Abstract
A new regional dataset comprising 425 intertidal diatom taxa from 175 samples from 11 ecologically diverse Oregon and Washington estuaries illustrates the importance of compiling a large modern dataset from a range of sites. Cluster analyses and detrended correspondence analysis of the diatom assemblages identify distinct vertical zones within supratidal, intertidal and subtidal environments at six of the 11 study sites, but the abundance of some of the most common species varies widely among and within sites. Canonical correspondence analysis of the regional dataset shows relationships between diatom species and tidal exposure, salinity and substratum (grain size and organic content). Correspondence analyses of local datasets show higher values of explained variation than the analysis of the combined regional dataset. Our results emphasize that studies of the autecology of diatom species require many samples from a range of modern environments to adequately characterize species–environment relationships.
Introduction
Diatoms are unicellular, photosynthetic algae that are important primary producers in coastal environments, occupying almost all coastal habitats, different species showing different sensitivity to tidal exposure, salinity and substratum (e.g., Sherrod Citation1999, Weckström & Juggins Citation2005). Diatom assemblages are readily preserved, easily detectable, and dense (∼500 to 15 000 cells per mL) in sediment and water, thereby providing a practical and widely applicable basis for ecological and palaeoecological interpretation (Round Citation1988, Smol & Stoermer Citation2010).
The utility of diatoms as environmental indicators is underpinned by comprehensive ecological classifications, particularly those based on salinity preference (Hustedt Citation1930, Citation1957, van der Werff & Huls Citation1958–Citation1966, Juggins Citation1992, Citation2013, Snoeijs Citation1993, Vos & de Wolf Citation1993, Van Dam et al. Citation1994). Coastal diatoms can also be classified by their mode of attachment to, and mobility in, sediment (Kosugi Citation1987, Vos & de Wolf Citation1993, Cunningham & McMinn Citation2004). Epipsammic species are immobile and attach to sand grains; epiphytic species use mucilage to attach themselves to the surface of aquatic plants, particularly macrophytes in the intertidal zone; and epipelic species are highly motile. A considerable body of literature discusses the distribution of modern species of diatoms and their habitats along the Pacific coast of central North America (e.g., McIntire & Overton Citation1971, McIntire Citation1973, Riznyk Citation1973, Main & McIntire Citation1974, Moore & McIntire Citation1977, Amspoker & McIntire Citation1986, Laws Citation1988, Hemphill-Haley Citation1993, Citation1995, Nelson & Kashima Citation1993, Sherrod Citation1999) and elsewhere (e.g., Aleem Citation1950, Admiraal & Peletier Citation1979, Citation1980, Admiraal et al. Citation1984, Sabbe Citation1993, Zong & Horton Citation1998, Sawai Citation2001, Sawai et al. Citation2004, Horton et al. Citation2007).
Multivariate analyses have been used to quantify the relationships between diatom species assemblages and environmental variables (e.g., McIntire Citation1978, Sullivan Citation1982, Whiting & McIntire Citation1985, Busse & Snoeijs Citation2003, Cunningham & McMinn Citation2004, Haubois et al. Citation2005, Ulanova & Snoeijs Citation2006, Horton et al. Citation2006, Rovira et al. Citation2012). However, a prerequisite for the success of such analyses is the development of a modern training set combining species assemblage and environmental data to develop the quantitative relationships of interest (e.g., Snoeijs Citation1993, Snoeijs & Vilbaste Citation1994, Snoeijs & Potapova Citation1995, Snoeijs & Kasperoviciene Citation1996, Snoeijs & Balashova Citation1998). Most such studies have focused on the distribution of assemblages at a single site, estuary, or type of depositional environment. Along the Pacific coast of North America, studies have identified intertidal diatom assemblages that are vertically zoned with respect to the tidal regime, corresponding to variations in both salinity and substratum (Amspoker & McIntire Citation1978, McIntire Citation1978, Nelson & Kashima Citation1993, Hemphill-Haley Citation1995, Sherrod Citation1999, Roe et al. Citation2009). However, this narrow spatial and environmental focus fails to reveal the full ecological tolerance of individual taxa or assemblages (e.g., Cunningham & McMinn Citation2004, Watcham et al. Citation2013). To provide appropriate analogues for fossil diatom assemblages, studies that characterize the modern distribution of diatoms should thoroughly sample a broad range of coastal environments, including supratidal forests, salt marshes, and tidal flats (e.g., Hemphill-Haley Citation1993, Citation1995, Zong & Horton Citation1998, Citation1999, Watcham et al. Citation2013). Such a broad spatial and environmental focus is most likely to capture the full environmental range and ecological tolerance of assemblages necessary to make quantitative interpretations of present and past environments.
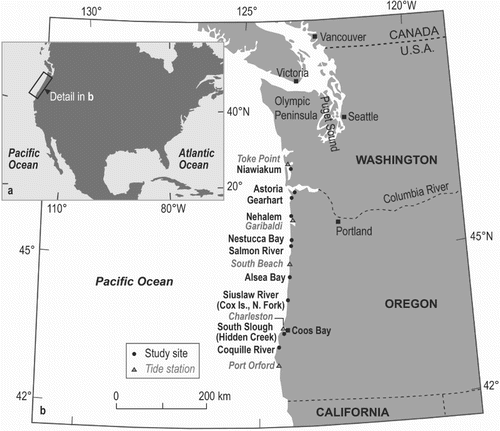
In this paper, we describe and quantify the distribution of intertidal diatoms using 175 samples from 11 estuarine sites along the Oregon and Washington coast (). These samples represent a wide range of habitats within and among sites that together constitute a spatially and ecologically comprehensive summary of the diversity of diatom assemblages in the region.
Materials and methods
Geographical background of the sites
Much of the coast of Oregon and southern Washington consists of resistant volcanic headlands separating low-relief embayments (Komar Citation2004). The embayments are characterized by uplifted marine terraces, valleys where rivers emerge at the shoreline, and associated estuaries, sand spits, beaches, and dunes. Extensive sand spits along the northern Oregon coastline protect large back-barrier estuaries. To the south, a continuous beach extends 100 km from Coos Bay north to Heceta Head near Alsea Bay and is backed by the largest coastal dune complex in the United States (Komar Citation2004). Many of the estuarine wetlands were historically diked, primarily to create pasture (Adamus Citation2005). In southern Washington, dune-backed beaches extend up to 100 km north of the Columbia River mouth where they join the steep, rocky coast of the mountainous Olympic Peninsula. The most extensive tidal wetlands in Washington fringe shallow estuaries inland of the dune-backed beaches (e.g., Atwater & Hemphill-Haley Citation1997).
Low-energy depositional settings in coastal Oregon and Washington are characterized by salt marshes and tidal flats. Our 11 sites (), spanning ∼400 km of coastline, cover a wide range of physiographic settings characterized by a wide diversity in environmental parameters, such as salinity, substratum (grain size and organic content) and tidal exposure. Vascular plant species (e.g., Weinmann et al. Citation1984, Pojar & MacKinnon Citation1994, Nelson & Kashima Citation1993, Cooke Citation1997) at our sites generally form distinctive communities that define broad vertical ecological zones as a result of the varied tolerance of particular species to tidal exposure, although zones overlap, such as high-middle and middle-low marshes. The most common floral zones identified are the tidal flat (i.e., vascular plants absent or Zostera), low salt marsh (Carex lyngbyei, Salicornia depressa, and Triglochin maritima), middle salt marsh (Juncus arcticus, Deschampsia caespitosa, and Triglochin maritima) and high salt marsh (Juncus arcticus, Agrostis sp., Argentina egedii, and Deschampsia caespitosa) zones (e.g., Frenkel et al. Citation1981, Weinmann et al. Citation1984, Nelson & Kashima Citation1993, Atwater & Hemphill-Haley Citation1997). Upland areas above the highest tides are dominated by freshwater plants including trees or dwarfed trees (e.g., Picea, Alnus, Abies, Tsuga). The dominant vascular plant species and salinity ranges in each floral zone at our study sites are summarized in Online Appendix Table A1.
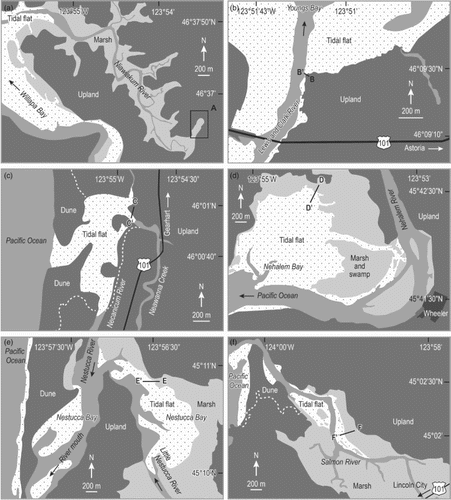
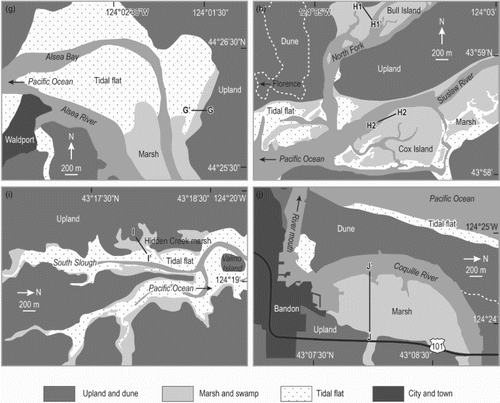
Fig. 2. Detailed maps of the 11 study sites. (a) Niawiakum River. (b) Astoria. (c) Gearhart. (d) Nehalem Bay. (e) Nestucca Bay. (f) Salmon River. (g) Alsea Bay. (h) North Fork of Siuslaw River and Siuslaw River (Cox Island). (i) South Slough (Hidden Creek), Coos Bay. (j) Coquille River. Lines with paired letter labels show locations of sampled elevational transects. Samples at the Niawiakum River were collected at random sites in the wetland area.
