Abstract
Purpose
The degree of disability that is acceptable to patients following traumatic brain injury (TBI) continues to be debated. While the dichotomization of outcome on the Glasgow Outcome Score (GOSE) into ‘favourable’ and ‘unfavourable’ continues to guide clinical decisions, this may not reflect an individual’s subjective experience. The aim of this study is to assess how patients’ self-reported quality of life (QoL) relates to objective outcome assessments and how it compares to other debilitating neurosurgical pathologies, including subarachnoid haemorrhage (SAH) and cervical myelopathy.
Method
A retrospective analysis of over 1300 patients seen in Addenbrooke’s Hospital, Cambridge, UK with TBI, SAH and patients pre- and post- cervical surgery was performed. QoL was assessed using the SF-36 questionnaire. Kruskal-Wallis test was used to analyse the difference in SF-36 domain scores between the four unpaired patient groups. To determine how the point of dichotomization of GOSE into ‘favourable’ and ‘unfavourable’ outcome affected QOL, SF-36 scores were compared between GOSE and mRS.
Results
There was a statistically significant difference in the median Physical Component Score (PCS) and Mental Component Score (MCS) of SF-36 between the three neurosurgical pathologies. Patients with TBI and SAH scored higher on most SF-36 domains when compared with cervical myelopathy patients in the severe category. While patients with Upper Severe Disability on GOSE showed significantly higher PC and MC scores compared to GOSE 3, there was a significant degree of variability in individual responses across the groups.
Conclusion
A significant number of patients following TBI and SAH have better self-reported QOL than cervical spine patients and patients’ subjective perception and expectations following injury do not always correspond to objective disability. These results can guide discussion of treatment and outcomes with patients and families.
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity worldwide. It is associated with prolonged hospitalisation, followed by challenges in access to specialist rehabilitation and community reintegration.Citation1 Patients are often left with sequelae of chronic physical, cognitive and behavioural deficits.Citation2 Investigators and centres worldwide use metrics such as the Extended Glasgow Outcome Scale (GOSE) for prognostication and assessment of treatment success.Citation3,Citation4 However, such tools may fail to account for the widespread effects of the condition and may not capture the patients’ and families’ subjective experience of long-term consequences of the disease.
Quality of life (QOL) instruments, such as the SF-36, attempt to quantify outcomes by measuring subjective ratings of health and have been validated in numerous neurological pathologies.Citation5–11 From a clinical, patient, and health economic perspective, it is the quality of life that is key to measuring the success of treatment. Such questionnaires have the added benefit of highlighting the important areas of physical, psychological and social deficits that impact patients’ overall health and recovery. This is particularly the case for chronic conditions such as those afflicting the brain due to their wide spectrum of morbidity.Citation5
Despite the consensus that TBI leads to detrimental effects on patients’ cognitive and physical well-being, what constitutes a ‘good’ QOL outcome following TBI continues to be debated. The Glasgow Outcome Score (GOSE) is a widely used scale to determine outcomes in TBI patients. It has become a convention to dichotomise the scale into ‘favourable’ and ‘unfavourable’ outcomes to help guide assessment and clinical decisions. However, as mentioned previously, this may not reflect an individual’s experience and the cut-off for ‘favourable’ outcome (GOSE 4, upper severe disability) may fail to consider subjective QOL rating.
It is also not clear whether QOL as an outcome measure is specific to TBI patients or relates more generally to common chronic neurosurgical pathologies. When examined alongside patients with non-brain trauma, there have been mixed findings in the literature with some long-term studies indicating that there is no significant difference in the QOL between TBI and non-TBI trauma.Citation12,Citation13 This has led to the theory that health may be related not only to brain injury but also to the subjective experience of trauma.Citation14–16 This is particularly important when counselling patients and their families about interventions in the acute setting such as decompressive craniectomy, which may improve survival at the expense of additional morbidity.Citation3
Subarachnoid haemorrhage (SAH) is another form of acute brain injury that accounts for 25% of the quality-adjusted life years lost after stroke, although it is only responsible for 5% of cases.Citation17 We have therefore considered SAH as an appropriate acute brain injury comparator to TBI. Cervical Myelopathy is a common condition that is responsible for considerable morbidity with a wide spectrum of outcomes, and it provides a comparator between cerebral and spinal pathologies.
