Abstract
Background
The lateral supraorbital approach (LSO) provides an optimal access corridor for various skull bases lesions, including olfactory groove meningiomas (OGMs). The aim of this study is to describe the authors’ experience with the management of large and giant OGMs utilizing the LSO approach and describe the technical nuances of the procedure.
Methods
A retrospective review of seven patients with large and giant OGMs managed with the LSO approach between 2013 and 2019 was performed. Radiographic and clinical data were recorded and analyzed.
Results
Seven patients with large and giant OGMs underwent surgical resection via the LSO approach. Six patients were female, with a median age of 56 years. Patients commonly presented with altered mentation, anosmia, and headaches. The average tumor volume was 120.6 ± 64.7 cm3 with five cases of vascular encasement. Simpson grade II resection was achieved in four patients while Simpson grade IV resection was achieved in three patients. The median length of stay was 2.0 days. The median preoperative Karnofsky Performance Scale (KPS) score was 70, improving to 100 at last postoperative follow-up visit. Two complications were encountered in the form of postoperative cerebrospinal fluid leak in one patient and a transient diplopia in another patient. Tumor recurrence/progression was identified in two patients during a median follow-up time of 65.5 months. Both cases have been managed with adjuvant radiosurgery.
Conclusion
The LSO approach is a safe and effective minimally invasive transcranial corridor for the management of OGMs that should be part of the armamentarium of skull base neurosurgeons.
Introduction
Olfactory groove meningiomas (OGMs), arise in the anterior cranial fossa (ACF), extending from the cribriform plate to planum sphenoidale, and account for approximately 10% of intracranial meningiomas.Citation1,Citation2 These tumors may present insidiously depending on their size and impingement on adjacent neurovascular structures. Patient may present with anosmia, visual deficits, mental disturbances, headache, and occasionally seizures.Citation1,Citation3,Citation4 Transcranial approaches for OGMs include bicoronal approaches or unilateral frontal, pterional, and lateral supraorbital (LSO) approaches.Citation1,Citation3,Citation5 With advancements in surgical techniques and endoscopic instrumentations, the endoscopic endonasal approach (EEA), has been increasingly reported in the management of these lesions.Citation6 Both endonasal and transcranial approaches, however, offer distinct advantages and inherent limitations. However, the superiority of one approach over the other remains controversial.Citation2,Citation5,Citation7–10
With the increased utilization of endonasal techniques for ACF pathologies, the use of transcranial approaches has significantly decreased. However, such transcranial approaches remain instrumental in the armamentarium of skull base neurosurgeons. The LSO approach is a minimally invasive technique commonly employed for intracranial aneurysms and selected anterior cranial fossa tumors.Citation3,Citation11–13 Its utility in the management of OGM and other ACF tumors is scarcely reported in the literature.Citation10,Citation11,Citation13,Citation14 The LSO approach is a minimally invasive technique associated with high efficacy rate, low complication profile, and good aesthetic outcomes. In this study, we retrospectively reviewed our single center experience with large and giant OGMs managed through the LSO approach. We also discuss the surgical nuances in the management of these lesions.
Methods
Patient selection
With the approval of the Institutional Review Board at the University of Pittsburgh Medical Center, we retrospectively reviewed the medical records of all patients who underwent surgical resection via the LSO approach for large and giant OGMs between October 2013 and October 2019 by the senior author (R.F.S). The diagnosis of OGM was based on clinical history, physical examination, gadolinium-enhanced magnetic resonance imaging (MRI), and pathological confirmation. All cases were large or giant OGMs, defined as tumor diameter of >3 cm or tumor volume of >10 cm3 for large, and tumor diameter >6 cm for giant OGMs. Patients were excluded if they were <18 years of age or lacked complete clinical and radiographic data. The records were identified by detailed review of clinical records. A manual chart review was performed to verify patients’ inclusion and to obtain clinical variables. The following variables were collected from the patients’ medical records: age, gender, clinical presentation, tumor volume, WHO grade, extent of resection using Simpson grade,Citation15 length of stay (LOS), follow-up duration, adjuvant therapy, and complications. Continuous variables are reported as the means ± standard deviations, whereas percentages were used for categorical variables.
