Data will be collected using standardised assessment tools available in a range of languages including English, Spanish, German, Swedish, Portuguese, Dutch, Norwegian, Greek, Finnish and Maltese, where appropriate adaptations exist. Where adaptations are required, teams within each country will explore available options for adaptation prior to commencement.
The primary outcomes are the Amsterdam Nijmegen Everyday Language Test (ANELT (Blomert, Citation1992)/Scenario Test (Van Der Meulen et al., Citation2010)) at 12 months following stroke.
Secondary outcome measures are the Therapy Outcome Measure (TOMS), Subjective Index of Physical and Social Outcome; SIPSO (Trigg & Wood, Citation2000), Aphasia Severity Rating Scale (ASRS) of the Boston Diagnostic Aphasia Examination (Goodglass et al., Citation2019)/Western Aphasia Battery-Aphasia Quotient (WAB-AQ) (Crary & Gonzalez Rothi, Citation1989), European Quality of Life Scale; EQ-5D-5 L) (“European Quality of Life Scale,” Citation2011), Stroke and Aphasia Quality of Life Score (SAQoL-39) (Hilari et al., Citation2003), General Health Questionnaire (GHQ-12 (GL Assessment, Citation2019)), modified Rankin Scale [mRS (Bonita & Beaglehole, Citation1988)], and satisfaction with therapy provision (Revicki et al., Citation2008) at 6 and 12 months post-stroke (: Description of assessment tools; : Schedule of measurements).
Baseline assessment
Following receipt of consent to participate, demographic data will be recorded. This will include age, sex, medical history, stroke and aphasia severity, functional communication, languages spoken, years of education, handedness, ethnicity, employment status, living arrangements, presence of cognitive impairment and presence of motor impairment; time since stroke to inclusion in I-PRAISE will also be documented (; schedule of assessments).
Commencement of therapy
Therapy commencement will be defined as the point at which routine aphasia therapy commences. We will also note whether advice and education were provided prior to this session. Consent for inclusion in the study can occur after the commencement of therapy.
We will collect data on the treatment received at each site and across each health service as people move through their recovery. Therapy data will be collected by the person delivering the treatment at each session. This will describe the intervention, duration, frequency, outcome measurements and discharge destination. Language assessments will be carried out by a SLT/trained research assistant, as appropriate.
Completion of treatment will be defined as the point at which the participant is discharged from standard SLT.
Discharge
Where discharge from standard SLT occurs prior to the first scheduled follow-up (6- month post- index stroke), we will collect data on the discharge destination, ASRS & Bedside WAB-AQ, ANELT/Scenario Test & TOMS. Where the 6-month assessment occurs within 4 weeks of SLT discharge, a single 6-month assessment will be conducted. Should the participant be re-referred for speech and language therapy, the subsequent therapy will be added to their original records whilst retaining the original follow up assessment schedule.
Follow up: 6 and 12 months post-stroke
Where applicable, participants will be followed up in the site as part of a study-specific speech and language therapy review. The assessor will make a note of the status of the participant (alive and participating/alive but unable to participate at this follow-up point/alive but withdrawn from study/lost to follow up/deceased). At both follow-up time points, the assessor will systematically document ASRS/WAB-AQ, ANELT/Scenario Test & TOMS. ANELT/Scenario Test will be audio-/video recorded for offline blinded analysis, where appropriate and according to individual site ethical permissions. Where centralised adjudication is not possible, the assessor will complete the scoring locally, and this will be noted. Presence of cognitive impairment (using standard practice at each site) and functional dependency (mRS) will be described. A research assistant attached to the study will contact the participant/carer in writing and follow up with a phone call to collect data on participants’ use of support/third sector local services. We will request permission to contact the group for a description of the services that they provide. We will issue a survey to third-sector organisations to ask about the components of support that they provide. Information relating to resource utilisation will be obtained from the participant and linked administrative medical records, when available. This will include general practitioner visits, hospital and rehabilitation admissions, outpatient and community service use, medication use for anxiety and depression, speech and language therapy, use of speech aids and devices, employment status, respite care and informal care as a result of stroke via a standardised resource utilisation questionnaire. Unit prices from each country for the different resource use items will be obtained. Finally, we will capture impact of, and participant satisfaction with therapy at 6 and 12 months using a Likert scale (Revicki et al., Citation2008).
