Abstract
A listing of the accepted freshwater diatom genera worldwide is presented, indicating the distribution of the genera by continent. Out of a total of 249 genera, 63 (25%) of those genera are endemic to a single continent. The continent with the largest number of endemic genera is Asia, hosting 35 endemic genera. While Asia is also the continent with the most reported genera, a regression analysis showed there is no relationship between the continental richness of genera and generic endemism. Disjunct genera, those found on two continents, represent distributions found in other groups of organisms such as in higher plants, including North America-Europe, North America–Asia and Southern Hemisphere disjunctions. There are certain lineages of freshwater diatoms where the endemic genera are restricted to specific continents: both thalassiosiroid and cymbelloid diatoms have endemics in Asia, while eunotioids have endemics in South America. Future research is suggested combining distributions over space and time related to phylogenetic relationships of diatoms.
Background
While historically there have been researchers who identified and described biogeographic patterns of freshwater diatoms (e.g. Ehrenberg Citation1849, Skvortzow Citation1937, Hustedt Citation1938–Citation1939), ideas about rampant dispersal and wide variation within species (such as those espoused by Lotsy Citation1916 on ‘syngameons’), the dogma of ‘Everything is Everywhere’ developed in Bacteriology by Baas-Becking (Citation1934) and rekindled by Finlay and colleagues (Finlay Citation2002, Finlay & Estaban Citation2007, Fenchel & Finlay Citation2003, Citation2004a, Citationb, see Williams’ Citation2011 review of this in relation to diatoms) kept a strong grip on the thinking about freshwater diatom distributions. That grip was perhaps most pronounced in the development of the now classic ‘Süsswasserflora von Mitteleuropa’ and the way species concepts in that work were developed (Krammer & Lange-Bertalot Citation1986). The concept was a self-fulfilling prophecy, since research projects in areas without detailed taxonomic works began to ‘shoehorn’ forms into European taxon names, since these floras were the only ones available.
Beginning in 2000, a series of papers (Kociolek & Spaulding Citation2000, Vanormelingen et al. Citation2007, Vyverman et al. Citation2007) started to analyse and summarize the literature on freshwater diatom distributions, concluding that endemism was found at different levels of taxonomic hierarchy amongst the freshwater diatoms around the world. The model that endemism exists and can be (has been!) applied to a substantial number of taxa around the world, has been adopted by most workers. Molecular methods have supported the splitting of morphologically similar entities at the level of species, suggesting shoe-horning of taxa into names whose valve morphology was quite different was not justified. The morphological measures for splitting out forms may actually be too conservative relative to the genetic differences identified, as ‘pseudo-cryptic’ and ‘cryptic’ species have been documented (Vaneslander et al. Citation2009, Poulícková et al. Citation2010, Lundholm et al. Citation2012, Vanormelingen et al. Citation2013).
The diatom tree of life
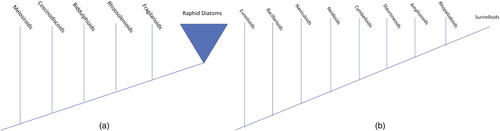
Nearly three centuries of work with light, scanning and transmission electron microscopy has yielded the description of over 75,000 diatom taxa (Kociolek et al. Citation2018), and there have been numerous attempts to organize that diversity into groupings that reflect evolutionary relationships. Williams (Citation2007) has documented many of the systems that emerged from those observations and proposals, with most of them choosing a single idea on which to base a system (modes of sexual reproduction; ability for active movement; size and number of chloroplasts, etc.). More integrative systems were proposed, ranging from ‘ideas’ (inexplicit review of certain facts) to formal analyses across the entire diatom group. The morphology-based descriptions were fully resolved but not repeatable (Round & Crawford Citation1981, Citation1984, Simonsen Citation1987, Round et al. Citation1990), and formal analyses of morphological data across broad taxonomic scales yielded unresolved polytomies (e.g. Cox & Williams Citation2006). Explicit, formal analyses of the overall diatom tree of life were ushered in with the development of molecular techniques, yielding evidence-based, testable hypotheses. Sometimes the evidence was not always retrievable (Theriot Citation2008, Journal of Phycology Citation2009, Citation2010, Kaczmarska & Medlin Citation2009), branching patterns of the trees have differed based on taxon sampling and/or genes sequenced and analysed (Sörhannus et al. Citation1995, Sörhannus Citation2004, Sims et al. Citation2006, Theriot et al. Citation2010), and there have been vigorous discussions about how evolutionary trees can be converted into classifications (see discussions about the value of monophyly towards that end; Williams & Kociolek Citation2007, Citation2010a, b). A 2015 paper by Theriot et al. focused on the information content of molecular data, yielded a tree of over 200 terminal taxa based on sequence data from seven genes. Based on this analysis, a phylogenetic analysis of the overall diatom tree of life could be developed, like the one in a and b, which here includes only those clades with freshwater genera.
Figure 1. Showing the phylogenetic relationships of major groups of freshwater diatoms. a. Overall relationships of non-raphid diatom groups with freshwater representatives. After Theriot et al. (Citation2015). b. Overall relationships of raphid diatom groups with freshwater representatives. After Theriot et al. (Citation2015).
Endemism of freshwater diatom genera
Several analyses of endemism have centred on the taxonomic level of genus. Kociolek & Spaulding (Citation2000) and Khursevich & Kociolek (Citation2012) provided reviews of endemic freshwater diatom genera, based on over a century of reports. Vyverman et al. (Citation2007), using genera as the finest taxonomic category, assuming data in their analysis of over 600 lakes worldwide would be consistent at this level of taxonomic hierarchy. From that study, Vyverman et al. (Citation2007, p. 1924) concluded that ‘At regional to global scales, historical factors explain significantly more of the observed geographic patterns in genus richness than do contemporary environmental conditions.’ It is clear that different authors have viewed the level ‘genus’ of the Linnaean hierarchy differently (and not just in diatoms, see Laurin Citation2010), the monophyly of many diatom genera has yet to be verified (Kociolek & Williams Citation2015), and there might be other rationales for comparisons (those lineages of the same age, lineages with similar number of taxa, etc. see Avise & Johns Citation1999, Avise & Mitchell Citation2007). However, reflecting the current state of knowledge for the group, it seems that the level of genus may be the best place to start for the freshwater diatoms.
How many endemic freshwater genera are there, and where do you find them?
Tables and provide the first comprehensive listing of currently recognized and used freshwater diatom genus names worldwide, both fossil and recent, and the distribution of those genera by continent. In Table the genus names are listed within broad phylogenetic groupings, indicated in the phylogeny illustrated in a and b, while in Table they are presented alphabetically. These tables are derived from summaries provided in Fourtanier & Kociolek (Citation1999) and Kociolek et al. (Citation2018), and listed individual papers.
Table 1. A worldwide listing of recognized freshwater diatom genus names in use, including fossil and recent taxa, listed within major clades as outlined in and their distributions.
Table 2. Overall genus listing_alphabetical by continent.
In several cases, there are genera that have disjunct distributions between two continents. If a genus has a distribution across three continents, it is listed as ‘widely distributed’, and if it has been reported across four or more continents it listed as ‘cosmopolitan’. These are conservative summaries, since in other groups, an organism's distribution across an entire continent might be considered ‘cosmopolitan’. In Table those genera endemic to, or with disjunct distributions between, two continents are presented by continent.
Table 3. Endemic and disjunct freshwater diatom genera listed by continent.
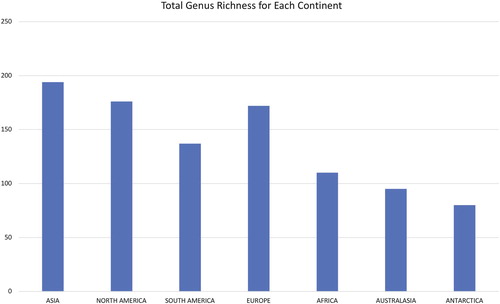
A total of 249 genera are presented in the overall listing. Several groups, notably the eunotioids and cymbelloids, are almost exclusively freshwater, and the thalassiosiroids have a majority of freshwater genera, while all the other main groups of diatoms have a significantly larger representation (at the level of genus) in brackish or marine waters. Of those that are not endemic or disjunct, most are considered cosmopolitan. Twenty-seven (just over 10%) genera are considered ‘widely distributed’.
With respect to generic endemism, 63 freshwater diatom genera have distributions restricted to a single continent (25% of the total number of genera) and another 27 are found disjunct between two continents (a total of 90 genera being limited to one or two continents, over 36% of all the recognized genera). If we exclude genera that have the vast majority of their species in marine systems (the 11 genera indicated by an asterisk in Table ), the overall percentages of endemics (26%) and endemics + genera that are disjuncts (37%) increase only slightly, relative to the freshwater genera.
Nearly all the major groups of diatoms have a least one endemic genus, the exceptions being the non-thalassiosiroid biddulphioids, and amphoroids. There are some very large groups, such as the bacillarioids, the gomphonemoids, those diatoms in a morphological grade with a single raphe system (‘monoraphids’), and the ‘fragilarioid’ diatoms, that have few endemic genera. It is possible that treatment of these groups with finer taxonomic circumscription might yield interpretable biogeographic patterns or reveal endemism (see below).
Are endemic genera evenly distributed across the globe?
Put another way, we can ask, ‘Based on current knowledge, which continents have the greatest number or greatest percentage of endemic freshwater diatom genera?’ Table summarizes the number and percent of those genera with limited distributions.
The data from Table show that 56% of the endemic genera of freshwater diatoms are found in Asia, and the remaining 44% are found elsewhere around the world. Asia has four times the number of endemic genera of South America and North America, the continents with the second highest levels of generic endemism (eight and seven genera, respectively). The high level of endemism in Asia is driven in part by the number of endemic genera described from Lake Baikal. That long-lived (ca. 30 million years) water body has 13, living and fossil genera that are endemic to the lake. While other genera were described from Lake Baikal, (Kulikovskiy et al. Citation2012, Citation2015), some of them have newly-proposed representatives outside the lake. Another five genera (all fossil) have been described from sediments from the Baikal region, if not from the lake itself. Even if we were to exclude the Baikal genera, Asia would still have nearly twice the number of endemics found in the more heavily-studied regions of either North America or Europe.
Table 4. Listing of continents with the number of endemic genera versus the total number of endemic genera (63), and the percentage of endemic genera.
Generic endemism and genus richness
Would we expect a proportional number of endemic genera to be related to the overall number of genera for a continent? In Table , genus richness and generic endemism are listed by continent. With the information currently in hand, we can estimate the number of genera in the most studied areas of the world. For example, of the most studied regions, North America and Europe have176 and 172 freshwater diatom genera in their respective floras. For the North American flora, with eight endemic genera, and Europe with four endemic genera, there is a very low percentage of endemism (ca. 4.5% and 2.0%, respectively) at the level of genus. Based on the work of Kulikovskiy et al. (Citation2016) and Kociolek et al. (Accepted), we can total the number of freshwater diatom genera in Asia to be 194. This yields a level of endemism at the rank of genus at ca. 18%. Ninetyfive freshwater diatom genera comprise the Australasian flora, making the level of endemism (3%) similar to that of Europe (2%) and Africa (2%), but lower than South America (5.8%) and Antarctica (3.8%) (Table , and ). Thus, based on the total number of genera, the level of endemism in freshwater diatom genera in Asia is more than four times greater than any other continent in the world, and more than three times greater as a percent of the total number of genera.
