?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Russo and Williamson [2007. “Interpreting Causality in the Health Sciences.” International Studies in the Philosophy of Science 21: 157–170] put forward the following thesis: in order to establish a causal claim in medicine, one normally needs to establish both that the putative cause and putative effect are appropriately correlated and that there is some underlying mechanism that can account for this correlation. I argue that, although the Russo–Williamson thesis conflicts with the tenets of present-day evidence-based medicine (EBM), it offers a better causal epistemology than that provided by present-day EBM because it better explains two key aspects of causal discovery. First, the thesis better explains the role of clinical studies in establishing causal claims. Second, it yields a better account of extrapolation.
1. An Epistemological Thesis
Russo and Williamson (Citation2007, §§1–4) put forward an epistemological thesis that can be phrased as follows:
In order to establish a causal claim in medicine one normally needs to establish two things: first, that the putative cause and effect are appropriately correlated; second, that there is some mechanism which explains instances of the putative effect in terms of the putative cause and which can account for this correlation.
This epistemological thesis, which has become known in the literature as the Russo–Williamson thesis or RWT, has generated some controversy—see, e.g. Weber (Citation2007, Citation2009), Broadbent (Citation2011), Campaner (Citation2011), Clarke (Citation2011), Darby and Williamson (Citation2011), Gillies (Citation2011), Illari (Citation2011), Howick (Citation2011a, Citation2011b), Russo and Williamson (Citation2011a, Citation2011b), Campaner and Galavotti (Citation2012), Claveau (Citation2012), Dragulinescu (Citation2012), Clarke et al. (Citation2013, Citation2014) and Fiorentino and Dammann (Citation2015). The aim of this section is to explain what the thesis says, why it is true, and why it is controversial. In section 2, I argue that an approach to medical methodology based on RWT fares better than present-day evidence-based medicine (EBM) in explaining three basic facts about how clinical studies (CSs) can be used to establish causal claims in medicine. In section 3, I argue that RWT motivates a better account of extrapolation inferences too.
1.1. What the Thesis Says
First, let us clarify what the thesis says. This is important because RWT has occasionally been misinterpreted, particularly with respect to the following point.
RWT requires establishing the existence of a correlation and the existence of a mechanism, not the extent of the correlation, nor the details of the mechanism. In some cases, of course, establishing the extent of a correlation is a means to establishing its existence, and establishing the details of a mechanism is a means to establishing its existence, but these means are not the only means. We shall return to this point in section 2.
The second general point to make is that RWT is a purely epistemological thesis, concerning the establishing of causal relationships. Russo and Williamson (Citation2007) used the thesis to argue for a particular metaphysical account of causality—the epistemic theory of causality—but RWT itself does not say anything directly about the nature of causality. The thesis is intended to be both descriptive and normative: i.e. as capturing typical past cases of establishing causality in medicine (e.g. Clarke Citation2011; Gillies Citation2011), as well as characterising the logic of establishing causality.
Let us now clarify some of the terms that occur within the statement of the thesis.
1.1.1. Medicine
Here ‘medicine’ is to be construed broadly to include the health sciences as well as practical medicine. Causal claims of interest to medicine include claims about the effectiveness of drugs, medical devices and public health interventions, and claims about harms induced by such interventions or by pathogens or environmental exposures, for example. Henceforth, we will primarily be interested in generic claims (repeatably instantiatable or ‘type-level’ claims, such as the claim that taking aspirin relieves headache), but RWT may be taken to apply also to single-case claims (‘token-level’ claims, such as the claim that Bob's taking aspirin this morning relieved his headache).
1.1.2. Mechanism
In the statement of RWT above, ‘mechanism’ can be understood broadly as referring to a complex-systems mechanism, a mechanistic process, or some combination of the two. A complex-systems mechanism consists of entities and activities organised in such a way that they are responsible for some phenomenon to be explained (Machamer, Darden, and Craver Citation2000; Illari and Williamson Citation2012). An example is the mechanism by which the heart pumps blood. A mechanistic process is a spatio-temporally contiguous process along which a signal is propagated (Reichenbach Citation1956; Salmon Citation1998). An example is an artificial pacemaker's electrical signal being transmitted along a lead from the pacemaker itself to the appropriate part of the heart. A mechanism might also be composed of both these sorts of mechanisms: for example, the complex-systems mechanism of the artificial pacemaker, the complex-systems mechanism by which the heart pumps the blood and the mechanistic process linking the two.
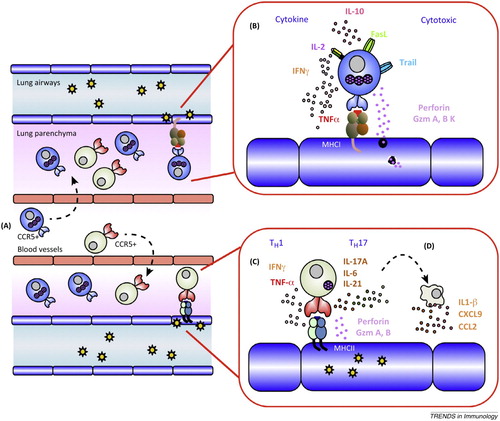
Note that a mechanism cannot in general be thought of simply as a causal network. A causal network can be represented by a directed graph whose nodes represent events or variables and where there is an arrow from one node to another if the former is a direct cause of the latter. On the other hand, a mechanism is typically represented by a richer diagram, such as is frequently found in textbooks and research articles in medicine. , for instance, exemplifies the fact that organisation tends to play a crucial explanatory role in a mechanism. Organisation includes both spatio-temporal structure and the hierarchical structure of the different levels of the mechanism.Footnote1
Figure 1. T cell effector mechanisms in a lung infected by influenza A virus (Gruta and Turner Citation2014).
Note also that high-quality evidence of mechanism can be obtained by a wide variety of means. provides some examples.
Table 1. Examples of sources of evidence of mechanisms in medicine (Clarke et al. Citation2014).
1.1.3. Establishing
A causal claim is ‘established’ just when standards are met for treating the claim itself as evidence, to be used to help evaluate further claims. This requires not only high confidence in the truth of the claim itself but also high confidence in its stability, i.e. that further evidence will not call the claim into question.
That establishing a proposition gives rise to evidence tells us something about establishing, but leaves open the question of what constitutes evidence. Evidence has variously been analysed as one's knowledge, or one's full beliefs, or those of one's degrees of belief which are set by observation, or one's information, or what one rationally grants (Williamson Citation2015). We need not settle the question of what constitutes evidence here. It is worth noting, though, that on some of these accounts evidence must be true, while others admit the possibility that some items of evidence are false. This has consequences for whether establishing is factive. For example, if, as argued by Williamson (Citation2015), one's evidence consists of the propositions that one rationally grants, then establishing a claim does not guarantee its truth, because not everything that one rationally grants need be true. That establishing is not factive is suggested by apparently true assertions such as, ‘Certain researchers had established that stress is the principal cause of stomach ulcers, but further investigations showed that it is not’. (One cannot substitute ‘knew’ for ‘had established’ in this sentence, because knowledge implies truth; one would need ‘thought they knew’ instead.)
Whether or not establishing is factive, it requires meeting a high epistemological standard. In particular, establishing a causal claim should be distinguished from acting in accord with a causal claim as a precautionary measure: in certain cases in which a proposed health action has a relatively low cost, or failing to treat has a high cost, it may be appropriate to initiate the action even when its effectiveness has not been established, so that benefits can be reaped in case it turns out to be effective.
1.1.4. Correlation
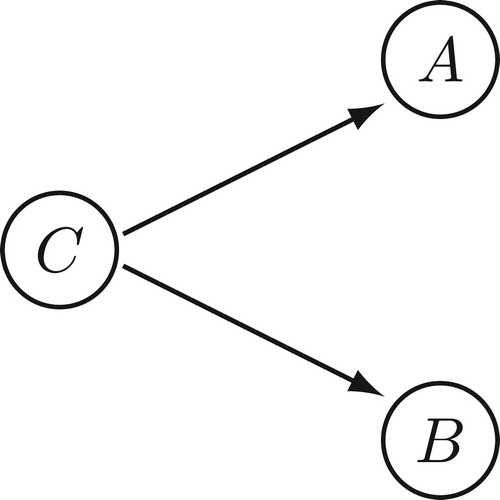
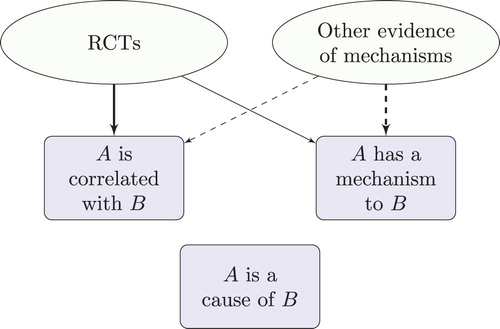
The epistemological thesis says that one needs to establish that the putative cause and effect are ‘appropriately correlated’. Here ‘appropriately correlated’ just means probabilistically dependent conditional on potential confounders, where the probability distribution in question is relative to a specified population or reference class of individuals.Footnote2 Thus, if A is the putative cause variable, B the putative effect variable and C is the set of potential confounder variables, one needs to establish that A and B are probabilistically dependent conditional on C, often written . A confounder is a variable correlated with both A and B, e.g. a common cause of A and B (). The dependence needs to be established conditional on confounders because otherwise an observed correlation between A and B might be attributable to their correlation with C, rather than attributable to A being a cause of B. The set of potential confounders should include any variable that plausibly might be a confounder, given the available evidence of the area in question.
