Abstract
The genus Entomoneis includes diatoms with an elevated bilobate keel, a sigmoid raphe canal and numerous girdle bands. It is known to inhabit various environments, from freshwater to marine, both plankton and benthos. During a phytoplankton investigation in the Kungälv estuary on the west coast of Sweden, we observed numerous cells belonging to Entomoneis. Further morphological investigations performed with light and electron microscopy allowed us to describe Entomoneis annagodhei, sp. nov., with a unique set of morphological characters. Most importantly, a still undocumented character proved to be an oblique transapical fascia across the centre of the valve. Other distinctive features of this species are a pronounced raphe canal with dense raphe fibulae, very fine striation, resolvable only with electron microscopy, external lanceolate slit-like opening of the central nodule, and fine, linear to undulate external ridges running more or less parallel and adjacent to the raphe and valve margin. The morphological characters and morphometry are discussed in comparison with similar taxa. Our results contribute to the under-appreciated diversity of Entomoneis, especially inhabiting the marine plankton.
Introduction
The diatom genus Entomoneis Ehrenberg includes species that can be readily distinguished in light microscopy due to several characteristic features, such as panduriform cells, numerous girdle bands and a sigmoid raphe positioned on a bilobate keel (a wing-like elevation) (Patrick & Reimer Citation1975, Round et al. Citation1990). Most Entomoneis species also possess a specific transition between the keel and valve body, discernible in various shapes, with basal fibulae that can be observed in electron microscopy (Patrick & Reimer Citation1975, Round et al. Citation1990, Mejdandžić et al. Citation2018). The history of the genus is somewhat complicated as most of the species were previously placed in Amphiprora Ehrenberg, which was found to be invalid. Patrick & Reimer (Citation1975) established the priority of Entomoneis as a valid name for species with panduriform cells, elevated bilobate keel, sigmoid raphe canal, numerous intercalary bands, bi- or multiseriate striae and a specific transition between valve body and elevated keel (previously known as ‘junction line’). Later research revised some of these ‘mandatory’ characters for Entomoneis identification, such as the presence of uniseriate striae (E. paludosa (W. Smith) Reimer) or hymenate striae with perforations (E. aequabilis K. Osada & H. Kobayasi, E. tenera Mejdandžić & Bosak, E. pusilla Bosak & Mejdandžić, E. gracilis Mejdandžić & Bosak, E. vilicicii Bosak & Mejdandžić, E. infula Mejdandžić & Bosak, E. adriatica Mejdandžić & Bosak, E. umbratica Mejdandžić & Bosak) or absence of basal fibulae within the transition between the valve body and keel (E. aequabilis, E. vertebralis Clavero, Grimalt & Hernández-Mariné) (Osada & Kobayasi Citation1991, Clavero et al. Citation1999, Mejdandžić et al. Citation2017, Citation2018). Later, electron microscopy and detailed morphological observations led to the transfer of many Entomoneis species from Amphiprora to genera such as Hamatusia Stidolph, Berkeleya Greville and Tropidoneis Cleve. There are 28 taxonomically accepted Entomoneis species according to AlgaeBase, compared to the 294 taxa listed under Amphiprora (Fourtanier & Kociolek Citation2005), clear evidence that most names were either invalid or incorrectly assigned to this genus (Guiry & Guiry Citation2019).
Entomoneis belongs to the group of diatoms with the raphe enclosed in a tubular canal supported by a set of siliceous braces called fibulae (Ross et al. Citation1979), placed within the Surirellales, characterized by a raphe canal elevated above the valve surface as a keel, with cell symmetry varying from an apical (e.g. Entomoneis) to transapical major axis of development (e.g. Surirella Turpin) with intermediate subcircular forms (e.g. Campylodiscus Ehrenberg ex Kützing) (Ruck et al. Citation2016). The complexity of valve structure and variation in the canal raphe system within this order have resulted in the creation of new genera such as Simonsenia Lange-Bertalot, Archibaldia Witkowski & Kociolek, Nagumoea Kociolek & Witkowski and Platichthys Lange-Bertalot, Kulikovskiy, Witkowski, Seddon & Kociolek. Although Entomoneis is not one of the most diversified diatom genera, new species are continuously being described from a variety of environments, freshwater to marine, plankton to benthos (John Citation1983, Osada & Kobayasi Citation1985, Citation1990a, Citation1990b, Citation1990c, Citation1991, Clavero et al. Citation1999, Reinke & Wujek Citation2013, Paillès et al. Citation2014, Mejdandžić et al. Citation2017, Citation2018, Liu et al. Citation2018). Recent studies describing new Entomoneis species used a multilayer approach, combining morphology and phylogeny, which has led to better species delimitation, revealing the under-appreciation of this genus, especially in the marine plankton (Mejdandžić et al. Citation2017, Citation2018).
