Abstract
We report a case of a 75-year-old patient with hypopituitarism, bitemporal visual field deficits and a parasellar mass on pituitary MRI. During surgery, suspicion was raised that a non-functioning pituitary adenoma was accompanied by an abutting diaphragm sellae meningioma, which was confirmed at pathological examination. In retrospect, the initial MRI suggested two separate tumours on the basis of differing densities but this distinction was not seen on the last preoperative MRI.
Introduction
Meningiomas and pituitary tumours are the two most prevalent benign tumours of the central nervous system (meningiomas: 35.9%, pituitary tumours: 15.5%)Citation1 but neither are common and without a history of radiotherapy their concomitant occurrence is extremely rare.Citation2 We describe a patient with a large sellar tumour and bitemporal hemianopsia. During surgery, two different types of tissue were recognized, which were pathologically diagnosed as an infradiafragmatic sellar pituitary non-functional adenoma with an abutting tuberculum sellae meningioma. During the sinonasal part of surgery a third tumour was identified and removed, which at pathologic examination showed a glomangiopericytoma. Surgical strategy and video are presented. In addition, a literature review of the coincidence of these tumours was conducted. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Case report
Clinical history and laboratory findings
A 75 year old woman without a history of prior malignancy or irradiation presented with symptoms of depression, fatigue and unintended weight loss. In the diagnostic work-up deficiencies of cortisol, TSH, LH and FSH, and a slightly elevated prolactin (55.5 µmol/L, normal range: 5.0–23.0 µmol/L) were found. The patient was treated with thyroxin and cortisol. Ophthalmologic examination showed homonymous temporal visual field deficits suggestive for chiasm compression indicating an MRI.
Imaging
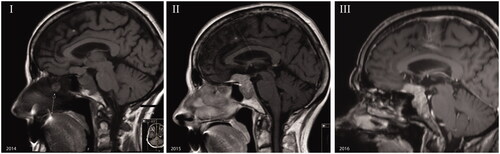
The MRI showed a sellar lesion extending into the sphenoidal sinus and a suprasellar lesion with a bilateral dural tail (). The sellar lesion showed right cavernous sinus invasion with encasement of the internal carotid artery. The pituitary stalk was deviated to the left. The optic chiasm was compressed and displaced cranially. The differential diagnosis consisted of meningioma, pituitary adenoma or a combination of both. The latter suggestion was based on the inhomogeneous densities of the sellar and suprasellar components of the tumour. A second preoperative MRI did not show this difference in density.
Surgical approach
The goal of surgery was to decompress the chiasm to restore visual fields, or at least halt further deterioration. Because of the suprasellar extension of the tumour, an extended endoscopic transplanum approach was chosen. Mucosal incisions for a right sided pedicled septal mucoperichondrium flap (HBF) were made.Citation3 In this process an incidentally found granulomatous anomaly on the right septum was radically resected and submitted for pathological examination. Bony resection of the sella wall and tuberculum and part of the sphenoidal planum was performed. The sellar dura was opened and typical pituitary adenoma tissue could be removed. Parts encasing the right internal carotid artery were intentionally left in place because of unwarranted risks. The pituitary gland could be identified on the left side. Even though adequate decompression of the sellar tumour was performed, the sellar diaphragm did not descend. Subsequentely the dura of the planum was opened and CSF drained. The suprasellar part of the tumour was notably firmer, more difficult to resect and completely separated from the sellar tumour by the diaphragm sellae. After internal tumour reduction with the CUSA the residual tumour could be removed leaving the arachnoid layer separating it from the optic nerves and chiasm intact…
Reconstruction of the skull base was performed with fat, inlay fascia lata and the HBF. All tissues were submitted separately for pathological examination. Surgical video of critical parts of the surgery is available online (Supplementary video 1).