Figure 3. Graph showing number of endemic genera and genera with disjunct distributions by continent.
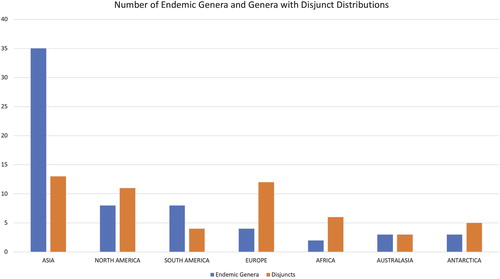
Table 5. Listing of endemic genera in relation to the total number of genera reported for each continent (with percent indicated).
A simple regression analysis of the number of endemic genera versus the total number of genera by continent (p > .11) suggests that, at the 95% confidence level, we cannot reject the null hypothesis. That is, there is no statistical relationship between the number of endemic genera and generic richness by continents.
Comparing endemism in diatoms to other organisms
We can compare the levels of endemism, and areas of higher endemism, to other groups of organisms. For other groups of freshwater organisms, a collection of papers from Hydrobiologia in 2008 was published called, ‘Freshwater Animal Diversity Assessment’ (Balian et al. Citation2008), which, among other things, looked at the number of genera and species of a wide range of taxonomic groups, and the levels of endemism, across geographic realms (Palaearctic, Nearctic, Neotropical, Afrotropical, Oriental and Australasian); nearly the same as the continental approach taken in the present report. In this volume, we can see groups that have very low generic endemism (rotifers have only 10 endemic genera out of a total of 128; nine of the 10 genera are monotypic; Segers Citation2008), while in freshwater amphipods nearly all of the 293 genera are endemics (Vainola et al. Citation2008). The freshwater amphipods, like the diatoms, have most of their diversity (genera and species) represented in marine systems, and the freshwater forms, like the freshwater diatoms, do not form a monophyletic group. ‘Genus level inter-continental distributions are rare even in the less strictly continental groups’ in freshwater amphipods (Vainola et al. Citation2008, p. 251), versus the 23% generic endemism seen in the freshwater diatoms.
For Angiosperms, Brummitt (Citation2005) reported that areas with ‘highest genus richness only had moderate degrees of generic endemism’ (p. 549), suggesting no relationship between the two, an outcome echoed by freshwater diatoms. Areas with Angiosperm endemism greater than 10% of the species include: Southern Africa (29%), Western Indian Ocean (29%) and Australia (35%); Malesia (10.8%), Mexico (11.8%), Caribbean (12.6%), Western South America (10.8%), Brazil (15.7%) and Southern South America (13.6%). Across freshwater animals, we can find groups with the greatest generic endemism in the Palaearctic (amphipods Vainola et al. Citation2008, Bryozoans, Massard & Geimer Citation2008), Nearctic (mussels, Bogan Citation2008), Neotropical (Odonates, Boxshall & Defaye Citation2008, Kalkman et al. Citation2008), Afrotropical (Ostracods; Martens et al. Citation2008), Australasian (Isopods, Wilson Citation2008) and Oriental (freshwater crabs, Yeo et al. Citation2008) regions. None of the groups surveyed showed highest generic endemism in the Antarctic region.
An analysis of the freshwater animal data published in Balian et al. (Citation2008) by Collen et al. (Citation2014) drew the interesting conclusion that ‘Pairwise analysis of geographical distribution between taxa showed that no single species group exhibited a consistent pattern of congruence with other taxa.’ This suggests that a group's evolutionary history may have more to do with their distributions than environmental or ecological conditions, echoing the conclusions by Vyverman et al. (Citation2007) for freshwater diatoms.
Disjunct taxa
Numbers of disjunct taxa are small (27 genera of the 249, or nearly 11%, have disjunct distributions), however, some patterns between disjuncts have been described for diatoms (Tables and ) and for a wide variety of other organisms. The greatest number of disjuncts is found between Europe and Asia, North America and Asia, and North America and Europe. Antarctica has connections via disjuncts with South America, Africa and Australasia, and these would seem biologically realistic given the proximity of these continents to Antarctica sensu lato (the definition we are using here). Likewise, some disjuncts across the southern hemisphere were reported earlier by Williams (Citation1996), Williams & Reid (Citation2006a, Citationb, Citation2009) and Williams & Kociolek (Citation2017). Disjunct distributions between North America and Asia have been reported for mosses (Crum Citation1972), freshwater invertebrates (Ross Citation1967, de Moor & Ivanov Citation2008), lichens (Sheard et al. Citation2017) and higher plants (e.g. Wen Citation1999, Qian Citation2002). Ehrenberg (Citation1849) noted the similarities between western North America and Asian diatom floras. And the disjunct distributions between North America and Europe have been noted at the species level of diatoms, as well as a wide range of other organisms (e.g. Sanmartin et al. Citation2001, Milne Citation2006, Reisch Citation2008, Garnica et al. Citation2011).
Table 6. Most common disjunctions between genera.
Phylogenetics and endemism in freshwater diatom genera
The concept of endemism of lineages is well known to students of biogeography of other organisms. The are many examples of this phenomenon, including the placental mammals of Australia (Holt et al. Citation2013), southern beeches (Nothofagus) for the flora of Australasia and South America (Veblen et al. Citation1996, Hill Citation2001) and the honeycreepers of Hawaii (Lerner et al. Citation2011). However, any analysis of endemism of lineages in diatoms is hindered by a still-primitive understanding of the phylogenetic relationships of large swaths of diatom groups that have freshwater representatives. For example, there is still tremendous work to do on the ‘naviculoids’. So, while lineages such as the Stauroneidaceae (Davidovich et al. Citation2017) have been well-established, the group of biraphid diatoms is, overall, extremely large and the number of taxa included in modern analyses is still relatively few. Therefore, an understanding of any phylogenetic context for endemism in the group is still rather primitive. That being said, there are some trends in the data presented in Tables and that can be discussed.
For example, within the thalassiosiroid diatoms, 11 of the 27 genera (over 40%) are endemics, and of those 11 endemics, nine are found in Asia. Of the Asian endemics, eight of the nine genera are exclusively fossil. Thus nearly 30% of the genera occur as fossils in Asia. The fossil endemic taxa overall, and particularly in Asia, are almost all of Miocene age (Khursevich & Kociolek Citation2012). Using fossil representatives Julius and colleagues have attempted to reconstruct the phylogeny of certain branches of this evolutionary lineage (e.g. Julius & Tanimura Citation2001, Tuji et al. Citation2014), but for the group overall no attempts have been made to incorporate fossils into phylogenetic reconstructions based on molecular data (Alverson et al. Citation2007). And given the important history of the lineage in Asia, it is surprising that no Asian freshwater representatives have been used in the molecular research.
Within the cymbelloids, (defined here as the freshwater cymbelloid and gomphonemoid diatoms sensu Kociolek & Stoermer Citation1988), seven of the 35 genera of this group (20%) are endemic to Asia. It is the only continent where endemic members of the group have thus far been documented, except for Angusticopula nom prov., a genus soon to be described from Antarctica. Also, in this group is the genus Geissleria Lange-Bertalot & Metzeltin (Kulikovskiy et al. Citation2014), and many species of this genus have been described from Lake Baikal. Bukhtiyarova & Pomazkina (Citation2013) described nearly 50 new species with features found in Geissleria and segregated them into nine new genera. These have not yet been included in this analysis, pending further review of their approach, but it points to the importance of Asia in the evolution of lineages within the freshwater cymbelloid diatoms.
The Miocene was a time of tremendous climate disruption in Asia, with the establishment of the monsoonal climate in the southcentral part of the country (Sun & Wang Citation2005, Favre et al. Citation2015). Such changes are suggested to help account for the establishment and diversity of the unique angiosperm flora of China (Lu et al. Citation2018) when 66% of the flora became established. Environmental changes in the late Miocene in the Baikal region were also documented with diatoms from long cores taken from the lake (Williams et al. Citation1997, BDP Members Citation2000). Uplift of the Tibetan Plateau in the Oligocene, around 40 mya, may have initiated changes in climate patterns across the northern hemisphere (Raymo & Ruddiman Citation1992), and accounted for the tremendous increase in continental freshwater ecosystems, productivity and taxon turnover (e.g. Bradbury et al. Citation1994, Bradbury & Krebs Citation1995, Khursevich Citation2006). De Bruyn et al. (Citation2014) describe Indochina and Borneo as major evolutionary hotspots for a wide range of groups, including plants, invertebrates and vertebrates, the result of several different geological collision events.
Another lineage that shows a high degree of endemism is the eunotioids of South America. This group has the highest proportion of endemism overall, with nine of 15 genera (60%) being either endemic or disjunct. There are seven endemic genera in the eunotioid lineage, and five of them occur in South America. Thus, 33% of the lineage (at the level of genus) is found only in South America. Williams & Reid (Citation2006a) showed a Pan-Pacific distribution for Amphorotia Williams & Reid.
On a finer taxonomic scale, the Orthoseiraceae has two of the three genera occurring as disjuncts, either between South America + Antarctica (Guarreraea Kociolek et al.), or between Australasia + Antarctica (Cavernosa S.R.Stidolph).
Extinctions and endemics
Fossil genera represent important proportions of endemics in certain lineages. For example, except for Actinocyclus normanii f. subsalsa (Juhlin-Dannfelt) Hustedt, all of the freshwater members of the genus are fossil. This was documented in North America by the work of Bradbury & Krebs (Citation1995). In addition, the three other genera that are representatives of the coscinodiscoids are also fossil. Fossil endemic genera can be found in North America (Fideliacyclus Siver et al.) and Asia (Lobodiscus Lupikina & Khursevich and Undatodiscus Lupikina).
A total of ten of the 11 endemic genera of the thalassiosiroids are known only as fossils. Conditions in which this group evolved in the Mio-Pliocene must have occurred where lacustrine systems were developed. Krebs et al. (Citation1987) suggested these formed a pattern of substitution (not necessarily evolution) in western North America. Khursevich and colleagues (Khursevich et al. Citation2000, Citation2002) described a sequence of genera in the sediments of Lake Baikal, where the pattern of substitution may have also been a pattern of evolutionary divergence and change.
In terms of continents, 43% of the endemic genera found in North America (three of seven) are fossils, while for Europe it is 25% (one of four) and for Asia nearly a third (11 of 34) are fossil endemics. While freshwater diatomites are known from every continent (Flower in Elias & Mock Citation2003), the insufficient study of these sediments from a taxonomic point of view is likely the primary reason that no fossil endemic genera have been recorded from other continents.