Establishing correlation is non-trivial for two reasons. First, because it requires establishing a probabilistic dependence in the data-generating distribution, rather than simply in the distribution of a sample of observed outcomes. The method of sampling and size of sample can conspire to render an observed sample correlation a poor estimate of a correlation in the population at large. Second, establishing correlation requires considering all potential confounders, and there can be very many of these.
To be clear, we shall use ‘observed correlation’ to refer to a correlation found in the data, ‘genuine correlation’ to refer to a correlation in the population from which the data are drawn, and ‘established correlation’ to refer to a claimed genuine correlation that has met the standards required for being considered established. If establishing is fallible, that a correlation is established does not guarantee that there is a genuine correlation, though it makes it very likely. Moreover, to establish a correlation between A and B, it is not necessary that every relevant dataset yields an observed correlation between A and B, although some observed correlation would typically be required.
1.1.5. Qualifications
RWT says that one ‘normally’ needs to establish both correlation and mechanism. This is because there are certain cases in which causality is apparently not accompanied by a correlation and there are also cases in which causality is apparently not accompanied by an underlying mechanism. If this is so, one cannot expect to establish both correlation and mechanism in these cases.
In cases of overdetermination, where the cause does not raise the probability of the effect because the effect will happen anyway, there is no actual correlation between the cause and the effect. In many such cases, one can expect a counterfactual correlation: if things had been different in such a way that the effect would not have happened anyway—e.g. had a second, overdetermining cause been eliminated—then the cause and effect would indeed be correlated. One might think, then, that one ought to be able to establish a counterfactual correlation for any causal claim, if not an actual correlation. However, there are cases in which the cause of interest and a second, overdetermining cause are mutually exclusive, so that it is not possible both to eliminate the second cause and allow the first cause to vary so as to establish a correlation. For example, an unstable atom may decay to one of two mutually exclusive intermediary states, B and , on the way to a ground state C; attaining either one of the intermediary states causes the particle to reach the ground state, even though there may well be no correlation,
; here one cannot eliminate
and vary B (see Williamson Citation2009, §10). Therefore, even the demand for a counterfactual correlation may be too strong.
Let us turn next to causality without mechanisms. Where the cause and/or the effect is an absence, it cannot be connected by an actual mechanism. In many such cases, one can expect a counterfactual mechanism. Suppose cause and effect are both absences: e.g. failing to treat causes a lack of a heartbeat. If things had been different in such a way that what was absent in the cause were present (e.g. the treatment is administered), then one would expect a mechanism from this presence to a presence corresponding to the effect (e.g. a heartbeat). One might think, then, that one ought to be able to establish the existence of a counterfactual mechanism for any causal claim, if not an actual mechanism. However, there are cases where one of the cause and effect is an absence and the other is a presence, and this strategy does not work. For example, suppose that failing to treat causes a blood clot. That the cause is an absence precludes a mechanism here, but the effect being an absence precludes a mechanism in the obverse case, namely, administering the treatment causes an absence of a blood clot.Footnote3
Now, establishing causality in these cases is not particularly problematic in practice. However, it is more subtle than simply establishing both correlation and mechanism, even where counterfactual correlations or mechanisms are admitted. The question as to how RWT needs to be modified to say something useful in such cases will be not be considered here, because it is not central to the following arguments. The use of ‘normally’ is intended to leave open the possibility that in certain cases of overdetermination or causation between absences one might not need to establish both correlation and mechanism.
1.2. Why the Thesis is True
Having clarified the statement of the epistemological thesis RWT, let us turn to its motivation.
To see why one ought to establish causality this way, consider that an observed correlation between two variables might be explained in a wide variety of ways, as depicted in . Some of these explanations provide reason to doubt that there is a genuine correlation in the underlying population. For example, one of the potential confounders might not have been adequately controlled for, or the sample may be rather small. On the other hand, some of these explanations provide reason to doubt that A is a cause of B, even where there is a genuine correlation between these variables. For example, there might be some variable that could not possibly be considered a potential confounder, given the evidence available, but nevertheless is a confounder, and has not been adequately controlled for. In such a case A and B can be genuinely correlated yet A may not be a cause of B—the correlation is attributable to a common cause. Or there may be a genuine correlation that is entirely non-causal, explained by a semantic relationship, for instance. Thus there are two forms of error: error when inferring correlation in the data-generating distribution from an observed correlation, and error when inferring that A is a cause of B from an established correlation. Evidence of mechanisms can help to eliminate both forms of error. For instance, it can help to determine the direction of causation, which variables are potential confounders, whether a treatment regime is likely to lead to performance bias, and whether measured variables are likely to exhibit temporal trends.Footnote4
Table 2. Possible explanations of an observed correlation between A and B.
The existence of the second kind of error—error when inferring that A is a cause of B from an established correlation—shows that it is not enough to simply establish correlation. If it is indeed the case that A is a cause of B, then there is some combination of mechanisms that explains instances of B by invoking instances of A and which can account for the correlation. Hence, in order to establish efficacy one needs to establish mechanism as well as correlation.Footnote5 This is enough to motivate RWT.
Let us consider an example. The International Agency for Research on Cancer (IARC) Monographs evaluate the carcinogenicity of various substances and environmental exposures. When evaluating whether mobile phone use is a cause of cancer, IARC found that the largest study (the INTERPHONE study) showed a correlation between the highest levels of call time and certain cancers. This correlation was confirmed by another large study from Sweden. However, evidence of mechanisms was judged to be weak overall, and certainly failed to establish the existence of an underlying mechanism. For this reason, chance or bias was considered to be the most likely explanation of the observed correlations, and while causality was not ruled out, neither was it established (IARC Citation2013, §§5–6).
Further discussion of the descriptive and normative adequacy of RWT can be found in the references provided at the start of this section. We will not revisit these arguments here. Instead, I shall argue here that RWT provides a better account of the epistemology of causality than a rival approach, namely the approach of present-day EBM. Let us now consider this rival approach.
1.3. Why the Thesis is Controversial
One reason why the epistemological thesis RWT is controversial is that it conflicts with the current practice of EBM.
EBM is concerned with making the evaluation of evidence explicit:
Evidence based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. (Sackett et al. Citation1996)
Of course, this goal is hardly controversial. What characterises present-day EBM is not the goal itself but the means by which it attempts to achieve this goal. EBM employs hierarchies of evidence in order to evaluate evidence and these hierarchies of evidence tend to favour clinical studies and statistical analyses of these studies over other forms of evidence. Clinical studies (CSs) measure the putative cause and effect, together with potential confounders. CSs include controlled experiments such as randomised controlled trials (RCTs) as well as observational studies such as cohort studies, case control studies, case series and collections of case reports.
Non-CS evidence of mechanisms, i.e. evidence of mechanisms obtained by means other than clinical studies, tends to be either ignored or relegated to the bottom of the hierarchy. For example, depicts an evidence hierarchy of SUNY (Citation2004), used for EBM training. This places animal research and in vitro research, which in the right circumstances can provide high-quality evidence of mechanisms, below ‘opinions’, and well below evidence obtained from clinical studies and statistical analyses of CSs. depicts the current evidence hierarchy of the Oxford Centre for Evidence-Based Medicine, which places ‘mechanism-based reasoning’ at the lowest level. Other approaches, such as the GRADE system, tend to overlook non-CS evidence of mechanisms entirely (Guyatt et al. Citation2011, ).
Figure 3. SUNY Downstate Medical Center EBM Tutorial (SUNY Citation2004).
Figure 4. Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence (OCEBM Levels of Evidence Working Group Citation2011).
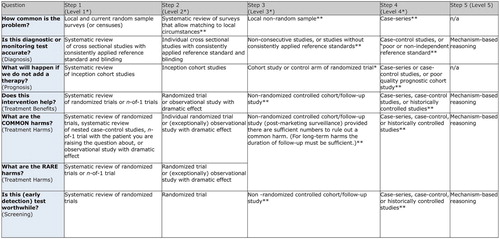
The main feature of contemporary EBM that is of relevance to this paper, then, is that it views non-CS evidence of mechanisms as either irrelevant to the process of evidence evaluation or as strictly inferior to evidence obtained from clinical studies and analyses of CSs. In the latter case, opinions differ as to whether or not clinical studies trump non-CS evidence of mechanisms, i.e. whether or not one should ignore non-CS evidence of mechanisms when clinical studies are available. Either way, however, clinical studies are viewed as superior to other kinds of investigation that provide high-quality evidence of mechanisms.
As a consequence, contemporary EBM stands in conflict with RWT. EBM prioritises clinical studies over evidence of mechanism that arises from other sources. RWT, on the other hand, treats all sources of evidence of mechanism equally.