The purpose of this study was to compare the difference in QOL between three different neurosurgical pathologies: TBI, SAH and cervical myelopathy in a total population of over 1300 patients. We have then considered the point of dichotomization between favourable and unfavourable outcomes in GOSE using SF36 as a quality of life comparator between these points of dichotomization. Understanding the relationship between physical impairment and subjective health rating may help guide treatment decisions and discussions with patients and families.
Methods
Participants
Four groups of patients were analysed in this retrospective study: TBI patients at follow-up, SAH patients at follow up and patients with cervical myelopathy before and after surgery. All patients were assessed at Addenbrooke’s Hospital, Cambridge, outpatient clinic 4-6 months following discharge and in the pre-admission clinic prior to surgery in the cervical spine pathology group. Consent was gained prior to administering tests of physical function and subjective health status. Patients scheduled for cervical surgery were first time operations and included anterior cervical discectomy (ACD) and cervical laminectomy. Only those 16 years or older were included in the final analysis. Data was stored in an approved electronic database as part of the Eastern Region Neurotrauma and Neurosurgery Audit. Descriptive statistics were used to summarise the demographic data. Statistical analysis was performed in SPSS 29.0 (IBM SPSS, Chicago, IL, USA) and graphs were generated in GraphPad Prism 9.4.1 (GraphPad Software, La Jolla, California, USA). Statistical significance was set at p < 0.05.
Assessment of QOL following TBI, SAH and cervical myelopathy
The SF-36 questionnaire was used to assess health-related QOL in TBI, SAH and cervical myelopathy patients. The test consists of 36 multiple choice questions that are grouped into eight domains: PF, physical functioning; RP, role limitation due to physical problems; BP, bodily pain; RE, role limitations due to emotional problems; VT, vitality; GH, general health perception; MH, mental health; SF, social functioning. The domain scores were calculated by transforming the raw data into a scale of 0–100 using Likert’s method of unweighted summed ratings.Citation18,Citation19 On the scale, the higher scores indicate better subjective health. Two summary scores, physical component summary (PCS) and mental component summary (MCS), were derived by taking unweighted means of the corresponding domains. The test has been validated in TBI patientsCitation20 and those with cervical spondylotic radiculomyelopathy.Citation21–23 Although no study has specifically assessed the contrast validity of SF-36 in SAH patients, it has been validated in stroke patients.Citation24
Disease-specific metrics included the Glasgow Coma Scale (GCS) score for TBI patients, World Federation of Neurosurgical Societies (WFNS) grading system for SAH, and Myelopathy Disability Index (MDI) for spinal surgery patients. Despite several criticisms of WFNS as a prognostication tool, it remains the gold standard during clinical assessment.Citation25 MDI is a commonly used questionnaire in cervical spine surgery with high reliability and sensitivity.Citation26,Citation27 It is the preferred functional outcome tool in the UK and is used for the British Spine Registry [https://www.britishspineregistry.com/].
To assess how QOL compared across the four groups, the disease-specific scores were first categorised into mild, moderate and severe. GCS was used to classify TBI into mild (13-15), moderate (9-12) and severe (3-8) categories.Citation28 Mild SAH was defined as a WFNS score of 1-2, moderate 3 and severe 4-5Citation29 and MDI was stratified into mild (<5), moderate (5-8), and severe (>8).Citation30 Kruskal-Wallis test was used to analyse the difference in the SF-36 domain scores across the stratified, unpaired groups and Dunn’s test was used for posthoc pairwise comparison.
Dichotomization of functional outcome following TBI and SAH
Functional outcome in the TBI group was assessed using GOSE, an eight-scale global measure,Citation31,Citation32 and included only patients who scored 3 or higher since the completion of SF-36 requires adequate communication skills. Disability following SAH was assessed using modified Rankin Score (mRS), a commonly used instrument validated in patients with stroke.Citation33 The conventional dichotomization of GOSE into ‘favourable’ (GOSE 5-8) and ‘unfavourable’(GOSE 1-4) has recently been challenged by the RESCUEicp trial, a large multicenter randomized trial, which included GOSE 4 (upper severe disability) in the ‘favourable’ category.Citation3 To provide justification for this inclusion, SF-36 scores for SAH patients with mRS 2 and mRS 3 (scale 0-6; unfavourable outcome defined as 3-6) were compared against TBI patients with GOSE 3 and GOSE 4.
