Abstract
We investigated the ultrastructure of Achnanthes delicatissima Simonsen in materials collected from the Swedish Baltic coast. Valve structure differs markedly from that of Achnanthes sensu lato, particularly in the absence of cribrate areolae which separates Achnanthes from all other members of the family Achnanthaceae. Comparing this species with those recently described achnanthoid genera, such as Scalariella Riaux-Gobin & Witkowski and Madinithidium Witkowski, Desrosiers & Riaux-Gobin revealed morphological differences that warrant the erection of a new genus, Navithidium. Owing to the small size of the frustules, it is difficult to identify Navithidium delicatissima in light microscopy due to similarities with some closely related taxa like Achnanthidium pseudochamaepinnularia (which is here transferred to Navithidium). A comparison with related genera is provided and discussed.
Introduction
The order Achnanthales (Bacillariophyta) comprises heterovalvar genera and was proposed by Silva (1962; see the Latin diagnosis in Round et al., Citation1990). It presently comprises three families: Achnanthaceae, Cocconeidaceae and Achnanthidiaceae. Several taxa originally assigned to Achnanthes Bory (the type of Achnanthales) have been revised and a number of new genera were established over the past three decades. These new genera reflect the revised taxonomy of a morphologically and genetically diverse group of monoraphid taxa. Genera presently included in the Achnanthales are: Achnanthes, Achnanthidium Kützing, Eucocconeis Cleve, Karayevia Round & Bukhtiyarova, Crenotia Wojtal, Kolbesia Round & Bukhtiyarova, Lemnicola Round & Basson, Madinithidium Witkowski, Desrosiers & Riaux-Gobin, Planothidium Bukhtiyarova & Round, Platessa Lange-Bertalot, Psammothidium Bukhtiyarova & Round, Astartiella Witkowski, Lange–Bertalot & Metzeltin, Pauliella Round et Basson, Pseudachnanthidium Riaux-Gobin, Rossithidium Round & Bukhtiyarova, Scalariella Riaux-Gobin & Witkowski and Majewskaea Van de Vijver et al. (see Krammer & Lange-Bertalot, Citation1991, Bukhtiyarova & Round, Citation1996, Round & Bukhtiyarova, Citation1996, Round & Basson, Citation1997, Krammer & Lange-Bertalot, Citation2004, Kulikovskiy et al., Citation2013, Citation2015, Riaux–Gobin et al., Citation2010, Citation2012, Van de Vijver et al., Citation2020). The characterization of these genera is based on frustule morphology but molecular studies of some of these species highlight characters not visible in ordinary microscopic examination and would lead to either the erection of new genera or the transfer (or combination?) of species from one genus to another. For instance, molecular analyses of Achnanthes parexigua Metzeltin & Lange-Bertalot, Achnanthes rostellata Cleve-Euler and Lemnicola uniseriata Yan Shi & Kim have led to their transfer to a newly erected genus Gregoria Kulikovskiy, Glushchenko, Maltsev & Kociolek (Kulikovskiy et al., Citation2020).
The Achnanthidiaceae D.G. Mann (Round et al., Citation1990) includes Eucocconeis Cleve and Achnanthidium Kützing. Although the latter was previously treated as a subgenus of Achnanthes (sensu Reimer in Patrick & Reimer, Citation1966, non sensu Hustedt, Citation1959), it was re-established as a genus by Round et al. (Citation1990), and subsequently emended by Round & Bukhtiyarova (Citation1996). Frustules of Achnanthidium are mainly characterized by being V-shaped in girdle view, with linear-lanceolate to lanceolate-elliptic valves, with subcapitate to subrostrate apices, and length to width ratios between 3:1 and 6:1, but not exceeding 30 μm in length. Their striae are denser at the apices, becoming widely spaced and shorter toward the valve middle, and the mantle is decorated with a row of areolae (Round et al., Citation1990, Round & Bukhtiyarova, Citation1996). However, several of these features are not easily resolved with light microscopy and in many cases identification becomes difficult without examining valve ultrastructure. The difficulties in working with small-celled heterovalvar species have been addressed in several works on monoraphid species (e.g. Lange-Bertalot & Krammer, Citation1989, Krammer & Lange-Bertalot, Citation1991, Riaux–Gobin et al., Citation2010, Citation2012).