Preoperative evaluation
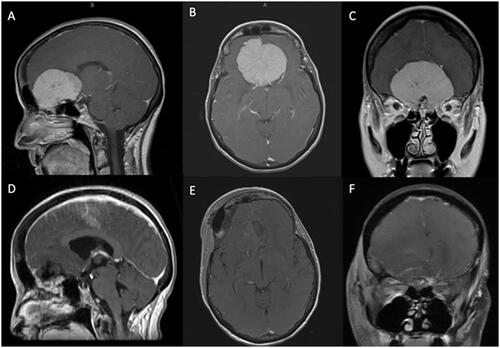
To facilitate surgical planning, all patients underwent preoperative computed tomography (CT) and magnetic resonance imaging (MRI) with contrast () to evaluate tumor size, lateralization, extension, involvement (i.e. encasement or impingement) of the surrounding neurovascular structures, and edema. Edema was assessed on preoperative fluid-attenuated inversion recovery (FLAIR) MRI. Extent of resection and tumor recurrence/progression were evaluated via postoperative and surveillance MRIs.
Figure 1. Preoperative sagittal (A), axial (B), and coronal (C) T1-weighted MRI with gadolinium depicting a large OGM with mass effect on the surrounding frontal lobes. The patient underwent a right LSO approach with Simpson grade II resection of the tumor as demonstrated on the postoperative sagittal (D), axial (E), and coronal (F) T1-weighted postcontrast MRI.
Surgical technique
The patient is placed under general anesthesia, intubated and positioned supine, with the head of the bed elevated at 20 degrees. The head is placed in pins, rotated 30 degrees away from the laterality of the tumor, and connected to a Mayfield (Integra LifeSciences, Billerica, MA) head-holder. A fronto-temporal or an eyebrow incision is planned (i.e. eyebrow incision is favored in patients for which the incision may be largely hidden by their eyebrows), and the site prepped and draped in the usual fashion. Neuromonitoring using somatosensory evoked potentials are recorded and monitored, and neuronavigation is registered and utilized throughout the procedure. An eyebrow or frontotemporal incision is made, and the orbital rim and zygoma are exposed. An oculoplastic surgeon was not utilized for any of the operations. Meticulous hemostasis is achieved. A burr hole caudal to the superior temporal line in the keyhole is made, and a 2 × 3 cm supraorbital craniectomy is created rostral to the orbital rim. The dura is pealed from the ACF floor, and the orbital roof is thinned to an egg-shell rim using a high-speed drill under microscopic magnification. Care is taken to not violate the periorbita.
The dura is opened in a C-shape fashion, reflected back, and rubber dams stacked on cotton patties are placed over the frontal lobe to facilitate egress of cerebrospinal fluid (CSF). Fixed retractors are avoided.Citation16,Citation17 The optico-carotid cistern is opened to permit CSF egress and brain relaxation. Tumor debulking is begun internally, and, over time, a plane is developed between the tumor and surrounding brain parenchyma. Ultrasonic aspiration, bipolar cautery, and suction are used to resect the tumor in a piecemeal fashion. This technique allows for easy identification and preservation of the olfactory tract once the majority of the tumor is resected. The tumor capsule is removed once sufficient debulking is completed. Once maximal safe tumor resection is achieved, the remainder of ACF dural attachments are cauterized with care to prevent injury to the optic nerves and surrounding structures. Removal of tumor involving the ethmoid sinuses is deliberately avoided to prevent postoperative CSF leak. Meticulous hemostasis is achieved. The dura is reapproximated primarily in a watertight fashion with 4-0 Surgilon braided nylon (Surgilon, Medtronic Inc., Dublin, Ireland) sutures. Cranioplasty with calcium phosphate cement is performed as previously described by the senior author.Citation2,Citation18 The wound is then closed in a multilayered fashion.
Follow-up
All patients were followed-up after discharge at 1 and 3 months, and thereafter for long-term follow-up at 1- to 2-year intervals to monitor for tumor recurrence/progression. Surveillance brain MRIs with and without contrast were obtained to evaluate for tumor recurrence/progression yearly in patients with Simpson grade II resection and biyearly in patients with higher Simpson grades. Tumor recurrence/progression was defined as radiographic evidence of new tumor growth or progression of residual tumor on follow-up MRI or contrasted CT scan. Time to recurrence/progression was defined as the interval between surgical resection and the first sign of recurrence/progression on imaging.