Pathology findings
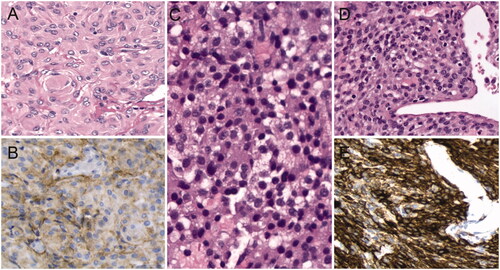
At pathological examination the septal tumour proved to be a sinonasal glomangiopericytoma. The pathologic examination of the sellar tissue showed a non-functioning adenoma of the pituitary gland. The suprasellar tumour proved to be a meningothelial meningioma ().
Postoperative outcomes
Initially, the patient suffered from headaches and vomiting, which was attributed to a pneumocephalus. These symptoms resolved spontaneous in the following days. The patient was discharged on postoperative day 7 in good clinical condition. The postoperative course was complicated by severe epistaxis due to bleeding from the left posterior branch of the sphenopalatine artery on day 12, for which she needed to be readmitted and surgically treated. During follow-up by the ophthalmologist her visual fields had improved, with no deficits attributable to chiasm compression. MRI 6 months and 1 year postoperative showed a small remnant in the right cavernous sinus. During follow-up the corticotropic axis restored and hydrocortisone could be discontinued.
Discussion
The diagnosis of an adjacent pituitary tumour and meningioma is not easy to make based on only a MRI. Even when the MRI suggests multiple tumours, identifying characteristics may change over time. In our case the only indicator for multiple tumours was the radiologic difference density between the sellar and suprasellar component on the initial MRI. This difference disappeared during follow-up. In our case not diagnosing the two tumours as separate entities did not result in a different treatment strategy. The extended endoscopic transsphenoidal approach is the standard procedure in our hospital for large suprasellar pituitary adenomas with a more anterior extension, as well as for tuberculum sellae meningiomas.
The endocrinological and visual symptoms are most likely caused by the pituitary adenoma and the meningioma, respectively. It is unlikely that the meningioma when solitary would have had intrasellar extension and cause hypopituitarism. Because the patient presented mainly with endocrinological, i.e. depression, fatigue and unintended weight loss, and not with visual complaints, it is improbable that the pituitary adenoma would have been able to grow suprasellarly and compress the optic chiasm before diagnosis.
In our literature search we found a total of 49 patients with both a meningioma and a pituitary tumour without a history of irradiation.Citation4–40 A preference for parasellar localization was found with 22 cases,Citation4–6,Citation10,Citation13,Citation14,Citation16,Citation22,Citation25–27,Citation29,Citation31,Citation33–36,Citation38–40 of which in 13 cases the tumours were contigious.Citation6,Citation10,Citation14,Citation22,Citation26,Citation27,Citation31,Citation33,Citation35,Citation38–40 In only three a distinction had been made between the tumours preoperatively.Citation26,Citation27,Citation32 When a meningioma or a pituitary adenoma becomes symptomatic and a MRI is made it is possible that an incidental meningioma or pituitary adenoma is seen. Because meningiomas can become symptomatic when parasellar due to chiasm compression, this could explain the higher prevalence of discovered co-occurrences of these meningiomas with pituitary adenomas. One may expect that with the advances in the quality of medical imaging the occurrence of two contiguous tumours mimicking one tumour would decrease. However, 5 of the 10 cases occurred in the last five years.Citation22,Citation26,Citation35,Citation40 Our literature review will be an underrepresentation of the real prevalence. During a retrospective study concerning pituitary adenomas seven other patients were found that were diagnosed with a pituitary adenoma and a concomitant meningioma in our hospital during the last seven years. Therefore, concomitant pituitary adenomas and menigiomas may be less rare than assumed.
