ABSTRACT
Background
Cognitive-linguistic interventions for aphasia are behavioural-based approaches to therapy that aim to treat language impairment skills post-acquired brain injury. The purpose of cognitive-linguistic intervention is to restore and rehabilitate language impairment skills through targeting phonologic, semantic and syntactic systems, which may support goals to improve everyday communication.
Aims
The aim of this systematic review was to investigate the effects of cognitive-linguistic interventions on language processing for aphasia in the first 90 days post-stroke. Secondary aims include the investigation of the effects of these interventions on functional communication and quality of life.
Methods
A systematic search was conducted across six databases. Twenty-one studies met the predefined eligibility criteria and were included in the review. Studies were rated for methodological quality and data extracted. A narrative synthesis was completed and conducted for all included studies. Four studies were suitable for meta-analysis.
Main Contribution
Evidence for the effects of cognitive-linguistic intervention for aphasia in the first 90 days post-stroke is inconclusive. Intervention approaches included constraint-induced intervention, melodic intonation therapy and study specific cognitive-linguistic intervention. Multiple studies investigated the use of computers as a mode of intervention delivery or to increase the frequency of intervention or session duration. Improvement on language outcomes was associated with positive effects on functional communication, regardless of the specific intervention. There were mixed results for quality-of-life outcomes.
Conclusions
Further research is required to guide aphasia intervention the first 90 days post stroke, a time critical period for recovery and rehabilitation. Research reports should include adequate description of participant characteristics and consistent use of intervention protocols and outcome measures. Providing a clear description of theoretical underpinnings and detailed information regarding the components of intervention will also facilitate future research synthesis.
Introduction
Aphasia can affect up to 40% of people following a stroke (Mitchell et al., Citation2021) and is associated with an increased length of hospital stay, increased mortality and disability, and reduced independence (Flowers et al., Citation2015; Ali et al., Citation2015). Aphasia is also linked to reduced social participation (Thayabaranathan et al., Citation2022) and increased risk of depression (Mitchell et al., Citation2017). The first 90 days post-stroke are an important period for people with aphasia as it is a critical time for brain recovery (Kiran & Thompson, Citation2019). Intervention during this early period of recovery provides an opportunity to access goal-directed rehabilitation services which improve independence and outcomes (Lynch et al., Citation2019). Research suggests it is also a phase when communication impairment may be associated with increased adverse events such as falls (Hemsley et al., Citation2019) and dissatisfaction with healthcare service (Hoffman et al., Citation2005).
In a recent scoping review of the management of communication disability in the first 90 days following a stroke, Baker et al. (Citation2021) recommended that people with aphasia receive appropriate and comprehensive intervention to reduce the incidence of preventable adverse events, address the impact of communication disability, and optimise communication outcomes. They concluded that whilst linguistic-behavioural interventions for aphasia formed the majority of research (34/129 studies), further high-quality research with purposive recruitment of people with aphasia during the early period of recovery post-stroke is required (Baker et al., Citation2021).
Cognitive-linguistic interventions for aphasia are behavioural-based approaches to therapy that aim to restore and rehabilitate language impairment skills through targeting phonology, syntax and semantic systems (de Jong-Hagelstein et al., Citation2011). Cognitive-linguistic interventions for aphasia do not include interventions targeting communication partner training, informational counselling, prescription of alternative and augmentative communication (AAC), modifying the patient’s healthcare environment to facilitate communication skills (Baker et al., Citation2021), or intervention targeting apraxia of speech or dysarthria. Aphasia rehabilitation is underpinned by cognitive and motor theories (Godecke et al., Citation2020), psycholinguistic models of language processing, neuropsychology and cognitive psychology concepts, and theories of language impairment based on linguistic models (Boyle et al., Citation2022).
Current cognitive-linguistic approaches described in the literature include constraint induced aphasia intervention (Pulvermüller et al., Citation2001), multimodality aphasia therapy (Rose et al., Citation2022), phonological or semantic therapy (e.g., Kristinsson et al., Citation2021), syntax therapy (e.g., Thompson et al., Citation2005,) and research-specific programs (e.g., the Very Early Rehabilitation in SpEech (VERSE) trial (Godecke et al., Citation2021)). Cognitive-linguistic interventions are commonly utilised by speech and language therapists in the treatment of aphasia within the acute and subacute hospital setting, and across the continuum of care (Verna et al., Citation2009; Hachioui et al., Citation2013).
Cognitive-linguistic interventions focus on treating underlying language impairments but can also have an impact on functional communication and quality of life outcome measures (Brady et al., Citation2016; Rose et al., Citation2022; Godecke et al., Citation2012). Functional communication is highly valued by researchers, clinicians, and participants alike as an important outcome of speech and language therapy (Worrall et al., Citation2011). The most recent Cochrane review of speech and language therapy for aphasia following stroke defined functional communication as “the ability to successfully communicate a message via spoken, written or non-verbal modalities (or a combination of these) within day-to-day interactions” (Brady et al., Citation2016 p.15). The Cochrane review found aphasia intervention can improve functional communication outcomes, reading, writing and expressive language compared to no intervention, SMD 0.28, 95% CI 0.06 to 0.49, p=0.01 (Brady et al., Citation2016). Brady et al. (Citation2016) also noted that the effect of cognitive-linguistic intervention on functional communication is not consistently measured or included in research. This is in part, due to a lack of consensus around the assessment and treatment of functional communication which has resulted in a large range of outcome measures and activities being considered as ‘functional’ (Doedens & Meteyard, Citation2022).
Improving quality of life (QOL) is another important outcome for people with aphasia, clinicians, and researchers. Evaluating change on QOL outcome measures can provide information about the impact of intervention on psycho-social wellbeing, health status and other aspects of life (Hilari et al., Citation2003; Thayabaranathan et al., Citation2022). The World Health Organisation (WHO) defines QOL as “individuals’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” (WHO, Citation2012 p.8). QOL can encompass emotional and social health, including the use of mood outcome measures to assess anxiety or depression (Cruice et al., Citation2003). As identified by Baker et al. (Citation2021) there is a paucity of evidence regarding the management of QOL in aphasia in the first 90 days post-stroke.
Cognitive-linguistic intervention is effective in the treatment of chronic aphasia with studies demonstrating improvements on language outcomes, functional communication, and QOL (Rose et al., Citation2022; Breitenstein et al., Citation2017). Chronic aphasia is defined as greater than six months post-stroke (Bernhardt et al., Citation2017). Aphasia research to date has largely focused on the chronic phase with fewer studies in the acute and subacute periods (Brady et al., Citation2016). The most recent Cochrane review reported less than a fifth (n=13, 18%) of studies involved participants within the first month post-stroke (Brady et al., Citation2016). Challenges associated with undertaking research in the early period of recovery post-stroke can include the impact of spontaneous recovery (Kirmess & Maher, Citation2010), ethical considerations when withholding treatment for a control arm (de Jong-Hagelstein et al., Citation2011) and potential treatment refusal for higher doses of intervention (Harvey et al., Citation2020). Additional factors such as the individualisation of therapy programs – “right combination of aphasia therapy for the right person, … at the right time” (Godecke et al., Citation2014, p.160), the variable responses to intervention in the context of aphasia heterogeneity (Menahemi-Falkov et al., Citation2021) and the lack of understanding of treatment dose in aphasia recovery (Harvey et al., Citation2022) have impacted on study findings in this early phase of recovery. In addition, researchers have reported various clinical barriers to implementing intervention in the acute and subacute setting, including the prioritisation of dysphagia treatment (and de-prioritisation of aphasia intervention) (Foster et al., Citation2016), patient fatigue (Kirmess & Maher, Citation2010) and reduced availability of patients due to competing commitments with other rehabilitation interventions (Nouwens et al., Citation2017).
However, there has been a recent shift away from relying on chronic aphasia research to guide clinical practice in the acute and subacute period. In their systematic review, Husak et al. (Citation2021) evaluated sixteen high quality studies (with a control or comparison group) targeting early aphasia intervention (up to four months post-stroke). Husak et al. (Citation2021) predominately evaluated change on impairment-based outcome measures but included several studies where the primary outcome was a functional communication outcome measure. They concluded that no particular treatment approach was more beneficial than another, that the evidence for providing intervention was inconclusive, and that more intervention (i.e., greater than 2-5 hours of intervention per week) did not lead to better outcomes. Husak et al. (Citation2021) findings are supported by the Brady et al. (Citation2022) recent network meta-analysis of individual participant data (the RELEASE Collaboration). Brady et al. (Citation2022) concluded that intervention in the first 3 months post-stroke is associated with the greatest gains in overall language outcomes, when combined with the right intensity, dosage, and frequency. The collaboration recommended a low intensity, moderate dosage of around two hours of speech and language therapy a week, totalling 20-50 hours of intervention for people with aphasia who can tolerate and engage in the therapy during the first three months post-stroke (Brady et al., Citation2022).
