Abstract
Specimens of an extremely long, heteropolar nitzschioid diatom from mud scraped from mangrove pneumorrhizae on two Micronesian islands were identified as a new species of Gomphotheca. This genus contains two other, rarely seen species, only one of which has been observed with SEM. Our SEM images showed that G. marciae n. sp. is structurally similar to G. sinensis, with very long, heteropolar, cuneate cells with a central keel and internal costate fibulae, and thick, chambered copulae with contrasting external and internal pore patterns. However, the raphe path of G. marciae lacks a central break, conopea are present along each side of the keel, the fibula height is different, pseudosepta are present at both poles, while valve cross section and stria density also differ. We also report the presence of single-walled pleurae.
Key words:
Introduction
Gomphotheca N.I. Hendey & P.A. Sims was established for two extremely long, narrow marine species formerly assigned to Gomphonitzschia Grunow, i.e. Gomphonitzchia chinensis (Skvortzow) Mills and Gomphonitzschia clevei Grunow, with Gomphotheca sinensis (Skvortzow) N.I. Hendey & P.A. Sims designated as the type species. Round et al. (Citation1990) also observed this species from material supplied by Sims. The genus definition included the following characters: valves extremely long and cuneate-clavate, costae and raphe fibulae joined in one system, costate fibulae often forked, ‘central’ raphe ending in the upper half of the valve, and the raphe bordered by stout siliceous ribs (Hendey & Sims Citation1982, Round et al. Citation1990). The position of the raphe was not defined to accommodate both G. sinensis, which has a central raphe, and G. clevei, which appears to have a marginal raphe. However, Hendey & Sims (Citation1982:200) noted that G. sinensis valves are so highly convex that many valves lie on their side, giving the impression of a marginal raphe; this could also be the case with G. clevei. Gomphonitzschia saigoni Meister (Citation1932) was described on the basis of a single incomplete valve and Hendey & Sims (Citation1982) synonymized it with G. sinensis.
There are few records of either species and scanning electron microscopy (SEM) only exists for the type species. Gomphotheca sinensis ( = Gomphonitzschia chinensis) was described from Fuzhou, China, by Skvortzow (Citation1931). It has also been reported from Mozambique; Tasmania; ‘South Seas;’ and a mangrove swamp near Cali, Colombia (summarized by Hendey & Sims Citation1982). The last was source material for Hendey & Sims’ (Citation1982) ultrastructural observations. They (Hendey & Sims Citation1982) remarked on several size groups and small morphological differences between their own collection and specimens on herbarium slides. Gomphotheca sinensis was later reported from the Persian Gulf by Tynni (Citation1983), who called his identification dubious, and Al-Handal (Citation2009), who described two morphologies of G. sinensis from the Shatt Al-Arab estuary.
We discovered a new Gomphotheca species in mangrove samples from Yap, Federated States of Micronesia (Oceania), and describe it from light and scanning electron microscopy, supporting the hypothesis that the characters of the type species are generally representative of this unusual genus.
Materials and methods
Samples of mud coating mangrove pneumorrhizae (air roots) were collected as follows (Table ).
Table 1. Sources of samples from Yap (Y) and Palau (PW) used in this study.
Yap. Samples Y34A–F collected 28 May 2014 by C.S. Lobban, M. Schefter and T. Gorong in the Maa’ mangrove, adjacent to Nimpal Marine Protected Area on Yap I., Wa’ab, Federated States of Micronesia. Samples from different elevations were scraped from the large conical roots of Sonneratia alba (white mangrove) and preserved in alcohol.
Palau. Sample PW1-1 collected 6 July 2021 by Kebang Ngiraklang from the slender air roots of Avicennia alba (black mangrove), nearshore in an urban mangrove area on the north shore of Koror I. Samples were sent damp to Guam and the aliquot for cleaning was taken before the remainder was preserved.