Results
Study population
During the study period, a total of seven patients with large and giant OGMs who underwent minimally invasive LSO approach by the senior author (R.F.S.) were included (). There were 6 females and 1 male with a median age of 56 ± 13.7 years. None of the patients had a prior craniotomy, endonasal resection, or radiation treatment.
Table 1. Patients and tumor characteristics.
The most frequently encountered presentations included altered mentation (i.e. blunted affect), substantial weight gain, anhedonia, headache, anosmia, and less frequently seizures, visual disturbances (including diplopia and blurry vision), syncope, and ataxia ().
Tumor characteristics
Tumor characteristics are outlined in . The mean tumor volume was 120.6 ± 64.7 cm3. Preoperative FLAIR MR images demonstrated edema in all seven patients. Vascular encasement was depicted in 5 (71.4%) patients. Histopathological evaluation revealed that 6 (85.7%) patients harbored WHO grade I, and 1 (14.3%) patient harbored WHO grade II meningioma. The mean Ki-67 proliferative index was 4.2%.
Outcomes
The mean estimated blood loss (EBL) was 1028.6 mL with a median operative time of 8.2 hours. Gross total resection (Simpson grade II) was achieved in 4 (57.1%) patients, with the remaining 3 (42.9%) having subtotal resection (Simpson grade IV) (). Residual tumor was deliberately left in 3 cases due to ethmoidal involvement, one of which (patient #2) grew from the left planum sphenoidale into the left olfactory groove, the second was located at the base of the sphenoid sinus, and the last was in the olfactory groove.
Table 2. Patients’ outcomes following LSO approach.
Postoperative improvement in patient’s clinical presentation was observed in all patients following OGM resection. Among the five patients who presented with anosmia/hyposmia, dysgeusia, or both, 4 (80.0%) patients reported, at least, partial improvement of their symptoms at the last follow-up visit.
The median hospital length of stay (LOS) was 2.4 days. Two (28.6%) patients were admitted to the intensive care unit (median ICU LOS of 1.8 days) postoperatively. All patients were discharged home following their hospitalization. The median preoperative Karnofsky Performance Scale (KPS) score was 70 and improved to 100 at last postoperative follow-up visit.
Complications
A total of two complications were encountered in the series (). The first complication involved technical errors whereby a breach of the lateral wall of the frontal sinus with the drill (i.e. error #1) underwent attempted repair with calcium phosphate cranioplasty alone (i.e. error #2). The patient returned two weeks following discharge with breakdown of the calcium phosphate cement and resultant CSF rhinorrhea and pneumocephalus, which required a reoperation. The reoperation consisted of primary repair of the dural defect, extension of the cranioplasty to cover the frontal sinus breach, and placement of a lumbar drain. The patient was subsequently discharged with no further issues. Almost 7 years later, the patient continues to do well with no neurological sequalae. The second complication involved a transient diplopia due to periorbital swelling following tumor resection, that recovered completely by the 3-month follow-up visit. No perioperative mortality was encountered in the series.
Follow-up and adjuvant therapy
All patients who underwent OGM resection were available for long-term follow-up. The median long-term follow-up duration was 65.5 months (). Tumor recurrence/progression was observed during long-term follow-up in 2 (28.6%) patients (both with WHO grade I meningiomas and had Simpson grade IV resection). The median interval between tumor resection and tumor recurrence/progression was 14.6 months. These two patients underwent adjuvant Gamma Knife® radiosurgery (GKRS). The median interval between resection and GKRS was 45.3 months. The median marginal dose of GKRS was 12.8 Gy at the 50% isodose line. Following adjuvant GKRS therapy, regression was observed in one patient, while the tumor remained stable in size in the other patient.
Table 3. Follow-up and adjuvant therapy.
Discussion
OGMs are uncommon tumors arising in the ACF of the skull base.Citation19 Meningiomas of the ACF are commonly grouped together, making, at times, distinction between OGM, planum sphenoidale, tuberculum sella, and dorsum sella meningiomas challenging. The initial description and characterization of OGMs may be credited to Cushing and Eisenhardt in their 1938 series of 29 patients treated surgically with a unilateral frontal and partial bifrontal lobectomy.Citation20 Since then, many modifications and improvements in surgical approaches and working corridors have been made to reach and resect these lesions.