Table 1. Comparison of morphological features of Entomoneis annagodhei to similar species.
In this study, we investigated planktonic Entomoneis species in samples collected from the Kungälv estuary on the west coast of Sweden. For the first time we show fascia structure, the external lanceolate slit-like opening of the central nodule, linear to undulate external ridges between the raphe canal and valve margin, in combination with morphometry and specific features based on light (LM) and scanning electron microscopy (SEM) support the description of Entomoneis annagodhei sp. nov. The similarities and differences of morphological features and morphometry with other species within the genus are discussed. This study contributes to the under-appreciated diversity of planktonic Entomoneis species.
Materials and methods
Sample collection
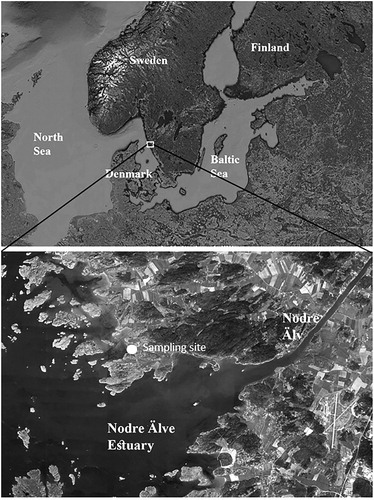
Samples containing cells of Entomoneis species were collected from the Nodre Älv Estuary (57° 47′ N, 11° 45′ E), to the north of Gothenburg city on the west coast of Sweden in April 2018 (Fig. 1). Plankton samples were obtained using a standard 20 µm mesh phytoplankton net and preserved with glutaraldehyde (final conc. 4%). The area is characterized by large number of fjords (archipelago) in which highly brackish water enters from the Skagerrak sea, which receives water from both the North Sea and the Baltic. The sampling site is affected by the Nodre Älv River, which is a branch of the major river in the region, Göta Älv. River water lowers salinity in the inner parts of the archipelago to ca 9.0 psu. The water among the islets of the inner archipelago is rather shallow, covering soft mud and silt sediments.
Cleaning of diatom frustules
Samples containing diatoms were firstly rinsed with distilled water to remove salt and glutaraldehyde. 20 mL of sample was then oxidized with 35% hydrogen peroxide for 15 min. After cooling, samples were again rinsed with deionized water to pH neutral. Finally, 1 mL of the cleaned material was mounted and dried on a coverslip at room temperature. Coverslips with dry material were heated at 150°C for few minutes for diatoms to adhere to the surface and then inverted on a microscope slide with Naphrax® (Brunel Microscopes Ltd., UK), which was then heated to eliminate air bubbles.
Microscopy
Light microscopical observations, morphometric measurements and imaging were carried out under a Zeiss Axioimager 2 microscope with differential interference contrast objectives equipped with a Canon Powershot 14 camera (Carl Zeiss, Oberkochen, Germany) at the Department of Biological and Environmental Sciences, Gothenburg University, Sweden. For scanning electron microscopy (SEM), a few drops of the cleaned diatom suspension were left to dry on an aluminium stub and examined in a Hitachi SU8010 Cold Field Emission SEM at the Botanischer Museum (BGBM), Berlin, Germany. Terminology follows Ross et al. (Citation1979) and Round et al. (Citation1990), with specific Entomoneis terminology following, Osada & Kobayasi (Citation1985) and Mejdandžić et al. (Citation2017, Citation2018).
In order to determine the frequency of E. annagodhei and other diatom taxa in the environment, phytoplankton samples were counted by the Utermöhl method (Utermöhl Citation1931, Citation1958). Finally, the relative abundances of those taxa constituting more than 2% of the diatom population in the sample were calculated (results are presented in %).