While studying surface sediment samples from the Baltic coast of Sweden, we found a population of Achnanthes delicatissima Simonsen (Hinz et al., Citation2012). This species is commonly found in Baltic waters (Witkowski et al., Citation2000) from where it was originally described (Simonsen, Citation1959). Examination of the fine structure of the frustule of this species shows differences from those belonging to Achnanthes sensu lato and other morphologically similar taxa such as Achnanthidium.
Assigning this species to any previously known genera within the Achnanthales is problematical as its morphological features do not fully match any of these genera. For this reason, we propose to establish the new genus Navithidium to accommodate A. delicatissima as well as including other taxa that share similar features. The new combination; Navithidium delicatissima is introduced based on examination with light (LM) and scanning electron microscopy (SEM). A comparison with similar Achanthidium taxa is presented.
Material and methods
Several diatom samples (benthic, epiphytic and pelagic) were collected on 15 September 2018 from Västervik, on the Swedish Baltic Sea coast (57° 44′ 21 N, 16° 40′ 04 E). The sampling site is a shallow impoundment connected to the Baltic Sea proper. The bottom sediments are silty to muddy and covered by patches of submerged macrophytes. Material for the present study was obtained from the red alga Ahnfeltia Fries. Parts of the alga were shaken vigorously to free the attached diatoms. The diatom suspension was preserved in glutaraldehyde (2.5%).
Diatom frustules were cleaned by boiling in 35% hydrogen peroxide followed by rinsing several times with deionized water. Permanent diatom slides were prepared by mounting cleaned frustules in Naphrax® mounting medium. A Zeiss Axioimager 2 microscope with differential interference contrast objectives (Nomarski) was used for LM observation and imaging. Samples and slides are stored at the Department of Biological and Environmental Sciences, University of Gothenburg (Gothenburg, Sweden). For SEM examination, part of the suspension was filtered through polycarbonate membrane filters with a pore diameter of 1 μm, pieces of which were fixed on aluminium stubs after air-drying. Stubs were examined and imaged in a Hitachi SU8010 Cold Field Emission SEM at the Botanischer Museum (BGBM), Berlin, Germany.
Morphological terminology follows Hendey (Citation1964), Round et al. (Citation1990) and Krammer & Lange-Bertalot (Citation1991). For comparison with selected taxa of the Achnanthales, the following publications were consulted: Krammer & Lange-Bertalot (Citation1991), Kobayashi (Citation1997), Potapova & Ponader (Citation2004), Potapova (Citation2006), Monnier et al. (Citation2004), Ponader & Potapova (Citation2007), Potapova & Hamilton (Citation2007), Riaux–Gobin et al. (Citation2010, Citation2012) and Desrosiers et al. (Citation2014). Sternum valve (rapheless) is abbreviated as SV, and the raphe sternum valve (raphe valve) is abbreviated as RV.
Results
Navithidium Al-Handal & Romero gen. nov.
Frustules heterovalvar and solitary. Valves elliptical with broadly rounded apices. SV flat and gently sloping to a narrow mantle. RV slightly concave with sharply inclined narrow mantle. Axial area of RV narrowly linear lanceolate, gradually widening around the central area. Raphe straight, central raphe endings somewhat distant, polar endings deflected in the same direction.
Etymology: The name refers to the naviculoid outline of the valve.
Navithidium delicatissima (Simonsen) Al-Handal, Romero & Wulff comb. nov. (Figs (LM), Figs (SEM)).
