Abstract
Purpose of the article: To determine whether intraoperative ventilation with pure oxygen during the last stage of surgery reduces the occurrence and volume of postoperative pneumocephalus when compared to conventional air/oxygen mixture in patients undergoing craniotomy.
Material and Methods: prospective randomized single-blinded study to compare the rate of occurrence and volume of postoperative pneumocephalus in patients undergoing craniotomy receiving intraoperative ventilation with pure oxygen (Group B) versus a conventional air/oxygen 1:1 mixture (Group A) during the last stage of surgery. This trial was registered in ClinicalTrials.gov #NCT02722928, protocol number 2015H0032.
Results: One hundred patients were randomized into group ‘A’ and group ‘B’. Seventy patients were included in the final analysis with 39 patients allocated in group ‘A’ and 31 patients in group ‘B’. Median and IQR were used for postoperative penumocephalus volume. Group A: 9.65 [3.61–23.20]; Group B: 7.06 [2.70–20.1]. Our study showed no prophylactic effect on postoperative pneumocephalus volume when using mechanical ventilation with higher oxygen concentrations than the standard FiO2 during the last stage of surgery in patients undergoing craniotomy (p = .47). No statistical difference was found in SICU LOS between groups (median 1,380 min [group A] versus 1,524 min [group B]; p = .18).
Conclusion: The use of intraoperative mechanical ventilation with pure oxygen was not associated with a prophylactic effect on the occurrence and extent of postoperative pneumocephalus in our patient setting. Published literature describing the extent of postoperative pneumocephalus is limited or highly variable among institutions.
Introduction
Pneumocephalus has been defined as a collection of air in the cranial cavity most commonly found during the radiologic examination after craniotomies.Citation1 Its onset may be spontaneous, associated with neurosurgery, craniofacial trauma, skull base tumors, and infections.Citation1–Citation3 Some of the perioperative factors contributing to the development of pneumocephalus are head positioning, intraoperative use of mannitol, nitrous oxide (N2O), hyperventilation, or continuous cerebrospinal fluid (CSF) drainage.Citation2,Citation4 Likewise, the size of the intracranial lesion, type of surgery, and surgical approach have been linked to the onset and extent of postoperative pneumocephalus. Moreover, its incidence has been reported as high as 100% after craniotomies, frontal area being the most commonly affected.Citation2,Citation4
Tension pneumocephalus is a life-threatening neurological condition that results from an extremely increased intracranial pressure (ICP) commonly associated with a large volume of intracranial air.Citation2 Two theories may explain the initiation of tension pneumocephalus. The ball-valve theory was based on the rapid accumulation of air entering across a skull defect through a dura flap resisting its spontaneous evacuation. The inverted bottle theory introduced the concept of sudden replacement of CSF by air through a fistula leading to a negative intracranial pressure and intracranial air entrapment.Citation3,Citation5–Citation7
The diagnosis of pneumocephalus is confirmed through imaging.Citation7 A computerized tomography (CT) scan is performed after craniotomies as a standard of care in the majority of medical centers in order to evaluate postoperative changes and the volume of intracranial air (if present). Reasoner et al. reported that 66% of CT scans performed after surgery showed moderate to large volume of intracranial air collection.Citation8 Likewise, Sloan et al. suggested that the volume of air accumulated may vary among patients, ranging from 6 to 280 cm3.Citation4 Patient positioning at 30° and avoiding Valsalva maneuvers are some of the conservative approaches for the treatment of postoperative pneumocephalus.Citation3,Citation6,Citation9
Recent scientific evidence supports supplemental oxygen therapy as a common practice to accelerate the resolution of pneumocephalus creating avenues for further research.Citation6 Increased inspired oxygen concentration will reduce alveolar nitrogen concentration and therefore, decrease blood nitrogen partial pressure. This change will affect nitrogen gradient between the entrapped intracranial air and blood, potentially promoting nitrogen exchange between these two compartments with subsequent faster pneumocephalus resolution. Considering the high incidence of postoperative pneumocephalus after intracranial surgery and the onset of potentially life-threatening complications such as tension pneumocephalus, we propose the following non-invasive bedside technique as a single prophylactic intervention that may significantly decrease postoperative pneumocephalus involving minimal associated risks for patients undergoing craniotomies.