Results
Descriptive statistics
A total of 513 patients with TBI, 656 with SAH, and 223 following cervical spinal surgery were reviewed at Addenbrooke’s Hospital 4-6 months following hospital discharge. In addition, 254 patients were seen in the pre-admission clinic prior to undergoing spinal surgery. A total of 514 patients who were seen in the pre-admission clinic prior to cervical spinal surgery were excluded from the analysis due to missing information on functional or QOL scores. Out of the remaining 254 patients, 220 underwent ACD, 1 patient underwent ACD plus lumbar laminectomy and 33 patients underwent cervical laminectomy. Patients seen in the clinic post-surgery included 194 following ACD, 1 ACD plus lumbar laminectomy and 28 following cervical laminectomy. A total of 31 patients were lost to follow-up after surgery. summarises the characteristics of the four cohorts.
Table 1. Descriptive statistics for the 4 cohorts. Condition severity was determined by GCS in TBI, WFNS in SAH and MDI in pre-and post-spinal surgery patients.
depicts the frequency distributions for the stratified disease-specific metrics. The median GCS score in the TBI group was 10 (Interquartile range (IQR) 7-14). The median WFNS score in SAH patients was 1 (IQR 1). Patients prior to spinal surgery had a median MDI score of 6 (IQR 1-12) while those post-surgery had a median score of 3 (IQR 10).
Comparison of QOL scores between TBI, SAH and spinal surgery patients based on disease severity
The Physical and Mental Component Summary scores of the SF-36 QOL test were compared within the mild, moderate and severe TBI, SAH and spinal myelopathy categories.
Kruskal-Wallis H test showed that the median PCS and MCS scores were significantly different between the 4 patient groups (TBI, SAH, pre- and post-spinal surgery) except for MCS scores in the moderate disease severity group ().
Table 2. Results of the Kruskal-Wallis H test assessing the difference in SF-36 PCS and MCS scores between TBI, SAH and myelopathy pre- and post-surgery patients in 3 categories of disease severity.
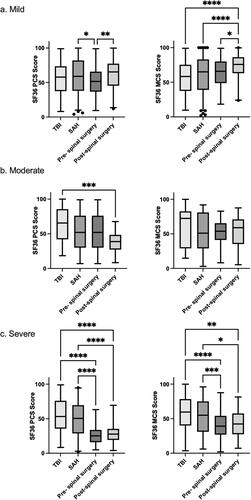
Pairwise comparisons of the groups (TBI, SAH, pre- and post-spinal surgery) within each severity category were performed using Dunn’s procedure with a Bonferroni correction for multiple comparisons. shows the median and distribution of scores for each cohort. Patients with severe TBI and SAH had significantly higher PCS and MCS scores relative to myelopathy patients (p < 0.05), indicating that despite significant brain trauma, this population have a higher self-reported QOL.
Figure 2. Box and whisker plots demonstrating SF-36 PCS and MCS scores in the mild (A), moderate (B) and severe (C) category. Dunn’s test with a Bonferroni correction for multiple comparisons was used to assess for differences in the group medians. (*p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001).
Dichotomisation of outcome following TBI: Comparison with SAH
GOSE scores are conventionally dichotomized with GOSE 1-4 considered ‘unfavourable’ and 5-8 ‘favourable’. However, the RESCUEicp trial challenged this subdivision by including GOSE 4, upper severe disability (criteria: patients are independent at home for at least 8 hours), in the ‘favourable’ cohort. To explore the basis for the inclusion of GOSE 4 in the ‘favourable’ group, the SF-36 scores corresponding to the two points of dichotomisation of GOSE were compared against those of mRS. MRS is a well-established functional outcome score used for patients with SAH and is frequently dichotomised to include favourable outcomes as 0-2 and unfavorable 3-6. (N.B.: no spinal myelopathy patients were included in this comparison).