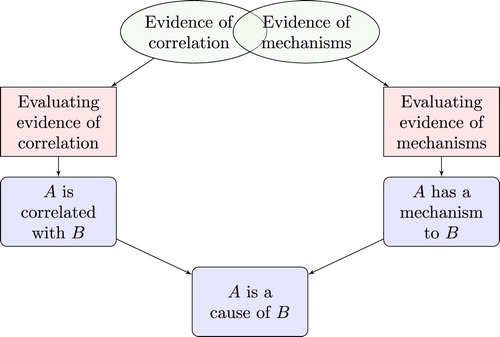
represents the approach motivated by RWT, as suggested by Clarke et al. (Citation2014). Evidence of correlation includes any evidence that is relevant to the claim that there is the appropriate sort of correlation between the putative cause and effect. Individual items of such evidence are likely to vary in quality and in the direction to which they point, so they need to be made explicit and evaluated in order to determine the extent to which the body of evidence as a whole confirms the correlation claim. Similarly, evidence of mechanisms includes any evidence relevant to the claim that the putative cause and effect are linked in the appropriate way by a mechanism. This evidence needs to be made explicit and evaluated to determine the extent to which it confirms the mechanistic claim. Finally, the extent to which evidence confirms the causal claim of interest depends on the extent to which it confirms the correlation and mechanistic claims. In particular, RWT says that if the evidence establishes both the latter claims then it establishes the causal claim.
Given the conflict between present-day EBM and RWT, and the fact that EBM is now widely championed, it is no wonder that RWT is controversial. However, we shall see that there are good reasons to prefer the RWT account of establishing causal claims to the EBM-motivated view. Next, in section 2, I shall argue that RWT better explains the role of clinical studies in establishing a causal claim. In section 3 I shall argue that RWT better explains the process of extrapolating a causal claim from a source population to a target population.
If these arguments are correct, present-day EBM fails to provide an adequate epistemology of causality. However, this does not imply that the whole enterprise of EBM is doomed. Current EBM provides a reasonable first approximation to the correct epistemology, and has led to numerous advances in patient care. The claim made here is that improvements can be made to contemporary EBM, and that the picture of provides a better approximation. This picture can thus be viewed as a way to develop ‘EBM+’, i.e. as a proposal to advance the methodology of EBM by taking better account of evidence of mechanisms (cf. ebmplus.org (http://ebmplus.org)). The main ideas behind EBM+ are (i) that it can be useful to explicitly scrutinise and evaluate all kinds of evidence of mechanisms, not just evidence arising from CSs (), and (ii) that this evidence needs to be considered alongside evidence of correlation—rather than as inferior to it—in order to establish effectiveness in medicine, as per .Footnote6 No claim is made that is the end of the story; further improvements can be made, no doubt.
The RWT-motivated EBM+ approach is thus in line with the goal of EBM, as stated by Sackett above, but not the practice of present-day EBM. While present-day EBM advances an essentially monistic account of causal evaluation, in terms of CSs, the RWT-motivated EBM+ approach is dualistic, treating evidence of mechanisms and evidence of correlation separately, but on a par. In this sense, RWT and EBM+ have a close affinity to the approach of Austin Bradford Hill, in which causal claims are established by means of a number of indicators, some of which provide good evidence of mechanisms and some of which provide good evidence of correlation (Hill Citation1965; Russo and Williamson Citation2007, §2; Clarke et al. Citation2014, §2.2). This sort of dualist approach can perhaps be traced back another century to Claude Bernard, who viewed it as essential to medicine in general:
Scientific, experimental medicine goes as far as possible in the study of vital phenomena; it cannot limit itself to observing diseases or content itself with expectancy or stop at remedies empirically given, but in addition it must study experimentally the mechanism of diseases and the action of remedies. (Bernard Citation1865, 207)
2. Explananda Concerning Clinical Studies
In this section, I shall argue that RWT can successfully explain three fundamental facts about the role of CSs in establishing a causal claim, and that the view motivated by present-day EBM cannot account for all of these facts (although it can account for the first fact). The three facts are these: (i) in some cases, CSs suffice to establish a causal claim; (ii) in some cases, randomised studies are not required to establish a causal claim; (iii) in some cases, randomised studies are trumped by other evidence of mechanisms. We shall examine each of these facts in turn.
2.1. In Some Cases, Clinical Studies Suffice to Establish a Causal Claim
Howick (Citation2011a) suggests that in a number of cases, medical interventions have been accepted on the basis of comparative clinical studies alone. He cites the following cases: the use of aspirin as an analgesic; the use of general anaesthesia; and the use of deep brain stimulation in treating patients with advanced Parkinson's disease or Tourette's syndrome. He argues that these cases are a problem for the epistemological thesis RWT, because the mechanisms of action were not—in some cases, still are not—known. Howick points out that these cases are quite compatible with contemporary EBM, which focuses overwhelmingly on clinical studies.
In response to this objection, one might question whether, in these examples, the causal claims really were established on the basis of comparative clinical studies alone. Cases such as aspirin and general anaesthesia pre-date EBM and their effectiveness was arguably established before they were tested in a systematic comparative clinical study. In all cases, background knowledge was important and it is far from obvious that the causal claims were established on the basis of comparative clinical studies alone.
However, I do not want to dwell on the particular examples here, because I want to accept the general principle that it is possible that clinical studies alone can be used to establish a causal claim in medicine. The point I want to make is that this general principle is quite compatible with RWT.
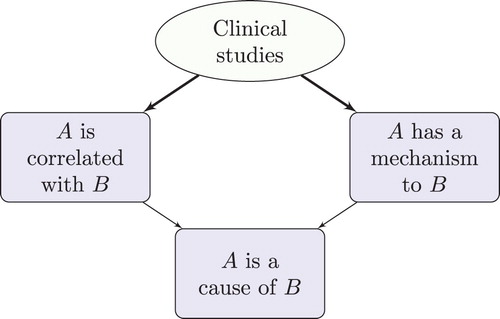
Consider the RWT-motivated picture of . Some of the total available evidence can be considered to provide evidence of correlation, in the sense that these items of evidence contribute to support or undermine the claim that the putative cause and effect are appropriately correlated. (An item of evidence contributes to support a claim if, when taken together with other items, it supports the claim, and the other items do not on their own support the claim to the same degree.) Some of the total available evidence can be considered to provide evidence of mechanisms, in the sense that these items of evidence contribute to support or undermine a claim that there is some mechanism which explains instances of the putative effect in terms of the putative cause and which can account for the extent of the correlation. There is no suggestion that an item of evidence cannot provide both evidence of correlation and evidence of mechanisms.
In particular, clinical studies not only provide evidence of correlation, they can also—in the right circumstances—provide high-quality evidence of mechanisms (). The inference here can be represented as follows:
This inference can be understood as follows. Suppose that there are sufficiently many independent clinical studies that sample the study population in question, they are of sufficient quality (e.g. they are sufficiently large, well-conducted RCTs), sufficiently many studies point in the same direction, and they observe a large enough correlation (aka ‘effect size’). Here ‘sufficiently’ is to be construed in such a way that the threshold is reached for establishing a genuine correlation, and that bias and confounding are ruled out as explanations of this correlation. Suppose further that available evidence rules out fishing, temporal trends and non-causal relationships such as semantic, constitutive, logical, physical and mathematical relationships (cf. ). Suppose, moreover, that there is no other evidence against the existence of an underlying mechanism of action: e.g. such a mechanism does not conflict with confirmed theory. Then, by a process of elimination, causation or reverse causation are the two remaining explanations (). Either way, there must be some underlying mechanism linking the putative cause and effect that explains this correlation. (Note that this inference scheme is non-deductive; there is no suggestion that the premisses guarantee the truth of the conclusions.)
In cases that satisfy the premisses of this inference, clinical studies can provide evidence of the existence of a mechanism even though they may fail to shed light on the details of the mechanism. If, in addition, temporal considerations rule out reverse causation, then one can reach the conclusion that the putative cause is indeed the cause of the putative effect. depicts this kind of inference, from the perspective of RWT. In this diagram, a thick arrow from node X to node Y signifies that X on its own would suffice to establish Y; a thin arrow is used if X is insufficient on its own to establish Y, but nevertheless contributes to support Y.
In sum, then, while Howick cites as counterexamples to RWT cases in which clinical studies have sufficed to establish causality, any such cases are in fact quite compatible with RWT. There are two separate distinctions at play here. The first is the distinction between evidence of correlation and evidence of mechanisms, which is invoked by RWT. The second is the distinction between clinical studies and evidence obtained by other means, which is central to present-day EBM. These distinctions do not coincide, and is only by erroneously conflating the two distinctions that one might think that instances of the above inference scheme refute RWT: by erroneously assuming that clinical studies provide only evidence of correlation and so inferring that RWT requires evidence obtained by other means. RWT requires evidence of two different kinds of connection—correlation and mechanism. It does not require two different kinds of evidence in the sense of requiring two independent sources of evidence—clinical studies and non-CS evidence of mechanisms.Footnote7
While the above inference scheme is compatible with RWT, it is important to observe that the conditions of the inference are very rarely met in practice. For example, instances of this form of inference are very hard to find in IARC evaluations: establishing the carcinogenicity of mists from strong inorganic acids may offer one rare example (IARC Citation2012a, 487–495). Thus, although non-CS evidence of mechanisms is not always essential to establishing causality, it is typically an important part of an inference to cause.