Samples were acid cleaned following our standard protocols (Lobban Citation2015). In brief, samples were boiled in nitric acid and rinsed ten times, centrifuging at 3000 rpm to recover cells from each wash. Slides were prepared in Naphrax and drops of the cleaned material dried onto cellulose nitrate filters for SEM. LM observation was performed with a Nikon 80i Eclipse microscope with differential interference contrast (DIC). SEM was performed with PhenonWorld G2Pro and ThermoFisher Phenom XL desktop SEMs. All SEM images shown here are from the XL instrument run at 10 kV.
Terminology follows Anonymous (Citation1975), Ross et al. (Citation1979) and Round et al. (Citation1990). However, there is ambiguity about the nature of the transverse ribs in relation to the raphe canal structure. Hendey & Sims (Citation1982) described the canal as being formed by two longitudinal ribs and opening to the interior of the cell via elongated portulae; they call the transverse structures costate fibulae, a term reflecting the ambiguity of the origin of these structures, i.e. whether derived from fibulae (normally bridging the raphe canal) and/or virgae. As we are unable to resolve the origin, we use their term.
Results
Gomphotheca marciae Lobban & Prelosky, n. sp. Figs 1–26.
Figs 1–8. Gomphotheca marciae n. sp., whole valves and external aspect (LM and SEM). Fig. 1. Preserved cell showing plastids. Fig. 2. Cleaned valve in LM (isotype). Figs 3, 4. Whole valves in SEM, external and internal aspects. Fig. 5. Detail of plastids. Figs 6, 7. Apical and basal poles, external aspect. Fig. 8. External detail showing raphe slit with lateral depressions along each side. Scale bars: Figs 1–4 = 50 µm, Fig. 5 = 10 µm, Figs 6–8 = 2 µm.
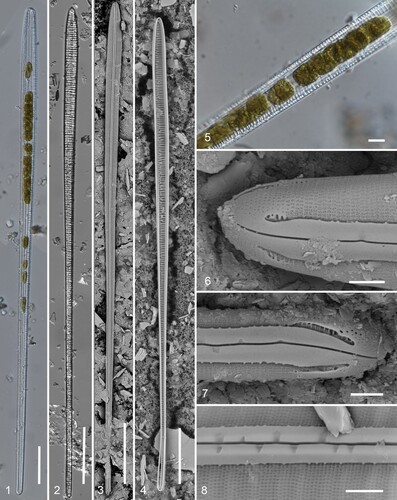
Figs 9–13. Gomphotheca marciae n. sp., internal aspect and valve structure (SEM). Figs 9, 10. Internal details of apical and basal poles showing pseudosepta (arrows). Fig. 11. Internal detail showing costate fibulae; white arrow points to row of pores in subraphe canal, black arrow to covering of canal between some fibulae. Interior of valvocopula seen across bottom of image. Fig. 12. Broken valve showing cross-sectional shape. Notice the pores passing through the attachment of the costate fibulae (long arrow) vs. basal silica layer (short arrow). Fig. 13. Valve broken near basal pole in oblique view, showing conopeum with pores beneath it, raphe slit with lateral depressions, subraphe canal, portion of pseudoseptum, and complexity of valve structure at the pole (black arrow). Scale bars: Figs 9–12 = 5 µm, Fig. 13 = 2 µm.