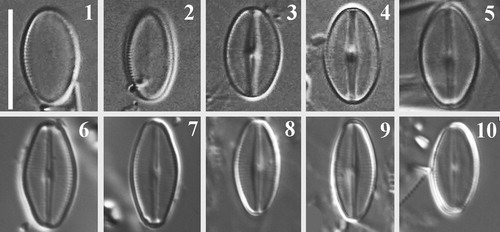
Figs 1–10. LM images of the SV (Figs 1, 2) and RV (Figs 3–10) of Navithidium delicatissima. Figs 1–5, LM images of material collected from Västervik, Swedish Baltic Sea coast. Figs 6–10. LM images of type material, Slide No. Di 208, Hustedt Collection.
Figs 11–16. SEM images of SV of Navithidium delicatissima. Figs 11, 12, 14. SV internal view of entire valve showing marginal striae and vestigial virgae. Fig. 13. External valve view showing areolae arranged on the mantle. Fig. 15. SV valvocopula. Fig. 16. Internal view of marginal striae composed of a single triangular areola with domed hymenes open only at the sides as very narrow slits which are only open on the peripheral ring.
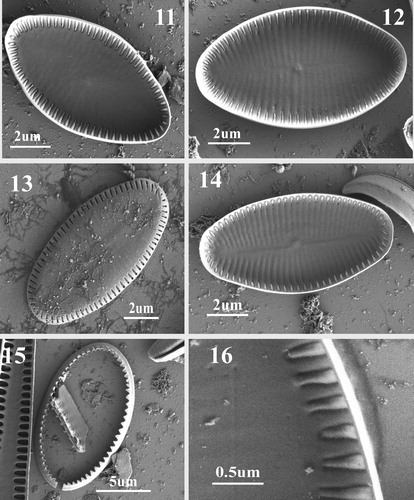
Basionym: Achnanthes delicatissima Simonsen in Hinz et al., Citation2012, p. 81, Simonsen, Citation1959, p. 75, pl. 10. figs 8–13.
Synonym: Achnanthes fogedii Håkansson, Citation1978, 407, fig. 1
Description light microscopy (Figs 1–10)
Valves elliptic with broadly rounded apices, 8.5–12 µm length (n = 40), 5–6.5 µm wide. SV with noticeably short marginal striae (Figs 1,2). Raphe straight, filiform. Axial area narrowly linear-lanceolate, central nodule raised above valve plane. Striae fine, appearing interrupted with two hyaline areas on both sides of the axial area (Fig. 4).
Description scanning electron microscopy (Figs 11–22).
SV: Externally, valve flat to weakly concave with narrow mantle (Fig. 13). Sternum very wide and occupying most of the valve surface. Striae uniseriate, 34–36 in 10 µm, equally spaced, short, and located on valve mantle, parallel in the middle and radiate towards the apices (Fig. 13). Each stria composed of a single elongated areola. Internally, with vestigial raphe evident, transapical striae separated by shallow virgae (Figs 11–12, 14). Single marginal areolae are narrowly triangular, with domed hymenes open only at the sides as very narrow slits (Fig. 16). Valvocopula open, fimbriae short and triangle-like (Fig. 15).
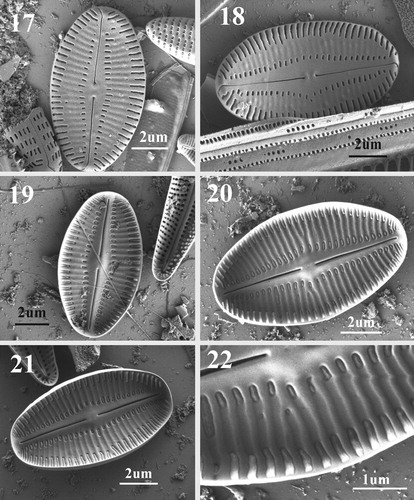
RV: Externally, valve face flat to slightly concave (Figs ). Axial area narrowly linear-lanceolate, expanded slightly at valve centre. Raphe filiform, straight, central endings small, coaxial, and very slightly expanded. Terminal raphe endings deflected on valve mantle in the same direction. Striae uniseriate, 26–30 in 10 µm, formed by dash-shaped areolae that are equidistant along valve margin (Figs ). Areolae arranged transapically in two rows, one marginal extending onto the mantle, and the other adjacent to the axial area, both separated by a relatively wide lunate hyaline area that does not extend to the valve apices. The hyaline area possesses vestigial striae. Inner areolae are short and of unequal length, marginal areolae wider. Internally, axial area slightly elevated with rounded central nodule (Figs ). Raphe slits wider than on the external side. Polar raphe endings terminate before mantle on weakly raised helictoglossae (Figs ). Mantle area above terminal raphe endings free of areolae. Virgae well developed (Fig. ). Areolae with domed hymenes open only at the sides as very narrow slits (Fig. ).