Results
Entomoneis annagodhei Al-Handal & Mucko sp. nov. (Figs LM 2–15, SEM 16-28)
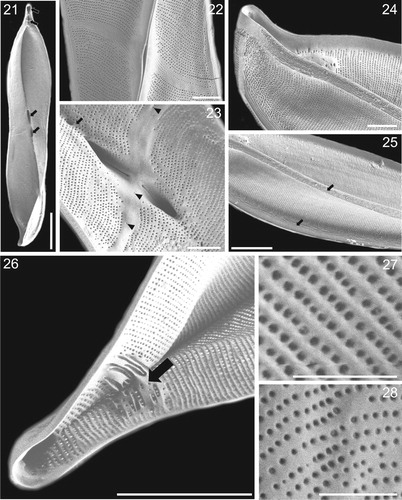
Figs 2–11. LM micrographs of holotype specimen of Entomoneis annagodhei sp. nov. at different foci showing general size diminution of valves. Note oblique transapical fascia (Figs 2–6; 8 and 11) and central area of the raphe canal with lanceolate slit-like opening (Figs 7, 9 and 10). Scale bar = 10 µm.
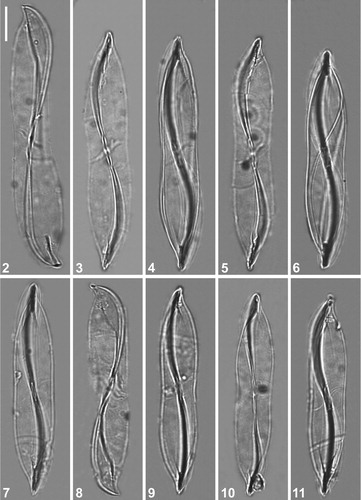
Figs 12–20. LM (Figs 12–15) and SEM (Figs 16–20) micrographs of holotype specimen of Entomoneis annagodhei sp. nov. Fig. 12. Panduriform cell containing two plate plastids and numerous intercalary bands and straight to slightly arcuate transition between valve body and keel (arrows). Figs 13–14. Linear to lanceolate valves of same specimen observed with DIC (Fig. 13) and without (Fig. 14) showing slightly concave valve margins at the centre of valve length and slightly protracted apices. Oblique transapical fascia (Fig. 13, arrow). Central area of raphe canal showing lanceolate slit-like opening (Fig. 14, arrow). Fig. 15. Girdle view of the valve with acuminate apices and pronounced narrow raphe canal separated with raphe fibulae in form of a line (arrowhead). Fig. 16. Valve view showing sigmoid raphe canal, dense transapical striae and lanceolate slit-like opening at the central raphe canal area (arrow). Fig. 17. Girdle view of the valve showing depressions at the transition between valve body and keel (arrows). Fig. 18. Girdle view of the valve showing inner structure and oblique transapical fascia (arrow). Fig. 19. Details of Fig. 16, showing fine valve striation and lanceolate slit-like opening (arrow). Fig. 20. Acuminate valve apex showing hooked terminal raphe fissure (arrow). Scale bar = 10 µm.
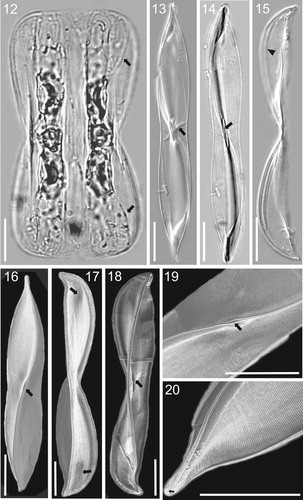
Figs 21–28. SEM microghraphs of Entomoneis annagodhei sp. nov. Fig. 21. Internal valve view showing apical and central sub-compartments (arrows). Fig. 22. External valve view showing oblique transapical fascia and lanceolate slit-like opening of deep raphe canal. Fig. 23. Internal view of central valve area showing oblique transapical fascia with few isolated areolae (arrowheads), central raphe fissures opening into fascia and dense raphe fibulae forming a line (arrow). Fig. 24. External valve view showing acuminate apex and dense smooth and parallel transapical striae and virgae. Fig. 25. External valve view showing linear to undulate external ridges running more or less parallel, and adjacent to the raphe (arrows). Fig. 26. Internal valve view of the apical sub-compartments and irregularly shaped and thickened basal fibulae (arrow). Figs 27–28. External and internal valve view with areola details. Scale bar = 10 µm (Fig. 21), 2 µm (Figs. 22–24), 5 µm (Figs. 25–26), 1 µm (Figs. 27–28).