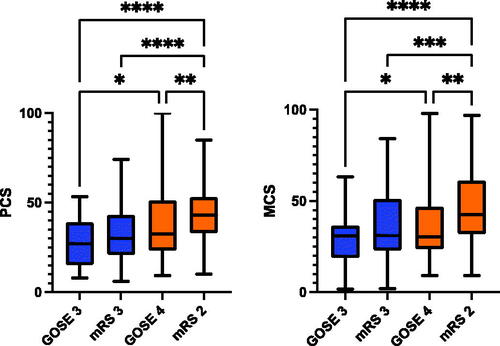
There was no statistically significant difference in the Physical and Mental Component scores between patients in the lower severe disability (GOSE 3) and those in mRS 3. Patients with upper severe disability (GOSE 4) had significantly better PC and MC scores than patients with GOSE 3. A summary of the mean SF-36 scores for each disease metric is shown in . The graphs demonstrate the degree of variability in SF-36 scores both within and across groups, highlighting that dichotomisation of scores may not be an optimal way to assess outcomes.
Figure 3. Mean SF-36 summary scores for TBI patients with upper (GOSE 4) and lower (GOSE 3) severe disability. The scores are compared to SF-36 summary scores of SAH patients with a) mRS 2 (upper end of ‘favourable’ outcome) and b) mRS 3 (lower end of ‘unfavourable’ outcome). Orange colours denote ‘favourable’ outcome while blue signify ‘unfavourable’. Significance: *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001.
Discussion
This study aimed to assess how QOL in the TBI population compares to other neurosurgical pathologies, both acute brain injury (SAH) and spinal disease (cervical myelopathy). When matched for initial disease severity, patients following TBI ranked significantly higher in the physical component score than spinal surgery patients, in moderate and severe categories. In the mental health domain, mild severity post-spinal surgery group ranked higher than TBI and SAH groups. However, in the severe category, this trend reversed, with TBI and SAH patients scoring higher than pre- and post-spinal surgery patients.
These findings suggest that a significant proportion of patients with severe TBI and SAH rate their physical and mental function as superior to those with cervical spine disease. These results are perhaps surprising, considering that psychological and cognitive sequelae are some of the most common deficits in brain injury.Citation34,Citation35 Similarly, SAH patients are well known to struggle with cognitive deficits secondary to factors such as pituitary dysfunction, stress and disturbances in their social structure.Citation36–38 These results resonate with the finding that TBI have similar physical and mental health rating to non-TBI trauma patients who are not expected to have any cognitive deficits.Citation39,Citation40
Several reasons may account for these findings. Firstly, cervical spine disease is a chronic pathology associated with complex and often poorly managed pain.Citation41,Citation42 Patients often complain of physical and psychological deficits with as many as 38% of these patients experience anxiety and another 29% depression.Citation43,Citation44 Some research indicates that patients with chronic pain suffer from poor self-efficacy (defined as perseverance and effort in difficult situations) and coping strategies leading to perpetuation of symptoms that affect their QOL.Citation45,Citation46 Even after surgery, over 60% of patients report ongoing daily pain and up to 80% report some form of neck disorders.Citation47 In addition, patients with nontraumatic spinal cord injury may not have the same access to rehabilitation services as those with traumatic etiologies, leading to reduced quality of life rating.Citation48,Citation49
TBI patients suffer from debilitating physical and mental deficits but are survivors of significant traumatic events, often with near death experience. Their expectation of outcome, and those of their family, may thus be very different. This psychological outlook may allow for better coping mechanisms and acceptance of restriction in participation. Between 15-21% of patients following TBI feel that their brain injury has not negatively impacted their life.Citation49 In addition, up to 62% of patients reported to acknowledge the anniversary of their brain injury with 1/10 marking the day as a positive event and celebration of achievement.Citation50 As previously demonstrated, subjective perception and expectations following brain injury do not always correspond to objective disability.Citation51 It is important to mention that cognitive impairment following TBI or SAH may lead to loss of insight (anosoagnosia) into personal deficits thus resulting in inflation of patient scores, particularly for those in the severe category.Citation52
In the second part of the study, the dichotomy point of GOSE was assessed by comparing it against mRS. By convention, GOSE 0-4 is considered ‘unfavourable’ outcome while 5-8 are deemed ‘favourable’.Citation53,Citation54 Patients with upper severe disability (GOSE 4) had significantly higher PC and MC scores than those with lower severe disability (GOSE 3). When compared against mRS 3, which is considered poor outcome in stroke, patients with upper severe disability (GOSE 4) had higher mental and physical domain scores on the SF-36, but these were not statistically significant. There was a large spectrum of SF-36 scores within both GOSE and mRS categories, highlighting that simple dichotomisation of outcome into favorable and unfavorable is not a valid approach to prognostication of patient reported outcome. As previously described in RESCUEicp trial, perceived outcome as favourable is highly subjective and despite severe handicap some patients have an acceptable QOL, especially at the severe end of the spectrum of injury.Citation3 This is consistent with the results previously reported by our group, which found a great degree of variability in the SF-36 scores for each GOSE category.Citation51
Several limitations were present in this study. Firstly, although each of the disease specific functional scores used in the paper have been validated for their respective pathology, comparison across diseases using different metrics may lead to incorrect pairing in functional outcome scores. Neither disease specific scores nor QOL questionnaires may appropriately capture the differences in the pathophysiology, evolution and natural history of TBI, SAH and cervical myelopathy. Thus, interpretation of SF-36 scores across 3 very different pathologies may fail to account for confounding factors that could impact QOL.