Several years prior, Harvey et al. (Citation2020) systematic review also suggested a similar maximum dose of between 20–60 hours of treatment during this acute and subacute period. Harvey et al. (Citation2020) outlined the four components of dose that should be considered: the therapeutic element (the basic unit of therapy), the session dose (quantitative measure of the therapeutic content, measured in minutes), the session intensity (number of therapeutic elements provided in a session), and session frequency (number of sessions per week). The review analysed whether time post-stroke impacted on dose effect and concluded that in the acute and early subacute phase a higher dose (i.e., more than 50 hours of therapy) of treatment provided over a short period may not be recommended over a lower dose of the same intervention (Harvey et al., Citation2020). This recommendation is based upon a reduced tolerance of higher-dose intervention and greater dropout rates.
Previous systematic reviews by Brady et al. (Citation2022) and Husak et al. (Citation2021) have provided evidence for the effect of early aphasia intervention on language and impairment-based outcomes. These systematic reviews have included Randomised Controlled Trials (RCTs) only, yet non-randomised controlled studies can complement evidence from RCTs and provide essential insights where RCTs are lacking. This review aims to investigate remaining gaps in understanding the impact of cognitive-linguistic intervention on language, functional communication and QOL outcomes, and to incorporate a range of study designs. The Baker et al. (Citation2021) scoping review supported the inclusion of high quality but smaller-scale studies whose results may provide more nuanced and meaningful findings. This review is a direct recommendation of Baker et al. (Citation2021) and aims to evaluate existing cognitive-linguistic intervention for aphasia in the first 90 days post-stroke via systematic review and meta-analysis.
Aim
The systematic review aimed to answer the following research questions:
Do structured cognitive-linguistic interventions for aphasia provided in the first 90 days after stroke, result in improvements in:
language processing,
functional communication, and/or
quality of life?
Method
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Page et al., Citation2021). The protocol for this review was registered a priori on PROSPERO 2022 (CRD42022339524).
Search strategy
The search terms included keywords and Medical Subject Headings developed with input from an academic librarian (see Supplementary Material A for example of Medline (OVID) search strategy). The terms included (stroke OR post stroke OR cerebrovascular accident) AND (subacute care OR hospital ward OR acute OR rehabilitation OR stroke rehabilitation OR subacute OR home OR community health) AND (aphasia OR dysphasia OR aphasia manag*) AND (rehabilitation OR speech OR language OR cognitive OR linguistic OR communication OR speech therapy OR language therapy). Six major databases were searched: Medline, CINAHL, PsycINFO, Cochrane Database, SpeechBITE and Google Scholar. The search was limited by English language. The review included eligible studies from the prior scoping review (Baker et al., Citation2021). A time limit from 2020 – current (12th June 2022) was used to capture studies published following the scoping review search. RCTs were limited from September 2015 onwards, and this time point was set to ensure this review evaluated RCTs published after the most recent Cochrane Review (Brady et al., Citation2016). Co-authors decided that repeat analysis of RCTs prior to September 2015 was not essential to the current study aim as details and outcomes of these studies are readily available in Brady et al. (Citation2016). Additional studies were identified by hand searching references. See PRISMA flow diagram .
Figure 1. PRISMA 2020 (Page et al., Citation2021) flow diagram for new systematic reviews which included searches of databases and other sources.
Selecting studies
The yield from the search was imported from Endnote (EndNote 20, Citation2013) to Raayan online software (Ouzzani et al., Citation2016). Duplicates were removed using the software and manual checking. Titles, abstracts, and full text articles from the systematic review search were screened independently by the first author (EE) and evaluated for eligibility based on the inclusion/exclusion criteria (see ). Secondary reviewers (CB and MR) were consulted for study eligibility and to reach a decision regarding inclusion. Screening and details of the scoping review methodology (including the literature search up until October 2020) are outlined in Baker et al. (Citation2021).
Table 1. Inclusion and exclusion criteria.
Data extraction and quality assessment
The research design of each study was identified and assigned a grade for level of evidence based on the National Health and Medical Research Council (NHMRC) guideline (NHMRC, Citation2009). Data were extracted and entered into a spreadsheet and fields included article details, study design, participant demographics, aphasia aetiology and severity, type of cognitive-linguistic intervention provided, primary and secondary outcome measures and results (see Supplementary Material B and C).
The methodological quality of each study was independently appraised by two reviewers (EE/AF/CB/EG/EL/JP/SD). RCTs and non-RCTs were assessed using the Physiotherapy Evidence Database (PEDro) scale (PEDro, Citation2020). The PEDro scale is an 11-point quality rating scale that has demonstrated reliability (Maher et al., Citation2003) and has been used in many systematic reviews, and in online databases outlining evidence-based practice (e.g., speechBITE). The methodological quality of single case experimental designs (SCEDs) was rated using the Risk of Bias in N-of-1 Trials (RoBiNT) scale (Tate et al., Citation2015). SCEDs are experimental designs that can include repeated measures, multiple baselines, sequential and/or randomised introduction of interventions, and specific data analysis and statistics (Krasny-Pacini & Evans, Citation2018). SCEDs provide considerably greater rigour than non-experimental single case designs (Tate et al., Citation2013). Case series and quasi-experimental designs were appraised using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series (Munn et al., Citation2020) and the JBI Critical Appraisal Checklist for Quasi-Experimental designs (Tufanaru et al., Citation2020) respectively. Case series and quasi-experimental designs were considered to have a lower level of evidence as per the NHMRC guidelines (NHMRC, Citation2009).
Presentation of the results and synthesising the evidence
A narrative synthesis was completed using descriptive summaries and tables to address the review questions. The intention was to complete a meta-analysis on all outcome measures, using the effect sizes from the mean change in scores of outcomes in the selected studies. Meta-analysis was completed by MB using the Review Manager Web (RevMan Web, Citation2020).
Results
Search results
The results of the study screening, eligibility, and inclusion are shown in a PRISMA flow diagram (Page et al., Citation2021), see . The search resulted in a yield of 547 studies and of these, four studies were eligible for inclusion. From the prior scoping review (Baker et al., Citation2021) 150 studies were identified and a further 21 identified via hand citation searching, with 17 studies eligible for inclusion (see ). A total of 21 studies were included in this study.
Study designs and NHMRC level of evidence
The study designs included RCTs (n=9), non RCTs (n=2), SCEDs (n=2), quasi-experimental design (n=6) and case series (n=2). The research design of the included studies was identified, and assigned a grade based on the NHMRC level of evidence (see Supplementary Material B).
Methodological quality
shows quality assessment ratings for RCTs and non-RCTs (n=11) using the PEDro scale. The cut off score for a moderate – high quality study is six points and above (PEDro statistics, 2022). Eight studies met this rating. Across the 11 studies, 90.90% (n=10) did not use blinding of participants and 100% (n=11) did not use blinding of therapistsFootnote1 Concealment of allocation occurred in 54.55% of studies (n=6). The total mean PEDro score for the group studies was 6.91 (range 3 to 9, SD=2.07) demonstrating adequate quality overall.
Table 2. PEDro scale quality assessment.
Currently, there is no agreed cut off score recommendation for the RoBiNT scale (Perdices et al., Citation2019) however other recent systematic reviews in aphasia rehabilitation and recovery have suggested a score of 12 or greater to be adequate quality (Harvey et al., Citation2020; Pierce et al., Citation2019). Both (n=2) SCED studies demonstrated lack of blinding of therapists and assessors, and reduced treatment adherence (see ).
Table 3. RoBiNT scale of quality assessment.
The quasi-experimental and case series study designs were evaluated using two different JBI Critical Appraisal Checklists, to assess and highlight the possibility of bias in the studies (Munn et al., Citation2020). Across the quasi-experimental studies there was insufficient information about concurrent treatment/care being received by the participants, no utilisation of a control group and reduced use of multiple measurement of the outcomes pre and post intervention. For the case series studies there was a lack of information about consecutive and complete inclusion of participants in the studies. Overall, these limitations contribute to possible bias within the studies, including bias in design and selection of participants, and instruction bias. See .
Table 4. Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Quasi-experimental Studies.
Table 5. Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series.
Due to insufficient homogeneity of outcome measures, study designs and intervention approaches in the included studies, it was only possible to complete meta-analysis on four studies using language assessment outcomes. Meta-analysis was completed evaluating the differences in means of language outcomes between cognitive-linguistic interventions, using a fixed effect model as heterogeneity was not detected (I2 = 0%).
Intervention characteristics
Across the 21 studies, six different types of cognitive-linguistic interventions were implemented, including if the intervention used a control group (refer to Supplementary Material C). The most common intervention approaches included constraint induced therapy (i.e. Constraint Induced Aphasia Therapy (CIAT)/Constraint Induced Language Therapy (CILT)) (Carpenter & Cherney, Citation2016; Ciccone et al., Citation2016; Godecke et al., Citation2014; Kirmess & Maher, Citation2010; Kirstensen et al., Citation2015; Woldag et al., Citation2017), and Melodic Intonation Therapy (MIT) (Fama et al., Citation2016; Lim et al., Citation2013; van de Sandt-Koenderman et al., Citation2018; Zhang et al., Citation2021). When a description of the cognitive-linguistic intervention was provided in the studies, it included lexical semantic therapy, semantic feature therapy, cued naming therapy, phonological feature mapping, writing, and reading (Ciccone et al., Citation2016; Godecke et al., Citation2014; Kesav et al., Citation2017; Liu et al., Citation2022; Wenke et al., Citation2018). Study-specific interventions were used in the VERSE trial and included personally salient stimuli in picture naming, spoken word-picture matching, phrase production and picture description tasks (Godecke et al., Citation2021). Approaches such as supported conversation for adults with aphasia (SCA) and augmentative and alternative communication (AAC) were also implemented in addition to the cognitive-linguistic intervention (Kesav et al., Citation2017; Fama et al., Citation2016). The study interventions were delivered in individual or group therapy settings, or a mixture of both, and five studies used computers to deliver intervention.