Description
Light microscopy: Cells solitary with well silicified frustules (Figs 2–15). Valves linear-lanceolate with slightly concave margins in the middle, 50-82 µm long, 10-12 µm wide at the central part (Figs 2–11). Two elongated plate-like plastids arranged parallel to each other in each cell (Fig. 12). Frustules panduriform in girdle view, constricted in the middle, with numerous girdle bands (Fig. 12). Valve apices more or less protracted in valve view (Figs 3–7; 9–11; 13–14) and acuminate in girdle view (Figs 2 and 8). Moderately elevated and narrow bilobate keel separated from the valve body, with the transition creating the impression of a straight to slightly arcuate line, in the apical area (Fig. 12, arrows). Raphe-bearing keel narrow and sigmoid (Figs 2–11; 13–14). An oblique, transapical fascia extending to valve margin, is visible at certain foci, especially using DIC (Fig. 13, arrow). Central area of raphe canal forming a lanceolate slit-like opening, visible when oblique transapical fascia out of focus (Fig. 14, arrow). Raphe canal pronounced with dense raphe fibulae in the form of a line (Fig. 15, arrowhead). Striae very fine, not discernible in light microscopy.
Scanning electron microscopy: External valve view reveals fine striation, as dense uniseriate transapical striae (Fig. 16). Small depressions near the valve apices correspond to the transition between the valve body and elevated keel (Fig. 17, arrows). Narrow, elevated raphe-bearing keel sigmoid in shape with lanceolate slit-like opening at the middle (Fig. 16 and 19, arrows). Central raphe fissures simple, flanked by an obliquely transapical fascia, particularly clear in internal valve view (Figs 18, arrow, and 23) Terminal raphe fissures hooked (Fig. 20, arrow). Internal valve view reveals sub-compartments or cavities at the valve apices and central nodule (Fig. 21, arrows). External valve view shows the deep raphe canal with a lanceolate slit-like opening in the middle, without visible central raphe fissures (Fig. 22). The marginal ends of fascia may have a few isolated areolae (Fig. 23, arrowheads). Internally the raphe canal is very deep, closed with dense, irregularly shaped fibulae in form of a line, 60–64 in 10 µm (Fig. 23, arrow). Transapical striae very fine and compact, formed of very small round areolae arranged in parallel rows, 52–58 striae in 10 µm (Figs 21–25). Internally and externally smooth and parallel dense transapical striae and virgae extending from the valve margin to raphe canal (Fig. 24). Many fine, linear to undulate ridges are present near the valve margin and raphe canal, more or less apically oriented, with frequent bifurcations (Fig. 25, arrows). Apical sub-compartments divided by irregularly shaped and basally thickened fibulae (Fig. 26, arrow). Areolae rounded, variable in size, irregularly spaced within a stria, 68–72 in 10 µm (Figs 27, 28; Fig. 27 external valve view; Fig. 28 internal valve view).
Holotype: Permanent slide containing frustules of E. annagodhei.sp. nov., deposited in the Botanischer Garten und Botanischer Museum (BGBM), Berlin, Germany under accession B 40 0045001 (holotype illustrated in Figs 3–5).
Isotype: Permanent slide containing frustules of E. annagodhei . sp. nov., deposited in the Croatian National Diatom Collection, University of Zagreb, Croatia under accession number HRNDC000436.
Type locality: Kungälv fjord, West coast of Sweden, North of Gothenburg city (57° 47′ N, 11° 45′ E).
Etymology: The epithet is named in honour of our late friend and colleague, Professor Dr Anne Godhe, whose scientific work contributed to the phylogeny of diatoms.
Ecology: Entomoneis annagodhei was one of the common species in the plankton of Kungälv fjord where it constituted 12.7% of the diatom population. Salinity at that location was 9.0 psu and temperature 3.4°C at time of collection. Associated taxa which appeared in large numbers were Chaetoceros Ehrenberg spp. (23.1%), Rhizosolenia setigera Brightwell (5.3%), Skeletonema marinoi Sarno & Zingone (4.8%), Entomoneis paludosa (4.8%), Entomoneis kjellmanii (Cleve) Poulin & Cardinal, Thalassiosira nordenskioeldii Cleve (3.5%), Thalassiosira eccentrica (Ehrenberg) Cleve (2.7%), Surirella brebissonii Krammer & Lange-Bertalot (3.1%) and Bacillaria paxillifer (O. F. Müller) Hendey (2.6%). A few specimens of E. annahodhei were also found in epipelic samples collected from sediments from the same site.