Furthermore, there are limitations in ascribing severity based on GCS and WFNS, depending on whether this is assessed before or after resuscitation. Nevertheless, these are the most commonly used tools for determining severity and pragmatically these metrics are used for stratification in both the research and clinical domains. There were also significantly fewer patients within the severe SAH and spinal groups as compared to TBI groups.
Our study included patients only seen at 4-6 months follow-up and thus results can only be interpreted as short-term QOL outcome. For instance, while TBI patients may undergo neurological recovery in the first several months following trauma, certain sequelae such as cognitive and psychiatric decline may not establish themselves until 1.5-2 years later.Citation55 Lastly, while SF-36 effectively combines several domains of mental and physical function, it is a composite global score that may fail to capture deficits in important elements such as cognition and psychology.
Conclusion
This study highlights the personal and subjective nature of patient reported outcome measures. As well as the objective level of disability quantified in the standard outcome assessments across neurosurgical pathologies (i.e., GOSE, mRS, MDI) there is a need to consider patients’ and families’ expectations of their outcome. Clinicians experienced in treating patients with neurosurgical pathologies recognise that neurological deficit is not a barrier to a good quality of life. Patients with severe TBI, and their families and carers, clearly recognise the threat to life of suffering an injury of this magnitude and may more readily accept morbidity. Conversely, many patients undergoing elective surgery, have an expectation that surgery will address the symptoms with which they present. This understanding has several important implications. Firstly, clinician judgements about what constitutes a ‘favourable’ or ‘unfavourable’ outcome have to be carefully calibrated against the expectations of the patient cohort in question. This is particularly relevant when considering withholding life saving treatment. Secondly, clinicians and researchers must recognize the inherent limitations of dichotomisation of outcomes, given that a wide overlap exists between these categories from the perspective of individual patients. Thirdly, surgeons must provide realistic expectations to their patients of what surgery can achieve and the natural history of disease is essential, particularly in the elective setting.
Consent/Ethical approval
Prospective clinical outcome data was collected across a range of neurosurgical pathologies. Data were stored in an approved electronic database together with demographic details as part of the Eastern Region Neurotrauma and Neurosurgery Audit, registered with the Trust Clinical Audit Department.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mendelow AD, Timothy J, Steers JW, et al. Management of patients with head injury. Lancet 2008;372:685–7.
- Menon DK, Schwab K, Wright DW, Maas AI, Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010;91:1637–40.
- Hutchinson PJ, Kolias AG, Timofeev IS, RESCUEicp Trial Collaborators, et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N Engl J Med 2016;375:1119–30.
- Cooper DJ, Rosenfeld JV, Murray L, Australian and New Zealand Intensive Care Society Clinical Trials Group, et al. Decompressive Craniectomy in Diffuse Traumatic Brain Injury. N Engl J Med 2011;364:1493–502.
- von Steinbuechel N, Richter S, Morawetz C, Riemsma R. Assessment of subjective health and health-related quality of life in persons with acquired or degenerative brain injury. Curr Opin Neurol 2005;18:681–91.
- Passier PECA, Visser-Meily JMA, Rinkel GJE, Lindeman E, Post MWM. Determinants of health-related quality of life after aneurysmal subarachnoid hemorrhage: a systematic review. Qual Life Res 2013;22:1027–43.
