ABSTRACT
Objectives: To compare the effect of time on cognitive impairments after Subarachnoid Haemorrhage and Traumatic Brain Injury and explore associations with baseline variables and global function.
Methods: Patients with a Glasgow Coma Scale score of 3–13, were assessed at 3, 6 and 12 months post injury by use of BNIS for cognitive impairment, RLAS-R to categorise cognitive and behavioural function, Barthel Index to assess performance of daily living, HADS to screen for depression and anxiety, and EuroQoL-5D, LiSat-11 and Glasgow Outcome Scale Extended to assess global function.
Results: BNIS T-scores did not differ significantly between groups and the proportion of patients with cognitive impairments was not significantly different at any time point. Cognition improved significantly between all time points in both groups except from 6 to 12 months after TBI. Generalised estimating equation showed non-significant signs of slower recovery of BNIS T-scores over time after SAH. Acute GCS scores were associated with BNIS T-scores after TBI but not after SAH. At 12 months, similar proportions of patients with SAH and TBI had good outcome.
Conclusions: Cognitive improvements after SAH and TBI exhibit similarities and correlate with global function. GCS scores are associated with outcome after TBI but not after SAH.
Background
Patients with non-traumatic subarachnoid haemorrhage (SAH) and moderate or severe, traumatic brain injury (TBI) often follow a similar pathway of care from neurointensive care units to rehabilitation services. While cognitive impairments and rehabilitation needs after TBI have been extensively studied, less is known for SAH.
Subarachnoid haemorrhage accounts for 5% of all strokes (Citation1) and 27% of all stroke-related years of potential life lost before the age of 65. The annual incidence of ruptured aneurysms, constituting 85% of all SAH (Citation2), rates from 9 in most European regions to 20 per 100 000 inhabitants in Finland (Citation3). Mortality rate is around 50% (Citation4). Of survivors, it has been estimated that 36–55% regain independence, defined as modified Rankin Scale (mRS) scores of 0–3, during the first year (Citation5).
Cognitive impairments are common after SAH and may impact on activities of daily living and quality of life (Citation6–Citation8). In a population-based interview survey of 230 participants at one year after SAH, 46% reported incomplete recovery and of these 50% reported memory problems (Citation9). No patient or disease characteristics that predicted good recovery were identified. In a prospective study of 32 patients, who were followed until12 months after SAH (Citation10), motor and psychomotor impairments recovered within 6 months while verbal memory did not improve significantly within this time period. Clinical parameters reflecting the impact of the bleed, as well as time on mechanical ventilation, were associated with neuropsychological outcome at one year (Citation10). However, even patients with severe SAH, presenting with a Hunt and Hess Grade V, may have a good recovery of cognitive function (Citation11). A recent multicentre study of 158 patients with aneurysmal SAH reported that cognitive impairment assessed by Montreal Cognitive Assessment (MoCA) early after the event correlated with functional outcome at 1 year but did not accurately predict functional outcome (Citation12).Thus, the need for further studies on the clinical course and prediction of long-term outcome after SAH remains (Citation13) as also highlighted in recent reviews (Citation14,Citation15). Impairments after SAH may depend not only on the primary lesion but also on aneurysm treatment method (Citation16,Citation17), on spasm related secondary ischemia (Citation18–Citation21) and other secondary insults (Citation22) and critical illness (Citation23,Citation24). In this respect and regarding the role of cognitive impairments for long term disability, SAH resembles TBI (Citation25). A comparison of the recovery of cognition after SAH and TBI may elucidate potentially common determinants of recovery and long-term outcome and support further prediction studies as well as rehabilitation planning.
The estimated total incidence rate of TBI in Europe ranges from 235 (Citation26) to 262 (Citation27) per 100.000 inhabitants and year. Most injuries are mild and the estimated proportion with moderate and severe TBI varies from around 2.5% to 29% (Citation27). There is a huge literature on cognitive function and outcome after TBI as illustrated by reviews on mild TBI (Citation28) and on moderate and severe TBI (Citation25) and prediction rules for moderate and severe TBI are evolving (Citation29–Citation31). Outcome is most often categorised by use of the Glasgow Outcome Scale (GOS) or the extended version (GOSE) (Citation32).