Neuroimaging
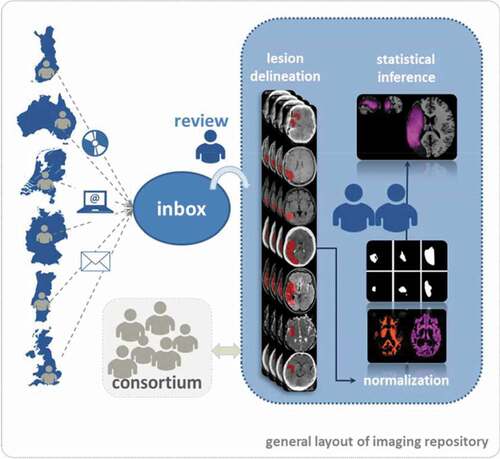
A repository of brain imaging data will accompany the clinical registry data, and will permit where available, an examination of the correlation between lesion size/pattern at the time of study inclusion with a number of linguistic variables. These include (i) aphasia severity and profile prior to therapy and (ii) language outcome at 6 and 12 months. All stroke patients undergo clinically motivated imaging on acute admission to hospital. Imaging modality varies across participating countries. An estimated 30% will receive additional follow-up scanning and MRI is performed in 30–40% of patients. Based on estimates from previous smaller scale randomised controlled studies with retrospective collections of routinely available brain images early after stroke (Baumgaertner et al., Citation2013; Breitenstein et al., Citation2017; Godecke et al., Citation2016) and respecting the fact that lesion delineation is not possible in very early acute standard CT-imaging (Gonzalez & Schwamm, Citation2016) we expect that lesion site can be determined in 60% of the patients included. This will yield a data bank containing the imaging of approximately 2800 patients of variable modalities (60/40% CT/MRI) with aphasia. The data will be collected within national framework structures where available (Germany, UK, Australia and Portugal); data analysis will be carried out by a central brain imaging data-managing team (Principal Investigator: Hellmuth Obrig, University Clinic Leipzig, Germany). Personal information on the patient will be anonymised prior to transfer. A local coordinator will retrieve images and transfer them to the central brain-imaging site in Leipzig (GER). Images will be reviewed, converted into NIfTI-format and transferred to the central data bank. The team at the central brain imaging site will process the data to yield a repository of lesion masks normalised to Montreal Neurological Institute (MNI) space (Collins et al., Citation1994) using statistical parametric mapping (SPM) (“NeuroImaging Tools & Resources Collaboratory,” Citation2019) and existing toolboxes (see ). This will allow for further analyses using pattern classification and correlation with behavioural data on patho-linguistic profile and therapy outcome measures. A consortium with representatives from all participating nations will steer the analysis strategy.
Pharmacological data
Medication data will be recorded from the participant, family member/carer or obtained from routinely collected, linked data, if feasible. Medication data prescribed after the stroke to treat comorbidities (e.g., epilepsy, depression) and the addition of pharmacotherapy or other treatment approaches (e.g., Transcranial Direct-Current Stimulation [tDCS]) to improve language deficits will be recorded.
Blinded outcome assessment
Where local ethical regulations permit, we will apply blinded adjudication of the primary outcome. For instances when ethical regulations prohibit centralised adjudication, assessments will be carried out by a trained assessor blinded to the nature/content of therapy, where appropriate.
Centralised adjudication
Where feasible, we will adapt an existing online platform (McArthur, Citation2014) for assessment of one of our primary outcomes (Amsterdam Nijmegen Everyday Language Test [ANELT]/or Scenario Test for non-verbal participants). Within this system, video/audio recordings of participant interviews can be anonymised and securely uploaded to the centralised system and distributed to blinded assessors to determine scores for each of the required assessment tools. These scores are then entered into the anonymised participant record. The aim is not to blind assessors to interventions as there is no active intervention, but to enable consistent scoring.
Data management & security
The Robertson Centre for Biostatistics (RCB), Glasgow, UK, will develop an electronic Case Report Form (e-CRF) for I-PRAISE. This will be available via a secure online portal where clinicians can enter data directly. Where local ethical regulations do not permit the use of non-departmental electronic devices, we will provide paper copies of the case report form (CRF) for data collection during the therapy session. These data will then be entered into the e-CRF by the clinician/research assistant, as appropriate. Only anonymised data will be entered into the analysis dataset. Data from each participating site will be uploaded directly using the e-CRF. Local coordinators will be granted access to data from their respective countries to check on completeness of data, acceptable ranges for variables and coding errors. The verified and cleaned data will be entered into a master dataset comprising all eligible participants from each country.
