Abstract
A chronic subdural haematoma (CSDH) is a collection of aged blood between the dura and the brain, typically treated with surgical evacuation. Many patients with CSDH have comorbidities requiring the use of antithrombotic medications. The optimal management of these medications in the context of CSDH remains unknown, as the risk of recurrence must be carefully weighed against the risk of vaso-occlusive events. To better understand these risks and inform the development of clinical practice guidelines, we conducted a systematic review and meta-analysis. A systematic review was conducted in accordance with the PRISMA guidelines, searching Medline and Embase databases. The study was registered with PROSPERO (CRD42023397061). A total of 44 studies were included, encompassing 1 prospective cohort study and 43 retrospective cohort studies. Pooled odds ratios (ORs) were calculated for CSDH recurrence and vaso-occlusive events in patients taking anticoagulant or antiplatelet medications compared to patients not receiving antithrombotic therapy. GRADE was used to assess the quality of evidence. In patients on anticoagulant therapy at CSDH diagnosis, the pooled OR for CSDH recurrence was 1.41 (95% CI 1.11 to 1.79; I2 = 28%). For patients on antiplatelet therapy, the pooled OR was 1.31 (95% CI 1.08 to 1.58; I2 = 32%). Patients taking antithrombotic medications had a significantly higher risk of vaso-occlusive events, with a pooled OR of 3.74 (95% CI 2.12 to 6.60; I2 = 0%). There was insufficient evidence to assess the impact of time to recommence antithrombotic medication on CSDH outcomes. We found that baseline antithrombotic use is associated with the risk of CSDH recurrence and vaso-occlusive events following surgical evacuation. The evidence base is of low quality, and decisions regarding antithrombotic therapy should be individualised for each patient. Further high-quality, prospective studies or registry-based designs are needed to better inform clinical decision-making and establish evidence-based guidelines.
Introduction
Chronic subdural haematoma (CSDH) is a collection of aged blood on the surface of the brain, hypothesized to result from either the progression of an acute subdural bleed, and/or due to an inflammatory processes at the dural cell border.Citation1 Collections can exert mass effect, leading to subacute neurological deterioration. In such instances, surgical evacuation achieves favourable outcomes in over 80% of patients.Citation2
Incidence estimates for CSDH range from 1.7/100,000/year to 48/100,000/year,Citation3,Citation4 with a median age of presentation of 79.Citation5 Demographic shifts mean that case numbers are projected to rise significantly in the coming decades.Citation6 While surgical intervention is effective,Citation7 elderly patients face a higher risk of perioperative morbidity and mortality,Citation8 along with a longer duration of inpatient stay.Citation9 Furthermore, the evidence base for the broader peri and non-operative aspects of CSDH care, such as pharmacological therapies and perioperative management, is weak.Citation10
A significant clinical concern is the perioperative management of antithrombotic therapy, particularly in the elderly, where at a population level over 10% of individuals take either anticoagulant or antiplatelet agents.Citation11,Citation12 Recent studies have shown that over 40% of patients with CSDH undergoing surgery may be taking such an agent.Citation13,Citation14 Antithrombotic therapy has been associated with both CSDH incidence and recurrence in retrospective cohort studies.Citation15,Citation16 Perioperatively, the management of such agents must balance the risk of bleeding (and thus potential CSDH recurrence or enlargement) with the risks of vaso-occlusive events (such as venous thromboembolism – VTE). This puts great importance on decisions relating to the accelerated reversal, recommencement, or withholding of such agents.