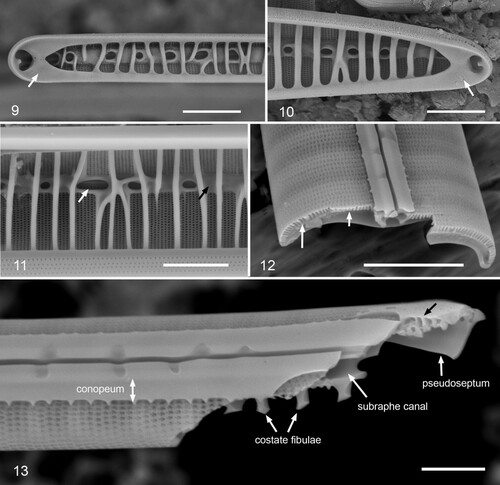
Figs 14–21. Gomphotheca marciae n. sp., girdle bands (LM and SEM). Fig. 14. Girdle view in LM showing valve with two attached copulae, arrows indicating ends of the pores/longitudinal chambers. Figs 15, 16. Whole mounts in SEM showing basal poles, with and without the hypotheca; copulae and pleurae labeled (VC = valvocopula, cop 2 = second copula, pl 1 = first pleura, etc.). In Fig. 15, pl 1 is intact and shows the wide apical cap that fits against cop 2, and a third pleura can be seen; in Fig. 16, the apex of the cingulum has broken off, revealing the internal relationship between cop 2 and pl 1. Inset in Fig. 15 (same scale) shows pole with cracked pl 1, showing thickness at apex (arrow). Fig. 17. Whole mount showing apical pole; in this image the external poration of the girdle bands is very clear. Fig. 18. Broken cingulum showing especially in internal aspect of valvocopula (VC), second copula (cop 2) and first pleura (pl 1). Figs 19, 20. Portions of second copula at two distances from apex showing inner and outer poration and reduction in rows from six to three toward base. Fig. 21. Broken second copula showing longitudinal canals. Scale bars: Fig. 14 = 10 µm, Figs 15–18 = 5 µm, Figs 19–21 = 2 µm.
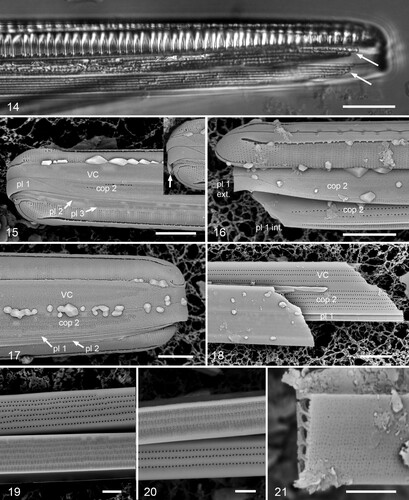
Figs 22–26. Gomphotheca marciae n. sp., copulae (SEM). Figs 22, 23. Basal and apical details of valvocopula in situ adjacent to the valve, showing that this band is closed and showing overlap of silica flaps on valvocopula with pseudoseptum on valve. Fig. 24. Oblique view of valvocopula showing silica flaps and ruffled groove of pars interior. Figs 25, 26. Open (basal) ends of a second copula showing external and internal poration. Scale bars 5 µm.
Diagnosis. Differing from G. sinensis in having a single raphe branch (no central interruption), narrow conopea along the keel, a pseudoseptum at each pole, a more flattened valve section, uniform areolae without pitting adjacent to the central ribs, and finer striae.
Holotype. Specimen at 13.5 mm E and 9.7 mm S of the mark on slide 3399 (location determined by method of Sterrenburg et al. Citation2012.), deposited at Academy of Natural Sciences Philadelphia (ANSP), accession # ANSP-GC20101.
Isotype. Slide 3038, GUAM Diatom Collection #GUD603038. Fig. 2.
Type locality. In submerged mud on mangrove (Sonneratia alba) pneumorrhizae, Ma’a, Yap Island, Wa’ab, Federated States of Micronesia, approximately 9°32′25.62′ N, 138° 5′14.45′ E, sample Y34A. C.S. Lobban, M. Schefter & T. Gorong, 28 April 2014.
Etymology. Named for the junior author’s grandmothers, Marcia D.L. Mendiola, and Mary Doris B. Prelosky.
Registration: http://phycobank.org/102927.
