Abstract
Background
Hyponatraemia is a common complication of aneurysmal subarachnoid haemorrhage (SAH). We aimed to determine current neurosurgical practice for the identification, investigation and management of hyponatraemia after SAH.
Methods
An online questionnaire was completed by UK and Irish neurosurgical trainees and consultant collaborators in the Sodium after Subarachnoid Haemorrhage (SaSH) audit.
Results
Between August 2019 and June 2020, 43 responses were received from 31 of 32 UK and Ireland adult neurosurgical units (NSUs). All units reported routine measurement of serum sodium either daily or every other day. Most NSUs reported routine investigation of hyponatraemia after SAH with paired serum and urinary osmolalities (94%), urinary sodium (84%), daily fluid balance (84%), but few measured glucose (19%), morning cortisol (13%), or performed a short Synacthen test (3%). Management of hyponatraemia was variable, with units reporting use of oral sodium supplementation (77%), fluid restriction (58%), hypertonic saline (55%), and fludrocortisone (19%).
Conclusions
Reported assessment of serum sodium after SAH was consistent between units, whereas management of hyponatraemia varied. This may reflect the lack of a specific evidence-base to inform practice.
Background
Subarachnoid haemorrhage (SAH) affects 6–12 persons per 100,000 population in the UK each year.Citation1 Hyponatraemia is found in 19–56% of patients with SAH,Citation2–4 and is often attributed to the syndrome of inappropriate antidiuretic hormone (SIADH), cerebral salt wasting syndrome (CSW) or vomiting.Citation4–6 Hyponatraemia due to SIADH may be compounded by high volume intravenous isotonic fluid supplementation administered to reduce risk of, or to treat, delayed cerebral ischemia where sodium and water excretion are not in equilibrium.Citation7 Hyponatraemia is reported to be associated with increased risk of seizures, length of hospital stay, mortality, and poor functional outcome.Citation6,Citation8–12
Guidelines for the investigation and management of hyponatraemia lack specific detail necessary for their application to hyponatraemia after SAH.Citation3 Guidance on the management of hyponatraemia is available from a collaboration between the European Society of Endocrinology, European Society of Intensive Care Medicine, European Renal Association-European Dialysis and Transplant Association (ESE) – however, its applicability to SAH patients is unclear.Citation13 The European Stroke Organisation (ESO) guidance on the management of SAH advises monitoring of urea and electrolytes at least every other day after SAH, maintaining daily fluid intake at 3L per day initially and targeting ‘normovolaemia’ in cases of hyponatraemia.Citation14 This is in contrast to the ESE who advocate investigation to ascertain the cause for hyponatraemia, and fluid restriction should findings be consistent with a diagnosis of SIADH.
We conducted a survey of practice at adult neurosurgical units from the UK and Ireland that participated in the Sodium after Subarachnoid Haemorrhage (SaSH) audit. We aimed to assess current practice for the identification, investigation and management of hyponatraemia after SAH.
Methods
Design and sampling strategy
An online 10 question questionnaire (Supplementary material) was completed by at least one registrar or consultant grade representative from every adult neurosurgical centre that participated in the Sodium after Subarachnoid Haemorrhage (SaSH) audit.
Data collection
Data were collected between August 2019 and April 2020 using the Google Forms online questionnaire.Citation15 Collected data were stored securely online, protected by password and only viewable by the steering committee.
Analysis
Data was exported to Microsoft Excel (v16.37). Where multiple respondents from the same unit reported incongruent data, all respondents were contacted by email to attain consensus. Units that reported having protocols for investigation and/or for management were analysed in comparison to those without. Analysis was conducted blind to both unit and respondent.
Results
A total of 43 responses were received representing 31 of the 32 (97%) neurosurgical units in the UK and Ireland. Among the seven units that had more than one respondent, six returned incongruent responses to at least one survey question. All of these units were able to achieve consensus for all questions. Units with multiple respondents reported a spread of responses which was similar to that of units with one respondent (). Four units reported having protocols for monitoring, investigation and management of hyponatraemia after SAH. Four other units reported having protocols for monitoring and investigation but not management and five had protocols for management only.