Sampling procedure
Surface sediments were collected at 175 locations from 11 study sites (Figs and ). Duplicate samples of foraminifera from six of the Oregon sites were collected and previously analyzed by Nelson et al. (Citation2008; Alsea Bay) and Hawkes et al. (Citation2010; the remaining five sites). At each site, samples were collected at stations positioned along a transect. The stations were at approximately evenly spaced vertical increments (usually every 10–20 cm) in order to sample diatom floras systematically along an elevational gradient. At the Niawiakum River site, sample stations were positioned randomly, because no clear elevation gradient was visible. At each station a sample was collected from the sediment-water interface (surface sediment 2–10 mm thick) using disposable 10-mL plastic syringes or a small spatula during low tides. After collection, samples were refrigerated.
Diatom identification
The 175 surface sediment samples for diatom identification and counting were prepared following Sawai & Nagumo (Citation2003). At least 300 diatom valves per sample were identified and counted under an oil-immersion microscope at 600× magnification. Fragments containing more than half a valve were included in the counts. Specimens of Planothidium delicatulum (Kützing) Round & Bukhtiyarova were counted separately, as frustules and non-raphid valves (P-valves, e.g., ), for comparison with a previous study (Sawai Citation2001). Sawai (Citation2001) recognized allochthonous occurrences of certain epiphytic taxa in the intertidal zone. For example, Cocconeis scutellum Ehrenberg is an epiphytic species commonly found attached to macrophytes in the intertidal zone. After death, the upper (rapheless) valve that is not attached to the macrophyte may detach and be transported by daily tides (Sawai Citation2001). As a result, rapheless valves of C. scutellum may be found in sediment across the entire tidal environment, although its habitat is limited to the macrophyte zone (Sawai Citation2001).
When needed, a scanning electron microscope was used for identification (Sawai & Nagumo Citation2003). Diatom abundance is expressed as a percentage of the total number of diatom valves counted (e.g., ). Identification of diatom species mainly followed Hendey (Citation1964), Patrick & Reimer (Citation1966, Citation1975), Krammer & Lange-Bertalot (Citation1986, Citation1988, Citation1991a, b), and Lange-Bertalot (Citation2000, Citation2001). Light and scanning electron microscopic images of the most common taxa are shown in Sawai & Nagumo (Citation2003).
Measurement of environmental variables
Tidal exposure, salinity and substratum (grain size and organic content) were measured for the 175 surface sediment samples from 11 study sites. Sample elevations were established by surveying to geodetic benchmarks using levelling instruments to calculate tidal exposure. Where there was no nearby geodetic benchmark, regular measurements (every 6 minutes) of tide levels were employed and sample elevations were estimated from the highest tide levels correlated with measurements made by a nearby tide-gauge (). To enable comparisons among sites with different tidal ranges, the duration of tidal inundation was expressed as the tidal exposure index (TEI), which represents the percentage of time that a sample location was submerged during the year 2007, based on astronomical tide predictions (Figs and A11). High TEI values indicate greater submergence and are found at lower elevations (e.g., tidal flat). Sampling sites above the upper limit of tidal inundation (e.g., supratidal) have a TEI value of zero. Mean tidal level has a TEI value of 50 (). To convert between orthometric (e.g., North American Vertical Datum of 1988; NAVD88) and tidal datums (e.g., Mean Higher High Water; MHHW) and to relate sample elevations to appropriate tidal datums we used values and predictions reported for nearby National Oceanic and Atmospheric Administration (NOAA) tide gauges (shown as triangles in ).
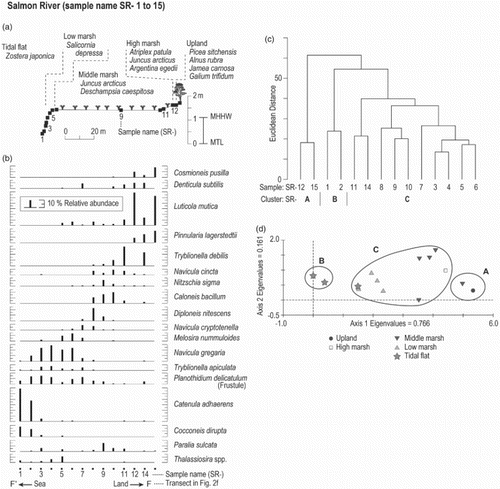
Fig. 3. Data analysis of diatom assemblages at the Salmon River (Oregon, USA). (a) A diagram of the studied transect at the Salmon River. Vertical and horizontal axes represent elevation (m) relative to MTL and distance (m), respectively. Surface samples were collected across four recognizable environmental zones: tidal flat, low marsh, middle marsh, high marsh, and supratidal. Dominant vascular plant species are listed for zones. (b) Changes in abundance (%) of dominant diatom species along the transect. (c) Result of agglomerative hierarchical cluster analysis. (d) DCA results for diatom distribution in the 14 surface samples.
Salinity was measured in pore-water using a calibrated refractometer at the time of sample collection. When the sample was not wet enough for measurement, pore-water was separated by centrifuge from the samples in the laboratory and its salinity measured.
Substratum (grain size and organic content) was measured on a sub-sample of the sediment used for diatom analysis. Grain-size distributions were measured with a laser particle-size analyzer (samples from Nehalem River) or gentle washing through 63 μm sieves (the other sites) after pretreatment with hydrogen peroxide to remove organic matter. The grain-size data were presented as percent mud content (silt plus clay, less than 63 µm). Organic content was measured using percentage loss on ignition (LOI). LOI was calculated by weighing and burning dry sediment in a furnace at 600°C for three hours (Ball Citation1964).
Data analysis
Agglomerative, hierarchical clustering (Euclidean distance) was used to identify distinctive groups of diatom assemblages. Cluster analysis was carried out on the samples from each transect. We used the cophenetic correlation between metrics (single, complete, average, and weighted average link, and Ward's Minimum) to select the metric causing the least distortion to the data. Reported results used an average link, and a broken stick plot was used to determine how many groups should be recognized. These analyses were executed using the cluster package in R (Maechler et al. Citation2005).
Detrended correspondence analysis (DCA), using supplementary environmental variables, was used to understand the relationships among the diatom assemblages from each transect (ter Braak & Šmilauer Citation2002). DCA, an ordination technique, represents assemblage samples as points in multi-dimensional space; similar assemblages are located close together and dissimilar assemblages further apart. Canonical correspondence analysis (CCA) using forward selection was applied to the regional dataset with measured environmental variables. CCA relates the distribution of diatoms to environmental factors (ter Braak Citation1986, ter Braak & Verdonschot Citation1995). The CCA extracts synthetic environmental gradients to illustrate the different habitat preferences (niches) of taxa via an ordination diagram. The variation partitioning (Økland & Eilertsen Citation1994) was performed to assess the relative contribution of different environmental variables for explaining the composition of assemblages. DCA and CCA were executed using CANOCO release 4.5 (ter Braak & Šmilauer Citation2002).
For clustering, CCA and DCA, rare species (those with a maximum abundance of <5% in a single sample) were excluded from the analysis of individual sites and the regional dataset. This reduced the number of species from 425 to 141 and gave the regional dataset the desirable statistical quality of having fewer species than samples. The CCA was performed for the regional dataset, but we excluded samples without salinity and LOI data.
Results
Diatom assemblages
Four hundred and twenty-five diatom taxa were identified in the 175 surface samples from the 11 study sites. Among them, 141 taxa have abundances greater than 5% in one or more samples and 77 taxa occur at a maximum abundance greater than 10%. illustrates how hierarchical agglomerative clustering and DCA grouped the samples from Salmon River. Equivalent diagrams for each of the other sites are in the Online Appendix (Figs A1-A10).
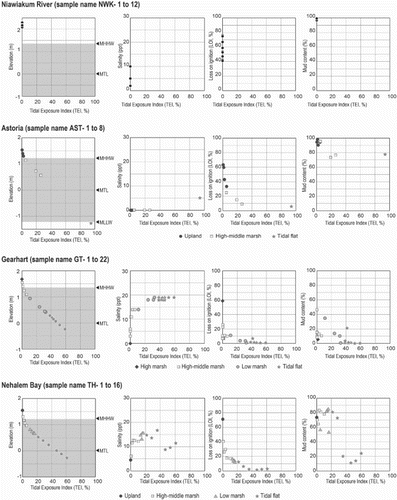
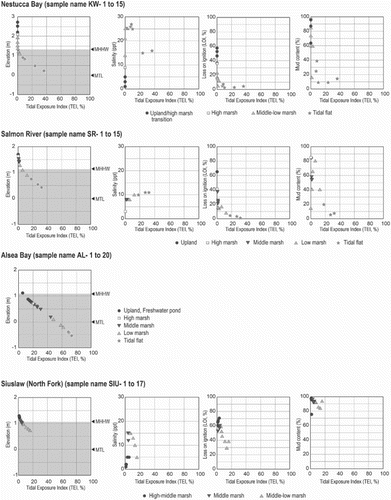
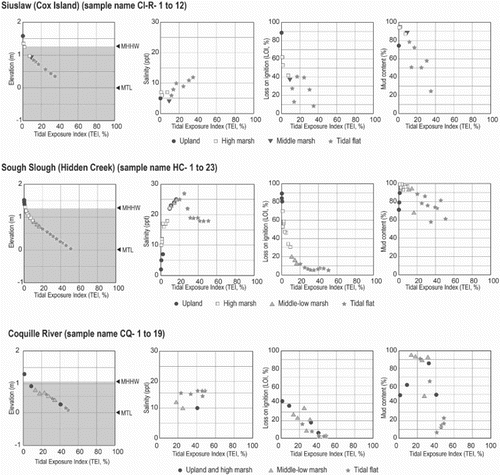
Fig. 4. Scatter plots between tidal exposure index (TEI) and elevation and three environmental variables at each site. MHHW; Mean Higher High Water, MLLW; Mean Lower Low Water, MTL; Mean Tidal Level. Gray zone in the left box at each site shows range of MHHW and MLLW.