- Hop JW, Rinkel GJ, Algra A, van Gijn J. Changes in functional outcome and quality of life in patients and caregivers after aneurysmal subarachnoid hemorrhage. J Neurosurg 2001;95:957–63.
- Greebe P, Rinkel GJE, Hop JW, Visser-Meily JMA, Algra A. Functional outcome and quality of life 5 and 12.5 years after aneurysmal subarachnoid haemorrhage. J Neurol 2010;257:2059–64.
- Carlozzi NE, Tulsky DS, Kisala PA. Traumatic brain injury patient-reported outcome measure: identification of health-related quality-of-life issues relevant to individuals with traumatic brain injury. Arch Phys Med Rehabil 2011;92:S52–S60.
- Polinder S, Haagsma JA, van Klaveren D, Steyerberg EW, van Beeck EF. Health-related quality of life after TBI: a systematic review of study design, instruments, measurement properties, and outcome. Popul Health Metr 2015;13:4.
- Dijkers MP. Quality of life after traumatic brain injury: a review of research approaches and findings. Arch Phys Med Rehabil 2004;85:S21–S35.
- Ouellet M, Sirois M, Lavoie A. Perceived mental health and needs for mental health services following trauma with and without brain injury. J Rehabil Med 2009;41:179–86.
- Curran CA, Ponsford JL, Crowe S. Coping strategies and emotional outcome following traumatic brain injury: a comparison with orthopedic patients. J Head Trauma Rehabil 2000;15:1256–74.
- Teasdale TW, Christensen AL, Willmes K, et al. Subjective experience in brain-injured patients and their close relatives: a European Brain Injury Questionnaire study. Brain Inj 1997;11:543–63.
- Martin C, Viguier D, Deloche G, Dellatolas G. Subjective experience after traumatic brain injury. Brain Inj 2001;15:947–59.
- Richmond TS, Thompson HJ, Deatrick JA, Kauder DR. Journey towards recovery following physical trauma. J Adv Nurs 2000;32:1341–7.
- Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998;50:1413–8.
- Ware JE, Snow KK, Kosinski M, Gandek B, F- 36 Health Survey: Manual and Interpretation Guide. Boston: Nimrod Press; 1993.
- Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995;33:AS264–79.
- Guilfoyle MR, Seeley HM, Corteen E, et al. Assessing quality of life after traumatic brain injury: examination of the short form 36 health survey. J Neurotrauma 2010;27:2173–81.
- King JT, Roberts MS. Validity and reliability of the Short Form-36 in cervical spondylotic myelopathy. J Neurosurg 2002;97:180–5.
- Latimer M, Haden N, Seeley HM, Laing RJ. Measurement of outcome in patients with cervical spondylotic myelopathy treated surgically. Br J Neurosurg 2002;16:545–9.
- Al-Tamimi YZ, Guilfoyle M, Seeley H, Laing RJ. Measurement of long-term outcome in patients with cervical spondylotic myelopathy treated surgically. Eur Spine J 2013;22:2552–7.
- Anderson C, Laubscher S, Burns R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke 1996;27:1812–6.
- Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988;51:1457.
- Casey AT, Bland JM, Crockard HA. Development of a functional scoring system for rheumatoid arthritis patients with cervical myelopathy. Ann Rheum Dis 1996;55:901–6.
- Singh A, Crockard HA. Comparison of seven different scales used to quantify severity of cervical spondylotic myelopathy and post-operative improvement. J Outcome Meas 2001–2002; 5:798–818.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81–4.
- Aggarwal A, Dhandapani S, Praneeth K, et al. Comparative evaluation of H&H and WFNS grading scales with modified H&H (sans systemic disease): A study on 1000 patients with subarachnoid hemorrhage. Neurosurg Rev 2018;41:241–7.
- Davies DM, Nourallah B, Venkatesh A, et al. Establishing mild, moderate and severe criteria for the myelopathy disability index in cervical spondylotic myelopathy. British Journal of Neurosurgery 2020;10:1–5.
- Peter Fayers RH, Assessing Quality of Life in Clinical Trials. Oxford: Oxford University Press; 2005.
- Nunnally JB, Psychometric Theory. New York: McGraw-Hill; 1994.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7.
- Seel RT, Kreutzer JS, Rosenthal M, Hammond FM, Corrigan JD, Black K. Depression after traumatic brain injury: A National Institute on Disability and Rehabilitation Research Model Systems multicenter investigation. Arch Phys Med Rehabil 2003;84:177–84.