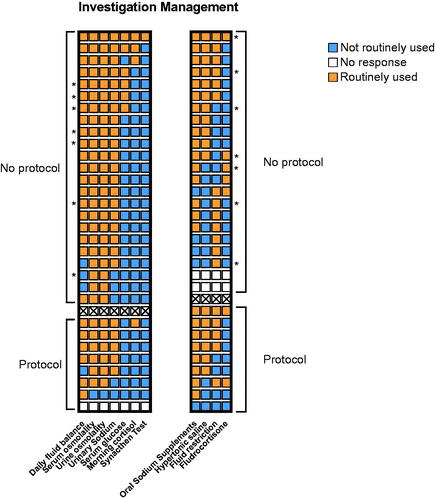
Figure 1. Heat map of reported investigation and management strategies for hyponatraemia after SAH. Respondents consistently reported the use of a core set of investigations used in patients with SAH. Management strategies were less consistent. Each row represents one neurosurgical unit. Blank boxes represent no response to this question. * Denotes consensus responses from units with >1 respondent. Units reporting protocols are displayed separate from those with no protocol.
Monitoring and investigation
All units monitored serum sodium after SAH every one to two days. In patients with hyponatraemia, all units measured serum sodium at least daily (). Investigation of hyponatraemia was reported to be triggered by serum sodium values below 135mmol/L for more than one day in 22 (71%) units (). Lower serum sodium values or greater durations would trigger investigation in a higher proportion of respondents ().
Table 1. Monitoring and threshold for investigation of hyponatraemia.
To investigate hyponatraemia after SAH 26 (84%) units report measuring daily fluid balance, 29 (94%) units routinely performed paired serum and urine osmolality, 26 (84%) units measured urinary sodium, 6 (19%) measured serum glucose, 4 (13%) measured morning cortisol levels, and 1 (3%) performed a short Synacthen test.
Reported practices for monitoring and investigation were not different between units with and without a protocol (; ).
Management
Management of hyponatraemia after SAH was less consistent between units, with 24 (77%) reporting routine use of oral sodium supplements, 18 (58%) reporting use of fluid restriction, 17 (55%) using hypertonic saline and 6 (19%) fludrocortisone (). Sixteen units (52%) routinely sought advice from an endocrinologist, and in 11 (35%) units this included clinical review. No difference in reported management strategy was observed between units with and without protocols ().
Discussion
Our questionnaire has demonstrated consistent strategies for monitoring of serum sodium and investigation of hyponatraemia after SAH across the UK and Ireland. The threshold for investigation of hyponatraemia varied across units with all units investigating a value of 130mmol/L for more than one day but only 35% investigating a value of 130–135mmol/L for one day. The reported routine management of hyponatraemia after SAH was variable, particularly for fluid restriction (58%) and hypertonic saline (55%). Use of a protocol was not associated with any particular investigation or management practice.