Confusingly, Howick also cites as evidence against RWT a range of cases in which evidence of mechanisms alone led to erroneous causal inferences; see also Howick (Citation2011b, chapter 10). These cases clearly confirm—rather than disconfirm—RWT, which says that causal claims cannot be established just by establishing mechanism since one needs to establish correlation as well. Moreover, these cases also support EBM+, which holds that evidence of mechanisms needs to made explicit and its quality scrutinised. This is because in many of these cases the evidence of mechanisms was rather weak.
2.2. In Some Cases, Randomised Studies are Not Required to Establish a Causal Claim
The second key fact that needs to be explained by an account of establishing causal claims in medicine is the fact that in some cases there is no need for RCTs when establishing causality. To see that this is so, consider three examples.
First, consider the tongue-in-cheek conclusions of Smith and Pell (Citation2003), who study ‘parachute use to prevent death and major trauma related to gravitational challenge’:
As with many interventions intended to prevent ill health, the effectiveness of parachutes has not been subjected to rigorous evaluation by using randomised controlled trials. Advocates of evidence based medicine have criticised the adoption of interventions evaluated by using only observational data. We think that everyone might benefit if the most radical protagonists of evidence based medicine organised and participated in a double blind, randomised, placebo controlled, crossover trial of the parachute. (Smith and Pell Citation2003, 1459)
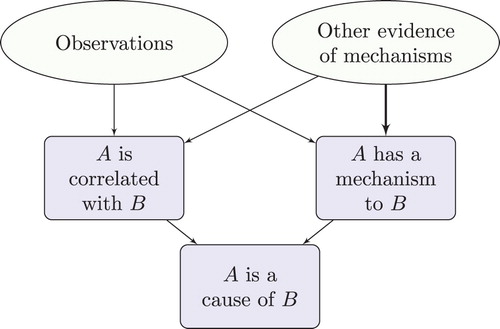
From the point of view of contemporary EBM, the evidence for the effectiveness of parachutes is very weak: no systematic studies, let alone RCTs, and some mechanistic evidence which sits at the bottom of the evidence hierarchy, if it features at all. It is hard to see how causality could be established on the basis of this evidence, if present-day EBM is right. From the point of view of EBM+, however, the evidence is strong: excellent evidence of mechanisms, and, although unsystematic, plenty of observational evidence relating to instances where parachutes were and were not used, and a very large observed effect size. From the point of view of EBM+, the evidence of mechanisms on its own suffices to establish the existence of a suitable mechanism, and, when combined with the unsystematic observations, the total evidence suffices to establish correlation too. Hence causality is established. This inference is depicted in . (Again, the thick arrow signifies that other evidence of mechanisms is sufficient to establish the existence of a mechanism.)
Having clarified the structure of this inference, let us consider a second example (see Worrall Citation2007). The question here is how to establish the effectiveness of extracorporeal membraneous oxygenation (ECMO) for treating persistent pulmonary hypertension (PPHS). With PPHS, immaturity of the lungs in certain newborn babies leads to poor oxygenation of the blood. ECMO oxygenates the blood outside the body (). Observational studies suggested that ECMO increases survival rate from about 20% to about 80% (Bartlett et al. Citation1982). However, under standard EBM procedures for evaluating evidence, the available evidence was viewed as insufficient to establish causality, and it was felt necessary to conduct an RCT (Bartlett et al. Citation1985). At least five subsequent RCTs were carried out, leading to loss of life in the control groups.
Figure 8. The ECMO mechanism, as depicted by Bartlett et al. (Citation1976).
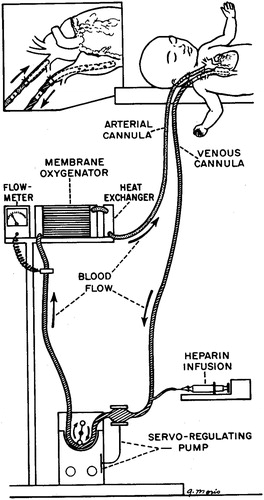
Conducting RCTs in such a case is considered standard EBM procedure. That non-RCT evidence is viewed as insufficient by contemporary EBM was confirmed by a recent Cochrane Review of ECMO, which explicitly disregarded any evidence that did not take the form of an RCT (Mugford, Elbourne, and Field Citation2010).
On the other hand, Worrall (Citation2007) suggests that RCTs were unnecessary in the ECMO case. This conclusion is supported by the RWT-motivated EBM+ approach. This case is analogous to the parachute case: before the first RCT there was strong observational evidence which indicated a large effect size, as well as excellent evidence of mechanisms. Indeed, as in the parachute case, the details of the mechanism of action were very well established. Thus captures the evidential situation in the ECMO case before the first RCT. There is little doubt that conducting RCTs led to yet greater surety; however, despite being mandated by EBM, RCTs were arguably unnecessary to establish causality.
As a third example, consider the case of establishing the carcinogenicity of aristolochic acid. When IARC originally investigated aristolochic acid in 2002, it found that, while there was observational evidence that Chinese herbs which contain aristolochic acid cause cancer, there was ‘limited’ evidence in humans concerning the carcinogenicity of aristolochic acid itself as an active ingredient, so carcinogenicity could not be established (IARC Citation2002, 69–128). IARC re-examined the question some years later and found that there was little in the way of further observational evidence in humans, so the study evidence involving humans was still ‘limited’. However, there was much more evidence of the underlying mechanisms available, to the extent that the mechanistic evidence could now be described as ‘strong’ and causality could be considered established (IARC Citation2012b, 347–361). The key point here is that the change in evidence that warranted establishing causality was a change in evidence of the underlying mechanisms.
These three cases instantiate the following form of inference:
In these cases, evidence of mechanisms obtained by means other than clinical studies provides evidence of correlation. When taken in conjunction with the observational studies, this can be sufficient to establish a genuine correlation. This correlation, when taken in conjunction with the established mechanism of action, can thereby establish causation (). Note that the observational studies do not need to be very systematic: this is so in the parachute example; it may also be true when establishing some adverse drug reactions (Aronson and Hauben Citation2006; Hauben and Aronson Citation2007), and it is also true of many interventions that pre-date EBM, such as the use of ileostomy surgery.
While this mode of inference clearly fits the EBM+ approach, motivated by RWT, it is harder for contemporary EBM to explain, because, as we saw in the ECMO case, much of the practice of present-day EBM demands randomised studies in order to establish causality. To be sure, some deny that randomised trials are required. For example, Glasziou et al. (Citation2007) argue that in cases where there is a large effect size, RCTs may be unnecessary. However, they struggle to explain from within the EBM paradigm how evidence of mechanisms can be treated on a par with observational studies to help establish causality. Instead they evoke Hill's indicators of causality, and Hill's approach is much more in line with RWT and EBM+ than with contemporary EBM (see section 1.3).
2.3. In Some Cases, Randomised Studies are Trumped by Other Evidence of Mechanisms
So far, we have seen that while present-day EBM can account for situations in which RCTs are sufficient to establish causality, it is doubtful whether EBM adequately handles cases in which RCTs are unnecessary. As we shall now see, it is clear that EBM cannot capture cases in which randomised studies are trumped by other evidence of mechanisms. This is because evidence of mechanisms obtained by means other than randomised studies is viewed—when it is considered at all—as strictly inferior to evidence arising from randomised studies (section 1).
There are two kinds of example here. One sort of example involves positive evidence of causality from randomised studies; this evidence is trumped by evidence that there is no mechanism by which causality can operate. To start with another tongue-in-cheek example, Leibovici (Citation2001) presented an RCT which observed a correlation between remote, retroactive intercessionary prayer and length of stay of patients in hospital. The patients in question had bloodstream infections in Israel during the period 1990–1996; the intervention involved saying ‘a short prayer for the well being and full recovery of the group as a whole’ in the year 2000 in the USA, long after recovery or otherwise actually took place. The study also found a correlation between the intervention and duration of fever. The author concludes:
No mechanism known today can account for the effects of remote, retroactive intercessory prayer said for a group of patients with a bloodstream infection. However, the significant results and the flawless design prove that an effect was achieved. (Leibovici Citation2001, 1451)
Present-day EBM clearly accords with this inference to an effect, because it views considerations to do with mechanisms as strictly inferior to evidence produced by clinical studies. However, the implicit conclusion is that this line of reasoning is ridiculous: no effect should be inferred. This contrary conclusion goes against EBM. It is not possible for present-day EBM to account for the possibility that a large, well-conducted RCT can be trumped by the fact that current science has no place for a mechanism between remote, retroactive intercessionary prayer and length of stay of in hospital. On the other hand, this is quite compatible with EBM+. depicts the inference here, from the perspective of RWT. Undermining evidence is represented by dashed arrows. The thick dashed arrow depicts an inferential connection that is enough on its own to rule out a mechanism. As before, the thick solid arrow depicts a connection that would normally be enough on its own to establish the conclusion (correlation): a significant result from a large well-conducted RCT. However, there is evidence which undermines this conclusion: well-confirmed scientific theory. The presence of this undermining evidence blocks any inference to either correlation or mechanism, and thereby blocks an inference to causation.