The most reported information on dose and intensity of intervention was for session dose (measured in minutes) reported in 90.48% studies (19 of 21 studies) and session frequency (number of sessions per week) reported in 90.48% (19 of 21 studies). A trend of more recent research providing increased detail and description of intervention (i.e., VERSE Supplementary Appendix) was evident. Further information about the mode of delivery, frequency and duration of intervention, treatment type and outcome measures are outlined in Supplementary Material C.
Study question results
Question 1: Do structured cognitive-linguistic interventions for aphasia provided in the first 90 days after stroke, result in improvements in:
a) language processing?
Overall, the results are mixed as to whether cognitive-linguistic interventions in the first 90 days post-stroke can improve language processing in people with aphasia. There was evidence that variation in the degree of post-intervention change was influenced by the type of intervention. There were a large variety of outcome measures used (greater than 25) across the studies. Participant characteristics and interventions were not always adequately described, including the intervention used in the control group. It is possible that elements of cognitive-linguistic intervention may have been provided in the comparison group when receiving traditional speech language intervention or usual care. These factors, combined with variable methodological quality of the studies impacted on the interpretation of the results. The following section will provide an overview of the studies based on intervention approaches and mode of service delivery.
Study specific cognitive-linguistic intervention
One large RCT (N=246), the VERSE trial (Godecke et al., Citation2021), reported a 51.7% maximal potential recovery at 12 weeks post-stroke (Western Aphasia Battery-Revised Aphasia Quotient (WAB-R AQ; Kertesz, Citation2007) but no significant difference between high and low intensity delivery. The study contributes to the evidence about intensity of intervention, with the low intensity group receiving 9.5 hours (SD=7.6) of therapy over 28 days compared to 22.7 hours (SD=8.4) of therapy for the high intensity group. The result suggests that more hours of cognitive-linguistic intervention do not necessarily enhance language recovery in this acute period. Another large RCT (N=153), the Rotterdam Aphasia Therapy Study (RATS-3; Nouwens et al., Citation2017), demonstrated no significant improvement from 4 weeks of high-intensity cognitive-linguistic intervention compared to no speech and language intervention. However, the majority of participants did not receive the intended dose of 28 hours of direct aphasia intervention (Nouwens et al., Citation2017). The per-protocol analysis demonstrated significant improvement for the intervention group on several primary outcomes (Amsterdam-Nijmegen Everyday Language Test A (ANELT-A) Blomert et al., Citation1994), adjusted difference = 5.41, 95% CI [1.52 to 9.31], p=0.007; Comprehensive Aphasia Test (CAT; Swinburn et al., Citation2004) word comprehension, adjusted difference= 3.64, 95% CI [0.58 to 6.69], p=0.020. Hoeg Dembrower et al. (2017) (N=118) demonstrated a relationship between prescribed intensive cognitive-linguistic intervention (45 minutes, five times a week for three weeks) and location of cerebral infarcts. They reported positive outcomes for people with aphasia with infarcts in the Wernicke’s and central areas but with preserved Broca’s area (Hoeg Dembrower et al., Citation2017). Seventy-eight percent (14 of the 18 participants) of the intervention group demonstrated significant improvement on the Amsterdam-Nijmegen Everyday Language Test (ANELT; Blomert et al., Citation1994) when compared to the control group (no speech and language therapy). This study supports the concept of high-intensity intervention in the acute phase for participants with infarcts in these areas.
Constraint induced intervention
Godecke et al. (Citation2014) demonstrated that the Very Early Rehabilitation (VER) program was effective when compared to a control group (18% greater recovery on Western Aphasia Battery – Aphasia Quotient (WAB-AQ; Kertesz, Citation1982), and 1.5% higher scores on discourse analysis (Kertesz, Citation1982; Nicholas & Brookshire, Citation1995)). The VER participants received either a modified group-based constraint induced intervention or individual cognitive-linguistic intervention (such as semantic feature therapy, lexical-semantic therapy, and phonological feature therapy). Carpenter and Cherney (Citation2016) single case experimental design study revealed a small-medium effect (greater than 15% increase in accuracy) for trained and untrained oral reading probes and untrained picture naming in the participants receiving usual care plus additional constraint induced intervention compared to usual care alone.
In contrast, Woldag et al. (Citation2017) and Ciccone et al. (Citation2016) reported both the constraint induced group and the comparison group improved regardless of intervention allocation. In a three-armed intervention study, Woldag et al. (Citation2017) compared high intensity (defined as three hours a day for 10 days) constraint induced group-based intervention versus high intensity communication group therapy versus low intensity individual and group intervention. There was no between group difference post-treatment on the Aachen Aphasia Test (AAT; Huber et al., Citation1983). Ciccone et al. (Citation2016) randomly allocated participants to either constraint induced therapy (n=12) or individual impairment-based intervention (n=8) for a total of 15-20 hours over 5 weeks. There was no significant difference between the two interventions, however there was a significant treatment effect from baseline to intervention completion and at 12 weeks post stroke on the WAB-AQ (Kertesz, Citation1982) (p<.001).
Melodic Intonation Therapy
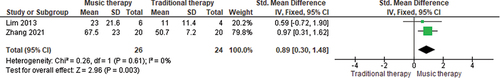
Several studies evaluating the use of melodic intonation therapy (MIT) reported positive results. Van de Sandt-Koenderman et al. (Citation2018) small pre-test/post-test design (N=9) demonstrated improvement on the AAT (repetition subtest) for 100% of subacute (<3 months post onset) participants (n=5) and improvement on everyday communication on the ANELT for 80% (four of five) subacute participants. Zhang et al. (Citation2022) RCT (N=42) reported significant differences between a Mandarin MIT group versus traditional speech and language therapy on a modified Boston Diagnostic Aphasia Examination (BDAE; Fong et al., Citation2019) subtests of spontaneous speech, naming, comprehension, and repetition, as well as summed BDAE scores at eight weeks, t2= 67.47 ±22.99, t=4.036, p=0.0001. Lim et al. (Citation2013) also reported their subacute neurological music therapy (NMT) group (n=10) made significant within group improvement in Korean WAB-AQ (Kim & Na, Citation2001) scores post treatment. However, there was no significant difference between the NMT group and the control (traditional speech and language intervention), and also no significant improvement post treatment for the control group. Meta-analysis of Lim et al. (Citation2013) and Zhang et al. (Citation2022) showed a statistically significant difference between MIT and traditional intervention, in favour of MIT with an overall large effect of SMD 0.89, 95% CI=0.30, 1.48, p=0.003 on the BDAE summed score (Zhang et al., Citation2022) and WAB-AQ (Lim et al., Citation2013) (see ).
Verbal-gestural therapy
In a small (N=4) SCED, Armour and Del Toro (Citation2021) reported improvement for 75% (n=3) of participants who received verbal-gestural therapy people with aphasia on the CAT and WAB-R. However methodological limitations were noted (see ).
Modes of delivery - group based intervention
In a case series study (N=10) involving people with severe aphasia, Fama et al. (Citation2016) reported improved initiation of communication following group-based versus individual therapy, Wilcoxon Z=2.045, p=.041. The group-based therapy included a range of intervention approaches such as MIT, multimodal communication, and naming therapy. This result is contrasted with Avent and Wertz (Citation1996) who concluded group treatment was no more effective than individual treatment when evaluating performance on a pragmatic protocol.
Modes of delivery – computer-based intervention
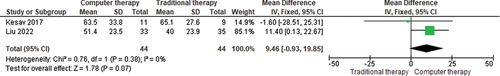
Utilisation of computers to increase the session duration and frequency of intervention was investigated by Kesav et al. (Citation2017) and Liu et al. (Citation2022) in randomised controlled trials. Both studies compared speech and language intervention provided in-person versus in-person plus via computer. Kesav et al. (Citation2017) study provided one hour of in-person intervention, three times a week for 4 weeks involving a variety of approaches (i.e., MIT, phoneme and grapheme therapy in writing and reading, and multiple input phoneme therapy) for a total of 12 hours of in-person intervention. This was versus in-person intervention plus additional 12 hours of computer-based intervention (one hour, three times a week, for four weeks) for a total of 24 hours of intervention. The computer program targeted cognitive-linguistic tasks such as auditory verbal comprehension, verbal fluency, naming, writing, reading and calculation (Kesav et al., Citation2017). Kesav et al. (Citation2017) reported improvements in the in-person group at 4 weeks, which contrasted with Liu et al. (Citation2022) who reported improvement in the computer-based group. Liu et al. (Citation2022) provided in-person cognitive-linguistic intervention (one hour a day, six days a week for four weeks) and the experimental group received additional (30 minutes) of computer-based intervention targeting cognitive skills (memory, reasoning, attention) using simulation and interactive games. A meta-analysis of these two studies, showed the overall advantage of computer-based intervention over in-person therapy, with a pooled mean difference 9.46, 95% CI -0.93, 19.85; p=0.07, on a Mandarin WAB-AQ (Ren et al., Citation2019) (see ).