The variation in management reported by respondents to our questionnaire is consistent with that reported by other studies of diagnosis and management of hyponatraemia after SAH.Citation16–18 Many of these studies have been observational, small, retrospective, at risk of bias, and used variable outcome measures which has hindered meta-analysis.Citation19
Guidelines
ESO guidelines for SAH recommend monitoring serum sodium at least every other day.Citation14 All respondents reported practices consistent with this in patients with or without hyponatraemia. European Society of Endocrinology (ESE) guidelines recommend serum glucose as the first investigation to exclude non-hypotonic hyponatraemia.Citation13 Hyperglycaemia occurs in approximately 30% of patients following SAH and is an independent predictor of poor prognosis.Citation20,Citation21 Few respondents reported routine measurement of serum glucose in our survey, perhaps because serum osmolality was used to exclude non-hypotonic hyponatraemia. In cases of hypotonic hyponatraemia with normal urinary osmolalities, and urinary sodium consistent with euvolaemia, morning cortisol or short Synacthen tests are recommended to assess hypothalamus-pituitary-adrenal axis function. Few respondents to our survey reported the routine measurement of morning cortisol. Cortisol measurement may be important SAH where hyponatraemia may indicate an inadequate hypothalamic-pituitary adrenal (HPA)-mediated stress response. Synacthen testing may be less appropriate following SAH as primary adrenal insufficiency is less likely to be the cause of HPA axis dysfunction. ESE guidance also suggests routine measurement of thyroid function, although hyponatraemia would only be observed in severe hypothyroidism and the results of these investigations may be difficult to interpret following SAH.Citation13 In general, investigation to rule out hypopituitarism as a cause of hyponatraemia after SAH is reasonable as hypopituitarism has been reported between 0–55% of patients after SAH.Citation22
In our survey, the most frequently reported management option for routine correction of hyponatraemia was oral sodium supplementation. This is included in the ESE guidelines for management of SIADH.Citation13 ESE’s first-line treatment for SIADH is fluid restriction, which was cited by 60% of respondents as a management option in their practice. For CSW, ESE guidelines recommended fluid resucitation.Citation13 ESO guidance for management of SAH recommends that normovolaemia be targeted in cases of hyponatraemia after SAH but provides no detailed recommendations on management according to aetiology or risk of vasospasm.Citation14 These recommendations, in addition to our questionnaire findings of variation in management strategies, may reflect uncertainty in differentiating aetiologies of hyponatraemia following SAH and thus the optimal fluid management strategy for patients with hyponatraemia after SAH. Fluid restriction is an important management option for SIADH within general medical patients without severe or acute features of hyponatraemia. However, the use of fluid restriction to manage hyponatraemia following SAH has been associated with increased risk of cerebral ischaemia.Citation16,Citation23 Consequently, intravenous volume expansion is widely used to treat and prevent delayed cerebral ischaemia after SAH, although efficacy has not yet been demonstrated.Citation24,Citation25
Our study has the advantage of a high response rate with representation from 31 of 32 (97%) adult neurosurgical units in the UK and Ireland. We did not assess intra-unit variability, but asked units to give a consensus of their usual practice and it is possible that there is within unit as well as between unit variability in investigation and management. Because not all units in our study provided multiple respondents, it is likely that there is variation in practice in single respondent units which is unaccounted for. Nonetheless, it is reassuring that a similar spread of responses was reported by both units with multiple respondents and those with single responses. Our study is limited by assessing reported practice rather than actual practice, but a national audit is underway to address that. Use of a protocol for investigation and management of sodium after SAH was not associated with any particular investigation or management strategy. However, we did not define what a formal protocol entails, and it may be that units follow standard practice for monitoring and investigation sodium following SAH without a formal written protocol. Our survey directly enquired about common tests and management options, leaving blank space text entry options for other approaches. Few units utilised these blank space sections and it is possible that further investigative or management options were not entered. For example, we did not directly enquire regarding the use of loop diuretics.
Conclusions
NSUs consistently reported measurement of serum sodium every one to two days after SAH. On diagnosis of hyponatraemia most NSUs report that they would undertake measurement of daily fluid balance, urine and serum osmolality and urinary sodium. Serum glucose, cortisol and Synacthen testing were consistently not used. Management practices were more variable particularly with regards to use of hypertonic saline and fluid restriction. Investigation and management of hyponatraemia after SAH may therefore not adhere to general guidelines for investigation of hyponatraemia, which may be appropriate in this setting. Further investigation of management strategies for hyponatraemia after SAH and correlation with clinical outcomes is needed to establish the evidence base and specific guideline development. We are addressing this through the Sodium after Subarachnoid Haemorrhage (SaSH) audit, which is a prospective national audit that will measure actual practice and provide a better understanding current practice and its relationship with patient outcomes.
ibjn_a_1859460_sm0194.docx
Download MS Word (16.2 KB)Disclosure statement
The study authors comprise the steering committee for the Sodium after Subarachnoid Haemorrhage audit.