Discussion
The general morphology of E. annagodhei is congruent with all the characteristic features of the genus, being panduriform in girdle view, having an elevated bilobate raphe-bearing keel, a sigmoid raphe canal and numerous intercalary bands. The unique morphological feature of E. annagodhei is the oblique transapical fascia located at the central nodule, a novel feature for Entomoneis.
Entomoneis annagodhei shares the possession of two plate-like plastids with other Entomoneis species such as E. paludosa, E. vertebralis and E. reimerii Reinke & Wujek (Osada & Kobayasi Citation1990c, Clavero et al. Citation1999, Reinke & Wujek Citation2013). However, the number of plastids varies within Entomoneis, from one (e.g. E. tenera, E. pusilla, E. gracilis, etc.) to two (e.g. E. paludosa, E. vertebralis), with multiple plastids yet undocumented (Osada & Kobayasi Citation1990c, Clavero et al. Citation1999, Mejdandžić et al. Citation2017, Mejdandžić et al. Citation2018) ().
Cell torsion was not observed in E. annagodhei, as in some other known taxa (E. alata var. japonica, E. centrospinosa Osada & Kobayasi, E. decussata (Grunow) Osada & Kobayasi, E. paludosa, E. punctulata, and E. pusilla), all sharing highly silicified frustules, probably making torsion difficult or impossible (Osada & Kobayasi Citation1985, Osada & Kobayasi Citation1990a, Osada & Kobayasi Citation1990b, Osada & Kobayasi Citation1990c, Mejdandžić et al. Citation2018). When comparing valve shape, E. annagodhei is most similar to E. alata var. japonica (Cleve) Osada & Kobayasi and E. punctulata (Grunow) Osada & Kobayasi whose valves are also linear-lanceolate with slightly concave valve margins at the centre (Osada & Kobayasi Citation1985, Osada & Kobayasi Citation1990c). Slightly protracted (valve view) and acuminate (girdle view) apices in E. annagodhei are also found in E. alata var. japonica and E. punctulata, but the taxa differ in valve length (75–150 µm in E. alata var. japonica and 18-53 µm in E. punctulata) (Osada & Kobayasi Citation1985, Osada & Kobayasi Citation1990c). Additionally, E. alata var. japonica and E. punctulata both have wide bilobate keels with pronounced and highly elevated wings proceeding over the valve margins in valve view, while E. annagodhei has a narrow bilobate keel with moderately elevated wings, which do not exceed valve margins in valve view (Osada & Kobayasi Citation1985, Osada & Kobayasi Citation1990c). The transition between valve body and elevated keel varies considerably within the genus, and there are currently two described species that do not show a transition (E. aequabilis and E. vertebralis) (Osada & Kobayasi Citation1991, Clavero et al. Citation1999). The transition in E. annagodhei creates the impression of a straight to arcuate line, but is restricted to the apical area, like the ultrastructure of E. punctulata (Osada & Kobayasi Citation1990c). However, the transition in E. punctulata is arcuate in shape and built from short row of puncta, while in E. annagodhei it is straight to slightly arcuate, built from irregularly shaped, variably long and thickened basal fibulae (Osada & Kobayasi Citation1990c).
Specific morphological characters, such as valve depression and sub-compartments or cavities, which are seen in E. annagodhei apices and the apical area between transitions (from valve body to elevated keel) and central nodule are congruent with the recently described E. triundulata, in which each valve is divided into five distinct parts, seen as swellings externally, or cavities internally (Liu et al. Citation2018). However, sub-compartments in E. annagodhei are less numerous and valve depressions less pronounced than in E. triundulata (Liu et al. Citation2018). A lanceolate external slit-like opening in the middle of raphe canal is inherent to E. annagodhei; no other species described so far possesses this feature. Terminal and central raphe fissures of E. annagodhei are similar to E. pusilla (hooked terminal fissure) and many other species (e.g. E. triundulata, E. tenera, E. adriatica, E. umbratica, etc.; straight central raphe fissures), but none of these species has a fascia (Mejdandžić et al. Citation2017, Citation2018, Liu et al. Citation2018).