Figure 3. Meta-analysis of computer-based intervention plus speech and language intervention versus traditional speech and language therapy (in-person).
In contrast to this result, Spaccavento et al. (Citation2021) reported that a novel Italian computer-based cognitive-linguistic program did not lead to improved results on an Italian AAT (Luzzatti et al., Citation1996), when compared to in-person intervention. Both groups received the same amount and type of cognitive-linguistic intervention and they both improved on post-test measures, with the exception of the AAT repetition, which favoured the in-person speech and language group, F(1,20)=9.46, p=0.006. Increasing the hours of naming intervention (from low intensity = four hours/week versus high intensity = eight hours/week) using a combined service delivery model (in-person, group and computer based) was evaluated by Wenke et al. (Citation2018). The study demonstrated statistically significant improvement post-treatment and on follow up for spoken naming subtest (p=.021) and aphasia severity (p=.018) of the CAT (Howard et al., Citation2004) (statistically significant when adjusted for alpha level of 0.25) for both groups combined (N=9). No formal between-group analysis was performed and the number of subjects within the 90-day period was small (n=3).
Question 2: Do structured cognitive-linguistic interventions for aphasia provided in the first 90 days after stroke result in improvements in:
b) functional communication
c) quality of life?
Overall, only a small number of studies (38.1%, eight of 21 studies) included measurements of either functional communication or QOL (or both). When utilised these outcome measures were mostly included as secondary outcomes (87.5%; seven of eight studies). Two studies included both functional communication and quality of life outcome measures (Spaccavento et al., Citation2021; Wenke et al., Citation2018). Four studies used QOL only (Godecke et al., Citation2021; Ciccone et al., Citation2016; Zhang et al., Citation2021; Nouwens et al., Citation2017) and two used a functional communication outcome only (Kristensen et al., Citation2015; Woldag et al., Citation2017).
Those measures that were completed by the participant with aphasia, were either a self-administered rating scale (Nouwens et al., Citation2017) or self-reported with communication support from a blinded assessor (Godecke et al., Citation2021), or in an interview style (Ciccone et al., Citation2016; Godecke et al., Citation2021). Three studies utilised a caregiver or team rating of the participant with aphasia (Kirstensen et al., Citation2015, Wenke et al., Citation2019; Spaccavento et al., Citation2018). One study completed a combination of self-report and caregiver-report via a questionnaire, depending on the participant’s ability to respond to the items (Spaccavento et al., Citation2021), and the questionnaire was administered by a speech and language therapist. Two studies utilised clinicians to complete outcome measures, including evaluation of mood by a neuropsychologist (Kristensen et al., Citation2015) and research assistants (Zhang et al., Citation2021).
The studies demonstrated that improvements on language outcomes generally led to positive change on functional communication, regardless of the intervention type or mode of delivery. Woldag et al. (Citation2017) reported significant improvement on the qualitative component of a functional communication measure (Communication Activity Log (CAL); Pulvermüller et al., Citation2001), for the constraint induced intervention arm of the study (15.2 + 0.9 x pre-treatment CAL qualitative -6.8, p=.049) compared to the conventional communication group. Kristensen et al. (Citation2015) reported significant improvement on the Danish adaptation of the Communication Effectiveness Index (CETI; Pedersen et al., Citation2001) (relative’s assessment) for both constraint induced and usual care interventions, but the improvement was not maintained at follow up. Wenke et al. (Citation2018) also reported improvement on the CETI (Lomas et al. Citation1989) for both the hybrid-4 and hybrid-8 groups (see Supplementary Material C for details of intervention), with maintenance at four weeks post-intervention for most participants. Spaccavento et al. (Citation2021) reported an improvement post-treatment for both the computer-based intervention and in-person intervention, on the Italian Version Functional Outcome Questionnaire for Aphasia (FOQ-A; Spaccavento et al., Citation2018), a caregiver functional communication questionnaire, F(1,20)=22.14, p=001.
Evidence for the impact of structured cognitive-linguistic intervention on QOL is mixed. Three studies demonstrated improvement in QOL measures regardless of intervention type (Spaccavento et al., Citation2021; Ciccone et al., Citation2016; Wenke et al., Citation2018) with no between-group differences. Nouwens et al. (Citation2017) reported no significant difference between groups for the cognitive-linguistic group or control group on the EuroQol Group outcome measure EQ-5D-3L (Rabin et al., Citation2011) for either the intention to treat or per protocol analysis. Godecke et al. (Citation2021) also found no significant change to depression (Aphasia Depression Rating Scale (ADRS) Benaim et al., Citation2004) or QOL (SAQOL-39g; Hilari et al., Citation2003) in any of the intervention arms. Zhang et al. (Citation2021) reported significant improvements in depression on the Hamilton Depression Scale (HAMD; Spreen & Risser, Citation2003) at 8 weeks, M=8.95 +/-1.97, compared to baseline, M=16.15 +/- 2.52, F=5.63, p=.0202, but not anxiety for the MIT intervention group, with the authors attributing the improved mood to the musical component of the program.
Discussion
The purpose of this systematic review was to investigate the effectiveness of structured cognitive-linguistic intervention to treat aphasia in the first 90 days post-stroke. The results regarding the effectiveness of intervention on language outcomes are unclear and the findings from the exploratory meta-analysis of four studies are mixed. Constraint induced aphasia intervention was utilised in the literature, but there is insufficient evidence to guide implementation, and based on the review findings no recommendations can be made with regards to therapy frequency, intensity, and dosage. Using computers as a mode of delivery or as an adjunct to in-person therapy to increase the frequency or session duration, was supported. When studies showed improvement in language outcomes, there was an associated positive effect on functional communication regardless of intervention type. Overall, there is a lack of evidence regarding the type of cognitive-linguistic interventions that offer superior outcomes for functional communication and QOL. However, it is important to acknowledge that improvement on outcome measures that are not the direct target of the intervention, cannot be assumed (i.e., providing structured cognitive-linguistic intervention in a study and then evaluating the impact on a functional communication or quality of life outcome measure).
Previous evidence within the chronic phases of rehabilitation highlights the need for a better understanding of optimal timing, amounts and frequency of treatments in the first 90 days after stroke (Brady et al., Citation2016). The findings of this review suggest that future research needs to specifically consider the planning of interventions, adequate description of people with aphasia, the careful selection of outcome measures and clear reporting. The following recommendations are made:
Adherence to clear planning, description, and reporting of aphasia intervention
The Aphasia Intervention description In Research (AsPIRE) project (Dipper et al., Citation2022) outlines several considerations for aphasia intervention studies. These include the use of the Template for Intervention Description and Replication (TIDieR) framework (Hoffman et al., Citation2014), with additional reporting items developed by the authors to assist with research replicability (Dipper et al., Citation2022; Behn et al., Citation2022). The AsPIRE project recommends that research should state how intervention is provided, session duration and intensity, modifications made, stimulus materials used, target response required, type of feedback provided, and fidelity and adherence to a program protocol (Dipper et al., Citation2022). Adequate reporting of these add to the validity of the research, provides opportunity for study replication, generalisation to the acute clinical setting and facilitates future synthesis and meta-analysis (Brady et al., Citation2020; Dipper et al., Citation2022). It also assists with documenting the details and ingredients provided in aphasia therapy, including the components of usual care or traditional speech language therapy (Brady et al., Citation2020).
Early aphasia intervention research is not always structured to demonstrate the relationship between the aspects of the therapy and its underlying neuropathology (Basilakos et al., Citation2022). Applying a framework, such as the Rehabilitation Treatment Specification System (RTSS; Boyle et al., Citation2022) or the Multidimensional Dose Articulation Framework (MDAF; Harvey et al., Citation2022) would assist in establishing clear components of intervention and the mechanism of action underlying improvement in the target outcomes. Examples of how RTSS and MDAF can be applied to aphasia interventions are discussed in Boyle et al. (Citation2022), Basiliakos et al. (Citation2022) and Cherney et al. (Citation2021) including retrospective application to published studies (Harvey et al., Citation2022).
Adequate participant descriptions and consistent use of core aphasia outcome measures
The most recent Cochrane review (Brady et al., Citation2016) noted that between-group comparisons in the included RCT’s were challenging due to variability in participant descriptions. The recent international DESCRIBE project (Wallace et al., Citation2022) outlines 14 items that should be included when reporting people with aphasia characteristics in research. Using this framework in aphasia research will add to validity and replicability of the studies.