Other inferences follow the same pattern. Some comparative studies for precognition have observed a significant correlation (see, e.g. Bem Citation2011), as have others in the case of homeopathy (e.g. Cucherat et al. Citation2000; Faculty of Homeopathy Citation2016). What are the options for resisting an inference to causality in such cases? EBM will point to the fact that the evidence base shows mixed results and is thus inconclusive. However, while this may be so for precognition and homeopathy in general, it is not the case for certain specific interventions which are instances of precognition or homeopathy; as the above references show, there are specific interventions for which only positive studies are available. A second possible way to resist an inference to causality in such cases is to invoke the machinery of Bayesianism: to argue that the prior probability of effectiveness is so low that the posterior probability remains low, despite confirmatory trials. This strategy is open to the charge of subjectivity. Clearly, the proponent of a subjective Bayesian analysis will have to admit that the choice of prior is subjective here. But even objective Bayesian analyses typically require a high prior probability of deception or experimental error (Jaynes Citation2003, §§5.1–2), and detractors can take issue with this presumption. A third alternative is to apply the RWT-motivated EBM+ approach. According to RWT, the inference in these cases follows the pattern of , and it is clear that causality has not been established, even in specific cases where trials would be sufficient in the absence of other evidence to establish correlation. Arguably, then, the RWT-motivated approach is the most promising of these three strategies.
In the kind of example considered above, positive evidence from randomised studies is trumped by evidence of absence of mechanism. But there is another sort of example, in which there is observational evidence, evidence from RCTs and other positive evidence of mechanisms, and in which the other evidence of mechanisms plays more of a role in establishing causality than do the RCTs. The ECMO case takes this form at the point after the first randomised trial. The first randomised trial provided weak evidence, because after the first baby was randomly assigned to the control arm of the trial and subsequently died, no more individuals were assigned to this arm. Thus the size of the trial was not sufficient to draw any strong conclusions. Arguably, at that point in time the evidence of mechanisms was stronger than the evidence arising from RCTs and it played more of a role in establishing causality. Indeed, if the analysis of section 2.2 is correct then the RCT evidence was redundant. The evidence of mechanisms trumps the RCT evidence in such a case.
2.4. Summary
To conclude, the causal epistemology motivated by RWT can validate all three facts about the role of clinical studies in establishing a causal claim. The EBM approach certainly captures the first fact (in some cases, clinical studies suffice to establish a causal claim). However, the practice of EBM goes against the second fact (in some cases, randomised studies are not required to establish a causal claim) and EBM certainly fails to explain the third fact (in some cases, randomised studies are trumped by other evidence of mechanisms).
The proponent of present-day EBM might object that one should not infer a normative thesis about appropriate methodology from a description of actual practice—i.e. from the three facts about the role of clinical studies in actual instances of causal discovery. It is no doubt true that some actual instances of causal discovery were methodologically flawed, and that in some cases researchers thought that they had established a causal claim when in fact they had failed to establish it. Thus one must be cautious when generalising from actual instances to normative claims. However, it is also beyond doubt that—in recent times—medicine has successfully discovered a great number of causal claims. Methods employed in actual medical examples work, by-and-large, and so they tell us something about appropriate methodology. Given this, the three facts do indeed admit a normative interpretation. It is thus incumbent upon the proponent of EBM who denies the normative interpretation of one (or more) of these facts to explain away all the apparent instances of causal discovery which seem to support it. Each of the three facts considered above, under a normative reading, says only that in some cases certain methods are appropriate, so in order to deny one of these facts the onus is on the proponent of present-day EBM to show that in all cases the corresponding methods are inappropriate.
3. Extrapolation
3.1. Three Approaches to Extrapolation
We now turn to the question of how a causal claim can be extrapolated from a source population to a target population of interest. This mode of inference is ubiquitous, because the population within which a typical clinical study establishes a correlation (e.g. hospital patients in a particular region who are not too young, not too old, not too ill and not pregnant) is almost never the same as the population within which the treatment is intended to be used. It is also very common—and particularly challenging—to extrapolate causal claims from animals to humans. Any adequate causal epistemology needs to explain how extrapolation is possible and needs to clarify the logic of extrapolation.
Here is a first approximation to the logic of extrapolation:
As Steel (Citation2008) points out, this explication faces two immediate problems. The first, which Steel calls the extrapolator's circle, is that ‘it needs to be explained how we could know that the model and the target are similar in causally relevant respects without already knowing the causal relationship in the target’ (78). The worry is that extrapolation seems redundant since the conclusion of the above rule of inference is apparently needed to establish the second premiss. The second problem, which we shall call the extrapolator's block, is that ‘any adequate account of extrapolation in heterogeneous populations must explain how extrapolation can be possible even when [causally relevant differences between the model and the target] are present’ (78–79). That is, the source and target population are rarely entirely similar in all causally relevant respects—particularly when extrapolating from animals to humans—and it needs to be made clear what sort of differences are permissible in order to prevent the second premiss of the above argument from failing and the inference thereby being blocked. Thanks to these two problems, this first attempt at a logic of extrapolation fails, and we must look further afield.
Note that a source population is chosen for investigation precisely because one can conduct more conclusive clinical studies on this population than on the target population. Thus the clinical studies that one can perform on the source population—typically, experimental studies—tend to be of a higher standard than those—typically, observational studies—which are directly obtained on the target population. Indeed, there would be no point extrapolating from source to target if the studies in the source population were less conclusive than those conducted on the target population. In the light of this point, one can sketch an approach to extrapolation motivated by contemporary EBM as follows:
This approach to extrapolation circumvents the aforementioned two problems very nicely. There is no extrapolator's circle because one does not need to know that the causal relationship holds in the target population to obtain observational studies in the target population. There is no extrapolator's block because this theory of extrapolation makes extrapolation possible even when there are substantial differences between the source and target populations.
That there may be substantial differences between the source and target populations points to two new problems that face the EBM-motivated approach. First, we have what we might call the extrapolator's fallacy: it needs to be explained how extrapolation is a reliable form of inference, rather than simply fallacious. The worry is that the EBM-motivated account will lead to lots of mistaken conclusions, because lower quality CSs in the target population, such as observational studies, typically provide weak evidence that the target population is similar to the source population in causally relevant respects. This problem may explain some recent scepticism about extrapolation amongst those interested in medical methodology (see, e.g. Ioannidis Citation2012). However, since almost every causal claim of interest has to be extrapolated from some source population, fallacious extrapolation is hardly a viable option.
The second, related problem is that the extrapolator's standards are slipping. In the EBM-motivated approach, there is a high standard for internal validity but a low standard for external validity: evidence deemed to be of high quality by EBM (such as that obtained from RCTs) is used to establish causality in a source population, while lower quality evidence (such as that obtained from observational studies) is used to establish causality in the target population. In general, an account of extrapolation should not have double standards—the burden of proof for causality should be similar in the source and target populations.
As Steel (Citation2008, chapter 5) suggests, in order to extrapolate a causal claim from a source population to a target population, one needs evidence that similar mechanisms operate in the two populations.Footnote8 This is particularly important in contexts where mechanisms are likely to differ, such as with extrapolations from animals to humans, or interventions involving long causal pathways. It turns out that this feature of extrapolation can be captured by the following RWT-motivated account.
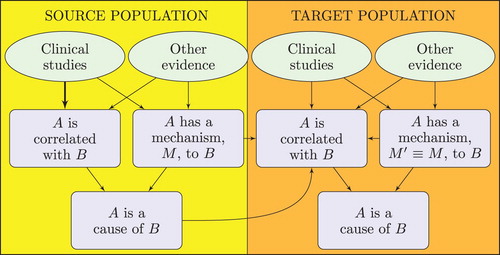
depicts an account of the logic of extrapolation that is motivated by RWT. In the source population, one can carry out clinical studies that normally cannot be carried out in the target population; these studies are often enough on their own to establish correlation. By also establishing mechanism, one can then establish causality in the source population. Let us turn to the target population. Clinical studies conducted on the target population, even when augmented by other evidence of the mechanisms of the target population, are insufficient to establish both correlation and mechanism—otherwise there would be no need for extrapolation. Extrapolation is possible when evidence of mechanisms in the target population is strong enough not only to establish the existence of a suitable mechanism in the target population, but also to establish that this mechanism is similar in key respects to the mechanism M inferred in the source population. The expression
in denotes this similarity claim. By means of this similarity of mechanisms, one can use the claim that A is a cause of B established in the source population to further support the correlation claim in the target population. In sum, where clinical studies and other mechanistic investigations in the target population are not jointly sufficient to establish correlation in the target, if the corresponding causal claim is established in the source population and it is also established that the mechanisms in the target population are sufficiently similar to those which underpin causation in the source population then this combination of evidence may be enough to establish correlation in the target population. If so, since mechanism in the target is also established, causality can be inferred.
As an extreme case, there may be no clinical studies in the target population; this in itself does not preclude extrapolation under the RWT-motivated account. For example, when IARC evaluated the carcinogenicity of benzo[a]pyrene, they found no human studies measuring exposure to benzo[a]pyrene together with relevant cancer outcomes. However, there were excellent animal studies and enough evidence of mechanisms in animals to establish carcinogenicity in the relevant animal models and to determine the details of the mechanism of action there. Furthermore, there was excellent evidence that the human mechanisms were similar to the mechanisms found in animals. This was considered enough to establish carcinogenicity in humans (IARC Citation2012a, 111–144). Note that this inference is not validated by the EBM-motivated account of extrapolation provided above, because there were no relevant clinical studies in humans. Thus the example favours the RWT-motivated account of extrapolation.