Some recent studies have applied the Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS) (Citation33) to screen for cognitive and affective disturbances after acquired brain injury. BNIS has documented validity (Citation34–Citation36), is easily applicable in clinical routine and has been used in some studies of TBI (Citation36–Citation38) as well as stroke (Citation39,Citation40). A recent study of patients with severe TBI with BNIS reported that most improvement occurred during the first three months after the injury while cognition was fairly stable between 3 and 12 months (Citation41).
The primary aim of our study was to compare the course and outcome of cognitive impairments by use of BNIS during the first year after non-traumatic SAH with corresponding data from patients with moderate and severe TBI admitted to the same neurointensive care unit at one university hospital and exposed to a similar neurorehabilitation and follow up program. A secondary aim was to explore relations between baseline characteristics and outcomes.
Materials and methods
Study design and participants
This was a prospective, observational study of patients with non-traumatic, aneurysmal subarachnoid haemorrhage and after moderate or severe traumatic brain injury. Study design has been described previously (Citation42,Citation43). In brief, inclusion required a SAH due to a ruptured aneurysm or moderate or severe TBI, a lowest GCS score during the first day after the event of 3–13, age ≥ 18 years, living in the Stockholm region and obtained informed consent. For patients, who were unconscious or otherwise unable to give informed consent, the closest relative was asked.
Patients were included at the neurointensive care unit (NICU) at the Department of Neurosurgery at Karolinska University Hospital from March 2009 until June 2012. Inclusion was not performed during holidays for logistic and administrative reasons.
After inclusion, acute (clinical and radiological parameters) and socioeconomic data were attained from medical records and via interview. Patients were assessed at three time points, 3, 6 and 12 months post injury/illness at the Department of Rehabilitation Medicine at Danderyd University Hospital, Stockholm, Sweden. Assessments included clinical examination and a battery of standardised assessment instruments and were performed by the same, experienced assessor (AT). In patients unable to communicate, the presence of a possible Disorder of Consciousness was evaluated by clinical examination and with the Coma Recovery Scale Revised.
The study was approved by the Regional Ethics Committee of Stockholm, Sweden (No: 2008/3:9 2008/1574–31/3) and complies with the Strobe checklist for observational studies.
Severity grading
In addition to the GCS score (Citation44) (3–8 severe injury, 9–13 moderate injury), clinical severity in patients with SAH severity was graded according to the Hunt-Hess scale (HH) (Citation45). The CT lesion after SAH was graded according to Fisher scale (FS) (Citation46). Aneurysms were verified by computed tomography angiography (CTA) or digital subtraction angiography (DSA). Aneurysms were divided into aneurysm from the anterior cerebral circulation and the posterior cerebral circulation. Computed tomography (CT) lesion (Citation46) grading of TBI was according to the CRASH model (Citation47) with addition of brain oedema, basilar skull fractures and facial fracture data. Coma Recovery Scale Revised (CRS-R) (Citation48) was used to assess disorders of consciousness (DOC) (Citation49).
Cognitive and affective function
The Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS) (Citation33) was used to screen cognitive and affective disturbances. The BNIS starts with three pre-screen items that assess level of arousal yielding maximally 3 points (p), basic communication level (3 p) and level of cooperation (3 p), to assess whether the patient is capable of participating in further assessment. The patients must achieve at least two points on each of the items to continue with items that assess the range of higher cerebral functions: speech and language (15 p), orientation (3 p), attention/concentration (3 p), visual and visuospatial problem solving (8 p), memory (7 p), affect (4 p) and awareness of own performance (1 p), corresponding to a total score of maximally 50 (sum of pre-screen and the 7 subscale scores). Total BNIS raw scores are converted to age-corrected standard Τ-points. Higher scores indicate better functioning. A cut-off score of < 47 was set for identifying brain dysfunction for patients < 60 years, < 46 for patients 60–69 years and ≤ 43 for patients > 70 years (Citation35,Citation36,Citation50). BNIS has good sensitivity (92%) to brain dysfunction and acceptable specificity (56%) (Citation34). In a Swedish study the sensitivity was 88% and the specificity 78% (Citation36). Cut-off for cognitive dysfunction for T-points was set at < 40 (i.e. <−1SD) (Citation51).