Each country will retain its own data and share a copy for the centralised I-PRAISE database. All data entered into the I-PRAISE database will be stored at the RCB, University of Glasgow, UK and will adhere to national/international data sharing regulations. These data will be backed up nightly according to RCB procedures. The primary datasets (as entered by respective clinicians) will be preserved unmodified. Primary datasets, the master dataset, any other datasets created for this project and analyses datasets will be accessed only by the I-PRAISE project management group. The centralised data will not be accessible to those outside of the project collaboration.
Monitoring
At each site quality assurance and verification of variables will be performed by cross-checking the dataset against the data sources, checking the acceptable ranges of variables and through correspondence with the principal investigators (PIs). This will be undertaken by local coordinators for each country. External verification will take place by examining data ranges, missing variables and through communication with PIs. Missing data and reasons for missing data will be verified by the local coordinating centres, and where possible we will attempt to retrieve missing data.
A Steering Committee will be formed comprising representatives from each participating country. The Steering Committee will be responsible for the day-to-day oversight of the study. The PIs for each site will be responsible for enrolment, data collection and follow-up each site. The Steering Committee will appoint a writing committee for dissemination activities.
Serious adverse events that are experienced by participants during the course of the study will be documented in the e-CRF for information only. The PIs for each country will monitor and report on recruitment within their country to inform progress and ensure that recruitment targets for each country are met.
Sample size calculations
We will enrol a minimum of 100 participants from each participating country, with the exception of smaller nations (such as Malta < 500,000 inhabitants), where sample sizes will take into consideration the size of participating centres; our aim is to be inclusive. Each site’s involvement will be guided by a PI from the local Higher Education Institution (HEI) collaborator or clinical setting, as appropriate. All participating sites will follow the I-PRAISE protocol, but may address additional objectives as will be outlined in their country-specific ethics application.
Based on existing annual referrals to participating sites for post-stroke aphasia rehabilitation, our liberal eligibility criteria and pragmatic enrolment estimates (estimating recruit over 2 years), our baseline sample size is expected to exceed 3000 IPD; exemplar estimates available include for the UK (n = 1000), Spain (n = 150), Germany (n = 1250), Finland (n = 450), Australia (n = 1167), Netherlands (n = 410), Chile (n = 250), Sweden (n = 350), and Portugal (n = 550).
Analysis
Our primary outcome is a change from baseline in the ANELT/Scenario Test at 12-months post-stroke. Secondary outcomes at 6 and 12 months include SIPSO, ASRS/WAB-AQ, SAQoL-39, EQ-5D, lesion size and site distribution, GHQ-12, resource use, and impact satisfaction with therapy provision (Revicki et al., Citation2008).
IPD meta-analyses will be performed and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, including data from each country as they become available. Continuous data will be reported as median [interquartile range/IQR], categorical data as N (%). A p-value of ≤ 0.05 will be taken as significant for each outcome. All tests will be two-sided. Details on population size, healthcare types and whether they are high- or low-middle income countries will be included as random or fixed effects within the modelling to account for differences due to setting. Where large differences in setting are detected, subgroup analyses will inform estimates within setting types.
Population who access aphasia therapy
We will extract participants’ population demographic data, presenting age, initial stroke severity, time between stroke and first assessment by a speech and language therapists, and initial severity of aphasia by ASRS/WAB-AQ as medians and IQRs. We will present all summary data (demography, co-morbidities, and living context) stratified by data acquisition source.
Clinical practice for treatment of aphasia
We will summarise the therapies delivered for aphasia, stratified by country and data acquisition source, describing demographic factors. This summary will include detailed descriptions of the components of care, the care pathways, the destination of referrals and the involvement/availability of community and third-sector support. Where third-sector support exists, we will also provide a description of the components of care and support that are provided by these third-sector organisations, describing demographic characteristics of participants who access these resources, where possible. We will formally examine these associations using regression analyses, adjusting for confounders. Summary statistics will include age, sex, time since stroke, duration, dosage and frequency of therapy for each participating site/country (median [IQR]). We will also consider the impact of treatment setting for example, using multilevel models.