Niawiakum River, Washington
Along the lower Niawiakum River, a tidal stream that flows into Willapa Bay, 82 taxa were identified in 12 samples (Fig. A1). Five species (Denticula subtilis Grunow, Tryblionella debilis Arnott, Cosmioneis pusilla (W.Smith) D.G.Mann & A.J.Stickle, Luticola mutica (Kützing) D.G.Mann, and Nitzschia brevissima Grunow) were dominant throughout this site. Four distinct groups of diatoms were recognized by hierarchical clustering. Cluster NWK-A is a single sample (NWK-11) dominated by Diadesmis contenta (Grunow ex Van Heurck) D.G.Mann. Cluster NWK-B consists of a single sample (NWK-3) characterized by Nitzschia scalpelliformis Grunow. Clusters NWK-C and NWK–D each comprise five samples. NWK-C is dominated mainly by C. pusilla and L. mutica. NWK-D is dominated by D. subtilis and D. contenta. Results of the DCA show that the sample assemblage of cluster NWK-B is an outlier due to the near absence of N. scalpelliformis in the other samples.
Astoria, Oregon
One hundred and six taxa were identified from eight samples along a transect where the Lewis and Clark River flows into Youngs Bay, southwest of the city of Astoria (Fig. A2). A benthic species (L. mutica) dominates assemblages at the landward edge of the transect, whereas freshwater planktonic forms (Aulacoseira granulata (Ehrenberg) Simonsen and Stephanodiscus Ehrenberg sp.) dominate the seaward edge of the transect, probably due to strong freshwater inflow from the Lewis and Clark, and Youngs Rivers. Dominant species in other samples are sample-specific. For example, the benthic Navicula rhynchocephala Kützing is dominant in assemblages AST-2 and AST-3, but absent in other samples. Hierarchical clustering reflected these sample-specific assemblages and produced six clusters, although DCA had showed two samples (clusters AST-A and AST-D) occupying similar ordination space. Benthic species, N. rhynchocephala and Navicula slesvicensis Grunow, dominate cluster AST-F, and the presence of Navicula cincta (Ehrenberg) Ralfs, D. subtilis, and Caloneis bacillum (Grunow) Cleve isolated AST-E from the other cluster.
Gearhart, Oregon
Near the mouth of the Necanicum River, south of the town of Gearhart on the outer Pacific coast, 122 taxa were identified from 22 samples (Fig. A3). Five species (L. mutica, C. pusilla, Pinnularia lagerstedtii (Cleve) Cleve-Euler, Pinnularia borealis Ehrenberg, and D. contenta) dominated assemblages from the high marsh. These species were gradually replaced in middle and low salt-marsh environments by T. debilis, Nitzschia bilobata W.Smith, and Diploneis smithii var. recta Peragallo. The tidal-flat assemblages are dominated by Navicula cancellata Donkin and Navicula palpebralis var. angulosa (Gregory) Van Heurck. Navicula gregaria Donkin is widespread throughout the transect, except in the upper high-middle marsh and supratidal. Hierarchical cluster analysis indicated the presence of two groups (Fig. A3). DCA shows that samples from clusters GT-A and GT-B, which are dominated by L. mutica, C. pusilla, and P. lagerstedtii, plot close together, implying that groups represented by the hierarchical cluster analysis may not be isolated from one another. Also, DCA suggests cluster GT-B may be subdivided into two subclusters (samples from tidal flat and others).
Nehalem Bay, Oregon
We identified 143 taxa from 16 samples along a transect on the north shore of Nehalem Bay (Fig. A4). Four species (D. subtilis, L. mutica, C. bacillum, and Navicula salinarum Grunow) characterize diatom assemblages in upland and high salt-marsh environments. Gyrosigma eximium (Thwaites) Boyer dominates the low salt marsh and upper tidal flat. Planothidium delicatulum and Catenula adhaerens (Mereschkowsky) Mereschkowsky dominate the remaining assemblages from the tidal flat. Navicula gregaria is widespread along the whole transect. Hierarchical clustering and DCA place the samples from Nehalem Bay in similar groups. The epipsammic species, P. delicatulum, dominates clusters TH-A and TH-C, whereas a high abundance of brackish species (e.g., D. subtilis) distinguishes cluster TH-B. The grouping of samples at Nehalem is influenced by counting P. delicatulum separately as frustules and non-raphid valves (P-valves).
Nestucca Bay, Oregon
Along a transect in the middle reaches of Nestucca Bay, 155 taxa were identified from 15 samples (Fig. A5). Diadesmis contenta dominates assemblages at the landward edge of the transect. Luticola mutica, C. pusilla and P. lagerstedtii are the most abundant taxa in high and middle salt-marsh environments. On the tidal flat, Navicula phyllepta Kützing, Amphora salina W. Smith, and Amphora luciae Cholnoky are characteristic species. At the seaward edge of the transect, two species of Melosira (Melosira moniliformis (O.F. Müller) C. Agardh and Melosira nummuloides C. Agardh) characterize diatom assemblages. Hierarchical clustering identified three clusters at Nestucca Bay. Cluster NB-A consists of a single supratidal assemblage. Cluster NB-B includes the high and middle salt-marsh samples; all remaining samples fall into cluster NB-C. DCA ordination places the clustered samples along axis one, but clusters A and B are not distinct.
Salmon River, Oregon
On a transect along the lower Salmon River, 151 taxa were identified from 14 samples (). Cosmioneis pusilla, D. subtilis, L. mutica, and P. lagerstedtii are dominant in landward assemblages. The dominant species in middle salt-marsh environments are C. bacillum and Diploneis nitescens (Gregory) Cleve. Navicula gregaria and M. nummuloides are restricted to the low marsh, while P. delicatulum appears to be distributed from the middle marsh to tidal flat. A benthic species (C. adhaerens) dominates samples from the sandy tidal flats. Hierarchical clustering and DCA identified three clusters at the Salmon River. Clusters SR-A and SR-B reflect high abundances of L. mutica and C. adhaerens, respectively. The DCA ordination is consistent with the cluster analyses and axis one is closely aligned with the environmental gradient from the tidal flat to the landward edge of the transect.
Alsea Bay, Oregon
We identified 148 taxa in 21 samples from a transect on the east shore of Alsea Bay (Fig. A6). Diadesmis contenta dominates the landward edge of the transect, whereas Pseudostaurosira perminuta (Grunow) Sabbe & Vyverman and P. delicatulum are dominant at the seaward edge. High and middle salt-marsh environments are dominated by C. pusilla, P. lagerstedtii and N. cincta. These species are replaced by G. eximium and N. gregaria in low salt-marsh samples. Hierarchical cluster analysis divides the samples from Alsea Bay into six clusters. However, DCA shows that some samples in clusters A, C, and D, which are dominated by C. pusilla, P. lagerstedtii and N. cincta, are similar.
North fork, Siuslaw River, Oregon
Along a transect on the North Fork of the Siuslaw River, 133 species were present in 16 samples (Fig. A7). Two benthic species, C. pusilla and P. lagerstedtii dominate the supratidal assemblages. Navicula gregaria appears in seaward samples, but the other brackish species (e.g., D. subtilis, L. mutica and T. debilis) are widely distributed among samples. Hierarchical clustering defined four clusters of samples. Cluster SIU-A is dominated by C. pusilla and P. lagerstedtii. Cluster SIU-B is distinguished by peaks of D. subtilis and Planothidium lanceolatum (Brébisson ex Kützing) Bukhtiyarova. Cluster SIU-C and SIU-D are distinct mainly due to their differences in the abundance of L. mutica and N. gregaria. DCA shows that clusters SIU-A and SIU-D, SIU-B, and SIU-C are distinct, but SIU-A and SIU-D are similar.
Cox Island, Siuslaw River, Oregon
At Cox Island in the middle tidal reaches of the Siuslaw River, we identified 124 taxa from 12 samples on a transect (Fig. A8). Samples from the landward end are dominated by L. mutica, P. lagerstedtii and T. debilis, whereas other assemblages are dominated by Fallacia tenera (Hustedt) A.J. Stickle & D.G. Mann, P. delicatulum, N. gregaria, and Navicula tenelloides Hustedt. Hierarchical clustering and DCA suggest that the samples fall into two clusters. Cluster CI-R-A consists only of the three most landward samples. CI-R-A is probably differentiated from CI-R-B mainly by the appearance of L. mutica and P. lagerstedtii and the disappearance of P. delicatulum and N. gregaria.