The pterional, and mini pterional, approaches were commonly reported for the management of OGMs.Citation1,Citation3,Citation21–24 The advantages of the pterional and the mini-pterional approaches are their immediate access to the sylvian fissure, exposure of the frontal and temporal lobes, avoidance of the frontal sinus and preservation of the superior sagittal sinus, and early visualization of neurovascular structures.Citation3,Citation4 However, the approach provided limited tumor exposure compared to the bifrontal approach and poor preservation of olfaction postoperatively.Citation3,Citation4 Therefore, the LSO approach, initially described by Hernesniemi et al., combined the advantages of the pterional and mini-pterional approaches for rapid and direct tumor exposure.Citation3,Citation25 The LSO approach allows for a more direct access to the ACF while minimizing frontal lobe retraction and post-operative complications.Citation26,Citation27 The LSO technique described here along with inside out tumor resection allows for easy identification and preservation of the olfactory tract once the bulk of the tumor is resected. However, the only pitfall of such technique is the increased blood loss associated with piecemeal tumor resection.
Endoscopic endonasal vs. transcranial approaches
With advancements in surgical techniques, endoscopic instrumentations, and increased understanding of the endonasal skull base anatomy, the EEA has been increasingly utilized in the management of various ACF meningiomas, including OGMs.Citation7 The EEA provides access to the tumor from below by which ethmoidal-based tumors may be, at least partially, resected while minimizing brain retraction.Citation1,Citation3,Citation7,Citation28 Despite the addition of nasoseptal flap reconstruction of the cranial base, however, the EEA remains associated with high rates of CSF leak and reduced olfaction compared with transcranial approaches.Citation2,Citation29 Further, there remains a lack of high level evidence supporting the notion that EEA for ACF meningioma resection is associated with a lower incidence of postoperative seizures. Romani et al.Citation30 reported new-onset postoperative seizures in only 1 of 66 patients with OGMs of all sizes. Banu et al.Citation31 reported equivalent rates of postoperative seizures in patients who underwent OGM resection via the EEA or the supraorbital eyebrow approach. None of the patients in our series experienced postoperative seizures. This work represents expansion of our prior report on this topic.Citation2
Although EEA permits early devascularization of the tumor by achieving early access to the ethmoidal feeding arteries in most OGMsCitation32, early tumor devascularization of OGMs can also be accomplished with the LSO as clearly demonstrated by Romani et al.Citation30 In their series of 66 patients, Romani et al.Citation30 reported a mean blood loss of 750 mL in tumors larger than 6 cm. In our series of OGMs treated via the LSO approach, the average blood loss was higher (mean 1028.6 mL), the elevated blood loss in our series is likely related to the large average tumor volume of 120.6 ± 64.7 cm3 but may also by differences in technique. While Romani et al. advocate for rapid coagulation of the anterior and posterior ethmoidal arteries, we favor deliberate and prolonged internal debulking of the tumor (i.e. to minimize manipulation of brain). The olfactory function can easily be harmed by excessive retraction, manipulation, or coagulation near the vascular supply of the olfactory tract.Citation30 Due to the importance of olfaction in patients’ quality of life, attempts to preserve olfactory function should be made during transcranial surgical resection. On the contrary, the EEA, by virtue, eliminates olfaction by traversing through the olfactory fibers to access the tumors. Some authors have suggested that preservation of olfaction is more likely in small and medium-sized OGMs compared to larger OGMs.Citation1,Citation33 In our patients, we were able to preserve olfaction in all of our patients despite the large tumor size, and among the ones who presented with anosmia, they had at least partial improvement in their olfaction. Removal of tumor with an “inside out” technique helps preserve olfaction. For example, once 1/2 to 2/3 of the tumor has been removed with the inside out technique, it is much easier to identify and preserve the olfactory nerves. The downside of “inside out” resection, however, is increased blood loss throughout operation.