Therefore, we hypothesized that intraoperative ventilation with pure O2 (100%) will have a positive prophylactic effect on occurrence and extent of postoperative pneumocephalus when compared to air/oxygen mixture with an inspired fraction of O2 (FiO2) of 60% in patients undergoing intracranial neurosurgeries. Increased intracranial air-blood nitrogen exchange during the last stages of the surgery will reduce postoperative intracranial entrapped air volume.
Material and methods
Objectives
Primary objective
To determine whether intraoperative ventilation with pure oxygen during the last stage of surgery (hemostasis and wound closure) reduces the occurrence and volume of postoperative pneumocephalus when compared to conventional air/oxygen mixture in patients undergoing craniotomy (hemispheric or posterior cranial fossa tumors and microvascular decompression)
Secondary objectives
To determine the incidence and volume of postoperative pneumocephalus in patients undergoing craniotomy in sitting and supine positions, the postoperative neurological outcomes, and intensive care unit length of stay among neurosurgical patients (posterior cranial fossa tumors, supratentorial tumors, and transsphenoidal/endoscopic procedures)
We compared the incidence and volume of postoperative pneumocephalus in patients undergoing surgery in sitting and supine positions. Furthermore, we compared postoperative neurological outcomes, and intensive care unit length of stay among neurosurgical patients (posterior cranial fossa tumors, supratentorial tumors, and transsphenoidal/endoscopic procedures)
Study design
We conducted a prospective randomized single-blinded study at The Ohio State University Wexner Medical Center enrolling patients (≥18 years) with an American Society of Anesthesiologists (ASA) physical status of II–IV undergoing neurosurgeries (supra- and infratentorial tumors such as astrocytomas and glioblastoma multiforme, and microvascular decompression) in sitting, prone, lateral, park bench, and other positions.
Sample size and statistical analysis
We estimated that a sample size of 80 patients per group would confer a greater than 80% power to detect a 20% decrease in volume in the intervention group with an alpha of 0.05 considering a mean pnemocephalus volume of 87 ml in the control group (standard deviation of 40). The sample size was estimated based on published data.Citation2 Our proposed intervention is considered harmless, being applied routinely in perioperative settings. No intervention-related adverse events were expected during the study.
Categorical variables were reported as frequencies and percentages and compared between randomization groups using Chi-square tests or Fisher’s exact tests where relevant. Continuous variables were reported as means and standard deviations or medians and interquartile ranges and compared between randomization groups using Student’s t-tests or Wilcoxon rank sum tests where relevant. Hypothesis testing was conducted at a 5% type 1 error rate (alpha = 0.05). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Randomization
The study was planned and conducted according to all the required institutional and state regulations related to clinical research. Patients were randomly distributed (random table) into two groups:
Control Group (A): received controlled ventilation throughout the surgery with a conventional air/oxygen 1:1 gas mixture (FiO2 60%)
Interventional Group (B): received controlled ventilation with a conventional air/oxygen 1:1 gas mixture from the beginning of the surgery and during the tumor removal phase. However, patients in group B were switched to controlled ventilation with 100% O2 once the tumor resection was completed and hemostasis started until extubation
Study procedures
Both groups received supplemental oxygen via nasal cannula as a standard of care during the early postoperative period. A standard CT scan was performed within 6 hours post-surgery for both groups. CT scans were assessed and interpreted by a blinded radiologist in order to diagnose the frequency and the extent of postoperative pneumocephalus (quantification). TeraRecon Intuition software version 4.4.11.265.8092 was used, retrieving the CT scan data from the system and loading it into the software program. All CT data maintained a 1 mm slice increment. The air in the skull was identified and circled using a region of interest (ROI) tool. The area defined for measurements applied in this study was within the skull in any space occupied by the brain. This excluded any air trapped between the bone from graft placement or between the skin and the skull. Once all the visually identified areas of ‘air’ were circled and then selected within the skull, a Hounsfield unit (HU) threshold tool was used to remove any excluded tissue. The setting of −150 HU or less was applied, and the selected areas were presented in 2D and 3D. Only the areas identified within the ROI accounted for the 3D image. The volume was calculated in cm3 using the software presented by clicking the ‘volume’ measurement tool.