South Slough (Hidden Creek), Coos Bay, Oregon
At the Hidden Creek marsh in the middle reaches of the South Slough arm of Coos Bay, we identified 124 taxa from 23 samples (Fig. A9). Some dominant species (C. pusilla, D. subtilis, M. nummuloides and P. perminuta) change their abundance gradually along the transect, but the abundances of other species change abruptly. For example, P. lagerstedtii peaks in the HC-18 assemblage, but is nearly absent in the adjacent samples along the transect. The results of the cluster analysis and DCA are inconclusive. For the DCA, the distribution of samples plotted along axis one suggests that these eigenvalues primarily reflect sample elevation along the gradient from tidal flat to upland.
Coquille River, Oregon
Along an 850-m-long transect near the mouth of the Coquille River, 128 taxa were identified in 16 samples (Fig. A10). Planothidium delicatulum and C. adhaerens dominate assemblages from the tidal flat. Gyrosigma eximium and N. scalpelliformis appear in the middle-low marsh samples. Navicula gregaria occurs in assemblages throughout the transect, but it peaks at the seaward edge of the tidal flat. Hierarchical cluster analysis groups the Coquille River samples into eight clusters. Because the clusters are not distinct on the DCA ordination diagram, we are uncertain of the significance of the cluster analyses.
Sediment analysis
The relationship between environmental variables and tidal exposure was investigated by plotting measured variables against the tidal exposure index (TEI) for all sites with the necessary data (Figs and A11). In general, salinity increases at low elevation samples near the sea and it declines in samples at higher elevations. But there is significant variability, e.g. high salinity values were measured at high elevations at Nehalem, Siuslaw (North Fork), Nestucca and South Slough), probably due to local evaporation. There are also fluctuations in salinity at low elevations at Nehalem, Nestucca and South Slough. Astoria has the lowest salinity because of the large freshwater discharge from the Lewis and Clark, and Youngs Rivers as well as the adjacent Columbia River (Figs and A11a). The low organic content of surface samples (LOI) corresponds with samples from lower TEI sites (Figs and A11b). Grain-size distributions for samples, represented by the percent mud content (silt plus clay, less than 63 µm), do not show curvilinear or linear relationships to TEI and may be location dependent (Figs and A11c).
Environmental gradients vary among sites because of differences in physiographic features. Differences in the amount of freshwater discharge result in different salinity gradients. We captured this diversity by sampling 11 sites with distinct physiographic features.
Statistical analysis of the regional dataset
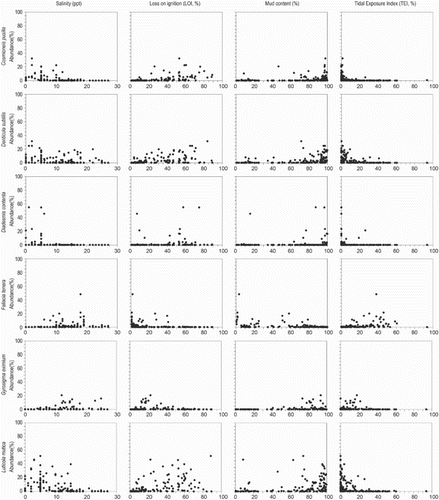
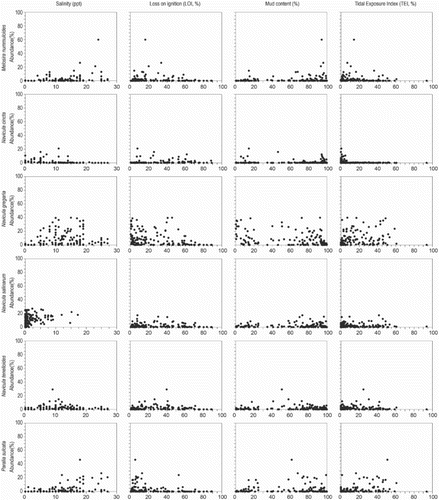
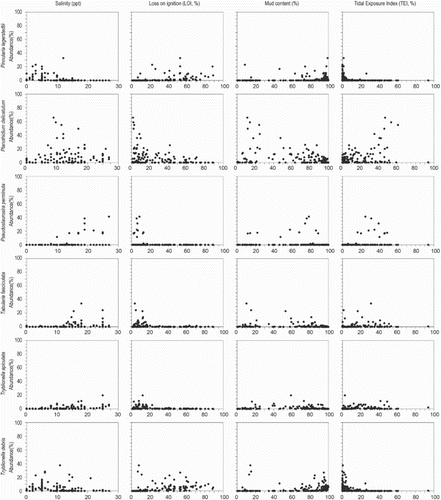
The relationship between the abundance of individual diatom species and environmental variables is shown in scatter plots (). Most species respond unimodally to salinity. Some brackish species have different responses to tidal exposure, either gradually (P. delicatulum) or exponentially (e.g., C. pusilla, D. subtilis and L. mutica) increasing with elevation, or showing a unimodal (F. tenera) relation. Planothidium delicatulum appears in samples from brackish water, with low organic content and long periods of tidal inundation (high TEI). The brackish diatom N. gregaria shows exceptionally high abundance along all environmental gradients, indicating that its distribution is unrelated to the measured environmental variables on the Oregon and Washington coast. Paralia sulcata (Ehrenberg) Cleve also shows a widespread distribution, but the latter is planktonic or tychoplanktonic in coastal waters, forming chains of cells with interlinking spines (Crawford Citation1979, McQuoid & Nordberg Citation2003). The cells are easily picked up into the water column by daily tides, dispersed throughout all tidal environments, and then widely distributed during ebb tides (Sawai Citation2001).
Fig. 5. Scatter plots of principal diatom species abundance (%) and four environmental variables. Principal species have >5% abundance in more than 10 samples.
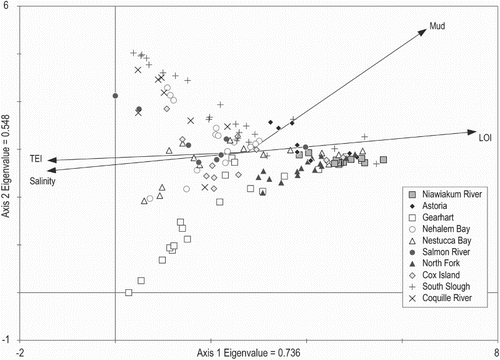
Detrended correspondence analysis (DCA) was used to investigate species-environmental relationships in the combined regional dataset (). DCA axis 1 is related positively to LOI and negatively to salinity and TEI. This probably demonstrates that elevation is the primary control on the distribution of intertidal diatoms at the regional scale in Oregon and Washington. DCA axis 2 partly reflects the influence of grain-size distribution, with sand-dominated samples plotting higher on DCA axis 2 than mud-dominated samples.
Fig. 6. Results of detrended correspondence analysis (DCA) showing patterns of variation in diatom sample assemblages for the combined regional dataset. The environmental variables were added as supplementary variables. Numbers of samples at each site are shown in Table A1.
Canonical correspondence analysis (CCA) supports the inferences drawn from cluster analysis and DCA (). The CCA axis 1 (eigenvalue = 0.612) is correlated with TEI, LOI and salinity, whereas axis 2 (eigenvalue = 0.312) is weakly correlated with percent mud content (; a). These two axes explain 7.3% and 69.0% of the species data and species-environment variance, respectively. The sample-environment biplots (a) show that axis 1 reflects an environmental gradient from the tidal flat plotted on the left (high salinity, long duration of tidal inundation with high TEI, and low LOI) to the high marsh/supratidal plotted on the right (low salinity, short duration of tidal inundation, and high LOI). The sample-environment biplots show that samples from multiple sites overlap one another in ordination space, indicating that distinctive assemblages of diatoms are found at more than one site, consistently co-varying along environmental gradients.
Fig. 7. Results of canonical correspondence analysis (CCA) for the combined regional dataset: (Left) sample-environment bi-plots. (Right) species-environment bi-plots. Species abbreviations are as follows: Cosmioneis pusilla (COSPU), Diploneis didyma (DIPDYD), Eunotia praerupta (EUPRA), Gyrosigma eximium (GYEX), Melosira moniliformis (MEMO), Navicula cancellata (NACAN), Navicula cincta (NACI), Navicula gregaria (NAGRE), Navicula palpebralis (NAPAL), Navicula salinicola (NASALI), Navicula salinarum (NASAL), Navicula slesvicensis (NASLE), Paralia sulcata (PASU), Pinnularia borealis (PINBOR), Pinnularia lagerstedtii (PINLAG), and Tryblionella salinarum (TRYSA).
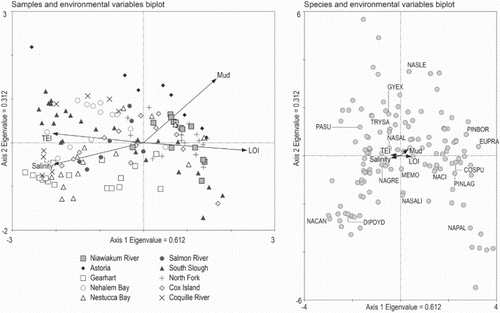
Table 1. Summary of CCA results for combined regional diatom dataset (species with maximum relative abundance greater than 5%).