Relationship between different therapies and aphasia outcomes
Where possible, we will categorise each identified aphasia therapy using stroke and aphasia care pathways, and informed by categorisations developed in previous work from the Collaboration of Aphasia Trialists (On behalf of the RELEASE Collaborators, Citation2021) as a guideline. We will describe participants according to verbal and non-verbal status at 12 months. Minimal detectable change and clinically important changes from baseline the ANELT or Scenario Test at 12-months post-stroke will be examined. Where possible, we will examine the trajectory of early spontaneous aphasia recovery using methods such as existing prognostic models (Godecke et al., Citation2013), multi-level models, or analysis of pre-treatment ANELT/Scenario Test scores from those who were included in I-PRAISE at various time points within one month of the index stroke. Outcomes will be assessed using for example, paired analyses of pre- and post-intervention measures to assess changes in the ANELT/Scenario Test at individual and population levels.
We will use Generalised Linear Models (GLM) to examine the associations between therapy, service level differences and various outcome measures at 6 months and 12 months following stroke, adjusting for potential confounding factors such as age, initial stroke severity, initial aphasia severity, co-morbidities and socio-economic status. Analyses will explore whether demographic factors add further predictive value to language outcome. Subgroup analyses will examine the association between engagement with third-sector services and aphasia outcomes.
Aphasia outcomes across different countries
We will describe demographics, therapy differences and outcomes across different countries using summary statistics, stratified by data acquisition source.
Participants’ perceptions of aphasia services and their ability to cope with post stroke aphasia at 12 months
Participants’ perceptions and satisfaction with care will be assessed using a 5-point Likert scale (Revicki et al., Citation2008). We will describe inter-country differences between participants and use descriptive statistics to summarise their perceptions of care.
Resource use and the costs of treatment
The costs associated with various aphasia intervention pathways will be described for each country and harmonised wherever possible using descriptions of the probability of different resources use items and use of a common currency based on conversion with appropriate purchasing power parities (Organisation for Economic Cooperation and Development, Citation2019) and reference year. Where possible, these data will then be used to determine different incremental costs per WAB-AQ improvement (e.g., net costs/Aphasia Quotient [AQ] score gained for effective interventions) for each broad pathway of care identified. One-way sensitivity analyses and probabilistic multivariable analyses will be used to account for variability in point estimates (e.g., clinical and resource use probabilities, prices) to determine their influence on the results. Consideration of local contexts, public and private sector funding and program delivery costs will be described.
Neuroimaging analysis
Lesion masks will be obtained from the available imaging and will be normalised to standard space (MNI-space). The masks will be correlated with linguistic and epidemiologic parameters. Based on the cohort size we aim to address previously recognised research questions for imaging-based predictions (Godecke et al., Citation2016). The lesion aphasia profile analysis will use standard lesion-symptom-mapping methodology (Rorden et al., Citation2007). Since different imaging modalities are implemented, a binary lesion mask will be used for the overall analyses. Lesion size and site will be correlated with lesion patterns. The overall goal is to identify brain lesion patterns correlating with long-term language functional outcomes (ANELT/Scenario Test) at 12 months post- index stroke. Secondary outcomes comprise linguistic measures as well as therapist’s and patient’s ratings of communicative performance. Secondary analyses will target differences of the predictive strengths depending on imaging modality (CT/different MRI protocols) and the time point of imaging (acute/subacute/early chronic stage). Subgroup analyses of different therapy regimens (e.g., intensive versus non-intensive) are also planned. Additionally, we expect to replicate previously reported lesion-aphasia profile correlations (Henseler et al., Citation2014), and provide data on differences between neuroimaging practice in the various national health care systems, to inform optimisation of imaging protocols for speech and language therapy service provision. The data will also be available to evaluate different analysis strategies (Turken & Dronkers, Citation2011; Yourganov et al., Citation2016).
Medication analysis
Multiple linear and multiple logistic regressions will be performed in order to identify significant predictors of language recovery in ANELT/Scenario Test, ASRS/WAB-AQ, EQ-5D as appropriate. The selection of these independent variables will be made based on existing literature, as well as assessment of interactions with demographic or comorbidity factors. Independent variables to be incorporated in the model include: medications with potentially detrimental effects (e.g., antihypertensives, clonidine, prazosin, anti-arrhythmics, adenosine agonists, digitalis glycosides, typical neuroleptics and antimuscarinic agents), medication with a potentially beneficial effect (e.g. nootropics, dopamine agonists, acetylcholinesterase inhibitors, and NMDA receptor antagonists), with adjustment for age, gender, depression score, drug administration, onset phase (acute or chronic), and initial aphasia severity.