Considering similarity of the groups and randomization, it was expected that the duration of the last part of surgery and the elapsing time from the end of surgery to postoperative CT acquisition to be the same for the control and treatment groups. The following data was considered for intergroup analysis and selection bias exclusion: demographics (age, gender, weight, and body mass index), oxygen inhalation time, the period of time elapsed from the end of surgery to CT scan completion. The occurrence of adverse events and neurological status were assessed before the CT scan and compared with the baseline evaluation. Stage 1 was defined as the interval of time (minutes) measured from skin incision to the onset of hemostasis (i.e. after tumor removal). Stage 2 was defined as the interval of time (minutes) measured from the onset of hemostasis to incision closure. We conducted a study intervention (for group B) during stage 2.
Results
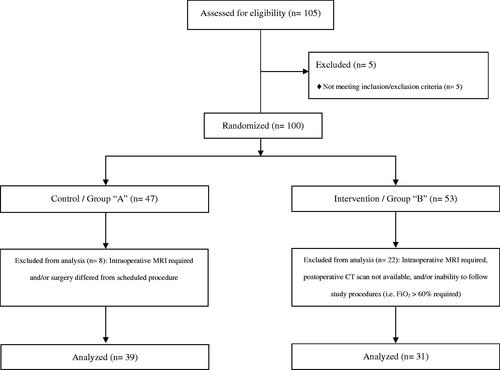
Consolidated Standards of Reporting Trials (CONSORT) 2010 was used in order to show our clinical trial flow-diagram ().Citation10 After eligibility was confirmed, 105 patients (n = 105) were enrolled in this study. Five patients (n = 5) required intraoperative magnetic resonance imaging (MRI) being transported to the MRI unit under mechanical ventilation using FiO2 of 100%; they did not qualify for randomization and were excluded from the analysis.
A total of 100 patients (n = 100) were randomized into group A and group B. However, 30 patients (n = 30) were excluded after randomization due to several reasons including protocol deviations (i.e. postoperative computerized tomography [CT] scan was not performed), or intraoperative MRI requirement. Therefore, only 70 patients (n = 70) were included in the final analysis with 39 patients (n = 39) allocated in group A and 31 patients (n = 31) in group B. Both groups were similar in regard to their demographic variables and perioperative diagnosis ().
Table 1. Demographic and clinical variables.
We estimated that our intervention would produce a 20% decrease in pneumocephalus volume for the interventional group. However, after enrolling 105 (n = 105) patients, an unanticipated blinded analysis showed that the variability relative to the mean pneumocephalus volume was much higher than initially assumed based on published data. Based on this observation, we requested the Data Safety Monitoring Committee (including an independent statistician) to carry out a blinded analysis. Therefore, it was determined that our study was underpowered, requiring an unfeasible number of patients to enroll (n > 400 patients/group) in order to detect a significant difference between groups.
The most common procedure performed in both groups was conventional anterior fossa surgery. Supine position was used in more than half of surgeries for both groups, and most of the patients were admitted to the surgical ICU (SICU) postoperatively ().
Table 2. Perioperative variables.
The following data in cm3 (median and IQR) was documented for postoperative pneumocephalus. A median of 9.65 [3.61–23.20] was reported for the control group in comparison with 7.06 [2.70–20.1] for the interventional group (p = .47) (). When considering conventional anterior fossa surgery, the following values (cm3) were reported for postoperative pneumocephalus in control group (A) and intervention group (B): 17.40 [6.89–24.40] and 13.95 [4.82–22.15] respectively (p = .47); for conventional posterior fossa surgery: 4.38 [0.81–10.50] and 4.00 [0.60–13.00] respectively (p = .87) (). Data collected from other types of surgeries such as minimally invasive procedures or conventional middle fossa surgery were not significant to allow further analysis.
Table 3. Postoperative pneumocephalus volume in cm3 (median and IQR) according to procedure performed.