The species-environment biplots (b) show which species characterize particular assemblages and environments. For example, typically freshwater taxa, such as Eunotia praerupta Ehrenberg (EUPRA) and P. borealis (PINBOR), have positive correlations with axis 1, with high LOI, low TEI, and low salinity. High-marsh taxa, such as C. pusilla (COSPU) and P. lagerstedtii (PINLAG), plot close to the freshwater species and, therefore, have similar correlations with these variables. In contrast, tidal-flat and low-marsh species, such as G. eximium (GYEX) and Tryblionella salinarum (Grunow) Pantocsek (TRYSA), have positive correlations with higher mud content, longer duration of tidal inundation and higher salinity. Navicula cancellata (NACAN) illustrates the influence of grain-size distribution because these species are found exclusively on sandy (low mud content) tidal flats that experience long periods of inundation.
Variation partitioning suggests that the four environmental variables that we measured explain 10.6% of the total variance observed in the regional diatom training set. This explained variance is attributed to percent mud content (22.6%), salinity (20.1%), TEI (17.0%) and organic content (17.9%; ) as unique contributions. Monte Carlo permutation tests indicate that each of these gradients accounts for a significant portion of the total variance in the diatom data (p = 0.001, 999 random permutations). Inter-correlations between environmental variables account for almost a quarter (21.7%) of the total explained variance within the diatom dataset.
Table 2. Environmental variables selected using manual forward selection within CANOCO.
Discussion
The majority of the diatom assemblages that we identified at the 11 sites in Oregon and Washington show relationships with tidal exposure, salinity and or substratum that are consistent with the conclusions of earlier studies of salt marshes and tidal flats, which reported that diatom assemblages are vertically zoned with respect to the tidal regime (e.g., Riznyk Citation1973, Sullivan Citation1975, Citation1978, Amspoker Citation1977, Whiting & McIntire Citation1985, Laird & Edgar Citation1992, Nelson & Kashima Citation1993, Hemphill-Haley Citation1995, Zong & Horton Citation1998, Sherrod Citation1999, Horton et al. Citation2006, Citation2007, Roe et al., Citation2009) (Figs and ). For example, the maximum abundances of L. mutica and D. contenta (common brackish or subaerial taxa) in our dataset occur in high salt-marsh environments. Other brackish diatoms, G. eximium and N. salinarum are abundant throughout middle and low salt-marsh environments. Epipsammic species, such as F. tenera and C. adhaerens, dominate sandy tidal flat environments.
However, we also noted differences in the distribution of salt-marsh species compared to those found in previous studies. For example, Nelson & Kashima (Citation1993) reported P. delicatulum to be widely distributed throughout the intertidal zone at Coos Bay and Coquille River, Oregon. However, apart from our South Slough site, this species dominated tidal-flat and low salt-marsh environments at our other sites. The widespread distribution of P. delicatulum reported by Nelson & Kashima (Citation1993) may be a result of counting both frustules and P-valves for this species. The P-valves removed from dead specimens are more susceptible to transport (allochthonous) and may distort ecological interpretations (Sawai Citation2001).
Comparisons of our local datasets show that diatom assemblages are not always zoned with respect to the same environmental variables (Tables A3 and A4). Environmental gradients are complicated by differences in physiographic features. For example, the steep slopes of tidal channel levees produce large gradients of tidal exposure, vegetation cover and substratum over short distances. Differences in the amount of freshwater discharge result in differences in salinity gradients. The variability of coastal environments explains the variation in abundance of some species among sites. Thus, our diatom dataset, which covers a wide range in environmental variables, helps us better understand the ecology of diatom species.
Our results emphasize that autecological studies of diatom species require many samples from a range of modern depositional environments. The abundance of some of the most common species varies widely among sites. For example, N. gregaria was common in tidal-flat, low salt-marsh and high salt-marsh settings at Nehalem Bay, but was only found in the tidal flat and low salt marsh at Nestucca Bay and Salmon River. Gyrosigma eximium achieved a maximum abundance in low marsh samples at Alsea Bay, but in middle-low marsh samples at Coquille River. Even within a local dataset, relative abundances of N. scalpelliformis and D. contenta vary among samples in near-identical environments only decimeters apart along the Niawiakum River. Such variability in the distribution of the most common taxa has been reported previously (e.g., Cunningham & McMinn Citation2004, Roe et al. Citation2009, Watcham et al. Citation2013).
Sampling season and methodology of sample collection may significantly affect assemblage composition, especially during local or seasonal blooms (e.g., Roe et al. Citation2009). Our samples were collected in spring or autumn (Table A1), periods when diatoms bloom (e.g., Graf et al. Citation1983, Muylaert et al. Citation2000, Villac & Kaczmarska Citation2011, Stonik et al. Citation2012), probably because temperatures are most favourable for cell division (McIntire Citation1978, Admiraal & Peletier Citation1980, Fogg & Bajpai Citation1988).
Counting methodology may also bias the relative abundance of diatoms. Benthic diatoms generally show large variations in size and shape (Round et al. Citation1990). For example, along Baltic Sea coasts the largest mean cell dimension varies between 4.2 and 653 μm (Snoeijs et al. Citation2002). Diatom analysis normally uses counting data based on a fixed number of diatom valves and frustules, and the probability of encountering larger species (e.g., Arachnoidiscus H. Deane & G. Shadbolt and Pleurosigma W. Smith) in the analysis may be low, due to differences in community density between large and small species. Our regional dataset does not include such large species, but large species might be under-represented if the dataset included them. Regardless of the cause of such differences, our results highlight the importance of employing a large regional dataset from the full range of tidal and adjacent environments to diminish the influence of site-specific assemblages.
The zonation of diatom assemblages based on the cluster analysis and ordination analyses (DCA and CCA) indicates that grain-size distribution is one of the most important controls on the distribution of diatom taxa across the upper intertidal zone in coastal estuaries, lagoons and embayments in Oregon and Washington. The abundance of attached taxa (e.g., epipsammic and epiphytic) is greatly affected by grain-size distribution and the appearance or disappearance of aquatic plants (e.g., macrophytes). The grain-size distributions are the result of proximally active sediment sources, such as eroding sea cliffs or discharge from coastal rivers and streams, and the hydrodynamic conditions, such as daily tides. Apart from samples in Gearhart, where daily tides expose the surface near mean tidal level, sand content increases and epipsammic and some epipelic species accumulate (Figs and ).
Some previous studies have investigated using similar approaches for local datasets. For example, Sullivan (Citation1982) employed CCA to understand the relationship between diatom distribution and ten environmental variables in a Mississippi salt marsh. He (Sullivan Citation1982) showed that the elevation and height of the spermatophyte canopy were related to the distribution of diatoms. Using CCA with diatom assemblages in Puget Sound, Washington, Sherrod (Citation1999) found that the species distributions primarily reflected salinity, and secondarily elevation. Zong & Horton (Citation1999) used CCA to study the relationships between diatom assemblages and several environmental variables from six salt marshes in southern Scotland and northern England. They identified three diatom assemblage zones related to the frequency of tidal exposure, salinity, and whether the substrata were muddy or sandy.
The variability of diatom assemblages among sites has been frequently recognized in regional studies of modern diatom distributions, particularly in higher intertidal environments (e.g., Nelson et al. Citation2008, Kemp et al. Citation2009, Woodroffe & Long Citation2009, Wilson & Lamb Citation2012). Our CCA shows that more than 89% of the total variation in the regional diatom training set is not explained by the environmental variables measured, either individually or in combination. We also performed CCA on local datasets (Tables A3 and A4), which show higher values of explained variation than CCA analysis on the combined regional dataset (e.g., Gearhart, 41.6%; South Slough, 33.8%; Coquille River, 52.6%). Although the inter-site variation in diatom assemblage composition is high, the percentage of explained variation is similar to the percentages found in many other biological datasets, with a large number of samples with many zero values (Jones & Juggins 1995, Zong & Horton 1999).
Whether this unexplained variance is due to some overlooked variable(s) or to a large amount of stochastic variation remains unclear. In this study, we tested only four environmental variables, but coastal diatoms are well known to be sensitive to other parameters including light intensity (Admiraal & Peletier Citation1980, Hudon & Bourget Citation1983), temperature (Amspoker & McIntire Citation1978, McIntire Citation1978, Fogg & Bajpai Citation1988), nitrogen and phosphorus concentration (Weckström & Juggins Citation2005), desiccation (Amspoker & McIntire Citation1978, McIntire Citation1978), and predation (Pillet et al. Citation2011).
Light intensity, which co-varies with tidal exposure time, can control the growth rate of diatom assemblages (Admiraal & Peletier Citation1980, Hudon & Bourget Citation1983). For example, when seasonal variations of light, temperature, and growth rate of benthic diatom species (e.g., N. salinarum) in the Wadden Sea were observed over six months, only differences in light period and irradiance values affected diatom cell division rates (Admiraal & Peletier Citation1980). Such variation in division rates was also reported from experimental studies on certain diatom species (Admiraal Citation1977a).
Nutrient concentration, which co-varies with salinity, is one of the most likely drivers for changing diatom distributions (Underwood et al. Citation1998). Local variability in freshwater input can induce differences in the nutrient levels in brackish environments within a small area (De Sève Citation1993). Ammonium, phosphorus, and silicate concentrations, for example, influence species compositions of diatom assemblages (Admiraal Citation1977b, Underwood et al. Citation1998, Sullivan Citation1999, Weckström & Juggins Citation2005, Snoeijs & Weckström Citation2010). Peletier (Citation1996) inferred changes in diatoms assemblages in coastal environments from reductions in nutrients over 10 years. Results from culturing diatoms confirm that nutrient variation also produces differential growth rates among brackish diatoms, such as N. phyllepta and N. salinarum (Underwood & Provot Citation2000).