Surprises
Islands are wonderful natural laboratories on which to observe and describe evolutionary and ecological processes. One of these processes is adaptive radiation, in which many descendants within a geographically-restricted monophyletic group exploit available niches (e.g. Givnish & Sytsma Citation1997, Ricklefs & Bermingham Citation2007, Grant & Grant Citation2007, Emlen et al. Citation2007). For diatoms, this phenomenon has not been documented for islands to any great degree, and certainly not at the level of genus. While many new taxa of a wide range of organisms have been described from the ‘island continent’ of Madagascar (Goodman & Benstead Citation2003), there are relatively few studies on the freshwater diatoms from Madagascar (Manguin Citation1941, Citation1952, Spaulding & Kociolek Citation1998, Metzeltin & Lange-Bertalot Citation2002). These studies have yielded no new or endemic genera. The total number of samples examined from this region, though, is likely less than 100, and the island has an area of over 575,000 km2. None of the largest rivers of the island have been sampled for diatoms. Similarly, the island of New Caledonia has perhaps been best studied, based on the works of Maillard (Citation1978), Manguin (Citation1962) and Moser et al. (Citation1995, Citation1998). Despite this effort, this ‘Island of endemics’ has yielded only one genus (Eileencoxia Blanco & Wetzel), though many new species have been recognized. The Hawaiian Islands have an endemic genus (Diprora Main), described from aerophilous environments (Main Citation2003), but currently, no other endemic freshwater diatom genera are recognized from these islands. In Tasmania and New Zealand, Eunophora Vyverman et al. was described as an endemic (Vyverman et al. Citation1998), though John (Citation2009a, Citationb, Citation2012a, Citationb, Citationc, Citation2015) subsequently reported it from Australia. Celebesia Kasputin et al. is known only from the island of Celebes (Kasputin et al. Citation2017). It is surprising given what we know of islands for other organisms, there has not been the divergence of freshwater diatoms, at the genus level, on islands to any great degree.
Can endemism of taxa/lineages give us new perspectives on phylogeny?
Phylogeography is the study of how history (evolutionary history through population genetics and phylogenetics) can lead to an understanding of the current spatial distribution of species and lineages (Avise Citation2000). This discipline demonstrates how phylogeny can help us interpret biogeographic patterns. We could, however, flip this relationship and pose the question, ‘How does the distribution of organisms affect our understanding of phylogeny?’ For example, if major families or groups that are endemic to certain places were absent from a dataset, would we recover the same groupings across that group's overall tree of life? In other words, does including biogeographically-limited taxa in the overall tree of life give us new perspectives on, or understanding of, the entire tree? For example, in the phylogeny of Theriot et al. (Citation2015), of the 207 freshwater terminal taxa, less than 1% are from Asia. Yet endemic Asian genera represent 13% of all freshwater diatom genera. Would including endemics from Asia give us new perspectives on the overall phylogeny of the diatoms?
Challenges of this type of analysis, and some directions for the future
This analysis is a summary of our current knowledge, of both the listing of genera with freshwater representatives, and the number of endemic genera and genera that appear to have disjunct distributions. There are biases in these data based on the levels of collection and taxonomic effort for different areas and approaches to taxonomic practice. There is also a heavy bias in the studies on recent freshwater versus fossil taxa. These will all have some effect on the data at hand, though the impacts of each is more a matter of speculation than being data-driven at the current time. Despite this, trends related to areas of endemism and phylogeography have been identified and are worthy of future research.
In freshwaters, estimates of the size of continental floras have ranged into the thousands of species (Kociolek Citation2005), yet worldwide there are a mere 248 genera. Thus, while ecologists have raised concerns about the increasing number of new taxa being recognized, diatom taxonomists have still been fairly (too?) conservative in recognizing genera (Kociolek & Williams Citation2015), compared to other groups of organisms. With a finer dissection of taxa at the level of genus, we should expect to see a greater number of genera that are endemic. Some areas of research that might start looking to divide up genera include:
Large genera that have not yet been broken up. Places to start might include Pinnularia Ehrenberg (over 3500 taxa), Eunotia Ehrenberg (almost 2400 taxa), Gomphonema Ehrenberg (nearly 2000 taxa), Stauroneis Ehrenberg (about 1200 taxa), Diploneis Ehrenberg ex Cleve and its relatives (around 1000 taxa) (taxon estimates from Kociolek et al. Citation2018). Several morphological groups are recognized within each of these genera (documented in Pinnularia by Patrick & Reimer Citation1966 and Krammer Citation2000, in Gomphonema by Kociolek & Kingston Citation1999, Kociolek et al. Citation2016, in Stauroneis by Hustedt Citation1964, and Diploneis by Pennesi et al. Citation2017).
Even though there was a major revision of Fragilaria Lyngbye and Synedra Ehrenberg in the late 1980s as well as work on other ‘araphid’ groups (Williams & Round Citation1986, Citation1987, Williams Citation1990), resulting in seven new genera from freshwaters, almost all of them are cosmopolitan. It is possible that finer-scale distributional patterns will be found within these groups.
With at least 3500 described species and subspecific taxa, the Bacillariales is one of the most species-rich taxonomic orders of diatoms (Kociolek et al. Citation2018), comparable to the entire Class Mammalia of metazoans. But whereas extant mammals are classified among some 1200 extant genera, Bacillariales are divided among less than 20 genera; there are only 11 genera found in freshwaters (the case is even more extreme if you include extinct taxa). Only two freshwater genera have been proposed within the Bacillariales over about the last 100 years, and all the freshwater genera except Gomphonitzschia Grunow are either widely distributed or cosmopolitan. This partly reflects the lack of formal treatment of the systematics of this group for almost 140 years (Cleve & Grunow Citation1880). While taxon sampling has been limited, initial phylogenetic analyses of this group with molecular data have yielded results that suggest some of the bacillarioid genera are non-monophyletic (Lundholm et al. Citation2002).
Recent phylogenetic studies by Ruck using morphology (Ruck & Kociolek Citation2004) and molecular data (Ruck & Theriot Citation2011) shed new light on relationships within the surirelloid diatoms, indicating that several of the commonly recognized genera are paraphyletic. Some endemic genera in this group (described by Jurilj Citation1949, Citation1954 from Lake Ohrid) have been resurrected (Ruck et al. Citation2016). However, a finer dissection of the group might point to additional endemics and disjunct distributions.
Although the monoraphid diatoms are not a natural group (Thomas et al. Citation2016), the 13 monoraphid genera found in freshwaters, are all either cosmopolitan or widely distributed. Given that the groups have been revised relatively recently, albeit in a piecemeal fashion and devoid of phylogenetic context (Round & Bukhtiyarova Citation1996, Round Citation1998), the biogeography of the genera suggests finer taxonomic distinctions might be forthcoming.
Conclusions
Endemic freshwater diatom genera comprise nearly a quarter of all genera in freshwaters across the globe. The number of endemic genera is not statistically related to the overall number of genera for a continent. Certain lineages of freshwater diatoms, including the cymbelloids and thalassiosiroids, have a significant number of endemics in Asia, while eunotioid diatoms have a large number of genera that are endemic to South America. Integrating phylogenetic information with levels of generic endemism can help identify research agendas for the study on freshwater diatoms, from phylogeny reconstruction to generic revisions. Genera with large numbers of species and subspecific taxa, usually coupled with cosmopolitan distributions, may be places to focus research. Revisionary studies of these groups can be hypothesis-based, including the evolutionary history of characters, taxa and biogeography, so that practitioners are not seen as focused on minute details and detached from broader studies of evolutionary biology, systematics and questions related to space and time (and the myriad of other questions correlated with these factors). These types of studies can lead to comparisons with other organisms and provide research questions related to distributions and endemism (Rosauer et al. Citation2009, Rosauer & Jetz Citation2015) as well as dispersal and the evolutionary ecology of food web interactions (e.g. Mathews & Heins Citation1987, Bohanak & Jenkins Citation2003).
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/0269249X.2019.1574243. References marked with an asterisk are cited in the supplemental data.
Supplementary_Table_7.___Genus_listing_Antarctica.xlsx
Download MS Excel (10.4 KB)Supplementary_Table_6.__Genus_Listing_Australiasia.xlsx
Download MS Excel (10.6 KB)Supplementary_Table_5.__Genus_listing_North_America.xlsx
Download MS Excel (12.3 KB)Supplementary_Table_4.__Genus_listing_Europe.xlsx
Download MS Excel (12.1 KB)Supplementary_Table_3.__Genus_listing_Asia.xlsx
Download MS Excel (13.6 KB)Supplementary_Table_2.__Genus_listing_South_America.xlsx
Download MS Excel (11.9 KB)Supplementary_Table_1.__Genus_listing_Africa.xlsx
Download MS Excel (10.9 KB)Acknowledgments
I am indebted to Dr. Silvia Sala, Facultad de Ciencias Naturales y Museo Universidad Nacional de La Plata, Argentina, for her help with literature, thoughts and a careful reading of the manuscript, as well as Drs David Williams, Life Sciences Department, The Natural History Museum, London, U.K., Maxim Kulikovskiy, Papanin's Institute for Biology of Inland Waters Russian Academy of Sciences, Borok, and Institute of Plant Physiology, Russian Academy of Sciences, Botanical Street 35, 127276, Moscow, Russia, and Rex Lowe, Bowling Green State University and University of Wisconsin, Madison, for their critical reading of the manuscript and helpful suggestions. Bart Van de Vijver, Botanical Garden, Meise, Belgium, kindly provided a list of genera from the Antarctic region.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- *Adesalu, T.A. & Julius, M.J. 2017. First observation of Spicaticribra from Africa, with a comment on nomenclatural and taxonomic status. Diatom Research 32: 359–362. doi: 10.1080/0269249X.2017.1358671
- Alverson A.J., Jansen R.K. & Theriot E.C. 2007. Bridging the Rubicon: phylogenetic analysis reveals repeated colonizations of marine and freshwaters by thalassiosiroid diatoms. Molecular Phylogenetics and Evolution 45: 193–210. doi: 10.1016/j.ympev.2007.03.024
- Avise J.C. 2000. Phylogeography. Harvard University Press, Cambridge.
- Avise J.C. & Johns G.C. 1999. Proposal for a standardized temporal scheme of biological classification for extant species. Proceedings of the National Academy of Sciences of the United States of America 96: 7358–7363. doi: 10.1073/pnas.96.13.7358
- Avise J.C. & Mitchell D. 2007. Time to standardize taxonomies. Systematic Biology 56: 130–133. doi: 10.1080/10635150601145365
- Baas-Becking L.G.M. 1934. Geobiologie of inleiding tot de milieukunde. W.P. Van Stockum & Zoon, The Hague, The Netherlands.
- *Bahls, L.L. 2009. A checklist of diatoms from inland waters of the Northwestern United States. Proceedings of the Academy of Natural Sciences of Philadelphia 158: 1–35. doi: 10.1635/053.158.0101
- Bahls L. 2015. Kurtkrammeria, a new genus of freshwater diatoms (Bacillariophyta, Cymbellaceae) separated from Encyonopsis. Nova Hedwigia 101: 165–190. doi: 10.1127/nova_hedwigia/2015/0263
- Balian E.V., Segers H., Lévèque C. & Martens K. 2008. The freshwater animal diversity assessment: an overview of the results. Hydrobiologia 595: 627–637. doi: 10.1007/s10750-007-9246-3
- BDP-Members. 2000. Paleoclimatic record in the late Cenozoic sediments of Lake Baikal (600 m deep-drilling data). Russian Geology and Geophysics 41: 3–32.
- *Bey M-Y. & Ector L. 2013. Atlas des diatomés des cours d’eau de la régioin Rhone-Alps. Tomes 1–6. Bonn’Impression 69300 Caluire. Available from: www.rhone-alpes.developpement-durable.gouv.fr.