A recent meta-analysis by Lu et al.Citation5 suggested that olfactory dysfunction and CSF leak are more likely to occur with EEA than transcranial approaches following OGM resection, but vision is less likely to be impacted with the EEA among all anterior skull base meningiomas. It is important to point out that resection of the ethmoidal component of the tumor is feasible through the LSO approach but results in high rates of CSF leak and accompanying morbidity (i.e. meningitis, extended length of stay, deep venous thrombosis) as the case with EEA of these lesions.Citation6,Citation7 Our philosophy of leaving tumor (i.e. ethmoid sinuses) is somewhat approach specific. For example, if we chose to use a bifrontal craniotomy to expose and remove a large or giant olfactory groove meningioma with extension into the ethmoid sinuses, in that case we would use a large and competent enough pericranial flap to likely prevent a postoperative leak. With a small supraorbital craniotomy or craniectomy, however, that type of repair would be more tenuous, and CSF leak would occur in, at least some cases, so that we avoid with the LSO approach. Therefore, the risks and benefits of each these approaches should be weighted on a case-by-case basis along with surgeon expertise and comfort.
Operative time and complications
In most instances, the minimally invasive LSO approach is associated with reduced operative time compared to the pterional, bifrontal, or other transcranial skull base approaches.Citation3 The median operating time in our series, however, was 8.2 hours, which is longer than operating times reported by Romani et al.Citation3 but similar to the operative time with EEA for OGMs. This might be due to the fact that our tumors are significantly larger than the ones in Romani’s series as well as our technique of internal debulking of the tumor, which we believe is associated with lower complication rates.
In our series, we did not encounter any mortalities, which aligns with published rates between 0% and 30% in the prior series.Citation1,Citation28,Citation34–36 Surgical mortality following OGM resection has been suggested to be related to sectioning of the superior sagittal sinus and draining midline veins.Citation1,Citation3 We did not experience any intraoperative sinus injury or injury to any major veins.
Reported complications of LSO for OGM resection include CSF leak, infection, bleeding, subdural hygroma, visual disturbances, motor deficits, and seizures.Citation3,Citation34,Citation36–38 In our cohort, we encountered two complications in the form of CSF leak and transient diplopia that recovered completely on follow-up. The patient with CSF leak required another operation for dural defect repair, sinus cranialization, and lumbar drain placement. The patient tolerated the procedure well and was discharged home on postoperative day 3 in a stable condition.
Breaching the frontal sinus during LSO approach presents a significant risk for CSF leak and subsequent infection.Citation1 Prior series of OGM resection via transcranial approaches report CSF leak rates of 3–16%.Citation1,Citation3,Citation36,Citation39,Citation40 This high rate of CSF leakage in OGM surgery can result from infiltration of the tumor into the ethmoidal bone and its drilling to prevent recurrences.Citation3 Therefore, we usually elect not to enter the ethmoids for tumor removal in order to prevent such complication, and accept a higher Simpson grade resection in such instances.
Outcomes
Tumor recurrence following OGMs resection has been reported to range between 5% and 41%.Citation1,Citation3,Citation28,Citation41–43 Recurrence appears to occur more frequently when tumor consistency is less solid, ethmoidal involvement is present, or if dural insertion is not adequately resected and coagulated.Citation28,Citation41 In our experience, the recurrence/progression rate was 28.6% during a median long-term follow-up of 62.3 months, in line with previously published reports. Both cases in our series had a WHO grade I meningiomas and had Simpson grade IV resection; they were both managed with GKRS with adequate tumor control. We believe the addition of GKRS following near total or subtotal resection is associated with improved tumor control rates, as documented in the literature.Citation40
In our experience, Simpson grade II resection was achieved in 57.1% of the patients, while Simpson grade IV resection was achieved in 42.9%. Romani et al. and Schwartz et al. achieved radiographic complete tumor resection in 92% and 100% of their cases, respectively.Citation3,Citation10 However, comparisons of Simpson grade resection between the LSO approach and EEA are controversial, given that some advocates of EEA argue that Simpson grade I resection via transcranial approaches are not possible and vice versa. In our series, the median preoperative KPS score was 70, improving to 100 at last postoperative follow-up visit. In our experience, we prefer not to drill into the ethmoid bone for complete surgical resection (Simpson grade I) of these tumors, reflecting that 4 out of the 7 patients in this series achieved Simpson grade II resection. We believe that the benefits of leaving small residual in the ethmoid outweigh the risks of CSF leak associated with drilling it.
Limitations
Our study is limited by its retrospective nature, single-site perspective, small sample size, and the lack of an endoscopic endonasal group. The tumors included in the study happened to be all large and giant OGMs, and none were small in size. During the study period, the senior author did refer two asymptomatic patients with smaller tumors (i.e. and no or minimal edema) for stereotactic radiosurgery. All of the consecutive surgeries in this study were performed by a single surgeon (R.F.S). Further, the results do not reflect any differences in surgical technique or postoperative management between surgeons or institutions. Finally, olfactory outcomes were assessed subjectively through patients reports, and objective assessment of olfaction using validated scoring systems was not performed.