This systematic review included a meta-analysis for only 17% (four out of 23 studies), partially due to heterogeneity of outcome measures. The Research Outcome Measurement in Aphasia (ROMA; Wallace et al., Citation2019) was developed in response to the heterogeneity evident in aphasia therapy research. ROMA provides clear information on best practice for outcome measurements, particularly impairment-based outcomes. The ROMA project included stakeholder consultation, a review of available aphasia outcome measures, and discussion and endorsement at an international level. The consistent use of outcome measures in future aphasia research will foster collaboration and increase the opportunity for secondary data analyses which in turn ensures more efficient and efficacious use of research results (Brady et al., Citation2020).
Clinical implications
Structured cognitive-linguistic interventions to treat aphasia in the first 90 days post-after stroke demonstrate some positive change on language processing, however study results are ambiguous. Cognitive-linguistic interventions can be provided in conjunction with other best practice recommendations such as the Australian Aphasia Rehabilitation Pathway (Citation2014), the Canadian Stroke Best Practices (Citation2019) or the National Institute for Health and Care Excellence Clinical Guidelines (Citation2019) to create a holistic programme of management. This study focused on cognitive-linguistic treatments, however, to address all aspects of living with aphasia, interventions such as Communication Enhanced Environments (D’Souza et al., Citation2021), supported communication partner training for family and staff (Clancy et al., Citation2020) and informational counselling (Stroke Foundation, Citation2022) are all important for people with aphasia and their carers early post-stroke. Clinicians are also encouraged to address activities and participation (such as person-centred goal setting) and personal factors (such as psychological care) to optimise healthcare, communication, and rehabilitation outcomes (Baker et al., Citation2021). These latter interventions also ensure the therapeutic potential during this acute and early subacute phase is being utilised, and they counteract a potential period of communication inactivity (Hersh, Citation2016).
Flexibility in providing structured cognitive-linguistic intervention is required in acute-subacute care, where there are unique barriers to providing therapy (Nouwens et al., Citation2017). Clinicians need to be confident to assess communication needs and deliver interventions that meet the goals of people with aphasia. It is vital for clinicians to understand the specific ingredients of an intervention, and the modifications required to deliver these at an appropriate dose in acute and subacute settings (Boyle et al., Citation2022; Harvey et al., Citation2022). It is also important that the intervention target matches the selected outcome measure. For example, if the goal is to improve naming skills, then an appropriate language outcome measure should be used. People with aphasia want intervention to improve their functional communication skills and aspects of their QOL (Worrall et al., Citation2011). Future research is required to design interventions that specifically aim to address these skills and include appropriate assessments to monitor progress and outcomes.
Limitations of this study
This study only included research published in English which limits the application of the findings for people with aphasia globally. It should be acknowledged that by purposively including a wide variety of study designs this review evaluated studies with lower quality ratings and less robust methodology, limiting both the generalisability of the findings and the ability to provide recommendations for clinical practice. However, including a wide variety of study designs was identified as an important component by the scoping review (Baker et al., Citation2021). It is worth noting that studies that did not use a control group in the acute phase were at risk of being unable to eliminate the variable of spontaneous recovery. This is an important consideration during this critical period as up to 60% of patients with mild-moderate aphasia will recover within the first 90 days (Ali et al., Citation2015).
Conclusion
Aphasia post-stroke can affect up to 40% of people and is associated with longer hospital stays and poorer psychosocial and rehabilitation outcomes (Ali et al., Citation2015; Flowers et al., Citation2015; Mitchell et al., Citation2021). The results of this review further highlight the need for high quality evidence to guide clinical practice in the acute and subacute phase of recovery and rehabilitation. Recommendations around dosage of intervention during this phase are emerging, but further information regarding the effectiveness of specific intervention approaches is required. This requires detailed research with robust description of participant characteristics, consistent use of outcome measures and clear theoretical guidelines regarding components of intervention and the underlying mechanisms of change.
Disclosure of interest
The authors declare there is no conflict of interest. There was no funding sources or sponsor.
Word count (inclusive of abstract, excluding reference list): 6790.
Supplemental Material
Download MS Word (55 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02687038.2023.2282659.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Notes
1. In behavioural interventions, blinding of participants and therapists is typically not possible.
References
- Ali, M., Lyden, P., & Brady, M. (2015). Aphasia and dysarthria in acute stroke: Recovery and functional outcome. International Journal of Stroke, 10(3), 400–406. https://doi.org/10.1111/ijs.12067
- Armour, M., & Del Toro, C. M. (2021). The effectiveness of verbal–gestural treatment on verb naming in acute inpatient rehabilitation. American Journal of Speech-Language Pathology, 30(2), 713–721. https://doi.org/10.1044/2020_AJSLP-20-00365
- Australian Aphasia Rehabilitation Pathway. (2014). Best practice statements comprehensive supplement to the Australian Aphasia Rehabilitation Pathway. http://www.aphasiapathway.com.au/flux-content/aarp/pdf/Aphasia-Rehabilitation-Best-Practice-Statements-15042015-comprehensive-BMJ-Suppl-file-b.pdf
- Avent, J., & Wertz, R. (1996). Influence of type of aphasia and type of treatment on aphasic patients’ pragmatic performance. Aphasiology, 10(3), 253–265.
- Baker, C., Foster, A. M., D’Souza, S., Godecke, E., Shiggins, C., Lamborn, E., Lanyon, L., Kneebone, I., & Rose, M. (2021). Management of communication disability in the first 90 days after stroke: A scoping review. Disability and Rehabilitation, 44(26), 8524–8538. https://doi.org/10.1080/09638288.2021.2012843
- Baker, C., Worrall, L., Rose, M., Hudson, K., Ryan, B., & O’Byrne, L. (2018). A systematic review of rehabilitation interventions to prevent and treat depression in post-stroke aphasia. Disability and Rehabilitation, 40(16), 1870–1892. https://doi.org/10.1080/09638288.2017.1315181
- Basilakos, A., Hula, W. D., Johnson, L. P., Kiran, S., Walker, G. M., & Fridriksson, J. (2022). Defining the neurobiological mechanisms of action in aphasia therapies: Applying the Rehabilitation Treatment Specification System framework to research and practice in aphasia. Archives of Physical Medicine & Rehabilitation, 103(3), 581–589.
- Behn, N., Harrison, M., Brady, M. C., Breitenstein, C., Carragher, M., Fridriksson, J., Godecke, E., Hillis, A., Kelly, H., Palmer, R., Rose, M. L., Thomas, S., Tippett, D., Worrall, L., Becker, F., & Hilari, K. (2022). Developing, monitoring, and reporting of fidelity in aphasia trials: Core recommendations from the collaboration of aphasia trialists (CATs) trials for aphasia panel. Aphasiology, 37(11), 1733–1755. https://doi.org/10.1080/02687038.2022.2037502
- Benaim, C., Cailly, B., Perennou, D., & Pelisser, J. (2004). Validation of the Aphasic Depression Rating Scale. Stroke, 35(7), 1692–1696.
- Bernhardt, J., Hayward, K. S., Kwakkel, G., Ward, N. S., Wolf, S. L., Borschmann, K., Krakauer, J. W., Boyd, L. A., Carmichael, S. T., Corbett, D., & Cramer, S. C. (2017). Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. International Journal of Stroke, 12(5), 444–450. https://doi.org/10.1177/1747493017711816
- Blomert, L., Kean, M.L., Koster, C.& Schokker, J. (1994). Amsterdam—Nijmegen everyday language test: Construction, reliability and validity. Aphasiology, 8(4), 381–407.
- Boyle, M., Gordon, J. K., Harnish, S. M., Kiran, S., Martin, N., Rose, M. L., & Salis, C. (2022). Evaluating cognitive-linguistic approaches to interventions for aphasia within the Rehabilitation Treatment Specification System. Archives of Physical Medicine and Rehabilitation, 103(3), 590–598. https://doi.org/10.1016/j.apmr.2021.07.816
- Brady, M. C., Ali, M., VandenBerg, K., Williams, L.J., Williams, L.R, Abo, M., Becker, F., Bowen, A., Brandenburg, C., Breitenstein, C., Bruehl, S., Copland, D.A., Cranfill, T.B., Pietro-Bachmann M.D., Enderby, P., Fillingham, J., Lucia Galli, F., Gandolfi, M., Glize, B., … Harris Wright, H. (2022). (RELEASE Collaborators). Precision rehabilitation for aphasia by patient age, sex, aphasia severity, and time since stroke? A prespecified, systematic review-based, individual participant data, network, subgroup meta-analysis. International Journal of Stroke, 17(10), 1067–1077. https://doi.org/10.1177/17474930221097477
- Brady, M. C., Ali, M., VandenBerg, K., Williams, L. J., Williams, L. R., Abo, M., Becker, F., Bowen, A., Brandenburg, C., Breitenstein, C., Bruehl, S., Copland, D. A., Cranfill, T. B., di Pietro-Bachmann, M., Enderby, P., Fillingham, J., Galli, F. L., Gandolfi, M., Glize, B., Hinckley, J. (2020). Communicating simply, but not too simply: Reporting of participants and speech and language interventions for aphasia after stroke International Journal of Speech Language Pathology, 22(3), 302–312. https://doi.org/10.1080/17549507.2020.1762000
- Brady, M., Kelly, H., Godwin, J., Enderby, P., & Campbell, P. (2016). Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews, 2016(6), CD000425.