Due to the very fine striation, valves of E. annagodhei appear hyaline in LM, like a few other taxa such as E. vertebralis, which has a hyaline valve (Clavero et al. Citation1999), E. tenera, E. pusilla, E. gracilis, E. vilicicii, E. infula, E. adriatica, and E. umbratica whose striation is apparent in EM, but with hymenate, finely perforated striae (Mejdandžić et al. Citation2017, Citation2018). Entomoneis annagodhei has dense uniseriate striae (52–58 stria in 10 µm) with round areolae which mostly resemble the ultrastructure of E. paludosa and E. triundulata Bing Liu & D.M. Williams, but whose areolae are elliptical or apically elongated and enclosed externally by a hymen (18-25 striae in 10 µm in E. paludosa, ca. 40 striae in10 µm in E. triundulata) (Osada & Kobayasi Citation1990c, Liu et al. Citation2018). Transapical valve and keel virgae that bifurcate towards valve or keel margin are common within Entomoneis (e.g. E. tenera, E. aequabilis, E. japonica, E. umbratica, E. gracilis, E. vilicicii, etc.), but no other species have the fine linear to undulate ridges beside the raphe canal and at the valve margin as in E. annagodhei (Osada & Kobayasi Citation1985, Osada & Kobayasi Citation1991, Mejdandžić et al. Citation2017, Citation2018).
The majority of described Entomoneis taxa are usually brackish to freshwater epipelic, although newly described marine planktonic species are being discovered, shifting Entomoneis diversity into the plankton realm (Paillès et al. Citation2014, Mejdandžić et al. Citation2017, Citation2018, this study). High relative abundance of E. annagodhei in phytoplankton samples reveals its co-dominance with Chaetoceros, however there are no long-term data series for this sampling site for further ecological discussion. What can be concluded from this example is that pennate planktonic diatoms can be an important constituent of the phytoplankton community, where panduriform species of Entomoneis clearly have advantages for inhabiting the water column compared to other pennate diatoms common in benthic habitats. A few specimens of E. annagodhei were also recorded in epipelic samples from the same site, indicating that this species can inhabit both plankton and epipelon, probably due to cell sinking (Bosak et al. Citation2016).
Recent research on Entomoneis has combined morphological observations with phylogeny, using nuclear SSU rDNA and plastid-encoded rbcL and psbC genes, as for other diatom genera such as Nitzschia Hassall (Al-Handal et al. Citation2019, Barkia et al. Citation2019, Lobban et al. Citation2019), Haslea Simonsen (Prasetiya et al. Citation2019), Proschkinia Karayeva (Majewska et al. Citation2019), and Craspedostauros E. J. Cox (Ashworth et al. Citation2017). This study did not combine morphology with phylogeny, since morphological features were sufficiently specific and unusual for the clear delimitation of E. annagodhei. However, this does not mean that future research should not focus on gathering molecular data of E. annagodhei, since molecular data is always welcome and usable in diatom research, especially evolutionary studies. Much work has focused on investigating planktonic Entomoneis species, but much remains to do. Under-appreciation of this genus in the plankton has already been stressed (Mejdandžić et al. Citation2017, Citation2018), and the current study contributes to reducing this knowledge gap.
Acknowledgments
The authors are grateful to Mr. Kim Govers and Miss Juliane Bettig of the Botanical Museum (BGBM), Berlin for their help with SEM observations. The first author expresses his gratitude to Dr. Regine Jahn and Dr. Jonas Zimmermann, BGBM, Berlin, for the kind invitation to use their institute’s facilities. Our thanks are due to Dr. Eileen Cox for her valuable comments and corrections to improve the quality of the paper. This work is part of a project funded by FORMAS, Sweden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Handal A.Y., Zimmerman J., Jahn R., Torstensson A. & Wulff A. 2019. Nitzschia biundulata sp. nov. a new sea ice diatom (Bacillariophyceae) from the Ross Sea, Antarctica. Nova Hedwigia 108:281–290. doi: 10.1127/nova_hedwigia/2019/0519
- Ashworth M.P., Lobban C.S., Witkowski A., Theriot E.C., Sabir M.J., Baeshen M.N., Ajarah N.H., Baeshen, N.A., Sabir J.S. & Jansen R.K. 2017. Molecular and morphological investigations of the stauros-bearing, raphid pennate diatoms (Bacillariophyceae): Craspedostauros EJ Cox, and Staurotropis TBB Paddock, and their relationship to the rest of the Mastogloiales. Protist 168: 48–70. doi: 10.1016/j.protis.2016.11.001
- Barkia I, Li C., Saari N. & Witkowski A. 2019. Nitzschia omanensis sp. nov., a new diatom species from the marine coast of Oman, characterized by valve morphology and molecular data. Fottea 19:175–184. doi: 10.5507/fot.2019.008
- Bosak S., Bošnjak I., Cetinić I., Mejdandžić M. & Ljubešić Z. 2016. Diatom community in the depths of the South Adriatic: an injection of carbon by biological pump. In 41st CIESM Congress.