Recent national guidelines in the USACitation17 and UKCitation18 provide general recommendations for perioperative management of antithrombotic agents; however, both documents emphasise the importance of assessing individualised bleeding risk in the context of non-elective procedures. Numerous systematic reviews have generated inconsistent conclusions regarding the risk of CSDH recurrence and vaso-occlusive events when taking antithrombotic medication.Citation19,Citation20 Consequently, there is no consensus on the optimal timing of recommencing either anticoagulant or antiplatelet agents.Citation21
The Improving Care in Elderly Neurosurgery InitiativeCitation22 (ICENI) was conceived to produce the first clinical practice guideline to guide the broader perioperative care of CSDH from the onset of symptoms to recovery. As part of this work, a synthesis of current evidence is required. Literature questions were created by several multidisciplinary working groups. These groups were formed to discuss the challenges in delivering care, highlight uncertainties, and form questions to pose in the scientific literature. Questions were structured as foreground PICO (Population, Intervention, Comparison and Outcome) questions, to facilitate GRADE evidence assessment. The results of which would then inform guideline statements.
Since the publication of the most recent systematic review investigating the association between antithrombotic therapy and CSDH recurrence,Citation23 primary studies have been published reporting on the outcomes of more than 2000 patients.Citation16,Citation24–27 Moreover, the first large analysis has since been published on the occurrence of vaso-occlusive events following operative management.Citation16 To inform contemporary clinical practice guidelines, we therefore performed an up-to-date systematic review and meta-analysis of studies reporting the clinical outcomes of patients taking antithrombotic medications at CSDH diagnosis.
The primary objective of this review was to systematically review evidence on key aspects of anti-thrombotic management in the perioperative care of cSDH, as identified by our multidisciplinary working groups.
Review questions
Review questions derived from our working groups were as follows:
In patients with CSDH undergoing surgery (P) do antithrombotic drugs (e.g., anticoagulants, antiplatelet agents) (I) increase the risk of treatment-related complications (O) compared to those who are not taking such drugs(C)?
In patients recovering from CSDH surgery (P), does late (I) vs early (C) recommencement of anticoagulation increase the risk of recurrence or other complications (O)?
In patients with cSDH who are not undergoing surgery (P) do antithrombotic drugs (e.g. anticoagulants, antiplatelets) (I) increase the risk of disease-related complications (e.g. expansion (O) compared to those who do not take such agents (C)?
In patients with cSDH who are not undergoing surgery (P) does discontinuation of antithrombotic agents (I) improve disease and safety-related outcomes (O) compared to continuing these agents (C)?
Material and methods
Guidelines and direction
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Supplementary Material 1). The study was registered with PROSPERO (registration number: CRD42023397061).
Definition of key clinical practice questions
Five multidisciplinary working groups were formed to explore the entirety of perioperative CSDH care.Citation28 Through facilitated discussion, key clinical practice questions were identified and expressed using the PICO format (Population, Intervention, Comparator, Outcome) and grouped into major themes. Coverage of these key themes by existing systematic reviews has been reported previously.Citation10
Search strategy
A comprehensive literature search was performed on 1st May 2022 of 2 databases: Medline, and Excerpta Medica Database (Embase), from inception. The exact search strategy for all databases and their results can be found in Supplementary Material 2. We reviewed the reference lists and bibliographies of included articles to attempt to identify additional studies. Papers were limited to those published in English.
Study screening and selection
Citations were transferred to the online platform Rayyan (Rayyan Systems Inc., Cambridge, USA) to facilitate de-duplication and paired abstract screening. Duplicates were then removed. A total of 11 reviewers screened 6,024 articles. Each article was screened by two blinded, independent reviewers. Articles were excluded based on the initial screening criteria outlined in Supplementary Material 2. Included articles were then categorised dependent on their title, journal, and abstracts, according to pre-defined ICENI categories (Supplementary Material 2).
A total of 116 articles labelled as category ‘Anticoagulant’ were identified. These 116 articles were then rescreened by abstract and full text against our specific criteria (Supplementary Material 2) by two independent, blinded reviewers (JB, GA). Criteria were generated and defined by our working group to review multiple issues with anticoagulation in CSDH care. However, we only identified evidence for review questions 1 and 2, as no included studies assessed patients managed non-operatively. We therefore included studies assessing the impact of antithrombotic medications in patients following surgical evacuation of CSDH. We excluded studies that were conference abstracts, case reports, and mixed populations where it was not possible to delineate CSDH-specific outcomes (e.g., a dataset containing both ASDH and CSDH data). Systematic reviews also were excluded at this stage; however, each was screened for studies not identified during the initial search.