Description. Frustules heteropolar, narrowly cuneate in valve and girdle view (Figs 1–4), with apparently about 20, large discoidal plastids, ca. 10 µm diameter (Figs 1, 5). Valves 348–624 µm long (mean = 410 ± 62, N = 20), tapering uniformly from widest part near apex, 9.5–12.3 µm, to 4.2–4.7 µm at base, a stout keel running centrally along the full length with no central nodule or break in the raphe. Raphe flanked by narrow conopea along each side (Figs 6–8, 13) with short marginal spines (Fig. 15). Striae 35–45 in 10 µm, fibulae 5.5–6.5 in 10 µm (Figs 6, 7, 9). Externally, raphe opens in a wide, straight furrow with small depressions in the raphe sternum at intervals along each side of the raphe fissure (Figs 8, 13). External terminal fissures slightly deflected, extending to the edge of the valve (Figs 6, 7, 15, 16). Striae continuing under conopea without change (Figs 12, 13), the most proximal line visible internally through portulae (Fig. 11). Areolae circular, closed by a membrane with several small pores, except at poles where there were simple pores (Figs 6, 7). Surface pitting at the poles (Figs 6, 7), where valve structure appeared thicker and more complex (Fig. 13).
Internally, pseudoseptum present at each pole with a portula through which the terminal raphe endings were seen (Figs 9, 10). Subraphe canal enclosed by longitudinal ribs with an irregular margin, forming rimmed portulae of various sizes but often covering the canal (Figs 9–11). Costate fibulae extended from the valve margin sometimes anastomosing or ending at or near the raphe canal, but no extra space between the striae was evident externally even though the costate fibulae seemed massive. As seen in the valve cross section (Fig. 12), costate fibulae curled or I-beam shape, not thick, the areolae penetrating through the base.
Cingulum includes two chambered copulae (Figs 14–26) and two to three narrow, single-walled pleurae (Figs 15–18). The valvocopula and second copula are thick and hollow with longitudinal chambers (Fig. 21), of similar width and tapering towards the base (Figs 14–18). Internal face of copulae with several rows of relatively large pores in uniform rows, clear even in LM (Figs 14, 18–20), 30 pores in 10 µm. External surface is densely perforated (Figs 17, 19, 20), with tiny pores arranged in rectangular groups aligned with the single pores of the inner face (Figs 18–20). The number of longitudinal chambers (Fig. 21) is apparently equal to the number of rows of large internal pores; no evidence of partitions across the longitudinal chambers. The number of rows of pores was different in the two copulae (Fig. 18) and greater toward the apex in both (Figs 19, 20). Valvocopula is a closed band with pair of internal flaps (partial septa) at each pole overlapping the valve pseudosepta (Figs 22–24). A wide groove along the advalvar edge, with some scalloping of the inner edge, matches the thick margin of the valve (Fig. 24). Three rows of pores near the apex are reduced to one at the base. Second copula open (Figs 15, 16, 25, 26) and lacking partial septa, wider than the valvocopula, up to six rows of pores at the apex, tapering to three at the open basal tips (Figs 19, 20, 25, 26), the open ends fitting against the first pleura (Figs 15, 16). The first pleura thickened and wide around the basal pole (Figs 15, 16), otherwise narrow and comprising only a single silica layer with a thickened shelf adjacent to the second copula (Figs 16, 18), a single row of pores in the pars interior, up to four rows in the pars exterior; band possibly open at the apical pole (not seen). One, sometimes two additional pleurae seen, the last-formed apparently more delicate and inconsistently visible (Figs 15, 17). No girdle bands were observed on the hypotheca, suggesting they form only close to the time of cell division or were hidden below the epitheca in specimens observed.
Distribution. YAP: rare in several samples from the inner and middle mangrove, above and below water at the time of collection. Y34B!, Y34C!, Y34D!, Y34E!. PALAU: rare in PW(2021)1-1! (intertidal).