To take another case where there were no clinical studies in the target population, consider the IARC evaluation of d-Limonene as a cause of cancer. In this case too, there were no studies available in humans. Carcinogenicity of d-Limonene was established in male rats, so this seemed to be a candidate for extrapolation. However, there were crucial dissimilarities between the mechanism of action in rats and the corresponding human mechanisms: in particular, a protein responsible for nephrotoxicity in male rats is specific to male rats. Thus no extrapolation was possible and carcinogenicity was not established (IARC Citation1999b, 317–327). This example, which is also in accord with the RWT-motivated account, shows how crucial it is to establish similarity of mechanisms.
Determining similarity of mechanisms can be rather tortuous. With regard to the question of the carcinogenicity of Di(2-ethylhexyl)phthalate (DEHP), causality was established in animals by 1982. In 2000, however, IARC downgraded its carcinogenicity rating in humans—to some controversy (Huff Citation2003)—because new evidence suggested that ‘DEHP caused liver tumours in rats and mice by a non-DNA-reactive mechanism involving peroxisome proliferation, which was considered not relevant to humans’ (Grosse et al. Citation2011, 329). In 2011, a third IARC working group had substantially more mechanistic evidence available, and this evidence suggested that there are other pathways in the cancer mechanism, some of which are relevant to humans. This led to the carcinogenicity rating to be upgraded again (Grosse et al. Citation2011). That the evaluation of carcinogenicity tracks evidence of mechanistic similarity simply cannot be explained by present-day EBM.
In some cases, new clinical studies in the target population can lead to a re-evaluation of a mechanistic similarity claim. IARC first examined acrylonitrile in 1979 (IARC Citation1979, 73–86), and in 1987 decided that carcinogenicity in rats was established and carcinogenicity in humans was likely (IARC Citation1987, 79–80). Carcinogenicity was not considered to be established in humans because studies in humans provided limited evidence of correlation and other evidence of similarity of mechanisms between rats and humans was also limited. Nevertheless, similarity of mechanisms was credible enough for carcinogenicity in humans to be considered likely. By 1999, further studies in humans had suggested that earlier observed correlations were probably due to confounding by smoking (IARC Citation1999a, 43–108). These studies cast doubt both on correlation and on similarity of mechanisms and led to a downgrading of the likelihood of carcinogenicity.
It is important to note that demonstrating mechanistic similarity requires showing that the whole structure of relevant mechanisms is sufficiently similar, not just that the mechanism M by which causality operates in the source population has an analogue in the target population. Thus, one needs to establish that any new counteracting mechanism in the target population is not so significant that it can cancel out (‘mask’) the action of the analogue of M. This masking problem was a stumbling block for Anitschkow when he tried to establish that dietary cholesterol causes atherosclerosis by appealing to animal experiments (Anitschkow Citation1933). He provided compelling evidence that the causal relationship holds in rabbits and that the mechanism responsible for this relationship also occurs in humans. However, various non-herbivorous animals, including rats, did not exhibit the correlation between dietary cholesterol and atherosclerosis that was found in rabbits. This lack of robustness suggests the presence of a counteracting mechanism in certain non-herbivorous species which masks the action of the positive mechanism of action that was found in rabbits. The presence of such a masking mechanism in humans would count as an important difference between the relevant mechanistic structures in rabbits and humans. Thus, similarity of mechanisms was not established, and causation in humans was rightly not considered established by Anitschkow's work (see Parkkinen Citation2016).
3.2. The Four Problems for Extrapolation
We shall now see that this RWT-motivated account of extrapolation survives the four problems for extrapolation identified above.
First, let us consider the extrapolator's circle. That there is no circle should be apparent from the fact that is acyclic: one does not need to have already established causality in the target population in order to meet any of the requirements for establishing causality. Of course, once these requirements are all met, causality in the target is thereby established, but there is no inferential circle here. See Steel (Citation2008, §5.4.2) for further discussion of how mechanism-based approaches can avoid the extrapolator's circle.
Turning next to the extrapolator's block, one might worry that we are lacking an account of how extrapolation is possible when mechanisms in the source and target populations are not identical. Similarity of mechanisms is a matter of degree, and the more similar the mechanisms, the more that causation in the source population confirms correlation in the target population. Steel (Citation2008, §5.3.2) discusses this question and presents comparative process tracing as a method for establishing similarity:
First, learn the mechanism in the model organism, by means of process tracing or other experimental means. For example, a description of a carcinogenic mechanism would indicate such things as the product of the phase I metabolism and the enzymes involved; whether the metabolite is a mutagen, an indication of how it alters DNA; and so on. Second, compare stages of the mechanism in the model organism with that of the target organism in which the two are most likely to differ significantly. For example, one would want to know whether the chemical is metabolized by the same enzymes in the two species, and whether the same metabolite results, and so forth. In general, the greater the similarity of configuration and behavior of entities involved in the mechanism at these key stages, the stronger the basis for the extrapolation. (Steel Citation2008, 89)
In fact, comparative process tracing is but one of several methods for establishing similarity of mechanisms. One can also establish similarity of mechanisms without determining the details of the mechanisms M and , by employing phylogenetic reasoning, robustness analysis or even enumerative induction (Parkkinen and Williamson Citation2017, §4). Thus there is a portfolio of methods for overcoming the extrapolator's block.
Let us consider the extrapolator's fallacy next. Unlike the EBM-motivated approach, the RWT-motivated analysis of extrapolation requires evidence that ensures that the source and target populations are similar in causally relevant respects. Mechanistic evidence plays a key role here, in ensuring that . By being more demanding than the EBM-motivated approach in terms of the evidence required in the target population, extrapolation promises to be more reliable under the RWT account than under the EBM account.
Finally, we can ask whether the extrapolator's standards are slipping. That this is not the case is apparent from : the inferential requirements—establishing correlation and mechanism—are the same in both the source and target populations. If anything, one might one worry that the standards of evidence are higher in the target population than in the study population since includes the extra requirement of establishing similarity of mechanism there. However, this is just an artefact of the diagram. Similarity of mechanisms concerns the relation between the source and target populations, not just the target population. Therefore, there is a genuine symmetry between what is required of the source and target populations.
That the RWT account of extrapolation overcomes the latter two problems, while the EBM approach does not, speaks in favour of the RWT approach and against the EBM approach.
3.3. Criticisms of Mechanistic Accounts of Extrapolation
Having developed the RWT-motivated theory of extrapolation, we shall now consider some criticisms of mechanistic accounts of extrapolation in the light of this theory.
Guala (Citation2010, §6) suggests that there are cases of extrapolation that do not proceed via comparative process tracing. Guala develops an example involving outer continental shelf auctions, which are used to sell oil leases in the Gulf of Mexico, to show that it is not always necessary to determine the details of the relevant mechanisms, as would be required by comparative process tracing. As noted above, however, the RWT-motivated account sees comparative process tracing as but one of several strategies for establishing similarity of mechanisms, and Guala's case is perfectly in accord with this. What is important to the RWT account is the inferential step : strategies for extrapolation seek to demonstrate similarity of mechanisms. As Guala notes,
This clearly falls short of a proper articulation of the mechanism … And yet, it is perfectly adequate for extrapolation purposes. Large parts of the mechanism can be “black boxed” as long as there are good reasons to believe that they are analogously instantiated in the laboratory and target system'. (Guala Citation2010, 1080)
One of the advantages of the RWT-motivated approach, then, is that by situating extrapolation in the inference scheme depicted in it covers much a broader range of scenarios than comparative process tracing does.
Howick, Glasziou, and Aronson (Citation2013a, Citation2013b) are broadly sceptical of mechanism-based extrapolation. They identify several problems for basing extrapolations on mechanistic evidence. First, our understanding of mechanisms is often incomplete. In response one can note that this is of course true, but insufficient knowledge of the details of M and for comparative process tracing does not always preclude establishing that
: one can often employ the other strategies mentioned above. Second, knowledge of mechanisms is not always applicable outside the tightly controlled laboratory conditions in which is gained. This is also true, but it is symptomatic of science in general: whatever approach one takes, one must make sure that one's conclusions are robust enough to extend to the application of interest. In particular, an EBM-motivated approach has to ensure that conclusions based on trials with strict exclusion criteria are transportable to the population to be treated. The third problem that they identify is that mechanisms can behave ‘paradoxically’, e.g. a drug can have opposite effects in different contexts. In response, observe that it is only by understanding the underlying mechanisms that one can explain these paradoxical effects and improve treatment. Moreover, clinical studies are crucial for identifying the presence of such effects. All this confirms the RWT-motivated account of extrapolation, which takes both clinical studies and non-CS evidence of mechanisms seriously. The fourth problem that Howick et al. pick out is the extrapolator's circle. Their worry is that the evidence of the target population required to establish that
makes the evidence on the source population redundant. As makes clear, this need not be the case: one can establish that
in the absence of evidence from clinical studies in the target population that would one their own be sufficient to establish causality. Howick et al. might respond by noting that under the EBM-motivated account of extrapolation, only weak evidence of the target population is required to establish causality in the target population and this evidence would be sufficient to establish causality there. However, as discussed above, this is a problem for the EBM-motivated account: it makes extrapolation too easy to be entirely credible—it is subject to the extrapolator's fallacy. That the RWT-motivated theory of extrapolation is more demanding in terms of the evidence required for extrapolation is an advantage over the EBM-motivated account.