Conclusion
The LSO approach is a safe and effective approach for the management of properly selected and large/giant OGMs. The approach combines the distinct advantages of minimally invasive techniques while maintaining olfaction and minimizing the rate of postoperative CSF leak. Further multicentric studies are needed to delineate its efficacy and limitations in the management of these challenging lesions.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee of University of Pittsburgh Medical Center and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Informed consent
The patients provided informed consent to participate in this study.
Disclosure statement
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. The senior author has no financial relationship with the manufacturer of the implant described herein.
References
- Nakamura M, Struck M, Roser F, Vorkapic P, Samii M. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery 2007;60:844–52.
- Gandhoke GS, Pease M, Smith KJ, Sekula RF. Supraorbital versus endoscopic endonasal approaches for olfactory groove meningiomas: a cost-minimization study. World Neurosurg 2017;105:126–36.
- Romani R, Lehecka M, Gaal E, et al. Lateral supraorbital approach applied to olfactory groove meningiomas: experience with 66 consecutive patients. Neurosurgery 2009;65:39–52. discussion 52–33.
- Feng AY, Wong S, Saluja S, et al. Resection of olfactory groove meningiomas through unilateral vs. bilateral approaches: a systematic review and meta-analysis. Front Oncol 2020;10:560706.
- Lu VM, Goyal A, Rovin RA. Olfactory groove and tuberculum sellae meningioma resection by endoscopic endonasal approach versus transcranial approach: a systematic review and meta-analysis of comparative studies. Clin Neurol Neurosurg 2018;174:13–20.
- Koutourousiou M, Fernandez-Miranda JC, Wang EW, Snyderman CH, Gardner PA. Endoscopic endonasal surgery for olfactory groove meningiomas: outcomes and limitations in 50 patients. Neurosurg Focus 2014;37:E8.
- Fernandez-Miranda J, Gardner P, Prevedello D, Kassam A. Expanded endonasal approach for olfactory groove meningioma. Acta Neurochir (Wien) 2009;151:287–8.
- Gardner P, Snyderman C, Fernandez-Miranda J, Wang E. Apples and oranges: proper comparison of costs - endonasal vs. transnasal. World Neurosurg 2017;106:984–5.
- Youngerman BE, Banu MA, Gerges MM, et al. Endoscopic endonasal approach for suprasellar meningiomas: introduction of a new scoring system to predict extent of resection and assist in case selection with long-term outcome data. J Neurosurg 2020;135:1–13.
- Youngerman BE, Shtayer L, Gerges MM, Larsen AG, Tomasiewicz HC, Schwartz TH. Eyebrow supraorbital keyhole craniotomy for olfactory groove meningiomas with endoscope assistance: case series and systematic review of extent of resection, quantification of postoperative frontal lobe injury, anosmia, and recurrence. Acta Neurochir (Wien) 2021;163:101–12.
- Romani R, Laakso A, Kangasniemi M, Niemelä M, Hernesniemi J. Lateral supraorbital approach applied to tuberculum sellae meningiomas: experience with 52 consecutive patients. Neurosurgery 2012;70:1504–18. discussion 1518–1509.
- Romani R, Laakso A, Niemelä M, et al. Microsurgical principles for anterior circulation aneurysms. Acta Neurochir Suppl 2010;107:3–7.
- Romani R, Laakso A, Kangasniemi M, Lehecka M, Hernesniemi J. Lateral supraorbital approach applied to anterior clinoidal meningiomas: experience with 73 consecutive patients. Neurosurgery 2011;68:1632–47. discussion 1647.
- Romani R, Elsharkawy A, Laakso A, Kangasniemi M, Hernesniemi J. Complications of anterior clinoidectomy through lateral supraorbital approach. World Neurosurg 2012;77:698–703.
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22–39.
- Romani R, Silvasti-Lundell M, Laakso A, Tuominen H, Hernesniemi J, Niemi T. Slack brain in meningioma surgery through lateral supraorbital approach. Surg Neurol Int 2011;2:167.