If any disagreements occurred at either stage of screening, an attempt was made to resolve this between two review authors, and if discussion failed to lead to consensus, senior authors (BMD, DJS) were consulted for clarification. If any data was not present or available in the articles identified, corresponding authors were contacted via email to request the data.
Data extraction and synthesis
Data extraction was conducted independently and in duplicate by two authors (JB, GA) using a standardised pre-piloted data collection proforma. Data extracted included baseline patient demographics, type of antithrombotic medication, number of vaso-occlusive events, CSDH recurrence rate, follow-up duration. CSDH recurrence rates and vaso-occlusive events were used as outcome measures. Vaso-occlusive events were defined to include venous thromboembolism (deep vein thrombosis and pulmonary embolus), ischaemic stroke, and myocardial infarction. Data were incorporated into a Microsoft Excel spreadsheet and exported to R (v4.0.5; R Core Team, 2020) for analysis.
Statistical analysis
Baseline demographics and study characteristics were summarised using descriptive statistics. Studies were eligible for inclusion in a meta-analysis whenever they reported either the number of cases of CSDH recurrence or vaso-occlusive events. A meta-analysis of binary outcomes was performed to compare the risk of CSDH recurrence and vaso-occlusive events between a pooled antithrombotic group and a pooled control group. Odds ratios (ORs) were computed by assuming a random effects model, and summary estimates were presented with a 95% confidence interval (CI). The I2 statistic was used to present between-study heterogeneity and funnel plots of effect size against standard error were used to assess for the presence of publication bias. Calculations and data visualisation were performed using R software (v4.0.5; R Core Team, 2020). A P value less than 0.05 was considered statistically significant; all tests and CIs were two-sided.
Quality of evidence
The assessment of evidence quality was performed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process (Supplementary Material 3). In this approach, evidence is initially assumed to be high certainty and can be downgraded according to the risk of bias, indirectness, imprecision, heterogeneity, and publication bias to obtain an overall certainty for each outcome as levels of moderate, low, and very low quality. The quality of the included studies was assessed by two authors (JB, CG).
Results
Study selection process
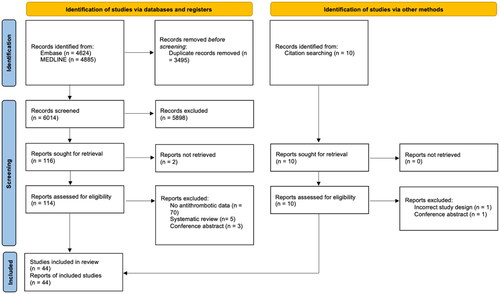
Following abstract and full-text screening of 6023 studies according to inclusion criteria, 49 total studies were included in the analysis ().
Study and patient characteristics
A total of 44 primary clinical studies were identified (Supplementary Material 4). This included one prospective cohort study and 43 retrospective cohort studies. Of the primary clinical studies, the mean patient age ranged from 62.1 to 84.4 and publication years were between 2001 and 2022. Using GRADE, overall evidence quality was rated low, due to indirectness and imprecision of study outcomes. Heterogeneity of study characteristics was high, with a wide range of follow-up durations and number of patients included (Supplementary Material 5). Studies also varied in their definition of CSDH recurrence and medication type. Some studies opted for a radiological definition of CSDH recurrence, whilst others defined recurrence only when repeat operation was necessary.
A total of 8 cohort studies were conducted across multiple centres, while 38 were single-centre studies. An evidence summary for the included studies is shown in Supplementary Materials 4.
In 23 primary studies, outcomes in patients taking anticoagulant medication at diagnosis were reported, along with a comparator group. Similarly, 25 primary studies reported outcomes in patients taking antiplatelet medication, against a comparator group. Six studies reported antithrombotic patient outcomes without dividing patients into antiplatelet or anticoagulant groups.
The number of vaso-occlusive events during admission was reported in six studies, along with antithrombotic status at diagnosis.