Missing data
Patterns and the extent of missing data will be explored. For each primary outcome, we will perform sensitivity analyses to examine the effects of missing data using multiple imputation. We will report on the proportion of outcome data that are genuinely missing at follow-up and describe the demographic and clinical factors of this population. We will compare this demography with the characteristics of those who completed follow up.
Protocol amendments
No changes (amendments) to the Protocol will be implemented without prior approval from the appropriate local Ethics Committee. If a Protocol amendment requires changes to the Informed Consent Form, the revised Informed Consent Form, prepared by the PI, will be approved by the Ethics Committee.
Discussion
I-PRAISE seeks to produce a high volume of structured data through coordinated, international application of a shared, standardised protocol. By taking a pragmatic, international approach to data acquisition and implementing a standardised protocol, I-PRAISE aims to capitalise on the wealth of data already being gathered for research and audit purposes. Generating high-quality, standardised international data will inform the delivery of interventions for aphasia, enable comparison of service delivery, and examination of associations between services, aphasia outcomes, resource utilisation/costs and difference among participant populations who engage and benefit from specific services across different countries. I-PRAISE will also contribute to our long-term ambition of establishing national clinical registries for post-stroke aphasia outcome and rehabilitation.
Leveraging the collection of patients’ and clinicians’ data is an important way of improving quality and efficiency of health care delivery and generating new knowledge (Murdoch & Detsky, Citation2013). Scaling this internationally has the potential to rapidly generate data on a population with pressing health needs(Everett et al., Citation2021) in a time efficient manner. Currently, rich data that are collected as part of routine care are often seen as a by-product of healthcare rather than as an asset to improve health care delivery (Murdoch & Detsky, Citation2013). I-PRAISE aims to capitalise on routinely collected data in the international context in the form of participant demographics, clinical measurements, intervention descriptions, neuroimaging and medication review, and supplement these with collection of data in a core outcome set immediately after the SLT intervention as well as at 6- and 12-months post-stroke. The cost of answering many clinical questions by conducting paired randomised comparisons and collecting structured data in relation to complex rehabilitation interventions, outcomes and population is prohibitive (Murdoch & Detsky, Citation2013). The establishment of an international registry comprising those with clinical aphasia in I-PRAISE will enable novel exploratory analyses, reflecting a much wider clinical population. Data-driven clinical decision support tools may eventually lead to improved use of limited resources or cost-savings and help with appropriate standardisation of care (Murdoch & Detsky, Citation2013). I-PRAISE offers the opportunity to integrate the traditional medical model with the social care available across multiple countries to improve and inform patient-directed interventions, and has the potential to inform clinical practice by using information generated in every-day clinical settings to inform the quality and efficiency of stroke care (Murdoch & Detsky, Citation2013).
Limitations include the availability of assessment tools in each of the required languages. Lack of availability has been highlighted by several countries and work is underway to adapt and validate several assessment tools. This has been informed by experienced colleagues from the Collaboration of Aphasia Trialists (CATs) (www.aphasiatrials.org), who are members of a working group specifically developed to facilitate adaptation of outcome assessments. We have also identified a need for staggered project initiation in order to accommodate such preparatory work, which will increase our timelines for analyses. However, staggered project initiation will enable experience to be shared, and processes to be refined as more countries commence data collection. Additional limitations include the availability of data storage and large-scale research support infrastructure across countries and the complexity of international collaboration. Concerns can be mitigated through the shared experience of I-PRAISE members, many of whom also collaborated on the large-scale RELEASE project (The RELEASE Collaboration, Citation2020), which collated and meta-analysed data from 174 international aphasia research datasets.
Advantages include (i) standardised description of the clinical aphasia population (demographics, language characteristics and comorbid stroke symptoms) (ii) description of the capacity for speech and language therapy provision and aphasia support across the recovery trajectory (components of care, care pathways, and involvement of third sector organisations) and across countries; (iii) identification of rehabilitation factors (type, delivery models, dosage) that are associated with good functional communication and participation outcomes, accounting for stroke and aphasia profiles (iv) identification of the subpopulations of people with aphasia who appear to have good outcomes and identification of those in whom more support is needed (those with poor outcomes despite access to support), (v) identification of the neuroanatomical correlates of good language outcome and (vi) description of the costs associated with current clinical interventions.