Figs 17–22. SEM images of SRV of Navithidium delicatissima. Figs 17, 18. External view showing striae interrupted by wide lateral areas on both side of the axial area, straight filiform raphe and linear lanceolate axial area. Note polar raphe ends curved to the same side of valve. Figs 19–21. Internal view of valve showing slightly elevated axial area and central nodule. Striae are separated by well-developed virgae. Fig. 22. Enlarged part of internal valve margin showing stria and areola structure.
Ecology and distribution: Navithidium delicatissima (as A. delicatissima) was originally found on sandy sediment in the Sahrensdorfer Binnensee (Burgstaaken, Germany), in the far south of the Baltic Sea. It has also been reported from sandy shores in the Kerguelen Islands, Antarctica (Riaux-Gobin et al., Citation2012). Without specifying its habitat, Witkowski et al. (Citation2000) stated that they found the species in several locations in the Mediterranean Sea. Not far from the location of the present finding, Håkansson (Citation1978) reported the species (as Achnanthes fogedii Håkansson) from sediment samples in Spälkö, Baltic coast, Southern Sweden. We observed this species epiphytic on the red alga Ahnfeltia, associated with Mastogloia elliptica (C. Agardh) Cleve, M. smithii Thwaites ex W.Smith, M. wulffiae Al-Handal & Pennesi and a dense population of Planothidium spp.
Discussion
Main morphological characters of Navithidium
Navithidium delicatissima was originally described as Achnanthes delicatissima by Simonsen (Citation1959) who found it in material collected from Sahrensdorfer Binnensee (Fehrman, Schleswig-Holstein, Germany). The taxon was later typified and validated by Hinz et al. (Citation2012). Type material of A. delicatissima was examined (slide No. Di 208, Hustedt Diatom Collection, Alfred Wegener Institute) and LM images were taken for comparison (Figs 6–10). Valve outline of specimens in the type material is slightly different from those observed in this study as our valves were slightly narrower with less rounded apices than in Hustedt’s material (Figs 6–10).
Riaux-Gobin et al. (Citation2012) examined the valve ultrastructure of the type material of A. delicatissima (Riaux-Gobin et al., Citation2012, p.22, figs 21-23). Although these authors followed Simonsen’s (Citation1959) generic assignment, they concluded that A. delicatissima does not share the features of Achnanthes sensu lato and may well belong to a different genus (Riaux-Gobin et al., Citation2012). According to Round et al. (Citation1990), one of the characteristic features differentiating Achnanthes from other achnanthoid taxa is the cribrate areolae. This feature is not observed in A. delicatissima (Simonsen, Citation1959), and therefore this species cannot be assigned to Achnanthes sensu lato.
Although N. delicatissima (=A. delicatissima) possesses single elongated areolae on its SV and interrupted striae on its RV, it is not possible to assign it either to Scaraliella or to Madithinidium as its broadly elliptic valves and areola structure are different from those of both genera. Hymenes of Navithidium areolae on both RV and SV are not reticulate and internally cover the whole areolae, with a raised middle part (i.e. the hymenes are domed) (Figs 16, 22). A distinguishing character that separates Navithidium from Scaraliella is the macroareola structure, with minute longitudinal slits surrounding the dome-like interior of the macroareola of Navithidium. In addition, marginal areolae on the interior face are triangular (Figs 16, 22) unlike those of all other genera. Since the characteristic features of Achnanthes are lacking in A. delicatissima and it does not share the features of the recently described Scalariella and Madithidinium, it is assigned to the new genus Navithidium.