- Thirumala P, Frederickson AM, Balzer J, et al. Reduction in high-frequency hearing loss following technical modifications to microvascular decompression for hemifacial spasm. J Neurosurg 2015;123:1059–64.
- Frederickson AM, Sekula RF. Jr. The utility of calcium phosphate cement in cranioplasty following retromastoid craniectomy for cranial neuralgias. Br J Neurosurg 2013;27:808–11.
- Fountas KN, Hadjigeorgiou GF, Kapsalaki EZ, Paschalis T, Rizea R, Ciurea AV. Surgical and functional outcome of olfactory groove meningiomas: lessons from the past experience and strategy development. Clin Neurol Neurosurg 2018;171:46–52.
- Cushing H, Eisenhardt L, Meningiomas, their classification, regional behaviour, life history, and surgical end results. Springfield, IL: Charles C Thomas; 1938.
- Cardali S, Romano A, Angileri FF, et al. Microsurgical anatomic features of the olfactory nerve: relevance to olfaction preservation in the pterional approach. Neurosurgery 2005;57:17–21. discussion 17–21.
- Hassler W, Zentner J. Surgical treatment of olfactory groove meningiomas using the pterional approach. Acta Neurochir Suppl (Wien) 1991;53:14–8.
- Schaller C, Rohde V, Hassler W. Microsurgical removal of olfactory groove meningiomas via the pterional approach. Skull Base Surg 1994;4:189–92.
- Turazzi S, Cristofori L, Gambin R, Bricolo A. The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery 1999;45:821–5. discussion 825–826.
- Figueiredo EG, Deshmukh P, Nakaji P, et al. The minipterional craniotomy: technical description and anatomic assessment. Neurosurgery 2007;61:256–64. discussion 264–255.
- Sekhar L, Janecka I, Munro I. Surgery of cranial base tumors. Ann Plast Surg 1993;30:572.
- Babu R, Barton A, Kasoff SS. Resection of olfactory groove meningiomas: technical note revisited. Surg Neurol 1995;44:567–72.
- Obeid F, Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery 2003;53:534–43.
- Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Oper Neurosurg 2008;63:ONS44–ONS53.
- Gerber M, Vishteh AG, Spetzler RF. Return of olfaction after gross total resection of an olfactory groove meningioma: case report. Skull Base Surg 1998;8:229–31.
- Banu MA, Mehta A, Ottenhausen M, et al. Endoscope-assisted endonasal versus supraorbital keyhole resection of olfactory groove meningiomas: comparison and combination of 2 minimally invasive approaches. J Neurosurg 2016;124:605–20.
- Beer-Furlan A, Vellutini E, Balsalobre L, Stamm AC. Endoscopic endonasal approach to ventral posterior fossa meningiomas: from case selection to surgical management. Neurosurg Clin N Am 2015;26:413–26.
- Welge-Luessen A, Temmel A, Quint C, Moll B, Wolf S, Hummel T. Olfactory function in patients with olfactory groove meningioma. J Neurol Neurosurg Psychiatry 2001;70:218–21.
- Bakay L, Cares H. Olfactory meningiomas. Acta Neurochir (Wien) 1972;26:1–12.
- Hallacq P, Moreau JJ, Fischer G, Béziat JL. Trans-sinusal frontal approach for olfactory groove meningiomas. Skull Base 2001;11:35–46.
- El Gindi S. Olfactory groove meningioma: surgical techniques and pitfalls. Surg Neurol 2000;54:415–7.
- Melamed S, Sahar A, Beller AJ. The recurrence of intracranial meningiomas. Neurochirurgia (Stuttg) 1979;22:47–51.
- Solero C, Giombini S, Morello G. Suprasellar and olfactory meningiomas. Report on a series of 153 personal cases. Acta Neurochir (Wien) 1983;67:181–94.
- Bassiouni H, Asgari S, Stolke D. Olfactory groove meningiomas: functional outcome in a series treated microsurgically. Acta Neurochir (Wien) 2007;149:109–21.
- Tuna H, Bozkurt M, Ayten M, Erdogan A, Deda H. Olfactory groove meningiomas. J Clin Neurosci 2005;12:664–8.
- Jääskeläinen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol 1986;26:461–9.
- Mathiesen T, Lindquist C, Kihlström L, Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery 1996;39:2–7; discussion 8-9.
- Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 1985;62:18–24.