SICU length of stay (LOS) was compared and no significant differences were found between groups among the subset of patients undergoing anterior fossa surgery and the subset of patients undergoing posterior fossa surgery respectively ().
Table 4. SICU LOS according to procedure performed (median min [IQR]).
No changes in neurological outcomes were reported at Postoperative Day 3 (POD3) when compared to baseline. No statistical differences were found between groups when considering neurological improvement or deterioration from preoperative findings ().
Table 5. Changes in neurological outcomes at POD 3 compared to preoperative evaluation.
Discussion
Our prospective randomized study is the first to examine the prophylactic effect of normobaric hyperoxygenation on limiting the extension of postoperative pneumocephalus volume in patients undergoing elective neurosurgery. A total of 70 patients were included in our final analysis. The study did not show pneumocephalus volume reduction when higher oxygen concentration (FiO2 100% vs 60%) was used prophylactically during the last stage of surgery in patients undergoing craniotomy (p = .47).
Postoperative pneumocephalus is usually asymptomatic with complete resolution within 2 to 3 weeks from onset. Nevertheless, symptoms such as a persistent headache, nausea, vomiting, and neurological deterioration may be present in some patients.Citation2,Citation4,Citation6,Citation9 Several conservative approaches such as avoiding Valsalva maneuvers, pain control, and preventing hyperthermia have been associated with the reduction of the extent of postoperative pneumocephalus.Citation9 The use of high oxygen concentrations during invasive and non-invasive ventilation has been commonly included among these conservative interventions.
Hong et al. reported faster resorption of postoperative pneumocephalus volume in 22 patients treated with normobaric hyperoxia after neurosurgery. Patients received mechanical ventilation at high-oxygen concentrations during 3 hours after surgery. The authors compared the volume of intracranial air performing two CT scans at a different time point after surgery.Citation2 It is relevant to mention that our study did not capture the pneumocephalus volume changes within 24 hours diagnosed by a second CT scan. Postoperative faster resorption of pneumocephalus has been explained by different theories, one of the most relevant proposing that nitrogen is replaced by oxygen.Citation11 Gore et al. observed a significant pneumocephalus reduction (65% vs 31%) in 6 neurosurgical patients receiving pure oxygen (100%) through a non-breather mask vs room air (21%) during 24 hours after neurosurgery.Citation12
Our intraoperative ventilation with pure oxygen was performed during stage 2 of the surgery. When comparing the length of stage 2 between groups, no statistical significance was found (median 49 min and 55 min respectively; p = .36). Recently, Siegel et al. reported the efficacy of administering high-flow oxygen (30 L/min) through a nasal cannula (HFNC) for the treatment of postoperative pneumocephalus.Citation6 After extubation, our patients received supplemental oxygen (3 L/min) through a conventional nasal cannula during the lapsing time between the end of the surgery and the postoperative head CT scan with no statistical differences between group A and B (median 89 min and 80 min respectively; p = .82).
Paiva et al. reported a decreased in hospital LOS and better clinical and radiological pneumocephalus outcome in patients receiving hyperbaric oxygen versus normobaric oxygen. The authors included mostly patients with traumatic brain injury and concluded that pneumocephalus may persist even after normobaric hyperoxygenation, with an increased risk of central nervous system (CNS) infections and hospital stay.Citation3 We found no statistical differences in SICU LOS between groups (median 1,380 min [group A] vs 1,524 min [group B]; p = .18). These results are consistent with data comparing patients undergoing conventional anterior fossa versus posterior fossa surgery (p = .20 and p = .79 respectively).
Siegel et al. postulated improvement of pneumocephalus is linked to improvement in neurological outcomes.Citation6 In our study, 25 control group patients (64.1%) and 15 intervention group patients (48.4%) showed no changes in neurological outcomes at POD 3 when compared to baseline. Clinical neurological improvement was reported in 8 control group patients (20.5%) and 6 intervention group patients (19.3%). Additionally, neurological deterioration was documented in 6 control group patients (15.4%) and 10 intervention patients (32.3%) (p = .23).