One of the defining valve features of Navithidium is the presence of macroareolae. The term macroareola (=stria formed of a single foramen) was originally coined by Bukhtiyarova (Citation2006) when the genus Karayevia was proposed. Within the Achnanthales, the occurrence of macroareolae is not only typical of N. delicatissima but it is also present in Karayevia carissima (Lange-Bertalot) Bukhtiyarova and Karayevia dornii (Lange-Bertalot) Bukhtiyarova (Bukhtiyarova, Citation2006). Striae composed of one, slightly depressed, macroareola on the valve face, is a characteristic shared by some achnanthoid as well as several biraphid genera (e.g. Diadesmis Kützing, Chamaepinnularia Lange-Bertalot & Krammer, and Microfissurata Lange-Bertalot, Cantonati & Van de Vijver).
Achnanthidium pseudochamaepinnularia Riaux-Gobin et al. (Citation2010) possesses a similar valve outline, and similar stria and areola arrangement and structure. This species, however, differs in having denser striae on both valves and longer striae on its SV, which extend over a third of the valve compared to the noticeably short and marginal ones in N. delicatissima. Since A. pseudochamaepinnularia does not possess any of the characteristic features of Achnanthidium, or of Madinithidium, but shares the diagnostic characters of Navithidium, it is here transferred to our new genus.
New combination
Navithidium pseudochamaepinnularia (Riaux-Gobin, Compère et Witkowski) Al-Handal, Romero & Wulff.
Basionym: Achnanthidium pseudochamaepinnularia Riaux-Gobin et al. (Citation2012), Vie et milieu-Life and Environment, p.166, figs 38–47.
Comparison of Navithidium with closely related achnanthoid genera
One of the morphologically closely related genera to Navithidium is Achnanthidium (Table ). Features of the latter include a V-shaped frustule in girdle view, a narrow valve outline in valve view, a linear-lanceolate to elliptic-lanceolate valve shape, and valves with subcapitate to subrostrate apices (Round et al., Citation1990). The only feature that links N. delicatissima to Achnanthidium is the uni-directional deflection of the polar raphe endings.
Table 1. Comparison of Navithidium gen. nov. with related genera
The widely elliptical valves with broadly rounded apices of Navithidium resemble those of Psammothidium. Several features in the latter genus, such as the convex sternum valve, raphe valve curvature, raphe fissures lying in channels (especially near the valve centre) and striae reaching the sternum (Bukhtiyarova & Round, Citation1996) are lacking in Navithidium (Table ).
Several species in the Achnanthales share one distinctive feature: their striae are composed of single, transapically elongated areolae, which may or may not be interrupted (macroareolae) (Bukhtiyarova, Citation2006, Riaux–Gobin et al., Citation2010). This feature is also found in Achnanthidium pseudodelicatissimum Riaux-Gobin Compère et Witkowski, Achnanthidium flexuistriatum Riaux-Gobin, Compère et Witkowski, A. pseudochamaepinnularia and Achnanthidium scalariforme Riaux-Gobin, Compère et Witkowski. Riaux-Gobin et al. (Citation2012) found two similar species, which differ in that their elongated RV areolae are split in two by a longitudinal semi-lunar hyaline area. Based on this difference, the authors erected Scalariella to accommodate them: Scalariella pseudofallacia (Witkowski, Metzeltin et Lange–Bertalot) Riaux–Gobin et Witkowski (previously Naviculadicta pseudofallacia Witkowski, Metzeltin et Lange–Bertalot), and Scalariella. oblongella Riaux–Gobin, Witkowski et Ruppel. More recently, Desrosiers et al. (Citation2014) found a similar species whose RV striae lack the typical interruption of Scalariella, a frustule feature used to erect Madinithidium. Areolae of Madinithidium are long and wide, occluded by reticulate hymenes formed of small, irregularly arranged pores (Desrosiers et al., Citation2014, p. 585, fig. 11). In addition to the uninterrupted striae on the RV, this feature is shared by the above mentioned Achnanthidium species which were transferred to Madinithidium.