Additional information
Funding
References
- Luoma A, Reddy U. Acute management of aneurysmal subarachnoid haemorrhage. Contin Educ Anaesth Crit Care Pain 2013;13:52–8.
- Qureshi AI, Suri MFK, Sung GY, et al. Prognostic significance of hypernatremia and hyponatremia among patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2002;50:749–55. discussion 755–6.
- Sherlock M, O'Sullivan E, Agha A, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol 2006;64:250–4.
- Chandy D, Sy R, Aronow WS, et al. Hyponatremia and cerebrovascular spasm in aneurysmal subarachnoid hemorrhage. Neurol India 2006;54:273–5.
- Rahman M, Friedman WA. Hyponatremia in neurosurgical patients: clinical guidelines development. Neurosurgery 2009;65:925–36.
- Mapa B, Taylor BES, Appelboom G, et al. Impact of hyponatremia on morbidity, mortality, and complications after aneurysmal subarachnoid hemorrhage: a systematic review. World Neurosurg 2016;85:305–14.
- Musch W, Decaux G. Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM 1998;91:749–53.
- Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med 2016;129:823–35.e4.
- Al Mawed S, Pankratz VS, Chong K, et al. Low serum sodium levels at hospital admission: outcomes among 2.3 million hospitalized patients. PLoS One 2018;13:e0194379.
- Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009;122:857–65.
- Berardi R, Caramanti M, Castagnani M, et al. Hyponatremia is a predictor of hospital length and cost of stay and outcome in cancer patients. Support Care Cancer 2015;23:3095–101.
- Glenn TC, Patel AB, Martin NA, et al. Subarachnoid hemorrhage induces dynamic changes in regional cerebral metabolism in rats. J Neurotrauma 2002;19:449–66.
- Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014;170:G1–G47.
- Steiner T, Juvela S, Unterberg A, European Stroke Organization, et al. European Stroke Organization Guidelines for the Management of Intracranial Aneurysms and Subarachnoid Haemorrhage. Cerebrovasc Dis 2013;35:93–112.
- Google LLC. Google Forms. https://docs.google.com/forms/u/0/. 2020.
- Vermeij FH, Hasan D, Bijvoet HWC, Avezaat CJJ. Impact of medical treatment on the outcome of patients after aneurysmal subarachnoid hemorrhage. Stroke 1998;29:924–30.
- Wijdicks EF, Vermeulen M, ten Haaf JA, et al. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol 1985;18:211–6.
- Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 2006;34:617–23. quiz 624.
- Shah K, Turgeon RD, Gooderham PA, Ensom MHH. Prevention and treatment of hyponatremia in patients with subarachnoid hemorrhage: a systematic review. World Neurosurg 2018;109:222–9.
- Juvela S, Siironen J, Kuhmonen J. Hyperglycemia, excess weight, and history of hypertension as risk factors for poor outcome and cerebral infarction after aneurysmal subarachnoid hemorrhage. J Neurosurg 2005;102:998–1003.
- Lanzino G, Kassell NF, Germanson T, Truskowski L, Alves W. Plasma glucose levels and outcome after aneurysmal subarachnoid hemorrhage. J. Neurosurg 1993;79:885–91.
- Khajeh L, Blijdorp K, Neggers SJ, et al. Hypopituitarism after subarachnoid haemorrhage, do we know enough? BMC Neurol 2014;14:205.
- Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol 1985;17:137–40.
- Loan JJM, Wiggins AN, Brennan PM. Medically induced hypertension, hypervolaemia and haemodilution for the treatment and prophylaxis of vasospasm following aneurysmal subarachnoid haemorrhage: systematic review. Br J Neurosurg 2018;32:157–64.
- Velly LJ, Bilotta F, Fàbregas N, Soehle M, Bruder NJ, Nathanson MH. Anaesthetic and ICU management of aneurysmal subarachnoid haemorrhage. Eur J Anaesthesiol 2015;32:168–76.