- Clavero E., Grimalt J.O. & Hernández-Marinne M. 1999. Entomoneis vertebralis sp. nov. (Bacillariophyceae): a new species from hypersaline environments. Cryptogamie Algologie 20: 223–234. doi: 10.1016/S0181-1568(99)80016-6
- Cleve P.T. 1894. Synopsis of the naviculoid diatoms. PA Norstedt & söner.
- Cox E.J. 2001. What constitutes a stauros? A morphogenetic perspective. In: Festschrift für H. Lange-bertalot. (Ed. R. Jahn, A. Witkowski & P. Compère), A.R.G. Gantner Verlag K.G, Ruggell, pp. 303–316.
- Cox, E.J. 2012. Ontogeny, homology, and terminology - wall morphogenesis as an aid to character recognition and character state definition for pennate diatom systematics. Journal of Phycology 48: 1–31. doi: 10.1111/j.1529-8817.2011.01081.x
- Fourtanier E. & Kociolek J.P. 2005. Catalogue of diatom names. California Academy of Sciences, San Francisco.
- Guiry M.D. & Guiry G.M. 2019. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available from: https://www.algaebase.org [Accessed 18 December 2019].
- John J. 1983. The diatom flora of the Swan river estuary, western Australia. Bibliotheca Phycologia 64: 1–359.
- Lange-Bertalot H. 1979. Simonsenia, a new genus with morphology intermediate between Nitzschia and Surirella. Bacillaria 2: 127–136.
- Lange-Bertalot H., Witkowski A., Kulikovski M.S., Seddon A.W.R. & Kociolek, P. 2015. Taxonomy, frustular morphology and systematics of Platichthys, a new genus of canal raphe bearing diatoms within the Entomoneidaceae. Phytotaxa 236: 135–149. doi: 10.11646/phytotaxa.236.2.3
- Liu B., Williams D.M. & Ector, L. 2018. Entomoneis triundulata sp. nov. (Bacillariophyta), a new freshwater diatom species from Dongting Lake, China. Cryptogamie, Algologie 39: 239–253. doi: 10.7872/crya/v39.iss2.2018.239
- Lobban C.S., Ashworth M.P., Calaor J.J. & Theriot E.C. 2019. Extreme diversity in fine-grained morphology reveals fourteen new species of conopeate Nitzschia (Bacillariophyta: Bacillariales). Phytotaxa 401: 199–238. doi: 10.11646/phytotaxa.401.4.1
- Majewska R., Bosak S., Frankovich T.A., Ashworth M.P., Sullivan M.J., Robinson N.J., Lazo-Wasem E.A., Pinou T., Nel R., Manning S.R. & Van de Vijver B. 2019. Six new epibiotic Proschkinia (Bacillariophyta) species and new insights into the genus phylogeny. European Journal of Phycology 54:609–631. doi: 10.1080/09670262.2019.1628307
- Mejdandžić M., Bosak S., Nakov T., Ruck E., Orlić S., Gligora Udovič M., Peharec Štefanić P., Špoljarić I., Mršić G. & Ljubešić Z. 2018. Morphological diversity and phylogeny of the diatom genus Entomoneis (Bacillariophyta) in marine plankton: six new species from the Adriatic Sea. Journal of Phycology 54: 275–298. doi: 10.1111/jpy.12622
- Mejdandžić M., Bosak S., Orlić S., Gligora Udovič M., Peharec Štefanić P., Špoljarić I., Mršić G. & Ljubešić Z. 2017. Entomoneis tenera sp. nov., a new marine planktonic diatom (Entomoneidaceae, Bacillariophyta) from the Adriatic Sea. Phytotaxa 292: 1–18. doi: 10.11646/phytotaxa.292.1.1
- Osada K. & Kobayasi H. 1985. Fine structure of the brackish water pennate diatom Entomoneis alata (Ehr.) Ehr. var. japonica (Cl.) comb. nov. Japanese Journal of Phycology 33: 215–224.