Acknowledgements
The authors are grateful to Mr. Kim Govers and Miss Juliane Bettig of the Botanischer Museum (BGBM), Berlin, Germany, for their help with SEM observations. The first author expresses his gratitude to Dr. Regine Jahn and Dr. Jonas Zimmermann, BGBM, Berlin, for the kind invitation to use their institute’s facilities. We are grateful to Mr. Mikael Hedblom, Department of Biological and Environmental Sciences, University of Gothenburg, for his help in providing field equipment with sampling. Our thanks to anonymous reviewers for their constructive comments and remarks.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bukhtiyarova L. 2006. Additional data on the diatom genus Karayevia and a proposal to reject the genus Kolbesia. Nova Hedwigia 130: 85–96.
- Bukhtiyarova L. & Round F.E. 1996. Revision of the genus Achnanthes sensu lato section Marginulatae Bukh. sect. nov. of Achnanthidium Kütz. Diatom Research 11: 1–30. doi: https://doi.org/10.1080/0269249X.1996.9705361
- Desrosiers C., et al. 2014. Madinithidium gen. nov. (Bacillariophyceae), a new monoraphid diatom genus from the tropical marine coastal zone. Phycologia 53: 583–592. doi: https://doi.org/10.2216/14-21R2
- Håkansson H.L. 1978. Achnanthes fogedii, a new subfossil diatom from Sweden. Botaniska Notiser 131: 407–408.
- Hendey N.I. 1964. An introductory account of the smaller algae of British coastal waters, part V: Bacillariophyceae (diatoms). Her Majesty’s Stationery Office, London, 317 pp.
- Hinz F., Simonsen R. & Crawford R.M. 2012. Validation of 42 names of diatom taxa from the Baltic Sea. Diatom Research 27: 81–89. doi: https://doi.org/10.1080/0269249X.2012.687562
- Hustedt F. 1959. Die Kieselalgen Deutschlands, Österreichs und der Schweiz unter Berücksichtigung der übrigen Länder Europas sowie der angrenzenden Meeresgebiete. In: Anon (Ed.) Rabenhorst's Kryptogamen Flora von Deutschland, Österreich und der Schweiz, pp. 737–845. Akademische Verlagsgesellschaft, Leipzig.
- Kobayashi H. 1997. Comparative studies among four linear-lanceolate Achnanthidium species (Bacillariophyceae) with curved terminal raphe endings. Nova Hedwigia 65: 147–164. doi: https://doi.org/10.1127/nova.hedwigia/65/1997/147
- Krammer K. & Lange-Bertalot H. 1991. Bacillariophyceae. III. Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H., Gerloff J., Heynig H. & Mollenhauer D. (Ed.) Süsswasserflora von Mitteleuropa. Band 2/3, pp. 1–576. Gustav Fischer Verlag, Stuttgart, Jena.
- Krammer K. & Lange-Bertalot H. 2004. Bacillariophyceae. 4. Achnanthaceae. In: Süßwasserflora von Mitteleuropa (Ed. by Ettl H., Gerloff J., Heynig H. & Mollenhauer D.), Spektrum Akademischer Verlag Heidelberg, Berlin. pp. 1–486.
- Kulikovskiy M., Lange-Bertalot H., and Witkowski A. 2013. Gliwiczia gen. nov. a new monoraphid diatom genus from Lake Baikal with a description of four species new for science. Phytotaxa 109: 1–16. doi: https://doi.org/10.11646/phytotaxa.109.1.1
- Kulikovskiy M.S., Lange-Bertalot H., and Kuznetsova I.V. 2015. Lake Baikal: hotspot of endemic diatoms II. Iconographia Diatomologica 26: 1–657.