Seasonal grazing may reduce the abundance of benthic diatoms. Diatoms in freshwater and brackish environments are grazed by meso- and macro-fauna; including foraminifera (Pillet et al. Citation2011, Pillet & Pawlowski Citation2013), polychaetes (Montagna Citation1984, Martin et al. Citation1996), insects (Hill & Knight Citation1987, Citation1988), and fish (Abe et al. Citation2003, Citation2006).
Despite the many studies on the relationships between environmental variables and diatom assemblages, it is still quite difficult to identify the primary controlling variables at most coastal sites. Primary environmental variables are commonly inter-correlated with the distribution of other organisms at coastal sites, for example, tidal exposure, salinity, and nutrients also control vascular plant distribution. In turn, vascular plant community composition affects the organic content (LOI in this study) of surface soils and light intensity at the soil surface.
A further complicating factor in understanding how diatom assemblages vary in response to environmental variables is that molecular markers now indicate that species identified through microscopy-based studies may comprise several semi-cryptic or cryptic species (e.g., Créach et al. Citation2006). For example, Créach et al. (Citation2006) and Vanelslander et al. (2009) showed that N. phyllepta sensu lato comprises different species with specific ecophysiological characteristics. A quantitative real-time polymerase chain reaction study revealed two clusters of N. phyllepta whose distributions and abundances differed along the Westerschelde estuary (Créach et al. Citation2006). Thus, molecular analyses may be required to fully understand the autecology of diatom species.
Conclusions
The relationships among diatom species in 141 assemblages along intertidal transects at 11 sites along the Oregon and Washington coast were evaluated against four environmental variables (tidal exposure, salinity, grain-size distribution and organic content). The results of CCA show that individual species abundances in the combined regional data set of sample assemblages were primarily correlated with percent mud content, and secondarily with salinity and tidal exposure. In general, the species composition of diatom assemblages at our sites was consistent with patterns documented for salt marshes and tidal flats elsewhere. Species with polyhalobion and mesohalobion preferences occupy the marine and brackish environments of tidal flats and salt marshes, and oligohalobion forms occupy the high marsh and freshwater environments. The site-specific diversity of diatom assemblages makes it necessary to compile a large modern training set from a range of ecologically diverse sites in order to adequately characterize species–environment relationships.
Supplemental data
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/0269249X.2015.1126359
Sawai_Appnedix-r6.pdf
Download PDF (545.7 KB)Acknowledgements
This research was supported by National Science Foundation award (EAR 0842728, 1419824 and 1419846), the Earthquake Hazards Program of the U.S. Geological Survey, the Society of Sedimentary Geology (SEPM – Sanders Student Research Fund), and the Japan Society for the Promotion of Science (JSPS Postdoctoral Fellowships for Research Abroad). We thank Rob Witter, Simon Engelhart, Brian Atwater and Emily Smith for assistance with fieldwork. Roger Lewis and Linda Gerson collected and surveyed elevations for the samples from Alsea Bay under the guidance of Eileen Hemphill-Haley. Eileen Hemphill-Haley and anonymous reviewers provided thorough, constructive comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abe S., Iguchi K., Ito S., Uchida Y., Ohnishi H. & Ohmori K. 2003. Habitat use of the grazing goby (Sicyopterus japonicus) in response to spatial heterogeneity in riparian shade. Journal of Freshwater Ecology 18: 161–167. doi: 10.1080/02705060.2003.9663962
- Abe S., Kiso K., Katano O., Yamamoto S., Nagumo T. & Tanaka J. 2006. Impacts of differential consumption by the grazing fish, Plecoglossus altivelis, on the benthic algal composition in the Chikuma River, Japan. Phycological Research 54: 94–98. doi: 10.1111/j.1440-1835.2006.00413.x
- Adamus P.R. 2005. Science review and data analysis for tidal wetlands of the Oregon coast. Part 2 of a Hydrogeomorphic Guidebook. Report to Coos Watershed Association, U.S. Environmental Protection Agency, and Oregon Dept. of State Lands, Salem.
- Admiraal W. 1977a. Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Marine Biology 39: 1–9. doi: 10.1007/BF00395586
- Admiraal W. 1977b. Salinity tolerance of benthic estuarine diatoms as tested with a rapid polarographic measurement of photosynthesis. Marine Biology 39: 11–18. doi: 10.1007/BF00395587
- Admiraal W. & Peletier H. 1979. Sulphide tolerance of benthic diatoms in relation to their distribution in an estuary. British Phycological Journal 14: 185–196. doi: 10.1080/00071617900650201
- Admiraal W. & Peletier H. 1980. Influence of seasonal variations of temperature and light on the growth rate of cultures and natural populations of intertidal diatoms. Marine Ecology Progress Series 2: 35–43. doi: 10.3354/meps002035
- Admiraal W., Peletier H. & Brouwer T. 1984. The seasonal succession patterns of diatom species on an intertidal mud flat: an experimental analysis. OIKOS 42: 30–40. doi: 10.2307/3544606
- Aleem A.A. 1950. Distribution and ecology of British marine littoral diatoms. Journal of Ecology 38: 75–106. doi: 10.2307/2256526
- Amspoker M.C. 1977. The distribution of intertidal epipsammic diatoms on Scripps Beach, La Jolla, California, USA. Botanica Marina 20: 227–232. doi: 10.1515/botm.1977.20.4.227
- Amspoker M.C. & McIntire C.D. 1978. Distribution of intertidal diatoms associated with sediments in Yaquina Estuary, Oregon. Journal of Phycology 14: 387–395. doi: 10.1111/j.1529-8817.1978.tb02457.x
- Amspoker M.C. & McIntire C.D. 1986. Effect sedimentary processes and salinity on the diatom flora of the Columbia River estuary. Botanica Marina 29: 391–399. doi: 10.1515/botm.1986.29.5.391
- Atwater B.F. & Hemphill-Haley E. 1997. Recurrence intervals for great earthquakes of the past 3,500 years at northeastern Willapa Bay, Washington. U.S. Geological Survey Professional Paper 1576, 108pp.
- Ball D.F. 1964. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. Journal of Soil Science 15: 84–92. doi: 10.1111/j.1365-2389.1964.tb00247.x
- Busse S. & Snoeijs P. 2003. Gradient responses of diatom communities in the Bothnia Sea (northern Baltic Sea), with emphasis on responses to water movement. Phycologia 42: 451–464. doi: 10.2216/i0031-8884-42-5-451.1
- Cooke S.S. 1997. A field guide to common wetland plants of western Washington and north western Oregon. Seattle Audubon Society and Washington Native Plant Society, Seattle. 417 pp.
- Crawford R.M. 1979. Taxonomy and frustular structure of the marine centric diatom Paralia sulcata. Journal of Phycology 15: 200–210. doi: 10.1111/j.0022-3646.1979.00200.x
- Créach V., Ernst A., Sabbe K., Vanelslander B., Vyverman W. & Stal L.J. 2006. Using quantitative PCR to determine the distribution of a semicryptic benthic diatom, Navicula phyllepta (Bacillariophyceae). Journal of Phycology 42: 1142–1154. doi: 10.1111/j.1529-8817.2006.00268.x
- Cunningham L. & McMinn A. 2004. The influence of natural environmental factors on benthic diatom communities from the Windmill Islands, Antarctica. Phycologia 43: 744–755. doi: 10.2216/i0031-8884-43-6-744.1
- De Sève M.A. 1993. Diatom bloom in the tidal freshwater zone of a turbid and shallow estuary, Rupert Bay (James Bay, Canada). Hydrobiologia 269–270: 225–233. doi: 10.1007/BF00028021
- Fogg G.E. & Bajpai S.P. 1988. Temperature effects on natural phytoplankton populations. In: Algae and the aquatic environment (Ed by F.E. Round), pp. 197–204. Biopress Ltd., Bristol.
- Frenkel R.E., Eilers H.P. & Jefferson C.A. 1981. Oregon coastal salt marsh upper limits and tidal datums. Estuaries 4: 198–205. doi: 10.2307/1351475
- Graf G., Schulz R., Peinert R. & Meyer-Reil L.-A. 1983. Benthic response to sedimentation events during autumn to spring at a shallow-water station in the Western Kiel bight. Marine Biology 77: 235–246. doi: 10.1007/BF00395812
- Haubois A.G., Sylvestre F., Guarini J.M., Richard P. & Blanchard G.F. 2005. Spatio-temporal structure of the epipelic diatom assemblage from an intertidal mudflat in Marennes-Oléron Bay, France. Estuarine, Coastal and Shelf Science 64: 385–394. doi: 10.1016/j.ecss.2005.03.004
- Hawkes A.D., Horton B.P., Nelson A.R. & Hill D.F. 2010. The application of intertidal foraminifera to reconstruct coastal subsidence during the giant Cascadia earthquake of AD 1700 in Oregon, USA. Quaternary International 221: 116–140. doi: 10.1016/j.quaint.2009.09.019
- Hemphill-Haley E. 1993. Taxonomy of recent and fossil (Holocene) diatoms (Bacillariophyta) from northern Willapa Bay, Washington. U.S. Geological Survey Open-File Report 93–289, 151p.
- Hemphill-Haley E. 1995. Intertidal diatoms from Willapa Bay, Washington: application to studies of small-scale sea-level changes. Northwest Science 69: 29–45.