Though pituitary tumours and meningiomas don’t have a common etiologic origin, they seem to coincide more frequently than chance would allow. Radiotherapy for pituitary adenomas has been accepted as a cause for meningiomas.Citation2 A relation between GH-producing adenomas and meningiomas has been suggested.Citation9,Citation15 Of all pituitary adenomas 8.5%–16.5% are GH-secreting.Citation34 In literature we indeed see a higher proportion of GH-producing adenomas with concomitant meningioma (30.6%).Citation4,Citation5,Citation9–11,Citation17,Citation20,Citation21,Citation24,Citation29,Citation30,Citation32,Citation35,Citation40 The mechanism through which the GH-adenomas would possibly influence the meningiomas is unclear, but 75% of meningiomas do express GH and IGF-I receptors.Citation41 Until now no overlapping genetic factors have been discovered in pituitary adenomas and meningiomas.Citation42,Citation43
Glomangiopericytomas are rare perivascular myoid tumours accounting for 0.5% of sinonasal neoplasms.Citation44,Citation45 The occurrence of a glomangiopericytoma together with a pituitary adenoma or a meningioma has not yet been reported in literature. One case reported a glomangiopericytoma localised inside the cavernous sinus, mimicking a meningioma.Citation46 The closely related, but more malignant, hemangiopericytoma can occur as a meningeal tumour and is reported to occur together with pituitary adenomas twice,Citation47,Citation48 both growth hormone-secreting, and with a meningioma once.Citation49 One of the cases reported a positive staining for IGF-I receptors in the hemangiopericytoma.Citation47 As is the case with meningiomas it remains unknown if IGF-I receptors play a part in its aetiology.
Simultaneous endonasal endoscopic surgery for a pituitary adenoma and a parasellar meningioma can be challenging. Prior to our case only one case, described by Prevedello et al., has been presented in the literature in which this was successfully performed.Citation33 In all other cases we found, either a craniotomy was performed or more than one operation was necessary. Prevedello et al. performed a re-exploration; however, in retrospect this was reported to be unnecessary. In another case presented by Karsy et al. the intrasellar meningioma and pituitary adenoma were resected during one endoscopic transsphenoidal procedure. However, this microscopic fibroepithelial meningioma was not diagnosed as such preoperatively and was coincidentally diagnosed through the pathologic findings.Citation22
In the extended endoscopic transsphenoidal approach epistaxis occurs in 0–6% of the patients. Most of the times, as in our case, the focus is a branch of the sphenopalatine artery.Citation50
In conclusion we present a case with a concomitant pituitary adenoma and a meningioma, in which also a glomangiopericytoma was resected. To our best knowledge, this is the first case report in which imaging characteristics of two adjacent skull-base lesions change during follow-up.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient described in the report.
Case_report.mp4
Download MP4 Video (85 MB)Acknowledgements
We would like to thank drs. J.W. Schoones for helping us with the literature search and dr. S.G. van Duinen for his help with the pathology imaging.
Disclosure statement
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Funding
References
- Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumours diagnosed in the United States in 2008–2012. Neuro Oncol 2015;17:iv1–iv62.
- Partington MD, Davis DH. Radiation-induced meningioma after treatment for pituitary adenoma: case report and literature review. Neurosurgery 1990;26:329–31.
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. The Laryngoscope 2006;116:1882–6.
- Abs R, Parizel PM, Willems PJ, et al. The association of meningioma and pituitary adenoma: report of seven cases and review of the literature. Eur Neurol 1993;33:416–22.
- Amirjamshidi A, Mortazavi SA, Shirani M, Saeedinia S, Hanif H. ‘Coexisting pituitary adenoma and suprasellar meningioma-a coincidence or causation effect: report of two cases and review of the literature'. J Surg Case Rep 2017;2017:rjx039.
- Basu A, Brabant G, Gnanalingham KK. More than a prolactinoma. Pituitary 2010;13:87–88.
- Ben Nsir A, Khalfaoui S, Hattab N. Simultaneous occurrence of a pituitary adenoma and a foramen magnum meningioma: case report. World Neurosurg 2017;97:748.e1–e2.
- Brennan TG, Jr., Rao CV, Robinson W, Itani A. Case report. Tandem lesions: chromophobe adenoma and meningioma. J Comput Assist Tomogr 1977;1:517–20.
- Bunick EM, Mills LC, Rose LI. Association of acromegaly and meningiomas. JAMA 1978;240:1267–8.