- Dikmen SS, Bombardier CH, Machamer JE, Fann JR, Temkin NR. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil 2004;85:1457–64.
- Robba C, Bacigaluppi S, Bragazzi N, et al. Clinical prevalence and outcome impact of pituitary dysfunction after aneurysmal subarachnoid hemorrhage: a systematic review with meta-analysis. Pituitary 2016;19:522–35.
- Can A, Gross BA, Smith TR, et al. Pituitary Dysfunction After Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2016;79:253–64.
- Persson HC, Törnbom K, Sunnerhagen KS, Törnbom M. Consequences and coping strategies six years after a subarachnoid hemorrhage – A qualitative study. PloS One 2017;12:e0181006.
- Dahm J, Ponsford J. Comparison of long-term outcomes following traumatic injury: what is the unique experience for those with brain injury compared with orthopaedic injury? Injury 2015;46:142–9.
- Dahm J, Ponsford J. Long-term employment outcomes following traumatic brain injury and orthopaedic trauma: A ten-year prospective study. J Rehabil Med 2015;47:932–40.
- Rezai M, Côté P, Cassidy JD, Carroll L. The association between prevalent neck pain and health-related quality of life: a cross-sectional analysis. Eur Spine J 2009;18:371–81.
- Paanalahti K, Holm LW, Magnusson C, Carroll L, Nordin M, Skillgate E. The sex-specific interrelationship between spinal pain and psychological distress across time in the general population. Results from the Stockholm Public Health Study. Spine J 2014;14:1928–35.
- Stoffman MR, Roberts MS, King JT. Cervical spondylotic myelopathy, depression, and anxiety: a cohort analysis of 89 patients. Neurosurgery 2005;57:307–13. Discussion 307–13.
- Maratos EC, Trivedi R, Richards H, Seeley H, Laing RJC. Psychological distress does not compromise outcome in spinal surgery. Br J Neurosurg 2012;26:466–71.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215.
- Jackson T, Wang Y, Wang Y, Fan H. Self-Efficacy and Chronic Pain Outcomes: A Meta-Analytic Review. J Pain 2014;15:800–14.
- Peolsson A, Vavruch L, Öberg B. Can the results 6 months after anterior cervical decompression and fusion identify patients who will have remaining deficit at long-term? Disabil Rehabil 2006;28:117–24.
- Van der Putten J, Stevenson V, Playford E, Thompson A. Factors affecting functional outcome in patients with nontraumatic spinal cord lesions after inpatient rehabilitation. Neurorehabil Neural Repair 2001;15:99–104.
- New PW. Non-traumatic spinal cord injury: what is the ideal setting for rehabilitation? Aust Health Rev 2006;30:353–61.
- Headway. A New Me: Experiences of Life after Brain Injury.; 2017.
- Tsyben A, Guilfoyle M, Timofeev I, et al. Spectrum of outcomes following traumatic brain injury—relationship between functional impairment and health-related quality of life. Acta Neurochir (Wien) 2018;160:107–15.
- Arnould A, Dromer E, Rochat L, et al. Neurobehavioral and self-awareness changes after traumatic brain injury: Towards new multidimensional approaches. Ann Phys Rehabil Med 2016;59:18–22.
- Nichol AD, Higgins AM, Gabbe BJ, Murray LJ, Cooper DJ, Cameron PA. functional and quality of life outcomes following major head injury: Common scales and checklists. Injury 2011;42:281–7.
- Jüttler E, Schwab S, Schmiedek P, DESTINY Study Group, et al. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY). Stroke 2007;38:2518–25.
- Dean PJA, Sterr A. Long-term effects of mild traumatic brain injury on cognitive performance. Front Hum Neurosci 2013;7:30.

![Figure 1. Frequency distribution of GCS (TBI), WFNS (SAH) and MDI (cervical myelopathy) scores. The disability scores were categorised as follows: GCS: mild [13-15], moderate [9-12], severe [3-8]; WFNS: mild [1-2], moderate [3], severe [4-5]; MDI: mild [<5], moderate [5-8], severe [>8].](/cms/asset/340c53ab-9af1-4320-981d-f0a16506c414/ibjn_a_2152777_f0001_c.jpg)