Analyses from this international, multilingual, multicultural dataset will help to inform research into two of the James Lind Alliance’s top 10 research priorities for life after stroke (Pollock et al., Citation2012) and will address pivotal issues surrounding aphasia recovery at an international level, including description of the amount of recovery observed, the conditions under which this recovery occurs, and the state of aphasia therapy internationally. International health and outcome data will be used to describe associations between aphasia interventions and quality of life (EQ-5D), inform calculation of international health utilities for post-stroke aphasia populations, and provide indications of costs and subsequent outcomes for currently used interventions. Analyses will enable both within and between-country examination of outcomes, indicating areas that need improvement, highlighting practices that may work well across similar settings, and identifying geographic factors that are associated with better quality of life following aphasia. We welcome further countries wishing to join in this collaboration.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Baumgaertner, A., Grewe, T., Ziegler, W., Floel, A., Springer, L., & Martus, P. (2013). Fcet2ec (from controlled experimental trial to = 2 everyday communication): How effective is intensive integrative therapy for stroke-induced chronic aphasia under routine clinical conditions? A study protocol for a randomized controlled trial. Trials, 14(1), 308. https://doi.org/https://doi.org/10.1186/1745-6215-14-308
- Blomert, L. (1992). The Amsterdam - Nijmegen Everyday Language Test (ANELT). In Neuropsychological rehabilitation. Springer, Berlin, Heidelberg.
- Bonita, R., & Beaglehole, R. (1988). Modification of rankin scale: Recovery of motor function after stroke. Stroke, 19(12), 1497–1500. https://doi.org/https://doi.org/10.1161/01.STR.19.12.1497
- Breitenstein, C., Grewe, T., Flöel, A., Ziegler, W., Springer, L., Martus, P., Huber, W., Willmes, K., Ringelstein, R. B., Haeusler, K. G., Abel, S., Glindemann, R., Domahs, F., Regenbrecht, F., Schlenck, K. J., Thomas, M., Obrig, H., De Langen, E., Rocker, R., Wigbers, F., … Baumgaertner, A. Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. (2017). Lancet, 389(10078), 1528–1538. FCET2EC Study group. https://doi.org/https://doi.org/10.1016/S0140-6736(17)30067-3
- Collins, D. L., Neelin, P., Peters, T. M., & Evans, A. C. (1994). Automatic 3-D intersubject reg- istration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography, 18(2), 192–205. https://doi.org/https://doi.org/10.1097/00004728-199403000-00005
- Crary, M. A., & Gonzalez Rothi, L. J. (1989). Predicting the Western aphasia battery aphasia quotient. Journal of Speech and Hearing Disorders, 54(2), 163–166. https://doi.org/https://doi.org/10.1044/jshd.5402.163
- Everett, E. A., Everett, W., Brier, M. R., & White, P. (2021). Appraisal of health states worse than death in patients with acute stroke. Neurology. Clinical Practice, 11, 43–48. https://doi.org/https://doi.org/10.1212/CPJ.0000000000000856
- GL Assessment (2019). General health questionnaire. https://www.gl-assessment.co.uk/products/general-health-questionnaire-ghq/.
- Godecke, E., Armstrong, E. A., Rai, T., Middleton, S., Ciccone, N., & Whitworth, A. (2016). A randomized controlled trial of very early rehabilitation in speech after stroke. International Journal of Stroke, 11(5), 586–592. https://doi.org/https://doi.org/10.1177/1747493016641116
- Godecke, E., Rai, T., Ciccone, N., Armstrong, E., Granger, A., & Hankey, G. J. (2013). Amount of therapy matters in very early aphasia rehabilitation after stroke: A clinical prognostic model. Seminars in Speech and Language, 34(3), 129–141. https://doi.org/https://doi.org/10.1055/s-0033-1358369
- Gonzalez, R. G., & Schwamm, L. H. (2016). Imaging acute ischemic stroke. Handbook of Clinical Neurology, 135, 293–315. https://doi.org/https://doi.org/10.1016/B978-0-444-53485-9.00016-7.