- Breitenstein, C., Grewe, T., Flöel, A., Ziegler, W., Springer, L., Martus, P., Huber, W., Willmes, K., Ringelstein, E., Haeusler, K., Abel, S., Glindemann, R., Domahs, F., Regenbrecht, F., Schlenck, K., Thomas, M., Obrig, H., de Langen, E., Rocker, R. … Baumgaertner, A. (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. The Lancet, 389(10078), 1528–1538. https://doi.org/10.1016/S0140-6736(17)30067-3
- Canadian Stroke Best Practices. (2019). In Canadian Stroke Best Practices. Chapter 10: Rehabilitation to improve language and communication (6th ed.). https://www.strokebestpractices.ca/recommendations/stroke-rehabilitation/rehabilitation-to-improve-language-and-communication
- Carpenter, J., & Cherney, L. R. (2016). Increasing aphasia treatment intensity in an acute inpatient rehabilitation programme: A feasibility study. Aphasiology, 30(5), 542–565. https://doi.org/10.1080/02687038.2015.1023695
- Cherney, L. R., DeDe, G., Hoover, E. L., Murray, L., Obermeyer, J., & Pompon, R. H. (2022). Applying the Rehabilitation Treatment Specification System to functional communication treatment approaches for aphasia. Archives of Physical Medicine and Rehabilitation, 103(3), 599–609. https://doi.org/10.1016/j.apmr.2021.10.016
- Ciccone, N., West, D., Cream, A., Cartwright, J., Rai, T., Granger, A., … Godecke, E. (2016). Constraint-induced aphasia therapy (CIAT): A randomised controlled trial in very early stroke rehabilitation. Aphasiology, 30(5), 566–584.
- Clancy, L., Povey, R., & Rodham, K. (2020). “Living in a foreign country”: experiences of staff-patient communication in inpatient stroke settings for people with post-stroke aphasia and those supporting them. Disability and Rehabilitation, 42(3), 324–334. https://doi.org/10.1080/09638288.2018.1497716
- Cruice, M., Worrall, L., Hickson, L., & Murison, R. (2003). Finding a focus for quality of life with aphasia: Social and emotional health, and psychological well-being. Aphasiology, 17(4), 333–353. https://doi.org/10.1080/02687030244000707
- D’Souza, S., Godecke, E., Ciccone, N., Hersh, D., Armstrong, E., Tucak, C., Janssen, H. (2021). Investigations of a communication enhanced environment model on an acute/slow stream rehabilitation ward: A before-and-after pilot study. Clinical Rehabilitation, 36(1), 15–39. https://doi.org/10.1177/026921552110326
- de Jong-Hagelstein, M., van de Sandt-Koenderman, W., Prins, N., Dippel, D., Koudstaal, P., & Visch-Brink, E. (2011). Efficacy of early cognitive–linguistic treatment and communicative treatment in aphasia after stroke: A randomised controlled trial (RATS-2). Journal of Neurology, Neurosurgery and Psychiatry, 82(4), 399–404. https://doi.org/10.1136/jnnp.2010.210559
- Dipper, L. T., Franklin, S., de Aguiar, V., Baumgaertner, A., Brady, M., Best, W., Bruehl, S., Denes, G., Godecke, E., Gil, M., Kirmess, C., Markey, M., Meinzer, C., Mendez Orellana, M., Norvik, M., Nouwens, F., Rose, M. L., van de Sandt, M., Whitworth, A., & Visch-Brink, E. G. (2022). An umbrella review of Aphasia Intervention descriPtion In Research: The AsPIRE project. Aphasiology, 36(4), 467–492. https://doi.org/10.1080/02687038.2020.1852001
- Doedens, W., & Meteyard, L. (2022). What is functional communication? A theoretical framework for real-world communication applied to aphasia rehabilitation. Neuropsychology Review, 32(4), 937–973. https://doi.org/10.1007/s11065-021-09531-2
- Elsner, B., Kugler, J., Pohl, M., & Mehrholz, J. (2019). Transcranial direct current stimulation (tDCS) for improving aphasia in adults with aphasia after stroke. Cochrane Library, 2019(5), CD009760–CD009760. https://doi.org/10.1002/14651858.CD009760.pub4
- Enderby, P., & Petheram, B. (2002). Has aphasia therapy been swallowed up? Clinical Rehabilitation, 16(6), 604–608. https://doi.org/10.1191/0269215502cr505oa
- EndNote 20. (2013). The EndNote Team. Clarivate Analytics.
- Fama, M., Baron, C., Hatfield, B., & Turkeltaub, P. (2016). Group therapy as a social context for aphasia recovery: A pilot, observational study in an acute rehabilitation hospital. Topics in Stroke Rehabilitation, 23(4), 276–283.
- Flowers, H., Skoretz, S., Silver, F., Rochon, E., Fang, J., Flamand-Roze, C., & Martino, R. (2015). Poststroke aphasia frequency, recovery, and outcomes: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation, 97(12), 2188–2201.e8.
- Fong, M., Van Patten, R., & Fucetola, R. (2019). The factor structure of the Boston Diagnostic Aphasia Examination, (3rd ed). Journal of the International Neuropsychological Society, 25(7), 772–776. https://doi.org/10.1017/S1355617719000237
- Foster, A., O’Halloran, R., Rose, M., & Worrall, L. (2016). “Communication is taking a back seat”: Speech pathologists’ perceptions of aphasia management in acute hospital settings. Aphasiology, 30(5), 585–608. https://doi.org/10.1080/02687038.2014.985185
- Godecke, E., Armstrong, E., Rai, T., Ciccone, N., Rose, M. L., Middleton, S., Whitworth, A., Holland, A., Ellery, F., Hankey, G. J., Cadilhac, D. A., & Bernhardt, J. (2021). A randomized control trial of intensive aphasia therapy after acute stroke: The Very Early Rehabilitation for SpEech (VERSE) study. International Journal of Stroke, 16(5), 556–572. https://doi.org/10.1177/1747493020961926
- Godecke, E., Ciccone, N., Granger, A., Rai, T., West, D., Cream, A., … Hankey, G. (2014). A comparison of aphasia therapy outcomes before and after a Very Early Rehabilitation programme following stroke. International Journal of Language & Communication Disorders, 49(2), 149–161.
- Greener, J., Enderby, P., Whurr, R., & Fraser, H. (2001). Pharmacological treatment for aphasia following stroke. Cochrane Library, 2010(5), CD000424–CD000424. https://doi.org/10.1002/14651858.CD000424
- Grönberg, A., Henriksson, I., & Lindgren, A. (2021). Accuracy of NIH Stroke Scale for diagnosing aphasia. Acta Neurologica Scandinavica, 143(4), 375–382. https://doi.org/10.1111/ane.13388
- Hachioui El, H., Lingsma, H., Koenderman, M., Dippel, D., Koudstaal, P., & Visch-Brink, E. (2013). Recovery of aphasia after stroke: A 1-year follow-up study. Journal of Neurology, 260(1), 166–171. https://doi.org/10.1007/s00415-012-6607-2
- Harvey, S., Carragher, M., Dickey, M. W., Pierce, J. E., & Rose, M. L. (2020). Dose effects in behavioural treatment of post-stroke aphasia: A systematic review and meta-analysis. Disability and Rehabilitation, 4(12), 2548–2559. https://doi.org/10.1080/09638288.2020.1843079
- Harvey, S., Rose, M. L., Brogan, E., Pierce, J. E., Godecke, E., Brownsett, S. L. E., Churilov, L., Copland, D., Dickey, M. W., Dignam, J., Lannin, N. A., Nickels, L., Bernhardt, J., & Hayward, K. S. (2022). Examining dose frameworks to improve aphasia rehabilitation research. Archives of Physical Medicine and Rehabilitation, 104(5), 830–838. https://doi.org/10.1016/j.apmr.2022.12.002
- Hemsley, B., Steel, J., Worrall, L., Hill, S., Bryant, L., Johnston, L., Georgiou, A., & Balandin, S. (2019). A systematic review of falls in hospital for patients with communication disability: Highlighting an invisible population. Journal of Safety Research, 68, 89–105. https://doi.org/10.1016/j.jsr.2018.11.004
- Hersh, D. (2016). Therapy in transit: Managing aphasia in the early period post-stroke. Aphasiology, 30(5), 509–516. https://doi.org/10.1080/02687038.2015.1137555
- Hilari, K., Byng, S., Lamping, D. L., & Smith, S. C. (2003). Stroke and Aphasia Quality of Life Scale-39(SAQOL-39): Evaluation of acceptability, reliability, and validity. Stroke, 34(8), 1944–1950. https://doi.org/10.1161/01.str.0000081987.46660.ed
- Höeg Dembrower, K., Von Heijne, A., Laska, A., & Laurencikas, E. (2017). Patients with aphasia and an infarct in Wernicke’s area benefit from early intensive speech and language therapy. Aphasiology, 31(1), 122–128.