- Kulikovskiy M., et al. 2020. Gogorevia, a new monoraphid diatom genus for Achnanthes exigua and allied taxa (Achnanthidiaceae) described on the basis of an integrated molecular and morphological approach. Journal of Phycology 56: 1601–1613. doi: https://doi.org/10.1111/jpy.13064
- Lange-Bertalot H. & Krammer K. 1989. Achnanthes eine Monographie der Gattung mit Definition der Gattung Cocconeis und Nachträgen zu den Naviculaceae. Bibliotheca Diatomologica 18: 1–393.
- Monnier O., et al. 2004. Achnanthidium atomoides sp. nov., a new diatom from the Grand-Duchy of Luxembourg. Vie et Milieu 54: 127–136.
- Patrick R.M. & Reimer C.W. 1966. The diatoms of the United States exclusive of Alaska and Hawaii. Volume 1: Fragilariaceae, Eunotiaceae, Achnanthaceae, Naviculaceae. Monographs of the Academy of Natural Sciences of Philadelphia 13: 1–688.
- Ponader K.C. & Potapova M.g. 2007. Diatoms from the genus Achnanthidium in flowing waters of the Appalachian Mountains (North America): Ecology, distribution and taxonomic notes. Limnologica 37: 227–241. doi: https://doi.org/10.1016/j.limno.2007.01.004
- Potapova M. 2006. Achnanthidium zhakovschikovii sp. nov. (Bacillariophyta) and related species from rivers of Northwestern Russia. . Nova Hedwigia 82: 399–408. doi: https://doi.org/10.1127/0029-5035/2006/0082-0399
- Potapova M.G. & Ponader K.C. 2004. Two common North American diatoms, Achnanthidium rivulare sp. nov. and A. deflexum (Reimer) Kingston: morphology, ecology and comparison with related species. Diatom Research 19: 33–57. doi: https://doi.org/10.1080/0269249X.2004.9705606
- Potapova M. & Hamilton P.B. 2007. Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. Journal of Phycology 43: 561–575. doi: https://doi.org/10.1111/j.1529-8817.2007.00332.x
- Riaux–Gobin C., Witkowski A. & Compère P. 2010. SEM survey and taxonomic position of small–sized Achnanthidium (Bacillariophyceae) from coral sands off Réunion Island (Western Indian Ocean). Vie Milieu 60: 157–172.
- Riaux-Gobin C., Witkowski A. & Ruppel M. 2012. Scalariella a new genus of monoraphid diatom (Bacillariophyta) with a bipolar distribution. Fottea 12: 13–25. doi: https://doi.org/10.5507/fot.2012.002
- Round F.E. & Bukhtiyarova L. 1996. Four new genera based on Achnanthes (Achnanthidium) together with a re-definition of Achnanthidium. Diatom Research 11: 345–361. doi: https://doi.org/10.1080/0269249X.1996.9705389
- Round F.E. & Basson P.W. 1997. A new monoraphid diatom genus (Pogoneis) from Bahrain and the transfer of previously described species A. hungarica and A. taeniata to new genera. Diatom Research 12: 71–81. doi: https://doi.org/10.1080/0269249X.1997.9705403
- Round F.E., Crawford R.M. & Mann D.G. 1990. The diatoms. Biology and morphology of the genera. Cambridge University Press, Cambridge, 747 pp.
- Simonsen R. 1959. Neue Diatomeen aus der Ostsee I. Kieler Meeresforschungen 15: 74–83.
- Vijver B.V.d., et al. 2020. Majewskaea gen. nov. (Bacillariophyta), a new marine benthic diatom genus from the Adriatic Sea. Fottea 20: 112–120. doi: https://doi.org/10.5507/fot.2020.001
- Witkowski A., Lange-Bertalot H. & Metzeltin D. 2000. Diatom flora of marine coasts 1. Iconographia Diatomologica 7: 1–925.