Comparative studies in patients who were not undergoing surgical management were not identified. Consequently, of the aforementioned PICO questions, evidence was only available to address questions 1. Risks associated with taking antithrombotic medication, and 2. Risks associated with early versus late recommencement after surgery.
Recurrence following CSDH drainage in patients taking anticoagulant medication at CSDH diagnosis
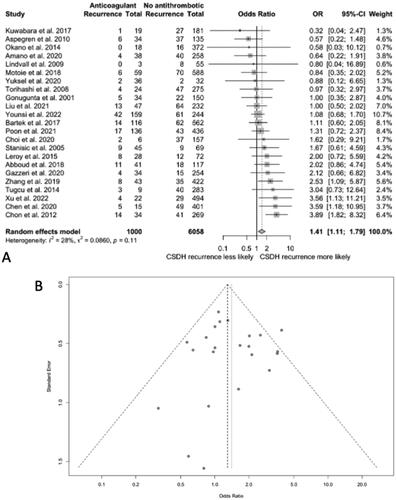
In the 23 studies comparing outcomes in patients taking anticoagulant drugs and those not on antithrombotic drugs, 11 found a higher risk of CSDH recurrence in the anticoagulated group, 6 found a lower risk, and 6 reported no difference in the level of risk between both groups.
The pooled OR of CSDH recurrence was 1.41 (95% CI 1.11–1.79; I Citation2 = 28%) for patients on anticoagulation at CSDH diagnosis compared to those on no antithrombotic drugs (). No publication bias was identified after plotting a funnel plot of OR values.
Recurrence following CSDH drainage in patients taking antiplatelet medication at CSDH diagnosis
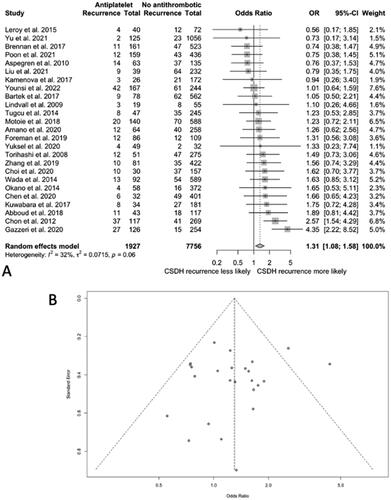
In the 25 studies comparing outcomes in patients taking antiplatelet drugs and those not on antithrombotic drugs, 15 found a higher risk of CSDH recurrence in the antiplatelet group, 6 found a lower risk, and 4 reported a similar risk for both groups.
The pooled OR of CSDH recurrence was 1.31 (95% CI 1.08–1.58; I Citation2 = 32%) for patients on antiplatelets at CSDH diagnosis compared to those not taking antiplatelet drugs (). No publication bias was identified after plotting a funnel plot of OR values.
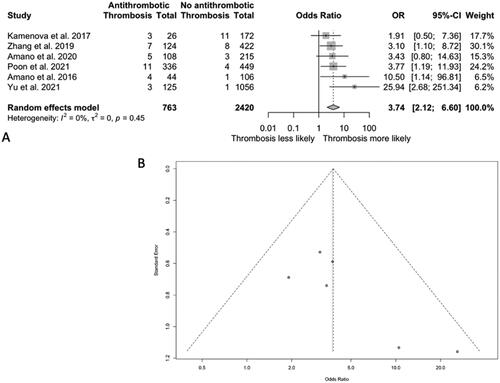
Occurrence of vaso-occlusive events following CSDH drainage in patients taking antithrombotic medications at CSDH diagnosis
In the six studies reporting the incidence of vaso-occlusive events in patients taking antithrombotic drugs and those not, all 6 found a higher risk of vaso-occlusive events in the antithrombotic group. The pooled OR of vaso-occlusive events was 3.74 (95% CI 2.12–6.60; I Citation2 = 0%) for patients on antithrombotic medications at CSDH diagnosis compared to those who were not (). No publication bias was identified after plotting a funnel plot of OR values.