- Bogan A.E. 2008. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 595: 139–147. doi: 10.1007/s10750-007-9011-7
- Bohanak A.J. & Jenkins D.G. 2003. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecology Letters 6: 783–796. doi: 10.1046/j.1461-0248.2003.00486.x
- Boxshall G.A. & Defaye D. 2008. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 595: 195–207. doi: 10.1007/s10750-007-9014-4
- Bradbury J.P., Bezrukova Ye. V., Chernyaeva G.P., Colman S.M., Khursevich G., King J.W. & Likoshway Ye. V. 1994. A synthesis of post-glacial diatom records from Lake Baikal. Journal of Paleolimnology 10: 213–252. doi: 10.1007/BF00684034
- Bradbury J.P. & Krebs W.N. 1995. The diatom genus Actinocyclus in the United States. United States Geological Survey Professional Paper 1543A: 1–47.
- Brummitt N.A. 2005. Patterns in the global distribution of flowering plant genera. In: Plant diversity and complexity patterns: local, regional and global dimensions. Biologiske Skrifter 55 (Ed. by I. Friis & H. Balslev), pp. 539–564.
- Bukhtiyarova L.N. & Pomazkina G.V. 2013. Bacillariophyta of Lake Baikal. Volume 1. Genera Baikalia, Slavia, Naviceia, Placogeia, Grachevia, Goldfishia, Nadiya, Cymbelgeia. Lviv, Lega-Pres. 184 pp.
- *Burliga, A.L., Kociolek, J.P., Salomoni, S.E. & Figueiredo, D. 2013. A new genus and species in the diatom family Eunotioiaceae Kützing (Bacillariophyceae) from the Amazonian hydrographic region, Brazil. Phytotaxa 79: 47–57. doi: 10.11646/phytotaxa.79.2.1
- *Cantonati, M., Van de Vijver, B. & Lange-Bertalot, H. 2009. Microfissurata gen. nov. (Bacillariophyta), a new diatom genus from dystrophic and intermittently wet terrestrial habitats. Journal of Phycology 45: 732–741. doi: 10.1111/j.1529-8817.2009.00683.x
- Cleve P.T. & Grunow A. 1880. Beiträge zue Kenntniss der Arctischen Diatomeen. Kongliga Svenska-Vetenskaps Akademiens Handlingar 17: 1–121.
- *Cocquyt, C., Vyverman, W. & Compere, P. 1993. A check-list of the algal flora of the East African Great Lakes. Scripta Botanica Belgica 8: 1–55.
- Collen B., Whitton F., Dyer E.E., Baillie J.E.M., Cumberlidge N., Darwall W.R.T., Pollock C., Richman N.I., Soulsby A.-M. & Bohm M. 2014. Global patterns of freshwater species diversity, threat and endemism. Global Ecology and Biogeography 23: 40–51. doi: 10.1111/geb.12096
- Cox E.J. & Williams D.M. 2006. Systematics of naviculoid diatoms (Bacillariophyta): a preliminary analysis of protoplast and frustule characters for family and order level classification. Systematics and Biodiversity 4: 385–399. doi: 10.1017/S1477200006001940
- Crum H.A. 1972. The geographic origins of the mosses of North America’s eastern deciduous forest. Journal of the Hattori Botanical Laboratory 35: 269–298.
- *Dalu, T., Taylor, J.C., Richoux, N.B. & Froneman, N.B. 2015. A re–examination of the type material of Entomoneis paludosa (W. Smith) Reimer and its morphology and distribution in African waters. Fottea, Olomouc 15: 11–25. doi: 10.5507/fot.2015.002
- Davidovich N., Davidovich O.I., Witkowski A., Li C., Dabek P., Mann D.G., Zglobicka I., Kurzydlowski K.J., Gusev E., Gorecka E. & Krzywda M. 2017. Sexual reproduction in Schizostauron (Bacillariophyta) and a preliminary phylogeny of the genus. Phycologia 56: 77–93. doi: 10.2216/16-29.1
- de Bruyn M., Stelbrink B., Morley R.J., Hall R., Caravalho G.R., Cannon C.H., van den Bergh G., Eijaard E., Metcalfe I., Boitani L., Maiorano L., Shoup R. & von Rintelen T. 2014. Borneo and Indochina are major evolutionary hotspots for Southeast Asian biodiversity. Systematic Biology 63: 879–901. doi: 10.1093/sysbio/syu047
- de Moor F.C. & Ivanov V.D. 2008. Global diversity of caddisflies (Trichoptera: Insecta) in freshwater. Hydrobiologia 595: 393–407. doi: 10.1007/s10750-007-9113-2
- Ehrenberg C.G. 1849. Über das mächtigste bis jetzt bekannt gewordene (angeblich 500 Fuss mächtige) Lager von mikroscopischen reinen Kieselalgen Süswasser-Formen am Wasserfall-Flusse im Oregon. Bericht über die zur Bekanntmachung geeigneten Verhandlungen der Königlich-Preussischen Akademie der Wissenschaften zu Berlin 1849: 76–87.
- *Ehrenberg, C.G. 1850. On infusorial deposits on the River Chutes in Oregon. American Journal of Science, 2nd series 9: 140.
- *Ehrenberg, C.G. 1854. Mikrogeologie. Das Erden und Felsen schaffende Wirken des unsichtbar kleinen selbstständigen Lebens auf der Erde. Leopold Voss, Leipzig. 374 pp.
- Elias S.A. & Mock C.J.. [Eds]. 2003. Encyclopedia of Quaternary Science. Elsevier, Amsterdam.
- Emlen D.J., Lavine L.C., & Ewen-Campen B. 2007. On the origin and evolutionary diversification of beetle horns. Proceedings of the National Academy of Sciences 104, Supplement 1: 8661–8668. doi: 10.1073/pnas.0701209104
- Favre A., Päckert M., Pauls S.U., Jähring S.C., Uhl D., Michalak I. & Muellner-Riehl A.N. 2015. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biological Reviews 90: 236–253. doi: 10.1111/brv.12107
- Fenchel T. & Finlay B.J. 2003. Is microbial diversity fundamental different from biodiversity of larger animal and plants? European Journal of Protistology 39: 486–490. doi: 10.1078/0932-4739-00025
- Fenchel T. & Finlay B.J. 2004a. The ubiquity of small species: patterns of local and global diversity. BioScience 54: 777–784. doi: 10.1641/0006-3568(2004)054[0777:TUOSSP]2.0.CO;2
- Fenchel T. & Finlay B.J. 2004b. Response from Fenchel and Finlay. BioScience 54: 884–885. doi: 10.1641/0006-3568(2004)054[0884:HATOE]2.0.CO;2
- Finlay B.J. 2002. Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063. doi: 10.1126/science.1070710
- Finlay B.J. & Esteban G.F. 2007. Body size and biogeography. In: Body size: the structure and function of aquatic ecosystems (Ed. by A. Hildrew, D. Raffaelli & R. Edmonds-Brown), pp. 167–185. Cambridge University Press, Cambridge.
- *Flower R.J. 2013. Diatomites: their formation, distribution and uses. In: Encyclopedia of quaternary sciences (Ed. by S.A. Elias & C.J. Mock, Second Edition), pp. 501–506. Elsevier, Amsterdam.
- Fourtanier E. & Kociolek J.P. 1999. Catalogue of the diatom genera. Diatom Research 14: 1–190. doi: 10.1080/0269249X.1999.9705462
- Frenguelli J. 1945. El Platense y sus diatomeas, Las diatomeas del Platense. Revista del Museo de La Plata (Nueva Serie). Sección Paleontologia 3: 77–221.
- *Furey, P.C., Lowe, R.L. & Johansen, J.R. 2012. Eunotia Ehrenberg (Bacillariophyta) of Great Smoky Mountains National Park, USA. Bibliotheca Diatomologica 56: 1–133.
- *Gallo-Sánchez, L.J., Sala, S.E., Guerrero-Tizzano, J.M. & Flórez, M.M.T. 2015. First report of the genus Spicaticribra Johansen, Kociolek and Lowe in a Columbian reservoir and revision of the infrageneric taxa present in South America. Actualidades Biológicas 37: 169–176.
- Garnica S., Spahn P., Oertel B., Ammirati J. & Oberwinkler F. 2011. Tracking the evolutionary history of Cortinarius species section Calochroi, with transoceanic disjunct distributions. BMC Evolutionary Biology 11: 213. doi:10.1186/1471-2148-11-213.
- *Gasse F., Barker P. & Johnson T.C. 2002. A 24,000 yr diatom record from the northern basin of Lake Malawi. In: The East African Great Lakes: limnology, palaeolimnology and biodiversity (Ed. by E.O. Odada & D.O. Olago), Advances in Global Change Research, Vol. 12, pp. 393–414. Springer, Dordrecht.
- *Gasse, F. & Fourtanier, E. 1991. African palaeoecology and biostratigraphy. Journal of African Earth Sciences (and the Middle East) 12: 325–334. doi: 10.1016/0899-5362(91)90081-9
- *Gell, P.A., Sonneman, J.A., Reid, M.A., Illman, M.A. & Sincock, A.J. 1999. An illustrated key to common diatom genera from Southern Australia. Cooperative Research Centre for Freshwater Ecology Identification Guide No. 26. Cooperative Research Centre from Freshwater Ecology, Thugoona. 63 pp.
- *Gligorga, M., Kralj, K., Plenkovic-Moraj, A., Hinz, F., Acs, E., Grigorszky, I., Cocquyt C. & Van de Vijver, B. 2009. Observations on the diatom Navicula hedinii Hustedt (Bacillariophyceae) and its transfer to a new genus Envekadea Van de Vijver et al. gen. nov. European Journal of Phycology 44: 123–138. doi: 10.1080/09670260802389783
- Givnish T.J. & Sytsma K.J. [Eds]. 1997. Molecular Evolution and Adaptive Radiation. Cambridge University Press, Cambridge.
- Goodman S. & Benstead J.P. 2003. The natural history of Madagascar. University of Chicago Press, Chicago. 1709 pp.
- Graeff C.L., Kociolek J.P. & Rushforth S.R. 2013. New and interesting diatoms (Bacillariophyta) from Blue Lake Warm Springs, Tooele County, Utah. Phytotaxa 153: 1–38. doi: 10.11646/phytotaxa.153.1.1
- Grant P.R. & Grant B.R. 2007. How and Why Species Multiply. The Radiation of Darwin's Finches. Princeton University Press, Princeton.
- *Guerrero, J.M., Vouilloud, A.A., Sala, S.E., Kociolek, J.P. & Van de Vijver, B. 2018. New species and a new genus of the Orthoseirales from Patagonia, Argentina, with comments on systematics of the Order. Phytotaxa 345: 119–132. doi: 10.11646/phytotaxa.345.2.3
- Hamsher S.E., Graeff C.L., Stepanek J.G. & Kociolek J.P. 2014. Variation in valve and girdle band morphology in freshwater Denticula (Bacillariophyceae) species: Implications for the systematic position of the genus including the description of Tetralunata gen. nov. (Epithemiaceae. Rhopalodiales). Plant Ecology and Evolution 147: 346–365. doi: 10.5091/plecevo.2014.990
- *Hartley, B., Ross, R. & Williams, D.M. 1986. A check-list of the freshwater, brackish and marine diatoms of the British Isles and adjoining coastal waters. Journal of the Marine Biological Association of the United Kingdom 66: 531–610. doi: 10.1017/S0025315400042235
- *Hickman, M. & Vitt, D.H. 1974. The aerial epiphyte flora of moss species from Subantarctic Campbell Island. Nova Hewigia 24: 443–458.