- Hoffman, J. M., Yorkston, K. M., Shumway-Cook, A., Ciol, M. A., Dudgeon, B. J., & Chan, L. (2005). Effect of communication disability on satisfaction with health care: A survey of medicare beneficiaries. American Journal of Speech-Language Pathology, 14(3), 221–228. https://doi.org/10.1044/1058-0360(2005/022)
- Hoffmann, T. C., Glasziou, P. P., Boutron, I., Milne, R., Perera, R., Moher, D., Altman, D. G., Barbour, V., Macdonald, H., Johnston, M., Lamb, S. E., Dixon-Woods, M., McCulloch, P., Wyatt, J. C., Chan, A.-W., & Michie, S. (2014). Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. British Medical Journal (BMJ), 348(mar07 3), g1687–g1687. https://doi.org/10.1136/bmj.g1687
- Howard, D., Swinburn, K., & Porter, G. (2004) Comprehensive Aphasia Test. Routledge.
- Huang, J., Qin, X., Shen, M., & Huang, Y. (2020). An overview of systematic reviews and meta-analyses on acupuncture for post-stroke aphasia. European Journal of Integrative Medicine, 37, 101133. https://doi.org/10.1016/j.eujim.2020.101133
- Husak, R., Wallace, S. E., Marshall, R. C., & Visch-Brink, E. G. (2021). A systematic review of aphasia therapy provided in the early period of post-stroke recovery. Aphasiology, 1–34. https://doi.org/10.1080/02687038.2021.1987381
- Kertesz, A. (1982). Western Aphasia Battery. Grune & Stratton.
- Kertesz, A. (2007). Western Aphasia Battery-Revised. The Psychological Corporation.
- Kesav, P., Vrinda, S., Sukumaran, S., Sarma, P., & Sylaja, P. (2017). Effectiveness of speech language therapy either alone or with add-on computer-based language therapy software (Malayalam version) for early post-stroke aphasia: A feasibility study. Journal of the Neurological Sciences, 380, 137–141.
- Kim H. H., & Na D. L. (2001). Paradise Korean version the Western Aphasia Battery. Paradise Welfare Foundation.
- Kiran, S., & Thompson, C. (2019). Neuroplasticity of language networks in aphasia: Advances, updates, and future challenges. Frontiers in Neurology, 10, 295–295.
- Kirmess, M., & Maher, L. (2010). Constraint induced language therapy in early aphasia rehabilitation. Aphasiology, 24(6–8), 725–736.
- Krasny-Pacini, A., & Evans, J. (2018). Single-case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Annals of Physical and Rehabilitation Medicine, 61(3), 164–179. https://doi.org/10.1016/j.rehab.2017.12.002
- Kristensen, L.F., Steensig, I., Pedersen, A.D., Pedersen, A.R., & Nielsen, J. F. (2015). Constraint-induced aphasia therapy in subacute neurorehabilitation. Aphasiology, 29(10), 1152–1163.
- Kristinsson, S., Basilakos, A., Elm, J., Spell, L. A., Bonilha, L., Rorden, C., den Ouden, D. B., Cassarly, C., Sen, S., Hillis, A., Hickok, G., & Fridriksson, J. (2021). Individualized response to semantic versus phonological aphasia therapies in stroke. Brain Communications, 3(3), fcab174–fcab174. https://doi.org/10.1093/braincomms/fcab174
- Laganaro, M., Di Pietro, M., & Schnider, A. (2006). Computerised treatment of anomia in acute aphasia: Treatment intensity and training size. Neuropsychological Rehabilitation, 16(6), 630–640.
- Lim, K.-B., Kim,Y.-K., Lee, H.-J., Yoo, J., Hwang, J.Y., Kim, J.-A., & Kim, S.-K. (2013). The therapeutic effect of neurologic music therapy and speech language therapy in post-stroke aphasic patients. Annals of Rehabilitation Medicine, 37(4), 556–562. https://doi.org/10.5535/arm.2013.37.4.556
- Liu, M., Qian, Q., Wang, W., Chen, L., Wang, L., Zhou, Y., Xu, S., Wu, J., Feng, T., Zhu, Z., & Xiang, J. (2022). Improvement in language function in patients with aphasia using computer‐assisted executive function training: A controlled clinical trial. PM & R, 14(8), 913–921. https://doi.org/10.1002/pmrj.12679
- Lomas, J., Pickard, L., Bester, S., Elbard, H., Finlayson, A., & Zoghaib, C. (1989). The communicative effectiveness index: Development and psychometric evaluation of a functional communication measure for adult aphasia. The Journal of Speech and Hearing Disorders, 54(1),113–24. doi: 10.1044/jshd.5401.113. PMID: 2464719.
- Luzzatti, C., Willmes, K., & De Bleser, R. (1996). L’Aachener Aphasie Test (AAT), Versione Italiana. Manuale e dati normative (2nd ed). Organizzazioni Speciali.
- Lynch, E., Mackintosh, S., Luker, J., & Hillier, S. (2019). Access to rehabilitation for patients with stroke in Australia. Medical Journal of Australia, 210(1), 21–26. https://doi.org/10.5694/mja2.12034
- Maher C.G., Sherrington C, Herbert R.D., Moseley A.M., Elkins M. (2003). Reliability of the PEDro Scale for rating quality of randomized controlled trials. Physical Therapy, 83(8),713–721.
- McCullough, K. C., McCullough, G. H., Ruark, J. L., & Rainey, J. (2006). Pragmatic performance and functional communication in adults with aphasia. The Journal of Speech and Language Pathology, Applied Behavior Analysis, 1(2), 164–178. https://doi.org/10.1037/h0100193
- Mead, G. E., Hsieh, C.-F., Lee, R., Kutlubaev, M., Claxton, A., Hankey, G. J., & Hackett, M. (2013). Selective serotonin reuptake inhibitors for stroke recovery: A systematic review and meta-analysis. Stroke, 44(3), 844–850. https://doi.org/10.1161/STROKEAHA.112.673947
- Menahemi-Falkov, M., Breitenstein, C., Pierce, J. E., Hill, A. J., O’Halloran, R., & Rose, M. L. (2021). A systematic review of maintenance following intensive therapy programs in chronic post-stroke aphasia: Importance of individual response analysis. Disability and Rehabilitation, 44(20), 5811–5826. https://doi.org/10.1080/09638288.2021.1955303
- Mitchell, A. J., Sheth, B., Gill, J., Yadegarfar, M., Stubbs, B., Yadegarfar, M., & Meader, N. (2017). Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. General hospital psychiatry, 47, 48–60. https://doi.org/10.1016/j.genhosppsych.2017.04.001
- Mitchell, C., Gittins, M., Tyson, S., Vail, A., Conroy, P., Paley, L., & Bowen, A. (2021). Prevalence of aphasia and dysarthria among inpatient stroke survivors: Describing the population, therapy provision and outcomes on discharge. Aphasiology, 35(7), 950–960. https://doi.org/10.1080/02687038.2020.1759772
- Munn, Z., Barker, T., Moola, S., Tufanaru, C., Stern, C., McArthur, A., Stephenson, M., & Aromataris, E. (2020). Methodological quality of case series studies, an introduction to the JBI critical appraisal tool. JBI Evidence Synthesis, 18(10), 2127–2133. https://doi.org/10.11124/JBISRIR-D-19-00099
- Murray, L., & Holland, A. (1995). The language recovery of acutely aphasic patients receiving different therapy regimens. Aphasiology, 9(4), 397–405.
- National Health and Medical Research Council (NHMRC). (2009). NHMRC levels of evidence and grades for recommendations for guideline developers. Canberra, Australia.
- National Institute for Health and Care Excellence (NICE). (2019). Stroke rehabilitation in adults clinical guideline. https://www.nice.org.uk/guidance/cg162/resources/stroke-rehabilitation-in-adults-pdf-35109688408261
- Nicholas, L. E., & Brookshire, R. H. (1995). Presence, completeness, and accuracy of main concepts in the connected speech of non-brain-damaged adults and adults with aphasia. Journal of Speech and Hearing research, 38(1), 145–156. https://doi.org/10.1044/jshr.3801.145
- Nouwens, F., de Lau, L. M., Visch-Brink, E. G., van de Sandt-Koenderman, W., Lingsma, H. F., Goosen, S., Blom, D. M., Koudstaal, P. J., & Dippel, D. W. (2017). Efficacy of early cognitive-linguistic treatment for aphasia due to stroke: A randomised controlled trial (Rotterdam Aphasia Therapy Study-3). European Stroke Journal, 2(2), 126–136. https://doi.org/10.1177/2396987317698327
- Ouzzani, M., Hammady, H., Fedorowicz, Z., & Elmagarmid, A. (2016). Rayyan a web and mobile app for systematic reviews. Systematic Reviews, 5(1), 210–210. https://doi.org/10.1186/s13643-016-0384-4
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Online), 372, n71–n71. https://doi.org/10.1136/bmj.n71
- Pedersen, P. M., & Vinter, K. (2001). Western Aphasia Battery (Danish version). Psykologisk Forlag.