- Goodglass, H., Kaplan, E., & Barresi, B. (2019). Boston diagnostic aphasia examination (Third ed.). Philadelphia: Lea & Febiger. https://www.parinc.com/Products/Pkey/16
- Henseler, I., Regenbrecht, F., & Obrig, H. (2014). Lesion correlates of patholinguistic profiles in chronic aphasia: Comparisons of syndrome-modality- and symptom-level assessment. Brain, 137(3), 918–930. https://doi.org/https://doi.org/10.1093/brain/awt374
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011 Dec;20(10), 1727–1736.
- Hilari, K., Byng, S., Lamping, D. L., & Smith, S. C. (2003). Stroke and Aphasia quality of life scale-39 (SAQOL-39). evaluation of acceptability, reliability, and validity. Stroke, 34(8), 1944–1950. https://doi.org/https://doi.org/10.1161/01.STR.0000081987.46660.ED
- McArthur, K. (2014). Improving efficiency in stroke trials: An exploration of methods to improve the use of the modified Rankin Scale in acute stroke trials. MD Thesis University of Glasgow.
- Murdoch, T. B., & Detsky, A. S. (2013). The inevitable application of big data to health care. JAMA, 309(13), 1351–1352. https://doi.org/https://doi.org/10.1001/jama.2013.393
- NeuroImaging Tools & Resources Collaboratory (2019). Clinical toolbox for statistical parametric mapping. https://www.nitrc.org/projects/clinicaltbx/.
- On behalf of the RELEASE Collaborators. (2021). RELEASE: An individual participant data meta-analysis, incorporating systematic review and network meta-analysis, of complex speech-language therapy interventions for stroke-related aphasia. National Institutes of Health Research: Health Services and Delivery Research.
- Organisation for Economic Cooperation and Development (2019). Purchasing power parities (PPP). https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm
- Pollock, A., St George, B., Fenton, M., & Firkins, L. (2012). Top 10 research priorities relating to life after stroke - consensus from stroke survivors, caregivers, and health professionals. International Journal of Stroke, 9(3), 313–320. https://doi.org/https://doi.org/10.1111/j.1747-4949.2012.00942.x
- Revicki, D., Hays, R. D., Cella, D., & Sloan, J. (2008). Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of Clinical Epidemiology, 61(2), 102–109. https://doi.org/https://doi.org/10.1016/j.jclinepi.2007.03.012
- Rorden, C., Karnath, H. O., & Bonilha, L. (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. https://doi.org/https://doi.org/10.1162/jocn.2007.19.7.1081
- The RELEASE Collaboration. (2020). REhabilitation and recovery of peopLE with Aphasia after StrokE (RELEASE): A protocol for a systematic review-based Individual Participant Data (IPD) meta- and network meta-analysis. Aphasiology, 34(2), 137–157. https://doi.org/https://doi.org/10.1080/02687038.2019.1643003
- Trigg, R., & Wood, V. A. (2000). The Subjective Index of Physical and Social Outcome (SIPSO): A new measure for use with stroke patients. Clinical Rehabilitation, 14(3), 288–299. https://doi.org/https://doi.org/10.1191/026921500678119607
- Turken, A. U., & Dronkers, N. F. (2011). The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience, 5, 1. https://doi.org/https://doi.org/10.3389/fnsys.2011.00001
- Van Der Meulen, I., Van De Sandt-koenderman, W. M., Duivenvoorden, H. J., & Ribbers, G. M. (2010). Measuring verbal and non-verbal communication in aphasia: Reliability, validity, and sensitivity to change of the Scenario Test. International Journal of Language & Communication Disorders, 45(4), 424–435. https://doi.org/https://doi.org/10.3109/13682820903111952
- Wallace, S. J., Worrall, L., Rose, T., LeDorze, G., Breitenstein, C., Hilari, K., Babbitt, E., Bose, A., Brady, M., Cherney, L. R., Copland, D., Cruice, M., Enderby, P., Hersh, D., Howe, T., Kelly, H., Kiran, S., Laska, A. C., Marshall, J., Nicholas, M., … Webster, J. (2019). A core outcome set for aphasia treatment research: The ROMA consensus statement. International Journal of Stroke, 14(2), 180–185. https://doi.org/https://doi.org/10.1177/1747493018806200
- Yourganov, G., Fridriksson, J., Rorden, C., Gleichgerrcht, E., & Bonilha, L. (2016). Multivariate connectome-based symptom mapping in post-stroke patients: Networks supporting language and speech. The Journal of Neuroscience, 36(25), 6668–6679. https://doi.org/https://doi.org/10.1523/JNEUROSCI.4396-15.2016