- Hendey N.I. 1964. An introductory account of the smaller algae of British coastal waters. V. Bacillariophyceae (Diatoms). Otto Koeltz Scientific Publishers, Koenigstein, 317 pp.
- Hill W.R. & Knight A.W. 1987. Experimental analysis of the grazing interaction between a mayfly and stream algae. Ecology 68: 1955–1965. doi: 10.2307/1939886
- Hill W.R. & Knight A.W. 1988. Concurrent grazing effects of two stream insects on periphyton. Limnology and Oceanography 33: 15–26. doi: 10.4319/lo.1988.33.1.0015
- Horton B.P., Corbett R., Culver S.J., Edwards R.J. & Hiller C. 2006. Modern saltmarsh diatom distributions of the outer banks, North Carolina, and the development of a transfer function for high resolution reconstructions of sea level. Estuarine, Costal and Shelf Science 69: 381–394. doi: 10.1016/j.ecss.2006.05.007
- Horton B.P., Zong Y., Hillier C. & Engelhart S. 2007. Diatoms from Indonesian mangroves and their suitability as sea-level indicators for tropical environments. Marine Micropaleontology 63: 155–168. doi: 10.1016/j.marmicro.2006.11.005
- Hudon C. & Bourget E. 1983. The effect of light on the vertical structure of epibenthic diatom communities. Botanica Marina 26: 317–3330. doi: 10.1515/botm.1983.26.7.317
- Hustedt F. 1930. Die Kieselalgen. In: Kryptogamen-Flora von Deutschland, Österreich und der Schweiz (Ed. by L. Rabenhorst), Band 7, Teil 1. 920 pp. Akademische Verlagsgesellschaft, Leipzig.
- Hustedt F. 1957. Die Diatomeenflora des Fluss-systems der Weser im Gebiet der Hansestadt Bremen. Abhandlungen des Naturwissenschaftlicher Verein zu Bremen 34: 181–440.
- Jones V.J. & Juggins S. 1995. The construction of diatom-based chlorophyll transfer function and its application at three lakes on Signy Island (marine Antarctic) subject to differing degrees of nutrient enrichment. Freshwater Biology 34: 433–445. doi: 10.1111/j.1365-2427.1995.tb00901.x
- Juggins S. 1992. Diatoms in the Thames estuary, England: ecology, palaeoecology, and salinity transfer function. Bibliotheca Diatomologica 25: 1–216.
- Juggins S. 2013. Quantitative reconstructions in palaeolimnology: new paradigm or sick science? Quaternary Science Reviews 64: 20–32. doi: 10.1016/j.quascirev.2012.12.014
- Kemp A.C., Horton B.P., Corbett D.R., Culver S.J., Edwards R.J. & van de Plassche O. 2009. The relative utility of foraminifera and diatoms for reconstructing late Holocene sea-level change in North Carolina, USA. Quaternary Research 71: 9–21. doi: 10.1016/j.yqres.2008.08.007
- Komar P.D. 2004. Oregon's coastal cliffs: processes and erosion impacts. In: Hampton MA, Griggs GB (eds.) Formation, evolution and stability of coastal cliffs – status and trends. U.S. Geological Survey Professional Paper 1693:65–80.
- Kosugi M. 1987. Limiting factors on the distribution of benthic diatoms in coastal regions – salinity and substratum. Diatom 3: 21–31.
- Krammer K. & Lange-Bertalot H. 1986. Süßwasserflora von Mitteleuropa. Bacillariophyceae 1. Teil:Naviculaceae. Gustav Fischer Verlag, Stuttgart. 876pp.
- Krammer K. & Lange-Bertalot H. 1988. Süßwasserflora von Mitteleuropa. Bacillariophyceae 2. Teil:Bacillariaceae, Epithemiaceae, Surirellaceae. Gustav Fischer Verlag, Stuttgart. 610pp.
- Krammer K. & Lange-Bertalot H. 1991a. Süßwasserflora von Mitteleuropa. Bacillariophyceae 3. Teil:Centrales, Fragilariaceae, Eunotiaceae. Gustav Fischer Verlag, Stuttgart. 576pp.
- Krammer K. & Lange-Bertalot H. 1991b. Süßwasserflora von Mitteleuropa. Bacillariophyceae 4. Teil:Achnanthaceae Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gustav Fischer Verlag, Stuttgart. 437pp.
- Laird K. & Edgar R.K. 1992. Spatial distribution of diatoms in the surficial sediments of a New England salt marsh. Diatom Research 7: 267–279. doi: 10.1080/0269249X.1992.9705219
- Lange-Bertalot H. 2000. Transfer to the generic rank of Decussata Patrick as a subgenus of Navicula Bory sensu lato. Iconographia Diatomologica 9: 670–673.
- Lange-Bertalot H. 2001. Diatoms of Europe. Diatoms of the European inland waters and comparable habitats. Volume 2. Navicula sensu stricto 10 genera separated from Navicula sensu lato. A.R.G. Ganter Verlag K.G., Ruggell. 526pp.
- Laws R.A. 1988. Diatoms (Bacillariophyceae) from surface sediments in the San Francisco Bay estuary. Proceedings of the California Academy of Sciences 45: 133–254.
- Maechler M., Rousseeuw P., Struyf A. & Hubert M. 2005. Cluster Analysis Basics and Extensions. R package version 1.14.4.
- Main S.P. & McIntire C.D. 1974. The distribution of epiphytic diatoms in Yaquina Estuary, Oregon (USA). Botanica Marina 17: 88–99. doi: 10.1515/botm.1974.17.2.88
- Martin D., Pinedo S. & Sardá R. 1996. Grazing by meroplanktonic polychaete larvae may help to control nanoplankton in the NW Mediterranean littoral: in situ experimental evidence. Marine Ecology Progress Series 143: 239–246. doi: 10.3354/meps143239
- McIntire C.D. 1973. Diatom associations in Yaquina estuary, Oregon: a multivariate analysis. Journal of Phycology 9: 254–259.
- McIntire C.D. 1978. The distribution of estuarine diatoms along environmental gradients: a canonical correlation. Estuarine and Coastal Marine Science 6: 447–457. doi: 10.1016/0302-3524(78)90023-3
- McIntire C.D. & Overton W.S. 1971. Distributional patterns in assemblages of attached diatoms from Yaquina Estuary, Oregon. Ecology 52: 758–777. doi: 10.2307/1936024
- McQuoid M.R. & Nordberg K. 2003. The diatom Paralia sulcata as an environmental indicator species in coastal sediments. Estuarine, Coastal and Shelf Science 56: 339–354. doi: 10.1016/S0272-7714(02)00187-7
- Moore W.W. & McIntire C.D. 1977. Spatial and seasonal distribution of littoral diatoms in Yaquina Estuary, Oregon (USA). Botanica Marina 20: 99–109. doi: 10.1515/botm.1977.20.2.99
- Montagna P.A. 1984. In situ measurement of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Marine Ecology Progress Series 18: 119–130. doi: 10.3354/meps018119
- Muylaert K., Sabbe K. & Vyverman W. 2000. Spatial and temporal dynamics of phytoplankton communities in a freshwater tidal estuary (Schelde, Belgium). Estuarine, Coastal and Shelf Science 50: 673–687. doi: 10.1006/ecss.2000.0590
- Nelson A.R. & Kashima K. 1993. Diatom zonation in southern Oregon tidal marshes relative to vascular plants, foraminifera, and sea level. Journal of Coastal Research 9: 673–697.
- Nelson A.R., Sawai Y., Jennings A., Bradley L., Sherrod B., Sabean J. & Horton B.P. 2008. Great-earthquake paleogeodesy and tsunamis of the past 2000 years at Alsea Bay, central Oregon coast, USA. Quaternary Science Reviews 27: 747–768. doi: 10.1016/j.quascirev.2008.01.001
- Økland R.H. & Eilertsen O. 1994. Canonical correspondence analysis with variation partitioning: some comments and an application. Journal of Vegetation Science 5: 117–126. doi: 10.2307/3235645
- Patrick R. & Reimer C. 1966. The Diatoms of United States. Exclusive of Alaska and Hawaii. Volume 1. The Academy of Natural Sciences of Philadelphia, Philadelphia. 688pp.
- Patrick R. & Reimer C. 1975. The Diatoms of United States. Exclusive of Alaska and Hawaii. Volume 2. The Academy of Natural Sciences of Philadelphia, Philadelphia. 213pp.
- Peletier H. 1996. Long-term changes in intertidal estuarine diatom assemblages related to reduced input of organic waste. Marine Ecology Progress Series 228: 47–56.
- Pillet L. & Pawlowski J. 2013. Transcriptome analysis of foraminiferan Elphidium margaritaceum questions the role of gene transfer in kleptoplastidy. Molecular Biology and Evolution 30: 66–69. doi: 10.1093/molbev/mss226
- Pillet L., de Vargas C. & Pawlowski J. 2011. Molecular identification of sequestered diatom chloroplasts and kleptoplastidy in foraminifera. Protist 162: 394–404. doi: 10.1016/j.protis.2010.10.001
- Pojar J. & MacKinnon A. (Compilers and Editors) 1994. Plants of the Pacific Northwest coast. B.C. Ministry of Forests and Lone Pine Publishing, Redmond, Washington, 526 pp.