4. Conclusion
We have seen that the epistemological thesis RWT motivates a view of medical methodology that stands in conflict with contemporary EBM. Although there is a tension between RWT and EBM, I have argued that RWT can better explain three key features of the use of CSs to establish causality, and that it yields a better account of extrapolation. Thus, I conclude that RWT and EBM+ offer a promising way forward in the controversy as to how best to improve EBM.
The EBM approach to causal inference has in recent years extended well beyond medicine, to public policy making and various areas of the social sciences, for example. While this paper has focussed on medicine, RWT can be interpreted as having a broader range of application, and similar conclusions to those drawn in this paper may apply beyond medicine. The broader scope of these conclusions is left as a question for further research.
Acknowledgments
I am very grateful to the Leverhulme Trust and to the UK Arts and Humanities Research Council for supporting this research, to Julian Reiss, Kurt Straif, and Michael Wilde for pointing me to useful IARC examples, and to Nancy Cartwright, Brendan Clarke, Donald Gillies, Phyllis Illari, Mike Kelly, Veli-Pekka Parkkinen, Christian Wallmann, Michael Wilde, and the anonymous referees for helpful comments.
Notes
1 To take an extreme example of the importance of organisation, a chimney mechanism is responsible for the extraction of smoke purely in virtue of its spatial organisation. No activities constitute the chimney mechanism itself—although smoke actively passes through the mechanism—and the only relevant properties of the entities that constitute the mechanism (e.g. bricks and mortar) are structural properties to do with their impermeability and their ability to support the load of the chimney. Kaiser (Citation2016) provides further evidence for the claim that a mechanism cannot always be identified with a causal network.
2 ‘Correlated’ is often used in weaker senses, e.g. meaning unconditionally probabilistically dependent, or unconditionally linearly dependent. Certain arguments of this paper also go through under these weaker interpretations of ‘correlated’: if, under a strong reading of ‘correlation’, it is not enough simply to establish correlation in order to establish causation, then that is also true under a weak reading.
3 Cases of disconnection (Schaffer Citation2000) or double-prevention (Hall Citation2004) may also be thought of as cases that involve absences.
4 Evidence of mechanisms can help in other respects too. For example, evidence of mechanisms is often essential in order to properly design a CS or interpret its results (Clarke et al. Citation2014).
5 These assertions hold ‘normally’, i.e. modulo the qualifications about underdetermination and causation between absences discussed above.
6 One might think that it would be very difficult to systematically consider evidence of mechanisms alongside evidence of correlation. However, as Parkkinen et al. (Citation2018) show, this is not the case. They put forward procedures for evaluating non-CS evidence of mechanisms and for combining this evaluation with a standard evaluation of CSs in order to provide an overall assessment of a causal claim.
7 This point was emphasised by Illari (Citation2011, §2). One might think that, by not requiring two different sources of evidence, RWT somehow becomes trivially true, or that it becomes compatible in general with present-day EBM. Subsequent sections of this paper show that this is not so, by highlighting points of disagreement with present-day EBM and arguing that these points of disagreement favour RWT.
8 Cartwright (Citation2011) is another proponent of the view that successful extrapolation requires evidence that goes beyond statistical studies.
References
- Anitschkow, Nikolaj. 1933. “Experimental Atherosclerosis in Animals.” In Arteriosclerosis, edited by E. V. Cowdry, 271–322. New York: Macmillan.
- Aronson, Jeffrey K., and Manfred Hauben. 2006. “Anecdotes that Provide Definitive Evidence.” British Medical Journal 333: 1267–1269. doi: 10.1136/bmj.39036.666389.94
- Bartlett, R. H., A. F. Andrews, J. M. Toomasian, N. J. Haiduc, and A. B. Gazzaniga. 1982. “Extracorporeal Membrane Oxygenation for Newborn Respiratory Failure: Forty-five Cases.” Surgery 92: 425–433.
- Bartlett, R. H., A. B. Gazzaniga, M. R. Jefferies, R. F. Huxtable, N. J. Haiduc, and S. W. Fong. 1976. “Extracorporeal Membrane Oxygenation (ECMO) Cardiopulmonary Support in Infancy.” ASAIO Journal 22: 80–92.
- Bartlett, Robert H., Dietrich W. Roloff, Richard G. Cornell, Alice French Andrews, Peter W. Dillon, and Joseph B. Zwischenberger. 1985. “Extracorporeal Circulation in Neonatal Respiratory Failure: A Prospective Randomized Study.” Pediatrics 76: 479–487.
- Bem, D. J. 2011. “Feeling the Future: Experimental Evidence for Anomalous Retroactive Influences on Cognition and Affect.” Journal of Personality and Social Psychology 100: 407–425. doi: 10.1037/a0021524
- Bernard, Claude. [1865] 1949. An Introduction to the Study of Experimental Medicine. New York: Dover.
- Broadbent, Alex. 2011. “Inferring Causation in Epidemiology: Mechanisms, Black Boxes, and Contrasts.” In Causality in the Sciences, edited by Phyllis McKay Illari, Federica Russo, and Jon Williamson, 45–69. Oxford: Oxford University Press.
- Campaner, Raffaella. 2011. “Understanding Mechanisms in the Health Sciences.” Theoretical Medicine and Bioethics 32: 5–17. doi: 10.1007/s11017-010-9166-5
- Campaner, Raffaella, and Maria Carla Galavotti. 2012. “Evidence and the Assessment of Causal Relations in the Health Sciences.” International Studies in the Philosophy of Science 26: 27–45. doi: 10.1080/02698595.2012.653113
- Cartwright, Nancy. 2011. “Predicting ‘It Will Work for Us’: (Way) Beyond Statistics.” In Causality in the Sciences, edited by Phyllis Illari, Federica Russo, and Jon Williamson, Chap. 35, 750–770. Oxford: Oxford University Press.
- Clarke, Brendan. 2011. “Causality in Medicine with Particular Reference to the Viral Causation of Cancers.” PhD diss., Department of Science and Technology Studies, University College London, London.
- Clarke, Brendan, Donald Gillies, Phyllis Illari, Federica Russo, and Jon Williamson. 2013. “The Evidence that Evidence-based Medicine Omits.” Preventive Medicine 57: 745–747. doi: 10.1016/j.ypmed.2012.10.020
- Clarke, Brendan, Donald Gillies, Phyllis Illari, Federica Russo, and Jon Williamson. 2014. “Mechanisms and the Evidence Hierarchy.” Topoi 33: 339–360. doi: 10.1007/s11245-013-9220-9
- Claveau, Francois. 2012. “The Russo–Williamson Theses in the Social Sciences: Causal Inference Drawing on Two Types of Evidence.” Studies in History and Philosophy of Biological and Biomedical Sciences 43: 806–813. doi: 10.1016/j.shpsc.2012.05.004
- Cucherat, M., M. C. Haugh, M. Gooch, and J.-P. Boissel. 2000. “Evidence of Clinical Efficacy of Homeopathy: A Meta-analysis of Clinical Trials.” European Journal of Clinical Pharmacology 56: 27–33. doi: 10.1007/s002280050716
- Darby, George, and Jon Williamson. 2011. “Imaging Technology and the Philosophy of Causality.” Philosophy & Technology 24: 115–136. doi: 10.1007/s13347-010-0010-7
- Dragulinescu, Stefan. 2012. “On ‘Stabilising’ Medical Mechanisms, Truth-makers and Epistemic Causality: A Critique to Williamson and Russo's Approach.” Synthese 187: 785–800. doi: 10.1007/s11229-011-0011-9
- Faculty of Homeopathy. 2016. “The Research Evidence Base for Homeopathy.” Accessed October 21, 2016. http://facultyofhomeopathy.org/wp-content/uploads/2016/03/2-page-evidence-summary-for-homeopathy.pdf.
- Fiorentino, Alexander R., and Olaf Dammann. 2015. “Evidence, Illness, and Causation: An Epidemiological Perspective on the Russo–Williamson Thesis.” Studies in History and Philosophy of Biological and Biomedical Sciences 54: 1–9. doi: 10.1016/j.shpsc.2015.09.010
- Gillies, Donald A. 2011. “The Russo–Williamson Thesis and the Question of Whether Smoking Causes Heart Disease.” In Causality in the Sciences, edited by Phyllis McKay Illari, Federica Russo, and Jon Williamson, 110–125. Oxford: Oxford University Press.