- Hill R. 2001. Biogeography, evolution and palaeoecology of Nothofagus (Nothofagaceae): the contribution of the fossil record. Australian Journal of Botany 49: 321–332.
- Holt B.G., Lessard J.-P., Borregaard M.K., Fritz S.A., Araújo M.B., Dimitrov D., Fabre P-H., Graham C.H., Graves G.R., Jensson K.A., Nogués-Bravo D., Wang Z., Whittaker R.J., Fjeldsa J. & Rahbek C. 2013. An update of Wallace’s zoogeographic regions of the world. Science 339: 74–78.
- Houk V. & Klee R. 2004. The stelligeroid taxa of the genus Cyclotella (Kützing) Brébisson (Bacillariophyceae) and their transfer into the new genus Discostella gen. nov. Diatom Research 19: 203–228.
- *Hustedt, F. 1938. Systematische und ökologische Untersuchungen über die Diatomeen-Flora von Java, Bali und Sumatra nach dem Material der Deutschen Limnologischen Sunda-Expedition. Allgemeiner Teil. II. Die Diatomeenflora der untersuchtn Gewässertypen. Tropische Binnengewässer, Band VII. Archiv für Hydrobiologie, Supplement 16: 1–155.
- Hustedt F. 1938–1939. Systematische und ökologische Untersuchungen über die Diatomeen-Flora von Java, Bali und Sumatra nach dem Material der Deutschen Limnologischen Sunda-Expedition. Allgemeiner Teil. I. Übersicht über das Untersuchengsmaterial und Charakteristik der Diatomeen flora der einzelnen Gebiete. Tropische Binnengewässer, Band VII. Archiv für Hydrobiologie, Supplement 15: 638–790.
- *Hustedt, F. 1939. Systematische und ökologische Untersuchungen über die Diatomeen-Flora von Java, Bali und Sumatra nach dem Material der Deutschen Limnologischen Sunda-Expedition. Allgemeiner Teil. III. Die ökologischen Faktoren und ihr Einfluß auf die Diatomeenflora. Tropische Binnengewässer, Band VII. Archiv für Hydrobiologie, Supplement 16: 274–394.
- Hustedt F. 1964. Die Kieselalgen Deutschlands, Österreichs und der Schweiz unter Berücksichtigung der übrigen Länder Europas sowie der angrenzenden Meeresgebiete (Ed. by L. Rabenhorst), pp. 349–556, Kryptogamen Flora von Deutschland, Österreich und der Schweiz. Akademische Verlagsgesellschaft m.b.h. Leipzig, Vol. 7, Issue: Teil 3, Lief. 3.
- *Joh G. 2012. Algal flora of Korea. Volume 3, Number 7. Chrysophyta: Bacillariophyceae: Pennales: Raphidineae: Acananthaceae. Freshwater diatoms V. National Institute of Biological Resources, Incheon. 134 pp.
- John J. 2009a. Diatom flora of tropical Australia: high lights and salient features. Scripta Botanica Belgica 45: 40.
- John J. 2009b. Eunophora in Western Australia and Queensland. Scripta Botanica Belgica 45: 41.
- John J. 2012a. A Beginner’s Guide to diatoms. A.R.G. Ganter Verlag K.G, Ruggell, Liechtenstein.
- John J. 2012b. Sand, dingos and diatoms. In: 22nd International diatom Symposium, Aula Academica, Ghent, 26– 31 August 2012, Abstracts (Ed. by K. Sabbe, B. Van de Vijver & W. Vyverman).VLIZ Special Publication 58, Ostend, Belgium, 176 pp.
- John J. 2012c. Diatoms in the Swan River Estuary, Western Australia: Taxonomy and ecology. Koeltz Scientific Books, Königstein, Germany. 456 pp.
- John J. 2015. A Beginner's Guide to Diatoms. O. Koeltz, Koenigstein.
- Journal of Phycology. 2009. Corrigendum. Journal of Phycology 45: 1416. doi: 10.1111/j.1529-8817.2009.00786.x
- Journal of Phycology. 2010. Corrigendum. Journal of Phycology 46: 1359. doi: 10.1111/j.1529-8817.2010.00917.x
- Julius M.L. & Tanimura Y. 2001. Cladistic analysis of plicated Thalassiosira (Bacillariophyta). Phycologia 40, 111–122. doi: 10.2216/i0031-8884-40-2-111.1
- Jurilj A. 1949. Nove Dijatomeje-Surirellaceae-iz Ohridskog Jezera i njihovo filogenetsko znacenje. Jugoslavenska Akademija Znanosti i Umjetnosti, Zagreb (Prirodoslovnih istrazivanja) 24: 171–259.
- Jurilj A. 1954. Flora i vegetacija Dijatomeja Ohridskog Jezera. Jugoslavenska Akademija Znanosti i Umjetnosti, Zagreb (Prirodoslovnih istrazivanja) 26: 99–190.
- Kaczmarska I. & Medlin L.K. 2009. Reply to comment by Theriot (2008) on Kaczmarska et al. (2006). Journal of Phycology 45: 987–994. doi: 10.1111/j.1529-8817.2009.00721.x
- Kalkman V.J., Clausnitzer V., Dijkstra K.-D.B., Orr A.G., Paulson D.R. & van Tol J. 2008. Global diversity of dragonflies (Odonata) in freshwater. Hydrobiologia 595: 351–363. doi: 10.1007/s10750-007-9029-x
- Kapustin D.A., Kulikovskiy M. & Kociolek J.P. 2017. Celebesia gen. nov., a new cymbelloid diatom genus from the ancient Lake Matano (Sulawesi Island, Indonesia). Nova Hedwigia, Beiheft 146: 147–155. doi: 10.1127/1438-9134/2017/147
- Khursevich G.K. 2006. Evolution of the extinct genera belonged to the family Stephanodiscaceae (Bacillariophyta) during the last eight million years in Lake Baikal. In: Advances in phycological studies (Ed. by N. Ognjanova-Rumenova & K. Manoylov), pp. 73–89. Sofia, Moscow: Pensoft Publishers and St. Kliment Ohridski University Press.
- Khursevich G.K., Fedenya S.A., Karabanov E.B., Williams D.F. & Kuzmin M.I. 2000. Stephanopsis Khursevich & Fedenya – new genus of class Centrophyceae (Bacillariophyta) from the Pliocene deposits of Lake Baikal. Algologia 10: 106–109.
- *Khursevich, G.K., Fedenya, S.A., Kuzmin, M.I., Karabanov, E.B., Williams, D.F. & Prokopenko, A.A. 2003a. Morphology of new taxa of the class Centrophyceae (Bacillariophyta) from the Pliocene and Pleistocene deposits of Lake Baikal, Siberia. Algologia 13: 305–321.
- *Khursevich, G.K., Fedenya, S.A., Kuzmin, M.I., Karabanov, E.B., Williams, D.F. & Prokopenko, A.A. 2003b. New species of Stephanodiscus (Bacillariophyta) from the Pleistocene sediments of Lake Baikal. Algologia 13: 389–401.
- Khursevich G.K. & Kociolek J.P. 2012. A preliminary, worldwide Inventory of the extinct, freshwater fossil diatoms from the orders Thalassiosirales, Stephanodiscales, Paraliales, Aulacoseirales, melosirales, Coscindiscales, and biddulphiales. Nova Hedwigia Beihefte 141: 315–364.
- Khursevich G.K., Kociolek J.P. & Fedenya S.A. 2002. A new genus of fossil freshwater diatoms (Bacillariophyta: Stephanodiscaceae) from the sediments of Lake Baikal. Proceedings of the California Academy of Sciences 53: 1–10.
- Kociolek J.P. 2005. A checklist and preliminary bibliography of the recent, freshwater diatoms of inland environments of the continental United States. Proceedings of the California Academy of Sciences 56: 395–525.
- Kociolek J.P., Balasubramanian K., Blanco S., Coste M., Ector L., Liu Y., Kulikovskiy M., Lundholm N., Ludwig T., Potapova M., Rimet F., Sabbe K., Sala S., Sar E., Taylor J., Van de Vijver B., Wetzel C.E., Williams D.M., Witkowski A. & Witkowski J. 2018. DiatomBase. Available from: http://www.diatombase.org [Accessed 15 March 2018].
- Kociolek J.P., Escobar L. & Richardson S. 1996. Taxonomy and ultrastructure of Stoermeria, a new genus of diatoms (Bacillariophyta). Phycologia 35: 70–78. doi: 10.2216/i0031-8884-35-1-70.1
- Kociolek J.P. & Kingston J.C. 1999. Taxonomy, ultrastructure and distribution of gomphonemoid diatoms (Bacillariophyceae: Gomphonemataceae) from rivers of the United States. Canadian Journal of Botany 77: 686–705. doi: 10.1139/b99-007
- Kociolek J.P., Liu Y., You Q., Wang Q., Fan Y. & Qi X. Accepted. Freshwater diatom genera of China: descriptions, keys, classification, ecology and guide to morphology. Science Press, Beijing.
- Kociolek J.P. & Spaulding S.A. 2000. Freshwater diatom biogeography. Nova Hedwigia 71: 223–241.
- Kociolek J.P. & Stoermer E.F. 1988. A preliminary investigation of the phylogenetic relationships of the freshwater, apical pore field-bearing cymbelloid and gomphonemoid diatoms (Bacillariophyceae). Journal of Phycology 24: 377–385. doi: 10.1111/j.1529-8817.1988.tb00187.x
- Kociolek J.P. & Williams D.M. 2015. How to define a diatom genus? Notes on the creation and recognition of taxa, and a call for revisionary studies of diatoms. Proceedings of the 8th Central European diatom Meeting. Zagreb, Croatia. Acta Croatica Botanica 74: 195–210. doi: 10.1515/botcro-2015-0018
- *Kociolek, J.P., Woodward, J.C. & Graeff, C. 2016a. New and endemic Gomphonema C.G. Ehrenberg (Bacillariophyceae) species from Hawaii. Nova Hedwigia 102: 141–171. doi: 10.1127/nova_hedwigia/2015/0296
- *Kociolek, J.P., Uyua, N.M., Sala, S.E., Santinelli, N.H. & Cefarelli, A. 2017. New species, new taxon report and biogeography of the diatom genus Gomphoneis Cleve (Bacillariophyceae) in Patagonia, Chubut Province, Argentina. Diatom Research 32: 439–450. doi: 10.1080/0269249X.2017.1393009
- *Kociolek, J.P., You, Q., Stepanek, J., Lowe, R.L. & Wang, Q. 2016b. A new freshwater diatom genus, Edtheriotia gen. nov. of the Stephanodiscaceae Glezer & Makarova (Bacillariophyta) from south-central China. Phycological Research 64: 274–280. doi: 10.1111/pre.12145
- Kociolek J.P., Woodward J.C., & Graeff C. 2016. New and endemic Gomphonema C.G. Ehrenberg (Bacillariophyceae) species from Hawaii. Nova Hedwigia 102: 141–171. doi: 10.1127/nova_hedwigia/2015/0296
- Krammer K. 1997. Die cymbelloiden Diatomeen. Ein Monographie der weltweit bekannten Taxa. Teil 2. Encyonema part., Encyonopsis and Cymbellopsis. Bibliotheca Diatomologica 37: 1–463.
- Krammer K. 2000. The genus Pinnularia. Diatoms of Europe 1: 1–703.