- Cannavo S, Curto L, Fazio R, et al. Coexistence of growth hormone-secreting pituitary adenoma and intracranial meningioma: a case report and review of the literature. J Endocrinol Invest 1993;16:703–8.
- Curto L, Squadrito S, Almoto B, et al. MRI finding of simultaneous coexistence of growth hormone-secreting pituitary adenoma with intracranial meningioma and carotid artery aneurysms: report of a case. Pituitary 2007;10:299–305.
- da Costa LB, Riva-Cambrin J, Tandon A, Tymianski M, Pituitary adenoma associated with intraventricular meningioma: case report Skull base. J North Am Skull Base Soc 2007;17:347–51.
- Deen HG, Jr, Laws ER. Jr., Multiple primary brain tumours of different cell types. Neurosurgery 1981;8:20–25.
- Della Puppa A, Del Moro G, Tosatto L, et al. Co-localisation of meningioma and craniopharyngioma mimicking a single skull base tumour in an elderly patient. J Neurooncol 2011;102:167–70.
- Furtado SV, Venkatesh PK, Ghosal N, Hegde AS. Coexisting intracranial tumors with pituitary adenomas: genetic association or coincidence? J Cancer Res Ther 2010;6:221–3.
- Gorge HH, Poll W, Gers B. Para- and suprasellar meningioma coincident with a hormonally active intrasellar hypophyseal adenoma–case report. Zentralbl Neurochir 1993;54:190–6.
- Guaraldi F, Corazzini V, Gallia GL, et al. Genetic analysis in a patient presenting with meningioma and familial isolated pituitary adenoma (FIPA) reveals selective involvement of the R81X mutation of the AIP gene in the pathogenesis of the pituitary tumour. Pituitary 2012;15:61–67.
- Hainer V, Krejcik L, Pelikan J, Tvaroh F, Urbanek J. Meningioma in contact with eosinophilic adenoma in a patient with acromegaly (author's transl). Cas Lek Cesk 1978;117:829–31.
- Honegger J, Buchfelder M, Schrell U, Adams EF, Fahlbusch R. The coexistence of pituitary adenomas and meningiomas: three case reports and a review of the literature. Br J Neurosurg 1989;3:59–69.
- Hyodo A, Nose T, Maki Y, Enomoto T. Pituitary adenoma and meningioma in the same patient (author's transl) Neurochirurgia (Stuttg) 1982;25:66–67.
- Irsy G, Goth M, Slovik F, et al. Growth hormone producing pituitary adenoma and meningioma. Zentralblatt Fur Neurochirurgie 1985;46:337–43.
- Karsy M, Sonnen J, Couldwell WT. Coincident pituitary adenoma and sellar meningioma. Acta Neurochirurgica 2015;157:231–3.
- Kitamura K, Terao H, Kamano S, et al.. Primary multiple brain tumours. No To Shinkei 1965;17:109–17.
- Kumaria A, Scott IS, Robertson IJ. An unusual pituitary adenoma coexistent with bilateral meningiomas: case report. Brit J Neurosurg 2017;1–2. doi:10.1080/02688697.2017.1386283
- Laun A, Lenzen J, Hildebrandt G, Schachenmayr W. Tuberculum sellae meningioma and hypophyseal adenoma in a woman. Zentralbl Neurochir 1993;54:119–24.
- Lim AC, Cerra C, Pal P, Kearney T, Gnanalingham KK. Visual loss from a pituitary mass: collision tumours of prostatic metastasis and suprasellar meningioma. J Neurol Surg A Cent Eur Neurosurg 2013;74:e81–4.
- Lim KZ, Goldschlager T, Chandra RV, Hall J, Uren B, Pullar M. Co-occurrence of pituitary adenoma with suprasellar and olfactory groove meningiomas. Basic Clin Neurosci 2016;7:361–5.
- Love JG, Blackburn CM. Association of intracranial meningioma with pituitary adenoma; report of successfully treated case. Minn Med 1955;38:335–6.
- Maiuri F, Cappabianca P, Iaconetta G, Esposito F, Messina A. Simultaneous presentation of meningiomas with other intracranial tumours. Br J Neurosurg 2005;19:368–75.