- Glasziou, Paul, Iain Chalmers,Michael Rawlins, and Peter McCulloch. 2007. “When are Randomised Trials Unnecessary? Picking Signal From Noise.” British Medical Journal 334: 349–351. doi: 10.1136/bmj.39070.527986.68
- Grosse, Yann, Robert Baan, Béatrice Secretan-Lauby, Fatiha El Ghissassi, Véronique Bouvard, Lamia Benbrahim-Tallaa, Neela Guha, Farhad Islami, Laurent Galichet, and Kurt Straif. 2011. “Carcinogenicity of Chemicals in Industrial and Consumer Products, Food Contaminants and Flavourings, and Water Chlorination Byproducts.” The Lancet Oncology 12: 328–329. doi: 10.1016/S1470-2045(11)70088-2
- Gruta, Nicole L. La, and Stephen J. Turner. 2014. “T Cell Mediated Immunity to Influenza: Mechanisms of Viral Control.” Trends in Immunology 35: 396–402. doi: 10.1016/j.it.2014.06.004
- Guala, Francesco. 2010. “Extrapolation, Analogy, and Comparative Process Tracing.” Philosophy of Science 77: 1070–1082. doi: 10.1086/656541
- Guyatt, Gordon, Andrew D. Oxman, Elie A. Akl, Regina Kunz, Gunn Vist, Jan Brozek, and Susan Norris, et al. 2011. “GRADE Guidelines: 1. Introduction—GRADE Evidence Profiles and Summary of Findings Tables.” Journal of Clinical Epidemiology 64: 383–394. doi: 10.1016/j.jclinepi.2010.04.026
- Hall, Ned. 2004. “Two Concepts of Causation.” In Causation and Counterfactuals, edited by John Collins, Ned Hall, and L. A. Paul, 225–276. Cambridge, MA and London: MIT Press.
- Hauben, Manfred, and Jeffrey K. Aronson. 2007. “Gold Standards in Pharmacovigilance: The Use of Definitive Anecdotal Reports of Adverse Drug Reactions As Pure Gold and High-Grade Ore.” Drug Safety 30: 645–655. doi: 10.2165/00002018-200730080-00001
- Hill, Austin Bradford. 1965. “The Environment and Disease: Association Or Causation?” Proceedings of the Royal Society of Medicine 58: 295–300. doi: 10.1177/003591576505800503
- Howick, Jeremy. 2011a. “Exposing the Vanities—and a Qualified Defence—of Mechanistic Evidence in Clinical Decision-making.” Philosophy of Science 78: 926–940. Proceedings of the Biennial PSA 2010. doi: 10.1086/662561
- Howick, Jeremy. 2011b. The Philosophy of Evidence-Based Medicine. Chichester: Wiley-Blackwell.
- Howick, J.,P. Glasziou, and J. K. Aronson. 2013a. “Can Understanding Mechanisms Solve the Problem of Extrapolating From Study to Target Populations (The Problem of ‘external Validity’)?” Journal of the Royal Society of Medicine 106: 81–86. doi: 10.1177/0141076813476498
- Howick, Jeremy, Paul Glasziou, and Jeffrey K. Aronson. 2013b. “Problems with Using Mechanisms to Solve the Problem of Extrapolation.” Theoretical Medicine and Bioethics 34: 275–291. doi: 10.1007/s11017-013-9266-0
- Huff, James. 2003. “IARC and the DEHP Quagmire.” International Journal of Occupational and Environmental Health 9: 402–404. doi: 10.1179/oeh.2003.9.2.138
- IARC. 1979. Some Monomers, Plastics and Synthetic Aelastomers, and Acrolein. Vol. 19 of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol1-42/mono19.pdf.
- IARC. 1987. Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. Vol. Supplement 7 of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/suppl7/Suppl7.pdf.
- IARC. 1999a. Re-Evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. Vol. 71 of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol71/mono71.pdf.
- IARC. 1999b. Some Chemicals that Cause Tumours of the Kidney or Urinary Bladder in Rodents and Some Other Substances. Vol. 73 of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol73/mono73.pdf.
- IARC. 2002. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Vol. 82 of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol100A/mono100A.pdf.
- IARC. 2012a. Chemical Agents and Related Occupations. Vol. 100F of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol100F/mono100F.pdf.
- IARC. 2012b. Pharmaceuticals. Vol. 100A of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol100A/mono100A.pdf.
- IARC. 2013. Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields. Vol. 102 of IARC Monographs Series. Lyon: International Agency for Research on Cancer. http://monographs.iarc.fr/ENG/Monographs/vol102/mono102.pdf.
- Illari, Phyllis McKay. 2011. “Disambiguating the Russo–Williamson Thesis.” International Studies in the Philosophy of Science 25: 139–157. doi: 10.1080/02698595.2011.574856
- Illari, Phyllis McKay, and Jon Williamson. 2012. “What is a Mechanism? Thinking About Mechanisms across the Sciences.” European Journal for Philosophy of Science 2: 119–135. doi: 10.1007/s13194-011-0038-2
- Ioannidis, John P. A. 2012. “Extrapolating From Animals to Humans.” Science Translational Medicine 4: 151ps15. doi: 10.1126/scitranslmed.3004631
- Jaynes, E. T. 2003. Probability Theory: The Logic of Science. Cambridge: Cambridge University Press.
- Kaiser, Marie I. 2016. “On the Limits of Causal Modelling: Spatially-structurally Complex Biological Phenomena.” Philosophy of Science 83: 921–933. doi: 10.1086/687875
- Leibovici, Leonard. 2001. “Effects of Remote, Retroactive Intercessory Prayer on Outcomes in Patients with Bloodstream Infection: Randomised Controlled Trial.” British Medical Journal 323: 1450–1451. doi: 10.1136/bmj.323.7327.1450
- Machamer, Peter, Lindley Darden, and Carl Craver. 2000. “Thinking About Mechanisms.” Philosophy of Science 67: 1–25. doi: 10.1086/392759
- Mugford, Miranda, Diana Elbourne, and David Field. 2010. “Cochrane Review: Extracorporeal Membrane Oxygenation for Severe Respiratory Failure in Newborn Infants.” Evidence-Based Child Health: A Cochrane Review Journal 5: 241–298. doi: 10.1002/ebch.522
- OCEBM Levels of Evidence Working Group. 2011. “The Oxford 2011 Levels of Evidence.” Oxford Centre for Evidence-Based Medicine, Oxford. http://www.cebm.net/index.aspx?o=5653.
- Parkkinen, Veli-Pekka. 2016. “Robustness and Evidence of Mechanisms in Early Experimental Atherosclerosis Research.” Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences 60: 44–55. doi: 10.1016/j.shpsc.2016.09.006
- Parkkinen, Veli-Pekka, Christian Wallmann, Michael Wilde, Brendan Clarke, Phyllis Illari, Michael P. Kelly, Charles Norrell, Federica Russo, and Jon Williamson. 2018. Evaluating Evidence of Mechanisms in Medicine: Principles and Procedures. Springer Briefs. Dordrecht: Springer.
- Parkkinen, Veli-Pekka, and Jon Williamson. 2017. “Extrapolating from Model Organisms in Pharmacology.” In Uncertainty in Pharmacology: Epistemology, Methods, and Decisions, edited by Adam La Caze and Barbara Osimani. Dordrecht: Springer.
- Reichenbach, Hans. 1956. The Direction of Time. 1971st ed. Berkeley and Los Angeles: University of California Press.
- Russo, Federica, and Jon Williamson. 2007. “Interpreting Causality in the Health Sciences.” International Studies in the Philosophy of Science 21: 157–170. doi: 10.1080/02698590701498084
- Russo, Federica, and Jon Williamson. 2011a. “Epistemic Causality and Evidence-based Medicine.” History and Philosophy of the Life Sciences 33: 563–582.
- Russo, Federica, and Jon Williamson. 2011b. “Generic Versus Single-case Causality: The Case of Autopsy.” European Journal for Philosophy of Science 1: 47–69. doi: 10.1007/s13194-010-0012-4
- Sackett, David L., William M. C. Rosenberg, J. A. Muir Gray, R. Brian Haynes, and W. Scott Richardson. 1996. “Evidence Based Medicine: What It Is and What It Isn't.” British Medical Journal 312: 71–72. doi: 10.1136/bmj.312.7023.71
- Salmon, Wesley C. 1998. Causality and Explanation. Oxford: Oxford University Press.
- Schaffer, Jonathan. 2000. “Causation by Disconnection.” Philosophy of Science 67: 285–300. doi: 10.1086/392776
- Smith, Gordon C. S., and Jill P. Pell. 2003. “Parachute Use to Prevent Death and Major Trauma Related to Gravitational Challenge: Systematic Review of Randomised Controlled Trials.” British Medical Journal 327: 1459–1460. doi: 10.1136/bmj.327.7429.1459
- Steel, Daniel. 2008. Across the Boundaries, Extrapolation in Biology and Social Science. Oxford: Oxford University Press.
- SUNY. 2004. “Suny Downstate Medical Center Evidence Based Medicine Tutorial.” The Medical Research Library of Brooklyn. http://library.downstate.edu/EBM2/contents.htm.
- Weber, Erik. 2007. “Social Mechanisms, Causal Inference, and the Policy Relevance of Social Science.” Philosophy of the Social Sciences 30: 348–359. doi: 10.1177/0048393107303814
- Weber, Erik. 2009. “How Probabilistic Causation Can Account for the Use of Mechanistic Evidence.” International Studies in the Philosophy of Science 23: 277–295. doi: 10.1080/02698590903197757
- Williamson, Jon. 2009. “Probabilistic Theories.” In The Oxford Handbook of Causation, edited by Helen Beebee, Christopher Hitchcock, and Peter Menzies, 185–212. Oxford: Oxford University Press.
- Williamson, Jon. 2015. “Deliberation, Judgement and the Nature of Evidence.” Economics and Philosophy 31: 27–65. doi: 10.1017/S026626711400039X
- Worrall, John. 2007. “Why There's No Cause to Randomize.” British Journal for the Philosophy of Science 58: 451–488. doi: 10.1093/bjps/axm024