Discussion
Structurally G. marciae is very similar to G. sinensis in having very long, heteropolar, cuneate cells with a central keel and internal costate fibulae; thick, hollow copulae with different pore patterns on exterior and interior. However, G. marciae differs in the raphe path lacking a central break, in the presence of conopea along each side of the keel, height of the fibulae, especially near the poles, presence of pseudosepta at both poles, and stria density (Table ). The ‘central’ nodule of G. sinensis is clearly shown by Hendey & Sims (Citation1982, figs 8, 11) and by Round et al. (Citation1990, p. 619), as are two longitudinal grooves that also break across the central area, whereas in G. marciae there is no trace of a central nodule either internally or externally. The conopea extending from the keel is clearly absent in G. sinensis (Hendey & Sims Citation1982, fig. 12 and Round et al. Citation1990, p. 619, fig. j). Conopea is seen in some species in several genera, e.g. Nitzschia Hassall and Amphora Ehrenberg, so their presence in one species of Gomphotheca and not another is not unusual; the presence of spines on conopea has been observed in a few species of conopeate Nitzschia (Sison & Lobban unpublished). Hendey & Sims (Citation1982) described G. sinensis as having the first row of poroids alongside the axial ribs (keel) transversely elongated; images in Round et al. (Citation1990) show that the poroids in G. sinensis are not elongated but rather the outer layer of the wall is not silicified, leaving a series of pits, with several internal openings visible in the bottom of each. The line of pitted areolae and the longitudinal grooves along the keel are not present in G. marciae, but there is substantial pitting at both poles, especially the basal. The costate fibulae in G. sinensis are strongly elevated (rather like microscope slides in a box) and the valvocopula has slots that fit against them. In our species, the fibulae are much lower and the extensions on the valvocopula (at both poles) are in the form of partial septa that overlap the valve pseudosepta. Although G. sinensis has a thickened, rib-like rim (Round et al. Citation1990), it has no pseudosepta; nevertheless, Hendey & Sims (Citation1982: 201) note extensions of the fibulae into the cell ‘almost forming pseudosepta’ (i.e. reminiscent of the pervalvar structures in Plagiogramma Greville or Cyclophora Castracane emend. Ashworth & Lobban, whereas pseudosepta in G. marciae are in the valvar plane like those of Grammatophora Ehrenberg and Hanicella Lobban & Ashworth). Hendey & Sims (Citation1982) noted that the valve of G. sinensis is highly convex – images there and in Round et al. (Citation1990) show an almost semicircular cross-section. However, other images in Round et al. suggest a more flattened profile, as seen in G. marciae.
Table 2. Comparison of the morphology of Gomphotheca spp.
Round et al. (Citation1990) commented that the girdle bands were incompletely known. We have confirmed what they showed, i.e. copulae thick, hollow structures, large pores through inner surface, dense pores through outer, but also show that there are two to three pleurae. In our species at least, the valvocopula is closed while the second band is open and is filled in at the basal pole by a thickened expansion of the first pleura. Differences in the valvocopula at the poles reflect the different valve structure; pseudosepta and relatively low fibulae in G. marciae vs. very high fibulae in G. sinensis; in G. sinensis, Hendey & Sims (Citation1982: 202) noted that the valvocopula has ‘digital extensions which correspond to and clasp the marginal ends of the costate fibulae at the head pole’.
The costate fibulae are extraordinary, massive reinforcing structures, comparable to the craticular bars of Climacosphenia Ehrenberg but developed on the valve rather than the valvocopula. Round et al. (Citation1990: 618) described them as consisting of massive fibulae connected with one or two transapical ribs and linked at their bases by thickened longitudinal ridges, the fibulae and ridges delimiting round or oval portulae into the subraphe canal. Their (Round et al. Citation1990) fig. e shows ribs extending partway from both the central ribs and the valve margin. The origin(s) of this architecture cannot be determined without an ontogenetic study; it is not clear whether the ribs are costae arising from the virgae, extensions of the raphe canal system, or further development of the pseudosepta seen at the apex. Nitzschia ventricosa Kitton also has ribs extending across the valve, but these are evidently thickened virgae connecting across the keel structure (Lobban et al. Citation2012, pl. 62, fig. 2).