Figure 4. A. Odds ratio (OR) of vaso-occlusive events after drainage in patients taking antithrombotic medication at diagnosis vs patients taking no antithrombotic drugs at CSDH diagnosis. B. A funnel plot representing the reporting odds ratio values for vaso-occlusive event risk in patients taking antithrombotic risk at baseline.
A lack of individual-level data on antithrombotic recommencement precluded meta-analysis of risk of recurrence with time.
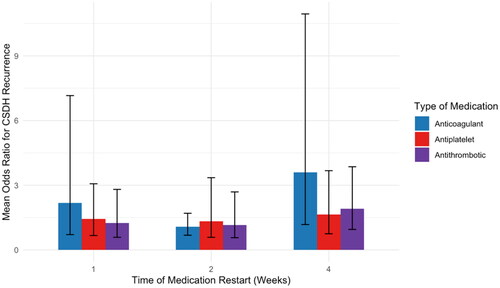
No association between time of medication restart and CSDH recurrence risk
A subanalysis was performed on studies (n = 10) reporting the times when antithrombotic medications were restarted (). This exploratory analysis revealed no significant association between the time of medication restart and CSDH recurrence risk.
Discussion
This systematic review and meta-analysis aimed to evaluate the impact of antithrombotic medications on treatment-related complications in patients undergoing surgical evacuation of CSDH. We identified 44 primary studies reporting outcomes after CSDH associated with antithrombotic drug use. The review found that patients taking anticoagulants (OR 1.41, 1.11–1.79) or antiplatelet (OR 1.31, 1.08–1.58) medications at the time of CSDH diagnosis had a higher risk of CSDH recurrence compared to patients not on these medications. Furthermore, patients on antithrombotic medications had a higher risk of vaso-occlusive events after CSDH drainage (OR 3.74, 2.12–6.60). Significant knowledge gaps were identified by the absence of evidence for guidance or outcomes of antithrombotic medications in patients undergoing non-operative management.
Notably, however, these findings are considered of low strength. The identified evidence base is limited by its quality and heterogeneity. Studies are primarily retrospective cohort studies, and are either inconsistent or lacking patient level outcomes (e.g. function or quality of life) to determine the implications of vaso-occlusive events or recurrence. This is notable for CSDH recurrence, with some studies using a radiological definition,Citation26 and other groups defining a case of recurrence by the requirement for revision surgery.Citation29 Variation was also identified in the type of antithrombotic medication, the duration of follow-up, and the time to restart antithrombotic drugs. There was insufficient data to definitively assess the impact of time to restart medication on CSDH outcomes, precluding a risk-benefit analysis. Only six studies with low event incidence reported the occurrence of vaso-occlusive events in patients following CSDH, limiting the interpretation of these results. It also remains unclear when recurrence events occurred in relation to antithrombotic reversal or medication washout, confounding the interpretation of our findings.
Despite this, to our knowledge, this is the largest systematic review and meta-analysis of antithrombotic-associated complications in patients following CSDH, reporting outcomes for 16,635 patients and including the first pooled analysis of vaso-occlusive events following operative management.Citation16
The association between antithrombotic medications and CSDH recurrence may be attributable to known features of CSDH pathophysiology. Although not yet fully understood, CSDH is characterised by a hyper-fibrinolytic state,Citation1 with evidence of raised tissue plasminogen activator and fibrinogen degradation products in CSDH fluid following drainage.Citation30 Higher levels of plasminogen have also been identified in patients prior to recurrence, suggesting markers of hyperfibrinolysis may be able to predict those at higher risk of recurrence.Citation31 This hyperfibrinolytic state is compounded by the existence of fragile blood vessels resulting from local angiogenesis.Citation32 When combined with antithrombotic agents, these factors drive the balance of haemostasis further towards a propensity for bleeding, increasing the risk of recurrence.