- Pedersen, P. M., Vinter, K., & Olsen, T. S. (2001). The communicative effectiveness index: Psychometric properties of a Danish adaptation. Aphasiology, 15, 787–802. doi:10.1080/02687040143000195
- Perdices, M., Tate, R. L., & Rosenkoetter, U. (2019). An algorithm to evaluate methodological rigor and risk of bias in single-case studies. Behavior Modification, 145445519863035. https://doi.org/10.1177/0145445519863035
- Physiotherapy Evidence Database (PEDro) scale. (2020). Accessed at https://pedro.org.au/wp-content/uploads/PEDro_scale.pdf
- Physiotherapy Evidence Database (PEDro). (2022). PEDro Statistics. https://pedro.org.au/english/learn/pedro-statistics/
- Pierce, J. E., Menahemi-Falkov, M., O’Halloran, R., Togher, L., & Rose, M. L. (2019). Constraint and multimodal approaches to therapy for chronic aphasia: A systematic review and meta-analysis. Neuropsychological Rehabilitation, 29(7), 1005–1041. https://doi.org/10.1080/09602011.2017.1365730
- Pulvermüller, F., Neininger, B., Elbert, T., Mohr, B., Rockstroh, B., Koebbel, P., & Taub, E. (2001). Constraint-induced therapy of chronic aphasia after stroke. Stroke, 32(7), 1621–1626. https://doi.org/10.1161/01.STR.32.7.1621
- Rabin, R., Oemar, M., Oppe, M., Janssen, B., & Herdman, M. (2011). EQ-5D-3L User guide: Basic information on how to use the EQ-5D-3L Instrument. EuroQol Group.
- Ren, C., Zhang, G., Xu, X., Hao, J., Fang, H., Chen, P., Li, Z., Ji, Y., Cai, Q., & Gao, F. (2019). The effect of rTMS over the different targets on language recovery in stroke patients with global aphasia: A randomized sham-controlled study. BioMed Research International, 4589056–4589057. https://doi.org/10.1155/2019/4589056
- Review Manager Web (RevMan Web). (2020). Version (5.4.1). The Cochrane Collaboration. https://revman.cochrane.org/
- Rose, M., Nickels, L., Copland, D., Togher, L., Godecke, E., Meinzer, M., Rai, T., Cadilhac, D. A., Kim, J., Hurley, M., Foster, A., Carragher, M., Wilcox, C., Pierce, J. E., & Steel, G. (2022). Results of the COMPARE trial of constraint-induced or multimodality aphasia therapy compared with usual care in chronic post-stroke aphasia. Journal of Neurology, Neurosurgery and Psychiatry. 93(6), 573–581. https://doi.org/10.1136/jnnp-2021-328422
- Spaccavento, S., Cafforio, E., Cellamare, F., Colucci, A., Di Palma, A., Falcone, R., Craca, A., Loverre, A., Nardulli, R., & Glueckauf, R. L. (2018). Italian adaptation of the functional outcome questionnaire – aphasia: Initial psychometric evaluation. Disability and Rehabilitation, 40(24), 2925–2930. https://doi.org/10.1080/09638288.2017.1362042
- Spaccavento, S., Falcone, R., Cellamare, F., Picciola, E., & Glueckauf, R. L. (2021). Effects of computer-based therapy versus therapist-mediated therapy in stroke-related aphasia: Pilot non-inferiority study. Journal of Communication Disorders, 94, 106158. https://doi.org/10.1016/j.jcomdis.2021.106158
- speechBITE. (2022). Speech Pathology Database for Best Interventions and Treatment Efficacy. https://speechbite.com/
- Spreen, O., & Risser, A. H. (2003). Assessment of Aphasia. Oxford University Press.
- Stroke Foundation. (2022). Clinical Guidelines for Stroke Management. Melbourne Australia. https://informme.org.au/guidelines/living-clinical-guidelines-for-stroke-management#
- Swinburn, K., Porter, G., & Howard, D. (2004). Comprehensive Aphasia Test. Psychology Press.
- Tate R. L., Rosenkoetter U., Wakim D., Sigmundsdottir L., Doubleday J., Togher L., McDonald S., Perdices M. (2015). The Risk of Bias in N-of-1 Trials (RoBiNT) Scale: An expanded manual for the critical appraisal of single-case reports. Author.
- Tate, R., Perdices, M., Rosenkoetter, U., Wakim, D., Godbee, K., Togher, L. & McDonald, S. (2013). Revision of a method quality rating scale for single-case experimental designs and n-of-1 trials: The 15-item Risk of Bias in N-of-1 Trials (RoBiNT) Scale. Neuropsychological Rehabilitation, 23(5), 619–638. https://doi.org/10.1080/09602011.2013.824383
- Thayabaranathan, T., Baker, C., Andrew, N., Stolwyk, R., Thrift, A., Carter, H., Moss, K., Kim, J., Wallace, S., Brogan, E., Grimley, N., Lannin, M., & Rose, D. (2022). Exploring dimensions of quality-of-life in survivors of stroke with communication disabilities – a brief report. Topics in Stroke Rehabilitation, 30(6), 603–609. https://doi.org/10.1080/10749357.2022.2095087
- Thompson, C., & Shapiro, L. (2005). Treating agrammatic aphasia within a linguistic framework: Treatment of underlying forms. Aphasiology, 19(10–11), 1021–1036. https://doi.org/10.1080/02687030544000227
- Tufanaru, C., Munn, Z., Aromataris, E., Campbell, J., & Hopp, L. (2020). Systematic reviews of effectiveness. In E. Aromataris, & Z. Munn (Eds.). JBI Manual for Evidence Synthesis. From https://synthesismanual.jbi.global
- van de Sandt-Koenderman, M., Mendez Orellana, C., Meulen, I., Smits, M., & Ribbers, G. (2018). Language lateralisation after melodic intonation therapy: An fMRI study in subacute and chronic aphasia. Aphasiology, 32(7), 765–783. https://doi.org/10.1080/02687038.2016.1240353
- Verna, A., Davidson, B., & Rose, T. (2009). Speech-language pathology services for people with aphasia: A survey of current practice in Australia. International Journal of Speech Language Pathology, 11(3), 191–205. https://doi.org/10.1080/17549500902726059
- Wallace, S. J., Isaacs, M., Ali, M., & Brady, M. C. (2022). Establishing reporting standards for participant characteristics in post-stroke aphasia research: An international e-Delphi exercise and consensus meeting. Clinical Rehabilitation, 37(2), 199–214. https://doi.org/10.1177/02692155221131241
- Wallace, S. J., Worrall, L., Rose, T., Le Dorze, G., Breitenstein, C., Hilari, K., Babbitt, E., Bose, A., Brady, M., Cherney, L. R., Copland, D., Cruice, M., Enderby, P., Hersh, D., Howe, T., Kelly, H., Kiran, S., Laska, A.-C., Marshall, J., … Webster, J. (2019). A core outcome set for aphasia treatment research: The ROMA consensus statement. International Journal of Stroke, 14(2), 180–185. https://doi.org/10.1177/1747493018806200
- Wenke, R., Cardell, E., Lawrie, M., & Gunning, D. (2018). Communication and well-being outcomes of a hybrid service delivery model of intensive impairment-based treatment for aphasia in the hospital setting: A pilot study. Disability and Rehabilitation, 40(13), 1532–1541.
- Woldag, H., Voigt, N., Bley, M., & Hummelsheim, H. (2017). Constraint-induced aphasia therapy in the acute stage: what is the key factor for efficacy? A randomized controlled study. Neurorehabilitation and Neural Repair, 31(1), 72–80.
- World Health Organisation (WHO). (2012). WHOQOL User Manual, Division of Mental Health and Prevention of Substance Abuse. https://www.who.int/tools/whoqol
- Worrall, L., Sherratt, S., Rogers, P., Howe, T., Hersh, D., Ferguson, A., & Davidson, B. (2011). What people with aphasia want: Their goals according to the ICF. Aphasiology, 25(3), 309–322. https://doi.org/10.1080/02687038.2010.508530
- Worrall, L. E., Hudson, K., Khan, A., Ryan, B., & Simmons-Mackie, N. (2016). Determinants of living well with aphasia in the first year poststroke: A prospective cohort study. Archives of Physical Medicine and Rehabilitation, 98(2), 235–240. https://doi.org/10.1016/j.apmr.2016.06.020
- Zhang, X-Y., Yu, W.-Y., Teng, W.-J., Lu, M.-Y., Wu, X.-L., Yang, Y.-Q., Chen, C., Liu, L.-X., Liu, S.-H., & Li, J.-J (2021). Effectiveness of melodic intonation therapy in chinese mandarin on non-fluent aphasia in patients after stroke: A randomized control trial Frontiers in Neuroscience, 15, 648724–648724. https://doi.org/10.3389/fnins.2021.648724