From the sampling locations, this appears to be an intertidal mangrove species. The Yap samples were from Sonneratia alba J. Smith in a marine protected area away from urban influence, the Palau sample from Avicennia alba Blume along the shore of the capital town. We have not yet observed it in other samples from these collection sites in Yap and Palau, nor from mangrove samples elsewhere in those islands, nor elsewhere in Micronesia (samples studied from Guam, Chuuk and Pohnpei). Hendey & Sims’ (1982) material of G. sinensis was also from mangroves (‘plankton material collected from a mangrove swamp’). The length and heteropolarity of these species suggest an epiphytic habit, like Licmophora C.A. Agardh, Synedrosphenia (H. Peragallo) Azpeitia and Climacosphenia, whereas the fluid muds of mangrove roots and mudflats are more typically habitats of strongly motile genera such as Gyrosigma Hassall, Pleurosigma Wm. Smith, Navicula Bory and Pinnunavis Okuno, the elongated epiphytic genera absent. We have not seen our species in situ to know if they are attached and Hendey & Sims (Citation1982) collected from the water column.
Our observations confirm the structure of the genus as described in G. sinensis. The most remarkable difference between G. sinensis and G. marciae is that the former has a central break in the raphe and the latter does not. Specimens that have been previously identified as G. sinensis [e.g. Al-Handal’s (Citation2009) material from the Gulf of Arabia] might be re-examined with SEM now that we are aware of two superficially similar species. The position of G. clevei must also await SEM observation, and for now, we have not emended the generic description, which is noncommittal on the location of the raphe/keel, but does specify ‘central’ raphe endings.
Acknowledgements
We thank Thomas and Berna Gorong for their help in collecting and storing the Yap samples and Bernadette Tharngan for arranging their export.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Handal A.Y. 2009. Littoral diatoms from the Shatt Al-Arab estuary, North West Arabian Gulf. Cryptogamie Algologie 30: 153–183.
- Anonymous. 1975. Proposals for a standardization of diatom terminology and diagnoses. In: Third symposium on recent and fossil marine diatoms. ((Ed. by Simonsen, R.), pp. 323–354. J. Cramer, Vaduz, Germany.
- Hendey N.I & Sims P.A. 1982. A review of the genus Gomphonitzschia Grunow and the description of Gomphotheca, gen. nov., an unusual marine diatom group from tropical waters. Bacillaria 5: 191–212.
- Lobban C.S. 2015. Grammatophora ornata (Fragilariophyceae: Grammatophoraceae), a new species with areolate valvocopulae, from a coral reef. Diatom 31: 12–17.
- Lobban C.S., Schefter M., Jordan R. W., Arai Y., Sasaki A., Theriot E. C., Ashworth M., Ruck E. C. & Pennesi C. 2012. Coral-reef diatoms (Bacillariophyta) from Guam: new records and preliminary checklist, with emphasis on epiphytic species from farmer-fish territories. Micronesica 43: 237–479.
- Meister F. 1932. Kieselalgen aus Asien. Gebrüder Borntraeger, Berlin.
- Ross R., Cox E.J., Karayeva N.I., Mann D.G., Paddock T.B.B., Simonsen R. & Sims P.A. 1979. An amended terminology for the siliceous components of the diatom cell. Beihefte zur Nova Hedwigia 64: 513–533.
- Round F.E., Crawford R.M. & Mann D.G. 1990. The diatoms. Cambridge University Press, Cambridge, U.K.
- Skvortzow B.W. 1931. Marine diatoms from Formosa Strait. Philippine Journal of Science 47: 151–161.
- Sterrenburg F.A.S., Hamilton P.B. & Williams D.M. 2012. Universal coordinate method for locating light-microscope specimens. Diatom Research 27: 91–94.
- Tynni R., 1983. Diatoms from the coast of Khawr Abdallah, Persian Gulf. Geological Survey of Finland, Report no. 60.