Likewise, association between antithrombotic medications and vaso-occlusive events is presumed to stem from their underlying co-morbidity, temporary cessation peri-operatively, and the known prothrombotic nature of undergoing surgery. Specific risks associated with antithrombotic medications may also be attributed to drug mechanism of action, such as in the case of anticoagulant medications, where warfarin antagonises vitamin K-dependent activation of clotting factors, and direct oral anticoagulants cause downstream inhibition of the coagulation cascade.
However, applying this information in practice is not straightforward. The aggregate data answers ‘background’ questions relevant to decision making but not specific ‘foreground’ questions. For example, should patients receiving anti-thrombotic management continue peri-operatively? Or if stopped, when or how should it be restarted? Such questions are directly applicable to clinical care. Clearly, these gaps need to be informed by targeted research, but in the interim, to help bridge the evidence gap, and support practice expert opinion is thus required. This review is part of a larger evidence synthesis conducted by the ICENI group,Citation22 which aims to develop clinical practice guidelines and recommendations for managing patients with CSDH, alongside prioritising key knowledge gaps.
Conclusion
Patients undergoing surgery for CSDH and receiving antithrombotic or anticoagulant medication have a 31–41% increased odds of recurrence but 274% increased odds of vaso-occlusive events. There was insufficient data to determine whether medication restart time is significantly associated with recurrence risk. Further research is required to better contextualise this risk and understand if and how it can be mitigated safely.
Supplemental Material
Download Zip (242.8 KB)Declaration of competing interests
Dr Brannigan reports consulting fees from Synchron.
Dr Thomas reports funding/consulting fees from Bayer, Pfizer, AstraZeneca, Daiichi Sankyo, Portola, Ablynx, Sanofi, LFB Biopharmaceuticals, Sobi, Grifols, NovoNordisk, CSL Behring and Takeda. Dr Uprichard reports fees for clinical research/educational meetings/scientific advisory boards from: AstraZeneca, Bayer, Daiichi Sankyo, Octaphama, Pharmacosmos, Pfizer, Portola, Shire, Sobi and Vifor Pharma.
Data availability statement
Our full dataset can be found in the supplementary materials.
Additional information
Funding
References
- Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation 2017;14:108.
- Ducruet AF, Grobelny BT, Zacharia BE, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev 2012;35:155–69; discussion 169.
- Fogelholm R, Heiskanen O, Waltimo O. Chronic subdural hematoma in adults. Influence of patient’s age on symptoms, signs, and thickness of hematoma. J Neurosurg 1975;42:43–6.
- Adhiyaman V, Chattopadhyay I, Irshad F, Curran D, Abraham S. Increasing incidence of chronic subdural haematoma in the elderly. QJM 2017;110:375–8.
- Rauhala M, Luoto TM, Huhtala H, et al. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg 2019;132:1147–57.
- Stubbs DJ, Vivian ME, Davies BM, Ercole A, Burnstein R, Joannides AJ. Incidence of chronic subdural haematoma: a single-centre exploration of the effects of an ageing population with a review of the literature. Acta Neurochir (Wien) 2021;163:2629–37.
- Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 2014;10:570–8.
- UNO M, TOI H, Hirai S. Chronic subdural hematoma in elderly patients: is this disease benign? Neurol Med Chir (Tokyo) 2017;57:402–9.
- Rauhala M, Helén P, Huhtala H, et al. Chronic subdural hematoma-incidence, complications, and financial impact. Acta Neurochir (Wien) 2020;162:2033–43.
- Gillespie CS, Fung KW, Alam AM, et al. How does research activity align with research need in chronic subdural haematoma: a gap analysis of systematic reviews with end-user selected knowledge gaps. Acta Neurochir (Wien) 2023;165:1975–86.
- Troy A, Anderson TS. National trends in use of and spending on oral anticoagulants among US medicare beneficiaries from 2011 to 2019. JAMA Health Forum 2021;2:e211693.
- Tan C, Cieslik L, Steele S, Warner V, Mariani J, Patel HC. Contemporary trends in antiplatelet prescription in Australia. Pharmacy Practice and Res 2020;50:366–8.
- Brennan PM, Kolias AG, Joannides AJ, et al. The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg 2017;127:732–9.