- Riznyk R.Z. 1973. Interstitial diatoms from two tidal flats in Yaquina estuary, Oregon, USA. Botanica Marina 16: 113–138. doi: 10.1515/botm.1973.16.3.113
- Roe H.M., Doherty C.T., Patterson R.T. & Swindles G.T. 2009. Contemporary distributions of saltmarsh diatoms in the Seymour-Belize Inlet complex, British Columbia, Canada: implications for studies of sea-level change. Marine Micropaleontology 70: 134–150. doi: 10.1016/j.marmicro.2008.12.001
- Round F.E. 1988. Algae and the aquatic environment: contributions in honour of J.W.G. Lund. Biopress Ltd., Bristol. 460 pp.
- Round F.E., Crawford R.M. & Mann D.G. 1990. The diatoms. Biology and morphology of the genera. Cambridge University Press, Cambridge. 747 pp.
- Rovira L., Trobajo R., Leira M. & Ibáñez C. 2012. The effects of hydrological dynamics on benthic diatom community structure in a highly stratified estuary: the case of the Ebro Estuary (Catalonia, Spain). Estuarine, Coastal and Shelf Science 101: 1–14. doi: 10.1016/j.ecss.2011.12.033
- Sabbe K. 1993. Short-term fluctuations in benthic diatom numbers on an intertidal sandflat in the Westerschelde estuary (Zeeland, The Netherlands). Hydrobiologia 269-270: 275–284. doi: 10.1007/BF00028026
- Sawai Y. 2001. Distribution of living and dead diatoms in tidal wetlands of northern Japan: relations to taphonomy. Palaeogeography Palaeoclimatology Palaeoecology 173: 125–141. doi: 10.1016/S0031-0182(01)00313-3
- Sawai Y. & Nagumo T. 2003. Diatoms from Alsea Bay, Oregon, USA. Diatom 19: 33–46.
- Sawai Y., Horton B.P. & Nagumo T. 2004. The development of a diatom-based transfer function along the Pacific coast of eastern Hokkadio, northern Japan—an aid in paleoseismic studies of the Kurile subduction zone. Quaternary Science Reviews 23: 2467–2483. doi: 10.1016/j.quascirev.2004.05.006
- Sherrod B.L. 1999. Gradient analysis of diatom assemblages in a Puget Sound salt marsh—Can such assemblages be used for quantitative paleoecological reconstructions? Palaeogeography, Palaeoclimatology, Palaeoecology 149: 213–226. doi: 10.1016/S0031-0182(98)00202-8
- Smol J.P. & Stoermer E.F. 2010. The diatoms. Applications for the environmental and earth sciences: Second edition. Cambridge University Press, Cambridge. 667 pp.
- Snoeijs P. 1993. Intercalibration and distribution of diatom species in the Baltic Sea. Volume 1. OPULUS Press, Uppsala. 129pp.
- Snoeijs P. & Balashova N. 1998. Intercalibration and distribution of diatom species in the Baltic Sea. Volume 5. OPULUS Press, Uppsala. 144pp.
- Snoeijs P. & Kasperoviciene J. 1996. Intercalibration and distribution of diatom species in the Baltic Sea. Volume 4. OPULUS Press, Uppsala. 126pp.
- Snoeijs P. & Potapova M. 1995. Intercalibration and distribution of diatom species in the Baltic Sea. Volume 3. OPULUS Press, Uppsala. 126pp.
- Snoeijs P. & Vilbaste S. 1994. Intercalibration and distribution of diatom species in the Baltic Sea. Volume 2. OPULUS Press, Uppsala. 126pp.
- Snoeijs P. & Weckström K. 2010. Diatoms and environmental change in large brackish-water ecosystems. In: The diatoms. Applications for the environmental and earth sciences: Second edition (Ed. by J.P. Smol & E.F. Stoermer), pp. 287–308. Cambridge University Press, Cambridge.
- Snoeijs P., Busse S. & Potapova M. 2002. The importance of diatom cell size in community analysis. Journal of Phycology 38: 265–281. doi: 10.1046/j.1529-8817.2002.01105.x
- Stonik I.V., Orlova T.Y., Propp L.N., Demchenko N.L. & Skriptsova A.V. 2012. An autumn bloom of diatoms of the genus Pseudonitzschia H. Peragallo, 1900 in Amursky Bay, the Sea of Japan. Russian Journal of Marine Biology 38: 211–217. doi: 10.1134/S1063074012030121
- Sullivan M.J. 1975. Diatom communities from a Delaware salt marsh. Journal of Phycology 11: 384–390.
- Sullivan M.J. 1978. Diatom community structure: taxonomic and statistical analyses of a Mississippi salt marsh. Journal of Phycology 14: 468–475. doi: 10.1111/j.1529-8817.1978.tb02471.x
- Sullivan M.J. 1982. Distribution of edaphic diatoms in a Mississippi salt marsh: a canonical correlation analysis. Journal of Phycology 18: 130–133. doi: 10.1111/j.1529-8817.1982.tb03166.x
- Sullivan M.J. 1999. Applied diatom studies in estuarine and shallow coastal environments. In The Diatoms: Applications for the Environmental and Earth Sciences (Ed. by E.F. Stoermer & J.P. Smol), pp. 334–351. Cambridge University Press, Cambridge.
- ter Braak C.J.F. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179. doi: 10.2307/1938672
- ter Braak C.J.F. & Šmilauer P. 2002. CANOCO Reference Manual and CanoDraw for Windows User's Guide: Software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca. 500 pp.
- ter Braak C.J.F. & Verdonschot P.F.M. 1995. Canonical correspondence analysis and relate multivariate methods in aquatic ecology. Aquatic Science 57: 153–187. doi: 10.1007/BF00877430
- Ulanova A. & Snoeijs P. 2006. Gradient response of epilithic diatom communities in the Baltic Sea proper. Estuarine, Coastal and Shelf Science 68: 661–674. doi: 10.1016/j.ecss.2006.03.014
- Underwood G.J.C. & Provot L. 2000. Determining the environmental preferences of four estuarine epipelic diatom taxa: growth across a range of salinity, nitrate and ammonium conditions. European Journal of Phycology 35: 173–182. doi: 10.1080/09670260010001735761
- Underwood G.J.C., Phillips J. & Saunders K. 1998. Distribution of estuarine benthic diatom species along salinity and nutrient gradients. European Journal of Phycology 33: 173–183. doi: 10.1080/09670269810001736673
- Van Dam H., Mertens A. & Sinkeldam J. 1994. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherland Journal of Aquatic Ecology 28: 117–133. doi: 10.1007/BF02334251
- Vanelslander B., Créach V., Vanormelingen P., Ernst A., Chepurnov V.A., Sahan E., Muyzer G., Stal L.J., Vyverman W. & Sabbe K. 2009. Ecological differentiation between sympatric pseudocryptic species in the estuarine benthic diatom Navicula phyllepta (Bacillariophyceae). Journal of Phycology 45: 1278–1289. doi: 10.1111/j.1529-8817.2009.00762.x
- Villac M.C. & Kaczmarska I. 2011. Marine planktonic diatoms, including potentially toxic species. In: The diatom world (Ed by J. Seckbach & J.P. Kociolek), pp. 467–490. Springer, New York.
- Vos P.C. & de Wolf H. 1993. Reconstruction of sedimentary environments in Holocene coastal deposits of the southwest Netherlands; the Poortvliet boring, a case study of palaeo-environmental diatom research. Hydrobiologia 269-270: 297–306. doi: 10.1007/BF00028028
- Watcham E.P., Shennan I. & Barlow N.L.M. 2013. Scale considerations in using diatoms as indicators of sea-level change: lessons from Alaska. Journal of Quaternary Science 28: 165–179. doi: 10.1002/jqs.2592
- Weckström K. & Juggins S. 2005. Coastal diatom-environment relationships from the Gulf of Finland, Baltic Sea. Journal of Phycology 42: 21–35. doi: 10.1111/j.1529-8817.2006.00166.x
- Weinmann F., Boulé M., Brunner K., Malek J. & Voshino V. 1984. Wetland plants of the Pacific Northwest: Seattle, Washington, U.S. Army Corps of Engineers, Seattle District. 85pp.
- van der Werff H. & Huls H. 1958–1966. Diatomeeënflora van Nederland. 8 parts, published privately by van der Werff, De Hoef, The Netherlands.
- Whiting M.C. & McIntire C.D. 1985. An investigation of distributional patterns in the diatom flora of Netarts Bay, Oregon, by correspondence analysis. Journal of Phycology 21: 655–661. doi: 10.1111/j.0022-3646.1985.00655.x
- Wilson G.P. & Lamb A.L. 2012. An assessment of the utility of regional diatom-based tidal-level transfer functions. Journal of Quaternary Science 27: 360–370. doi: 10.1002/jqs.1553
- Woodroffe S.A. & Long A.J. 2009. Salt marshes as archives of recent relative sea level change in West Greenland. Quaternary Science Reviews 28: 1750–1761. doi: 10.1016/j.quascirev.2009.02.009
- Zong Y. & Horton B.P. 1998. Diatom zones across intertidal flats and coastal saltmarshes in Britain. Diatom Research 13: 375–394. doi: 10.1080/0269249X.1998.9705456
- Zong Y. & Horton B.P. 1999. Diatom-based tidal level transfer functions as an aid in reconstructing Quaternary history of sea-level movements in the UK. Journal of Quaternary Science 14: 153–167. doi: 10.1002/(SICI)1099-1417(199903)14:2<153::AID-JQS425>3.0.CO;2-6