- Osada K. & Kobayasi H. 1990a. Entomoneis centrospinosa sp. nov., a brackish diatom with raphe-bearing keel. Diatom Research 5: 387–396. doi: 10.1080/0269249X.1990.9705128
- Osada K. & Kobayasi H. 1990b. Fine structure of the marine pennate diatom Entomoneis decussata (Grun) comb. nov. Japanese Journal of Phycology 38: 253–261.
- Osada K. & Kobayasi H. 1990c. Observations on the forms of the diatom Entomoneis paludosa and related taxa. In: Proceedings of the tenth international diatom symposium (Ed. by H. Simola), Koeltz Scientific Books, Koenigstein, pp. 161–172.
- Osada K. & Kobayasi H. 1991. Entomoneis aequabilis sp. nov. (Bacillariophyceae), a brackish species without junction lines. Japanese Journal of Phycology 39: 157–166.
- Paillès C., Blanc-Valleron M-M., Poulin M., Crémière A., Boudouma O. & Pierre C. 2014. Entomoneis calixasini sp. nov., a new fossil diatom from the Turkish Marmara Sea sediments. Diatom Research 29: 411–422. doi: 10.1080/0269249X.2014.921645
- Patrick R. & Reimer C.W. 1975. The diatoms of the United States. Vol. II, part I. Monographs of the Academy of Natural Sciences of Philadelphia 13: 213 pp.
- Prasetiya F.S., Gastineau R., Poulin M., Lemieux C., Turmel M., Syakti A.D., Hardivillier Y., Widowati I., Risjani Y., Iskandar I., Subroto T., Falaise C., Arsad S., Safitri I., Mouget J-L. & Leignel V. 2019. Haslea nusantara (Bacillariophyceae), a new blue diatom from the Java Sea, Indonesia: morphology, biometry and molecular characterization. Plant Ecology and Evolution 152: 188–202. doi: 10.5091/plecevo.2019.1623
- Reinke D.C. & Wujek D.E. 2013. Entomoneis reimeri sp. nov., a new saline diatom species from Kansas. Transactions of the Kansas Academy of Science 116: 113–118. doi: 10.1660/062.116.0302
- Ross R., Cox E.J., Karayeva N.I., Mann, D.G., Paddock T.B.B., Simonsen R. & Sims P.A. 1979. An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia 64: 513–533.
- Round F.E., Crawford R.M. & Mann D.G. 1990. The diatoms. Biology and morphology of the genera. Cambridge University Press, Cambridge. 747 pp.
- Ruck, E.C., Nakov, T., Alverson, A.J. & Theriot, E.C. 2016. Phylogeny, ecology, morphological evolution, and reclassification of the diatom orders Surirellales and Rhopalodiales. Molecular Phylogenetics and Evolution 103: 155–171. doi: 10.1016/j.ympev.2016.07.023
- Ruck, E.C. & Theriot, E.C. 2011. Origin and evolution of the canal raphe system in diatoms. Protist 162: 723–737. doi: 10.1016/j.protis.2011.02.003
- Stidolph S.R. 1993. Hamatusia nom. nov., a new generic name for the diatom Amphiprora reediana Stidolph. Diatom Research 8: 481–482. doi: 10.1080/0269249X.1993.9705278
- Utermöhl, H. 1958. Zur Vervollkommnung der quantitativen phytoplankton-methodik: mit 1 Tabelle und 15 Abbildungen im Text und auf 1 Tafel. Internationale Vereinigung für Theoretische und Angewandte Limnologie: Mitteilungen 9: 1–38.
- Utermöhl, V.H. 1931. Neue Wege in der quantitativen Erfassung des plankton. (Mit besonderer Berücksichtigung des Ultraplanktons.) Mit 4 Abbildungen im Text. Internationale Vereinigung für Theoretische und Angewandte Limnologie: Verhandlungen 5: 567–596.
- Witkowski A., Kociolek P. & Kuryzdlowski K. 2011. Valve ultrastructure of two new genera of marine canal-bearing diatoms (Bacillariophyceae). Phycologia 50: 170–181. doi: 10.2216/09-103.1