- Mathuriya SN, Vasishta RK, Dash RJ, Kak VK. Pituitary adenoma and parasagittal meningioma: an unusual association. Neurol India 2000;48:72–74.
- O'Connell JE. Intracranial meningiomata associated with other tumours involving the central nervous system. Br J Surg 1961;48:373–83.
- Ohata K. Simultaneous occurrence of a pituitary adenoma and a falcotentorial junction meningioma. Neurol Med Chir (Tokyo) 1985;25:680–6.
- Prevedello DM, Thomas A, Gardner P, Snyderman CH, Carrau RL, Kassam AB. Endoscopic endonasal resection of a synchronous pituitary adenoma and a tuberculum sellae meningioma: technical case report. Neurosurgery 2007;60:E401.
- Probst A. Combined occurrence of cushing-syndrome. Hypophyseal adenoma and suprasellar meningeoma. Case report. Zentralbl Neurochir 1971;32:75–82.
- Ruiz-Juretschke F, Iza B, Scola-Pliego E, Poletti D, Salinero E. Coincidental pituitary adenoma and planum sphenoidale meningioma mimicking a single tumour. Endocrinol Nutr 2015;62:292–4.
- Wild KvRH. Diagnostic problems and errors in suprasellar meningiomas vol 4. Amsterdam: ExcerptaMedica; 1974.
- Yamada K, Hatayama T, Ohta M, Sakoda K, Uozumi T. Coincidental pituitary adenoma and parasellar meningioma: case report. Neurosurgery 1986;19:267–70.
- Yu-Jen Lu C-CC, Shih-Ming Jung K-CW. Synchronous pituitary adenoma and tuberculum sellae meningioma. J Chinese Oncol Soc 2008;24:269–74.
- Zentner J, Gilsbach J. Pituitary adenoma and meningioma in the same patient. Report of three cases. Eur Arch Psychiatry Neurol Sci 1989;238:144–8.
- Zhao Y, Zhang H, Lian W, et al. Collision tumours composed of meningioma and growth hormone-secreting pituitary adenoma in the sellar region: case reports and a literature review. Medicine 2017;96:e9139.
- Friend KE, Radinsky R, McCutcheon IE. Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg 1999;91:93–99.
- Galani V, Lampri E, Varouktsi A, Alexiou G, Mitselou A, Kyritsis AP. Genetic and epigenetic alterations in meningiomas. Clin Neurol Neurosurg 2017;158:119–25.
- Zhou Y, Zhang X, Klibanski A. Genetic and epigenetic mutations of tumour suppressive genes in sporadic pituitary adenoma. Mol Cellular Endocrinol 2014;386:16–33.
- Higashi K, Nakaya K, Watanabe M, et al. Glomangiopericytoma of the nasal cavity. Auris Nasus Larynx 2011;38:415–7.
- Thompson LF-SJ, Wenig BM. Borderline and low malignant potential tumours of soft tissues. Lyon: IARC Press; 2005.
- Abou Al-Shaar H, Macdonald KI, Labib MA. Glomangiopericytoma simulating an intracavernous meningioma. Surg Neurol Int 2016;7:S142–S7.
- Elias WJ, Hussaina IM, Chadduck JB, Jane JA, Laws ER, Jr, Lopes MB. Hemangiopericytoma in the setting of acromegaly. Endocr Pathol 2002;13:251–61.
- Yokota M, Tani E, Maeda Y, Morimura T, Kakudo K, Uematsu K. Acromegaly associated with suprasellar and pulmonary hemangiopericytomas. Case Report J Neurosurg 1985;62:767–71.
- Binello E, Bederson JB, Kleinman GM. Hemangiopericytoma: collision with meningioma and recurrence. Neurol Sci 2010;31:625–30.
- Cappabianca P, Cavallo LM, de Divitiis O, de Angelis M, Chiaramonte C, Solari D. Endoscopic endonasal extended approaches for the management of large pituitary adenomas. Neurosurg Clin N Am 2015;26:323–31.