- Krammer K. 2003. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. Diatoms of Europe 4: 1–529.
- Krammer K. & Lange-Bertalot H. 1986. Bacillariophyceae. 1. Teil: Naviculaceae. In: Süsswasser flora von Mitteleuropa, Band 2/1. (Ed. by H. Ettl, J. Gerloff, H. Heynig & D. Mollenhauer). Gustav Fischer Verlag, Stuttgart & New York. 876 pp.
- *Krammer K. & Lange-Bertalot H. 1988. Bacillariophyceae. 2. Teil: Bacillariaceae, epithemiaceae, surirellaceae. In: Süsswasserflora von Mitteleuropa, Band 2/2 (Ed. by H. Ettl, J. Gerloff, H. Heynig & D. Mollenhauer). VEB Gustav Fischer Verlag, Jena, 596 pp.
- *Krammer K. & Lange-Bertalot H. 1991a. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Süsswasserflora von Mitteleuropa, Band 2/3 (Ed. by H. Ettl, J. Gerloff, H. Heynig & D. Mollenhauer). Gustav Fischer Verlag: Stuttgart & Jena. 576 pp.
- *Krammer K. & Lange-Bertalot H. 1991b. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis Teil 1–4. In: Süsswasserflora von Mitteleuropa, Band 2/4. (Ed. by H. Ettl, G. Gärtner, J. Gerloff, H. Heynig & D. Mollenhauer). Gustav Fischer Verlag: Stuttgart & Jena. 437 pp.
- *Krebs, W.N. & Bradbury, J.P. 1995. Geologic ranges of lacustrine species, Western North America. United States Geological Survey Professional Paper 1543B: 49–73.
- Krebs W.N., Bradbury J.P. & Theriot E. 1987. Neogene and Quaternary lacustrine diatom biochronology, western USA. Palaios 2: 505–513. doi: 10.2307/3514621
- Kulikovskiy M., Gluschchenko A.M., Genkal S.I. & Kuznetsova I.V. 2016. Identification book of diatoms from Russia. Filigran, Yaroslavl. 804 pp.
- Kulikovskiy M., Gusev E., Andreeva S. & Annenkova N. 2014. Phylogenetic position of the diatom genus Geissleria Lange-Bertalot & Metzeltin and description of two new species from Siberian mountain lakes. Phytotaxa 177: 249–260. doi: 10.11646/phytotaxa.177.5.1
- Kulikovskiy M., Lange-Bertalot H. & Kuznetsova I. 2015. Lake Baikal: hotspot of endemic diatoms II. Iconographia Diatomologica 26: 1–656.
- Kulikovskiy M., Lange-Bertalot H., Metzeltin D. & Witkowski A. 2012. Lake Baikal: hotspot of endemic diatoms. Iconographia Diatomologica 23: 1–607.
- *Kulikovskiy, M.S., Lange-Bertalot, H., Witkowski, A., Dorofeyuk, N.I. & Genkal, S.I. 2010. Diatom assemblages from Sphagnum bogs of the World. I. Nur bog in northern Mongolia. Bibliotheca Diatomologica 55: 1–326.
- Lange-Bertalot H. & Fuhrmann A. 2014. Ninastrelnikovia: a new genus of biraphid Bacillariophyceae. Nova Hedwigia Beiheft 143: 391–401.
- Lange-Bertalot H. & Genkal S.I. 1999. Diatoms from Siberia I – Islands in the Arctic Ocean (Yugorsky-Shar Strait) Diatomeen aus Siberien. I. Insel im Arktischen Ozean (Yugorsky-Shar Strait). Iconographia Diatomologica 6: 1–271.
- *Lange-Bertalot H., Hofmann G., Werum M. & Cantonati M. 2017. Freshwater benthic diatoms of Central Europe. over 800 common species used in ecological assessment. Koeltz Botanical Books, Koenigstein. 942 pp.
- *Lange-Bertalot, H. & Moser, G. 1994. Brachysira. Monographie der Gattung. Bibliotheca Diatomologica 29: 1–212.
- *Lange-Bertalot, H. & Wojtal, A.Z. 2014. Diversity in species complexes of Placoneis clementis (Grunow) Cox and Paraplaconeis placentula (Ehrenberg) Kulivoskiy, Lange-Bertalot & Metzltin. Nova Hedwigia Beiheft 143: 403–420.
- Laurin M. 2010. The subjective nature of Linnaean categories and its impact in evolutionary biology and biodiversity studies. Contributions to Zoology 79: 131–146.
- *Le Cohu, R., Gassiole, G. & Coste, M. 2014. Three new species of Cymbellales (Bacillariophyceae) from Réunion Island. Phytotaxa 156: 117–132. doi: 10.11646/phytotaxa.156.3.3
- Lerner H., Meyer M., James H., Hofreiter M. & Fleischer R. 2011. Multilocus resolution of phylogeny and Timescale in the extant adaptive radiation of Hawaiian honeycreepers. Current Biology 21: 1838–1844. doi: 10.1016/j.cub.2011.09.039
- Levkov Z., Metzeltin D. & Pavlov A. 2013. Luticola and luticolopsis. Diatoms of Europe 7: 1–697.
- Li Y., Lange-Bertalot H. & Metzeltin D. 2013. Sichuaniella Li Yanling, Lange-Bertalot et Metzeltin nom. nov. - a new name for Sichuania Li Yanling et al. In: Diatoms of Europe. Vol. 7. Diatoms of the European inland waters and comparable habitats. Luticola and Luticolopsis (Ed. by Z. Levkov, D. Metzeltin & A. Pavlov), p. 698. A.G. Ganter, Oberreifenberg.
- Liu Q., Wu W., Wang J., Feng J., Lu J., Kociolek J.P. & Xie S. 2017. Valve ultrastructure of Nitzschia shanxiensis nom. nov., stat. nov. and N. tabellaria (Bacillariales, Bacillariophycaeae) with comments on their systematic position. Phytotaxa 312: 228–236. doi: 10.11646/phytotaxa.312.2.5
- *Liu, Y., Kociolek, J.P., Fan, Y. & Kulikovskiy, M. 2018. A new genus of Eunotiales (Bacillariophta, Bacillariophyceae: Peroniaceae) from Southeast Asia, exhibiting remarkable phenotypic plasticity, and evidence for another lineage of monoraphid diatoms. Phycologia 57: 147–158. doi: 10.2216/17-21.1
- Lotsy J.P. 1916. Evolution by Means of Hybridization. Martinus Nijhoff, The Hague.
- Lowe R.L., Morales E. & Kilroy C. 2006. Frankophila biggsii (Bacillariophyceae), a new diatom species from New Zealand. New Zealand Journal of Botany 44: 41–46. doi: 10.1080/0028825X.2006.9513004
- Lu L.-M., Mao L.-F., Yang T., Ye J.-F., Liu B., Li H.-L., Sun M., Miller J.T., Mathews S., Hu H-H., Niu Y.T., Peng D-X., Chen Y-H., Smith S.A., Chen M., Xiang K-L., Le C-T., Dang V-C., Lu A-M., Soltis P.S., Soltis D.E., Li J-H. & Chen Z.-D. 2018. Evolutionary history of the angiosperm flora of China. Nature 554: 234–238. doi: 10.1038/nature25485
- *Luchini L. & Verona C.A. 1972. Catalogo de las Diatomeas argentinas. I. Diatomeas de aguas continentales. Comision de Investigaciones Científicas de la Provincia de Buenos Aires. La Plata Argentina. 304 pp.
- Lundholm N., Bates S.S., Baugh K.A., Bill B.D., Connell L.B., Léger C. & Trainer V.L. 2012. Cryptic and pseudo-cryptic diversity in diatoms-with descriptions of Pseudonitzschia hasleana sp. nov. and P. fryxelliana sp. nov. Journal of Phycology 48: 436–454. doi: 10.1111/j.1529-8817.2012.01132.x
- Lundholm N., Daugbjerg N. & Moestrup Ø. 2002. Phylogeny of the Bacillariaceae with emphasis on the genus pseudo-nitzsschia (Bacillariophyceae) based on partial LSU rDNA. European Journal of Phycology 37: 115–134. doi: 10.1017/S096702620100347X
- *Mahoney, R.K. 1989. Observations on the diatom Gomphopleura nobilis Reichelt ex Tempère. Proceedings of the Academy of Natural Sciences of Philadelphia 141: 251–261.
- *Mahoney R.K. & Reimer C.W. 1986. Studies on the genus Brebissonia (Bacillariophyceae). I. Introduction and observations de B. lanceolata comb. nov. In: Proceedings of the 8th international diatom symposium. (Ed. by M. Ricard), pp. 183–190. Koenigstein, Koeltz.
- Maidana N.I., Morales E.A., Bradbury J.P., Schäbitz F. & Houk V. 2017. A new order and family of diatoms: Arcanodiscales, Arcanodiscaeae (Bacillariophyta) to accommodate Arcanodiscus plattii gen. nov. et sp. nov. from the Argentinian Patagonia. Nova Hedwigia Beiheft 146: 63–72. doi: 10.1127/1438-9134/2017/063
- Maidana N.I. & Round F.E. 1999. Corbellia contorta gen. & sp. nov. (Bacillariophyceae). A new diatom genus from Santa Cruz Province (Argentina). Diatom Research 14: 331–336. doi: 10.1080/0269249X.1999.9705474
- Maillard R. 1978. Contribution à la connaissance des diatomées d'eau douce de la Nouvelle-Calédonie (Océanie). Cahiers O.R.S.T.O.M. Série Hydrobiologie 12: 143–172.
- Main S.P. 2003. Diprora haenaensis gen. et sp. nov., a filamentous, pseudoaerial, araphid diatom from Kaua’i (Hawaiian Islands). Diatom Research 18: 259–272. doi: 10.1080/0269249X.2003.9705591
- Manguin E. 1941. Contribution à la Flore des Diatomées d'eau douce de Madagascar. Revue Algologique 12: 153–157.
- Manguin E. 1952. Les Diatomées fossiles du bassin thermominéral d'Antsirabe, Ramanofana II. Mémoires de L'Institut Scientifique de Madagascar, séries B 4: 1–57.
- Manguin E. 1962. Contribution à la connaissance de la flore diatomique de la Nouvelle-Calédonie. Mémoires du Museum National d'Histoire Naturelle, nouvelle série, série B, Botanique 12: 1–40.
- *Manrique, J.M., Uyua, N.M., Bauer, G.A., Santinelli, N.H., Ayestarán, G.M., Sala, S.E., Sastre, A.V., Jones, L.R. & Whitton, B.A. 2017. Nuisance Didymosphenia geminata blooms in the Argentinean Patagonia: status and current research trends. Aquatic Ecosystem Health & Management 20: 361–368.
- Martens K., Schön I., Meisch C., & Horne D.J. 2008. Global diversity of ostracods (Ostracoda, Crustacea) in freshwater. Hydrobiologia 595: 185–193. doi: 10.1007/s10750-007-9245-4
- Massard J.A. & Geimer G. 2008. Global diversity of bryozoans (Bryozoa or Ectoprocta) in freshwater. Hydrobiologia 595: 93–99. doi: 10.1007/s10750-007-9007-3
- Matthews W.J. & Heins D.C. 1987. Community and evolutionary ecology of North American stream fishes. University Oklahoma Press, Norman.