- Stubbs DJ, Davies BM, Bashford T, et al. Identification of factors associated with morbidity and postoperative length of stay in surgically managed chronic subdural haematoma using electronic health records: a retrospective cohort study. BMJ Open 2020;10:e037385.
- Lindvall P, Koskinen LO. Anticoagulants and antiplatelet agents and the risk of development and recurrence of chronic subdural haematomas. J Clin Neurosci 2009;16:1287–90.
- Poon MTC, Rea C, Kolias AG, Brennan PM, British Neurosurgical Trainee Research C. Influence of antiplatelet and anticoagulant drug use on outcomes after chronic subdural hematoma drainage. J Neurotrauma 2021;38:1177–84.
- Hornor MA, Duane TM, Ehlers AP, et al. American College of Surgeons’ Guidelines for the Perioperative Management of Antithrombotic Medication. J Am Coll Surg 2018;227:521–36.e1.
- Keeling D, Tait RC, Watson H, British Committee of Standards for Haematology. Peri-operative management of anticoagulation and antiplatelet therapy. Br J Haematol 2016;175:602–13.
- Chari A, Clemente Morgado T, Rigamonti D. Recommencement of anticoagulation in chronic subdural haematoma: a systematic review and meta-analysis. Br J Neurosurg 2014;28:2–7.
- Nathan S, Goodarzi Z, Jette N, Gallagher C, Holroyd-Leduc J. Anticoagulant and antiplatelet use in seniors with chronic subdural hematoma: systematic review. Neurology 2017;88:1889–93.
- Licci M, Kamenova M, Guzman R, Mariani L, Soleman J. Influence of postoperative thrombosis prophylaxis on the recurrence of chronic subdural hematoma after burr-hole drainage. Crit Care Med 2018;46:e26–e32.
- Stubbs DJ, Davies B, Hutchinson P, Menon DK, Improving Care in Elderly Neurosurgery Initiative (ICENI). Challenges and opportunities in the care of chronic subdural haematoma: perspectives from a multi-disciplinary working group on the need for change. Br J Neurosurg 2022;36:600–8.
- Wang H, Zhang M, Zheng H, et al. The effects of antithrombotic drugs on the recurrence and mortality in patients with chronic subdural hematoma. Medicine (Baltimore))). 2019;98:e13972.
- Amano T, Matsuo S, Miyamatsu Y, Yamashita S, Nakamizo A. Impact of antithrombotic therapy on surgical treatment in patients with chronic subdural hematoma. J Clin Neurosci 2020;74:55–60.
- Oh HJ, Seo Y, Choo YH, et al. Clinical characteristics and current managements for patients with chronic subdural hematoma : a retrospective multicenter pilot study in the Republic of Korea. J 2022;65:255–68.
- Xu M, Tan W, Wang W, Wang D, Zeng W, Wang C. Minimally invasive surgery in chronic subdural hematoma: prognosis and recurrence factors of 516 cases in a single center. J 2022;11:28.
- Younsi A, Riemann L, Habel C, et al. Relevance of comorbidities and antithrombotic medication as risk factors for reoperation in patients with chronic subdural hematoma. Neurosurg Rev 2022;45:729–39.
- Protocol for the development of a … | Wellcome Open Research. Accessed September 16, 2023. https://wellcomeopenresearch.org/articles/8-390
- Kamenova M, Nevzati E, Lutz K, et al. Burr-Hole drainage for chronic subdural hematoma under low-dose acetylsalicylic acid: a comparative risk analysis study. World Neurosurg 2017;100:594–600.
- Weir B, Gordon P. Factors affecting coagulation: fibrinolysis in chronic subdural fluid collections. J Neurosurg 1983;58:242–5.
- Katano H, Kamiya K, Mase M, Tanikawa M, Yamada K. Tissue plasminogen activator in chronic subdural hematomas as a predictor of recurrence. J Neurosurg 2006;104:79–84.
- Hohenstein A, Erber R, Schilling L, Weigel R. Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and -2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma 2005;22:518–28.