Regardless of postoperative neurological improvement, airline travel should be carefully considered in patients who recently underwent intracranial and certain spine surgical procedures. Changes in airplane cabin atmospheric pressure may significantly affect the extension of pneumocephalus by increasing intracranial air volume.Citation13 Even though no neurological deterioration has been reported under these conditions, the Aerospace Medical Association recommends performing a lateral skull radiograph or a CT scan before traveling in order to rule out the presence of postoperative intracranial air. If images are unavailable, patients are advised to hold any air transportation for at least 7 days after the surgery.Citation14
Limitations
Were the difference observed between groups to be repeated in a larger study, >400 subjects per group would be required in order to reach significance. The sample size calculations were based on published data and resulted in the trial being under-powered.
Additionally, other limitations merit comment:
Our study design did not incorporate a second postoperative CT scan to evaluate the changes in pneumocephalus volume during the immediate postoperative period
Most of our patients (98.6%) were extubated in the operatory room after surgery and supplemental oxygen (3 L/min) was delivered through a nasal cannula without the possibility to receive pure oxygen for a longer period of time
Conclusion
The use of intraoperative mechanical ventilation with pure oxygen was not associated with a prophylactic effect on the occurrence and extent of postoperative pneumocephalus in our patient setting. Published literature describing the extent of postoperative pneumocephalus is limited or highly variable among institutions. Our study results confirmed that small tumor size and early surgical resection are associated with a decreased postoperative pneumocephalus volume.
Disclosure statement
The authors report no conflict of interest.
Data availability
De-identified data will be provided by the corresponding author upon request. This data remains encrypted in our research files in the Department of Anesthesiology at The Ohio State University Wexner Medical Center.
References
- Beppu T, Ogasawara K, Ogawa A. Alleviation of intracranial air using carbon dioxide gas during intraventricular tumor resection. Clin Neurol Neurosurg 2006;108:655–60.
- Hong B, Biertz F, Raab P, et al. Normobaric hyperoxia for treatment of pneumocephalus after posterior fossa surgery in the semisitting position: a prospective randomized controlled trial. PloS One 2015;10:e0125710.
- Paiva WS, De Andrade AF, Figueiredo EG, Amorim RL, Prudente M, Teixeira MJ. Effects of hyperbaric oxygenation therapy on symptomatic pneumocephalus. Ther Clin Risk Manage 2014;10:769.
- Sloan T. The incidence, volume, absorption, and timing of supratentorial pneumocephalus during posterior fossa neurosurgery conducted in the sitting position. J Neurosurg Anesthesiol 2010;22:59–66.
- Schirmer CM, Heilman CB, Bhardwaj A. Pneumocephalus: case illustrations and review. Neurocrit Care 2010;13:152–8.
- Siegel JL, Hampton K, Rabinstein AA, Mclaughlin D, Diaz-Gomez JL. Oxygen therapy with high-flow nasal cannula as an effective treatment for perioperative pneumocephalus: case illustrations and pathophysiological review. Neurocrit Care 2018;29:366–73.
- Coughlan F, Lam A, Honeybul S. Tension pneumocephalus following bilateral craniotomies. Cureus 2017;9:e1358.
- Reasoner DK, Todd MM, Scamman FL, Warner DS. The incidence of pneumocephalus after supratentorial craniotomy. Observations on the disappearance of intracranial air. Anesthesiology 1994;80:1008–12.
- Dabdoub CB, Salas G, Silveira EDN, Dabdoub CF. Review of the management of pneumocephalus. Surg Neurol Int 2015;6:155.
- Begg C, Cho, M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. Jama 1996;276:637–9.
- Dexter F, Reasoner DK. Theoretical assessment of Normobaric oxygen therapy to treat pneumocephalus recommendations for dose and duration of treatment. Anesthesiol: The J Am Soc of Anesthesiol 1996;84:442–7.
- Gore PA, Maan H, Chang S, Pitt AM, Spetzler RF, Nakaji P, 2008. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg 2008;108:926–9.
- Huh J. Barotrauma-induced pneumocephalus experienced by a high risk patient after commercial air travel. J Korean Neurosurg Soc 2013;54:142.
- Aerospace Medical Association Medical Guidelines Task Force. Medical guidelines for airline travel. Aviation, Space, and Environmental Medicine 2003;74:A1.