- *McBride, T.P. 2009. Freshwater diatoms on Sub-Antarctic Macquarie Island: an ecological survey of 14 lakes. Papers and Proceedings of the Royal Society of Tasmania 143: 73–80. doi: 10.26749/rstpp.143.2.73
- *Medvedeva L.A. & Nikulina T.V. 2014. Catalogue of freshwater algae of the southern part of the Russian Far East. Dalnauka, Vladivostok. 271 pp.
- Metzeltin D. & Lange-Bertalot H. 2002. Diatoms from the ‘island continent’ Madagascar. Iconographia Diatomologica 11: 1–286.
- Metzeltin D. & Lange-Bertalot H. 2007. Tropical diatoms of South America II. Special remarks on biogeography disjunction. Iconographia Diatomologica 18: 1–877.
- *Metzeltin, D., Lange–Bertalot, H. & Garcia–Rodriguez, F. 2005. Diatoms of Uruguay compared with other taxa from South America and elsewhere. Iconographia Diatomologica 15: 1–736.
- Milne R.I. 2006. Northern hemisphere plant disjunctions: a window on Tertiary land bridges and climate change? Annals of Botany 98: 465–472.
- *Montoya-Moreno, Y., Sala, S., Vouilloud, A., Aguirre, N. & Plata-Díaz, Y. 2013. Lista de las diatomeas des ambientes continentales de Colombia. Biota Colombiana 14: 13–78.
- Morales E.A. 2002. Studies in selected fragilarioid diatoms of potential indicator value from Florida (USA) with notes on the genus Opephora Petit (Bacillariophyceae). Limnologica 32: 102–113.
- Moser G., Lange-Bertalot H. & Metzeltin D. 1998. Insel der Endemiten Geobotanisches Phänomen Neukaledonien. Bibliotheca Diatomologica 38: 1–464.
- Moser G., Steindorf A. & Lange-Bertalot H. 1995. Neukaledonien Diatomeenflora einer Tropeninsel. Revision der collection Maillard und Untersuchung neuen materials. Bibliotheca Diatomologica 32: 1–340.
- *NIWA. 2012. A quick guide to the diatom genera in New Zealand fresh waters. Parts A-J. Available from: https://www.niwa.co.nz/sites/niwa.co.nz/files/diatom_key_2012_revisedb_0.pdf.
- Patrick R.M. & Reimer C.W. 1966. The diatoms of the United States. Vol. 1, Monograph 13. Academy of Natural Sciences of Philadelphia, Philadelphia. 688 p.
- Pennesi C., Caputo A., Lobban C.S., Poulin M. & Totti C. 2017. Morphological discoveries in the genus Diploneis (Bacillariophyceae) from the tropical west Pacific including the description of new taxa. Diatom Research 32: 195–228.
- Poulícková A., Veselá J. & Neustupa & Skaloud P. 2010. Pseudocryptic diversity versus cosmopolitanism in diatoms: a case study on Navicula cryptocephala Kütz. (Bacillariophyceae) and morphologically similar taxa. Protist 161: 353–369.
- Qian H. 2002. Floristic relationships between Eastern Asia and North America: Test of Gray’s hypothesis. The American Naturalist 160: 317–332.
- Raymo M.E. & Ruddiman W.F. 1992. Tectonic forcing of late Cenozoic climate. Nature 359: 117–122.
- *Reid, P.C., Lancelot, C., Gieskes, W.W.C., Hagmeier, E. & Weichart, G. 1990. Phytoplankton of the North Sea and its dynamics: a review. Netherlands Journal of Sea Research 26: 295–331.
- Reisch C. 2008. Glacial history of Saxifraga paniculata (Saxifragaceae): molecular biogeography of a disjunct Arctic-alpine species from Europe and North America. Biological Journal of the Linnaean Society 93: 385–398.
- *Rioual, P., Flower, R., Chu, G., Lu, Y., Zhang, Z., Zhu, B. & Yang, X. 2017. Observations on a fragilarioid diatom found in inter-dune lakes of the Badain Jaran Desert (Inner Mongolia, China), with a discussion on the recently erected genus Williamsella Graeff, Kociolek & Rushforth. Phytotaxa 329.
- *Rioual, P., Lu, Y., Chu, G., Zhu, B. & Yang, X. 2014. Morphometric variation of Seminavis pusilla (Bacillariophyceae) and its relationship to salinity in inter-dune lakes of the Badain Jaran Desert, Inner Mongolia, China. Phycological Research 62: 282–293.
- Ricklefs R.E. & Bermingham E. 2007. The West Indies as a laboratory of biogeography and evolution. Transactions of the Royal Society of London 363: 2393–2413. doi: 10.1098/rstb.2007.2068
- Rosauer D.F., Laffan S.W., Crisp M.D., Donnellan S.C. & Cook L.G. 2009. Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Molecular Ecology 18: 4061–4072. doi: 10.1111/j.1365-294X.2009.04311.x
- Rosauer D.F. & Jetz W. 2015. Phylogenetic endemism in terrestrial mammals. Global Ecology and Biogeography 24: 168–179. doi: 10.1111/geb.12237
- Ross H.H. 1967. Evolution and past dispersal of the Trichoptera. Annual Review of Entomology 12: 169–206. doi: 10.1146/annurev.en.12.010167.001125
- Ross R. & Sims P.A. 1978. Notes on some diatoms from the Isle of Mull, and other Scottish localities. Bacillaria 1: 151–168.
- Round F.E. 1998. Validation of some previously published achnanthoid genera. Diatom Research 13: 181. doi: 10.1080/0269249X.1998.9705442
- Round F.E. & Bukhtiyarova L. 1996. Four new genera based on Achnanthes (Achnanthidium) together with a re-definition of Achnanthidium. Diatom Research 11: 345–361. doi: 10.1080/0269249X.1996.9705389
- Round F.E. & Crawford R.M. 1981. The lines of evolution of the Bacillariophyta. I. Origin. Proceedings of the Royal Society of London, B 211: 237–260. doi: 10.1098/rspb.1981.0004
- Round F.E. & Crawford R.M. 1984. The lines of evolution of the Bacillariophyta. II. The centric series. Proceedings of the Royal Society of London, B 221: 169–188. doi: 10.1098/rspb.1984.0029
- Round F.E., Crawford R.M. & Mann D.G. 1990. The diatoms. Biology and morphology of the genera. Cambridge University Press, Cambridge. 747 p.
- Ruck E. & Kociolek J.P. 2004. A preliminary phylogeny of the family Surirellaceae. Bibliotheca Diatomologica 50: 1–236.
- Ruck E.C., Nakov T., Alverson A.J. & Theriot E.C. 2016. Phylogeny, ecology, morphological evolution, and reclassification of the diatom orders surirellales and rhopalodiales. Molecular Phylogenetics and Evolution 103: 155–171. doi: 10.1016/j.ympev.2016.07.023
- Ruck E.C. & Theriot E.C. 2011. Origin and evolution of the canal raphe system in diatoms. Protist 162: 723–737. doi: 10.1016/j.protis.2011.02.003
- *Rumrich, U., Lange-Bertalot, H. & Rumrich, M. 2000. Diatoms of the Andes. from Venezuela to Patagonia/Tierra del Fuego and two additional contributions. Iconographia Diatomologica 9: 1–673.
- Sanmartin I., Enghoff H. & Ronquist F. 2001. Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biological Journal of the Linnaean Society 73: 345–390. doi: 10.1111/j.1095-8312.2001.tb01368.x
- *Schoeman, F.R. 1982. The diatoms of the Jukskei Crocodile river system (Transvaal, Republic of South Africa): A preliminary check-list. Journal of South African Botany 48: 295–310.
- Segers H. 2008. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 595: 49–59. doi: 10.1007/s10750-007-9003-7
- *Shao, K.T. 2003–2014. TaiBNET(Catalogue of Life in Taiwan) http://taibnet.sinica.edu.tw. Taiwan.
- Sheard J.W., Ezhkin A.K., Galanina I.A. & Himelbrant D. 2017. The lichen genus Rinodina (Physciaceae, Caliciales) in north-Eastern Asia. The Lichenologist 49: 617–672. doi: 10.1017/S0024282917000536
- *Simonsen, R. 1979. The diatom system: ideas on phylogeny. Bacillaria 2: 9–71.
- Simonsen R. 1987. Atlas and Catalogue of the Diatom Types of Friedrich Hustedt. 3 Vols. J. Cramer, Berlin & Stuttgart.
- Sims P.A., Mann D.G. & Medlin L.K. 2006. Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45: 361–402. doi: 10.2216/05-22.1
- Siver P.A., Wolfe A.P. & Edlund M.B. 2016. Fideliacyclus wombatiensis gen. et sp. nov. – a Paleocene non-marine centric diatom from northern Canada with complex frustule architecture. Diatom Research 31: 397–408. doi: 10.1080/0269249X.2016.1256351
- Skvortzow B.W. 1937. Bottom diatoms from Olhon Gate of Baikal Lake, Siberia. Philippine Journal of Science 62: 293–377.
- Skvortzow B.W. 1976. Moss diatoms flora from River Gan in the northern part of Great Khingan Mts, China. With descriptions of a new genera Porosularia gen. nov. from northern and southern China, The second part. Quarterly Journal of the Taiwan Museum 29: 397–439.
- Sörhannus U. 2004. Diatom phylogenetics inferred based on direct optimization of nuclear-encoded SrRNA sequences. Cladistics 20: 487–497. doi: 10.1111/j.1096-0031.2004.00034.x
- Sörhannus U., Gasse F., Perasso R. & Baroin Tourancheau A. 1995. A preliminary phylogeny of diatoms based on 28s ribosomal RNA sequence data. Phycologia 34: 65–73. doi: 10.2216/i0031-8884-34-1-65.1
- Spaulding S.A. & Kociolek J.P. 1998. New Gomphonema (Bacillariophyceae) species from Madagascar. Proceedings of the California Academy of Sciences, 4th Series 50: 361–379.
- Stidolph S.R. 1990. Cavernosa kapitiana, a new diatom genus and species from Kapiti Island, New Zealand. Nova Hedwigia 50: 97–110. doi: 10.1127/nova.hedwigia/50/1990/97
- Sun X.J. & Wang P.X. 2005. How old is the Asian monsoon system? Palaeobotanical records from China. Palaeogeography Palaeoclimatology Palaeoecology 222: 181–222. doi: 10.1016/j.palaeo.2005.03.005
- *Taylor, J.C. & Cocquyt, C. 2016. Diatoms from the Congo and Zambesi basins – methodologies and identification of the genera. Abc Taxa, Brussels.
- Taylor J.C., Harding W.R. & Archibald C.G.M. 2007. An illustrated guide to some common diatom species from South Africa. Water Research Commission, Pretoria. 225 p.
- Taylor J.C. & Lange-Bertalot H. 2013. Cholnokyella aerophila J. C. Taylor & Lange-Bertalot gen. et spec. nov. A new diatom (Bacillariophyceae) from sandstone caves in South Africa. Nova Hedwigia 97: 295–304. doi: 10.1127/0029-5035/2013/0118
- *Tell, G. 1985. Catálogo de las algas de agua dulce de la República Argentina. Bibliotheca Phycologica 70: 1–283.
- Theriot E.C. 2008. Application of phylogenetic principles to testing evolutionary scenarios: a comment on Kaczmarska et al. molecular phylogeny of selected members of the order Thalassiosirales (Bacillariophyta) and the evolution of the fultoportula. Journal of Phycology 44: 821–833. doi: 10.1111/j.1529-8817.2008.00522.x
- Theriot E.C., Ashworth M., Ruck E., Nakov T. & Jansen R. 2010. A preliminary multi-gene phylogeny of diatoms (Bacillariophyta): challenges for future research. Plant Ecology and Evolution 143: 278–296. doi: 10.5091/plecevo.2010.418
- Theriot E.C., Ashworth M.P., Nakov T., Ruck E. & Jansen R.K. 2015. Dissecting signal and noise in diatom chloroplast protein encoding genes with phylogenetic information profiling. Molecular Phylogenetics and Evolution 89 28–36. doi: 10.1016/j.ympev.2015.03.012
- Thomas E., Stepanek J. & Kociolek J.P. 2016. Historical and current perspectives on the systematics of the ‘enigmatic’ diatom genus Rhoicosphenia (Bacillariophyta), with single and multi-molecular marker and morphological analyses and discussion on the monophyly of ‘Monoraphid’ diatoms. PLoS One 11(4): e0152797. doi: 10.1371/journal.pone.0152797
- *Tremarin, P.I., Freire, E.G. Bertolli, L.M. & Ludwig, T.A.V. 2009. Catealogo das diatomáceas (Ochrophyta-Diatomeae) continentais do estado do Paraná. Iheringia, Serie Botanica 64: 79–107.
- Tuji A., Mohri Y., Ki J.-S., Jung S.W. & Julius M.L. 2014. Phylogeny of Praestephanos gen. nov. (Thalassiosirales, Bacillariophyceae) based on Stephanodiscus suzukii, and related freshwater thalassiosiroid diatoms. Plankton and Benthos Research 9: 132–140. doi: 10.3800/pbr.9.132
- *Udovic, M.G., Cvetkoska, A., Zutinic, P., Basak, S., Stankovic, I., Spoljaric, I., Mrsic G., Borojevic K.K., Cukurin A. & Plenkovic-Moraj, A. 2017. Defining centric diatoms of most relevant phytoplankton functional groups in deep karts lakes. Hydrobiologia 788:169–191. doi: 10.1007/s10750-016-2996-z
- Väinölä R., Witt J.D.S., Grabowski M., Bradbury J.H., Jazdzewski K. & Sket B. 2008. Global diversity of amphipods (Amphipoda: Crustacea) in freshwater. Hydrobiologia 595: 241–255. doi: 10.1007/s10750-007-9020-6
- Vaneslander B., Créach V., Vanormelingen P., Ernst A., Chepurnov V.A., Sahan E., Muyzer G., Stal L.J., Vyverman W. & Sabbe K. 2009. Ecological differentiation between sympatric pseudocryptic species in the estuarine benthic diatom Navicula phyllepta (Bacillariophyceae). Journal of Phycology 45: 1278–1289. doi: 10.1111/j.1529-8817.2009.00762.x
- Vanormelingen P.V., Evans K.M., Chepurnov V.A., Vyverman W. & Mann D.G. 2013. Molecular species discovery in the diatom Sellaphora and its congruence with mating trials. Fottea 13: 133–148. doi: 10.5507/fot.2013.012
- Vanormelingen P., Verleyen E. & Vyverman W. 2007. The diversity and distribution of diatoms: from cosmopolitanism to narrow endemism. Biodiversity and Conservation 17: 393–405. doi: 10.1007/s10531-007-9257-4
- Veblen T., Hill R. & Read J. 1996. Ecology and biogeography of Nothofagus Forests. Yale University Press, New Haven. 414 pp.
- *Vouilloud A.A. 2003. Catalogo diatomeas Argentina. Privately published. 310 pp.
- *Vyverman, W. & Compère, P. 1991. Nupela giluwensis gen. & spec. nov. a new genus of naviculoid diatoms. Diatom Research 6: 175–179. doi: 10.1080/0269249X.1991.9705156
- Vyverman W., Sabbe K., Mann D.G., Kilroy C., Vyverman R., Vanhoutte K. & Hodgson D. 1998. Eunophora gen. nov. (Bacillariophyta) from Tasmania and New Zealand: description and comparison with Eunotia and amphoroid diatoms. European Journal of Phycology 33: 95–111. doi: 10.1080/09670269810001736593
- Vyverman W., Verleyen E., Sabbe K., Vanhoutte K., Sterken M., Hodgson D.A., Mann D.G., Juggins S., Van de Vijver B., Jones V., Flower R., Roberts D., Chepurnov V.A., Kilroy C., Vanormelingen P. & De Wever A. 2007. Historical processes constrains patterns in global diatom diversity. Ecology 88: 1924–1931. doi: 10.1890/06-1564.1
- Wen J. 1999. Evolution of Eastern Asian and Eastern North American disjunct distributions in flowering plants. Annual Review of Ecology and Systematics 30: 421–455. doi: 10.1146/annurev.ecolsys.30.1.421
- Wetzel C. & Kociolek J.P. 2018. Burliganiella gen. nov. (Bacillariophyta, Eunotiales) another case of raphe reduction based on the type material of Fragilaria siolii Hustedt. Cryptogamie, Algologie 39: 1–11. doi: 10.7872/crya/v39.iss2.2018.255
- *Wetzel, C.E., Lange-Bertalot, H., Morales, E.A., Bicudo, D.C., Hoffmann, L. & Ector, L. 2012. Bicudoa amazonica gen. nov. et sp. nov. (Bacillariophyta) – a new freshwater diatom from the Amazon basin with a complete raphe loss in the Eunotioid lineage. Phytotaxa 75: 1–18. doi: 10.11646/phytotaxa.75.1.1
- *Wetzel, C.E., Van de Vijver, B., Cox, E.J., Bicudo, D.C. & Ector, L. 2012. Tursiocola podocnemicola sp. nov., a new epizoic freshwater diatom species from the Rio Negro in the Brazilian Amazon Basin. Diatom Research 27: 1–8. doi: 10.1080/0269249X.2011.642498
- Williams D.M. 1990. Distrionella D.M. Williams, nov. gen., a new araphid diatom (Bacillariophyta) genus closely related to Diatoma Bory. Archiv für Protistenkunde 138: 171–177. doi: 10.1016/S0003-9365(11)80159-4
- Williams D.M. 1996. Fossil species of the diatom genus Tetracyclus (Bacillariophyta, ‘ellipticus’ species group): morphology, interrelationships and the relevance of ontogeny. Philosophical Transactions of the Royal Society, London, Series B 351: 1759–1782. doi: 10.1098/rstb.1996.0156
- *Williams, D.M. 2004. On diatom endemism and biogeography: Tetracyclus and Lake Baikal endemic species. In: Proceedings of the 17th International diatom Symposium (Ed. by F.E. Round), pp. 433–459. BioPress, Bristol.
- Williams D.M. 2007. Classification and diatom systematics: the past, the present and the future. In: Unravelling the Algae: the past, present and future of Algal systematics (Ed. by J. Brodie & J. Lewis), pp. 57–91. CRC Press, Boca Raton.
- Williams D.M. 2011. Historical biogeography, microbial endemism and the role of classification: everything is endemic. In: Biogeography of microscopic organisms: is everything small everywhere? (Ed. by D. Fontanneto), pp. 11–31. Cambridge: Cambridge University Press.
- Williams D.M. & Kociolek J.P. 2007. Pursuit of a natural classification of diatoms: history, monophyly and the rejection of paraphyletic taxa. European Journal of Phycology 42: 313–319. doi: 10.1080/09670260701419921
- Williams D.M. & Kociolek J.P. 2010a. Towards a comprehensive diatom classification and phylogeny (Bacillariophyta). Plant Ecology and Evolution 143: 265–270. doi: 10.5091/plecevo.2010.401
- Williams D.M. & Kociolek J.P. 2010b. Classifications of convenience: the meaning of names. Diatom Research 25: 213–216. doi: 10.1080/0269249X.2010.9705840
- Williams D.M. & Kociolek J.P. 2017. Historical biogeography of diatoms in Australasia: a preliminary assessment. In: Handbook of Australasian biogeography (Ed. by M. Ebach), pp. 17–46. Boca Raton: CRC Press.
- Williams D.F., Peck J., Karabanov E.B., Prokopenko A.A., Kravchinsky V., King J. & Kuzmin M.I. 1997. Lake Baikal record of continental climate response to orbital insolation during the past 5 million years. Science 278: 1114–1117. doi: 10.1126/science.278.5340.1114
- Williams D.M. & Reid G. 2006a. Amphorotia nov. gen., a new genus in the family Eunotiaceae (Bacillariophyceae), based on Eunotia clevei Grunow in Cleve et Grunow. Diatom Monographs 6: 1–153.
- Williams D.M. & Reid G. 2006b. Fossils and the tropics, the Eunotiaceae (Bacillariophyta) expanded: the Upper Eocene fossil diatom Eunotia reedi and the recent marine diatom Amphora reichardtiana from the tropics. European Journal of Phycology 41: 147–154. doi: 10.1080/09670260600628564
- *Williams, D.M. & Reid, G. 2006c. Large and species rich taxa: diatoms, geography and taxonomy. In: Reconstructing the tree of life: taxonomy and systematics of species rich taxa (Ed. by T.R. Hodkinson & J.A.N. Parnell), 299–316. Boca Raton: CRC Press.
- Williams D.M. & Reid G. 2009. New species in the genus Colliculoamphora Williams and Reid with commentary on species concepts in diatom taxonomy. Beihefte zur Nova Hedwigia 135: 185–200.
- Williams D.M. & Round F.E. 1986. Revision of the genus Synedra Ehrenb. Diatom Research 1: 313–339. doi: 10.1080/0269249X.1986.9704976
- Williams D.M. & Round F.E. 1987. Revision of the genus Fragilaria. Diatom Research 2: 267–288. doi: 10.1080/0269249X.1987.9705004
- Wilson G.D.F. 2008. Global diversity of Isopod crustacean (Crustacea: Isopoda) in freshwater. Hydrobiologia 595: 231–240. doi: 10.1007/s10750-007-9019-z
- Wolfe A.P. & Edlund M.B. 2005. Taxonomy, phylogeny, and paleoecology of Eoseira wilsonii gen. et sp. nov., a Middle Eocene diatom (Bacillariophyceae: Aulacoseiraceae) from lake sediments at Horsefly, British Columbia, Canada. Canadian Journal of Earth Sciences 42: 243–257. doi: 10.1139/e04-051
- Wojtal A.Z. 2013. Species composition and distribution of diatom assemblages in spring waters from various geological formations in Southern Poland. Bibliotheca Diatomologica 59: 1–436.
- *Yanagisawa, Y. & Tanaka, H. 2014. Mesodictyon japonicum, a new fossil diatom species from the Miocene marine sediments distributed in the Niigata area, central Japan. Diatom 30: 147–156.
- Yeo D.C.J., Ng P.K.L., Cumberlidge N., Magalhäes C., Daniels S.R. & Campos M.R. 2008. Global diversity of crabs (Crustacea: Decapoda: Brachynura) in freshwater. Hydrobiologia 595: 275–286. doi: 10.1007/s10750-007-9023-3
- *Zidarova, R., Kopalova, K. & Van de Vijver, B. 2016. Diatoms from the Antarctic region. I. Maritime Antarctica. Iconographia Diatomologica 24: 1–509.