Rancho Los Amigos Cognitive Scale-Revised (RLAS-R) (Citation52) was used to categorise level of cognitive and behavioral function. RLAS-R is a clinical scale with scores from 1 to 10, describing 10 stages of recovery after brain injury by assessing responsiveness to stimuli, ability to follow commands, presence of non-purposeful behavior, cooperation, confusion, attention to environment, verbal ability, memory, orientation and higher cognitive ability. RLAS-R levels were dichotomised into ‘inferior functioning’ (RLAS-R 1–8) and ‘superior functioning’ (RLAS-R 9–10).
The Hospital Anxiety and Depression Scale (HADS) (Citation53) was used to screen for depression and anxiety. HADS comprises 14 items (7 items in each subscale) which are assessed on a four point scale (range 0–3), where the total score is the sum of each subscale (range 0–21). Severity was classified using the two subscales as normal (0–Citation7), mild (Citation8–Citation10), moderate (Citation11–Citation14) or severe (Citation15–Citation21,Citation54).
Activities of daily living
The Barthel Index (BI) was used to measure performance in activities of daily living (ADL) (Citation55). The BI scale allow evaluation of functional independence in 10 activities of daily and yields a sum score from 0 to 100. BI was dichotomised into two categories ‘dependence’ (BI 0–95) and ‘independence’ (BI 96–100).
Global measures
Global outcome and independence was assessed by use of the Glasgow Outcome Scale Extended (GOSE) (Citation56,Citation57). GOSE has demonstrated good validity interrater reliability when applied by standardised interview (Citation32,Citation58).The eight categories span from ‘Dead’ (score 1) to ‘Upper Good Recovery’ (score 8). GOSE scores were dichotomised into ‘unfavourable outcome (GOSE 1–4) and ‘favourable outcome’ (GOSE 5–8).
Quality of life (QoL) was assessed by use of the EuroQoL 5D (EQ-5D) (Citation59). EQ-5D index estimates health in five dimensions and each dimension is scored as one of three levels allowing for 243 permutations of unique health states and are revised into an index with a range from −0.594 to 1, with 1.00 indicating full health. The EQ-5D also includes a visual analogue scale (VAS) ranging from 0 to 100.
Satisfaction with life was assessed by use of the 11-item version of Life Satisfaction Questionnaire (LiSat-11) (Citation60,Citation61). The LiSat-11 is a generic self-reported checklist comprising an estimate of satisfaction with life as a whole as well as for 10 specific domains. Each question is scored on a six-grade ordinal scale. Item scores was dichotomised into unsatisfied (Citation1–Citation4) and satisfied (Citation5,Citation6) to report results on item level.
Statistics
Statistical analysis was performed using IBM SPSS Statistics version 22 (IBM Corporation, Armonk, New York, USA). This observational study reports descriptive data with central measures (mean, median or percent) and measures of spread (SD, percentile, min-max). Nonparametric methods were used as data were not normally distributed according to Shapiro-Wilk’s test of normality. Wilcoxon signed ranks test was used for analysis of BNIS T-scores, RLAS-R levels, EQ-5D index, EQ-5D VAS, Barthel Index, HADS depression and anxiety over time. The Z-test for proportions was used to for analyses of populations’ proportion. Friedmans test was used for comparisons of LiSat.
The Mann-Whitney U test was used for non-response analysis with respect to these variables: age and GCS. The chi-squared test was used for non-response analysis with respect to gender. Generalised estimating equation (GEE) was used to explore the effect of time on cognitive function according to BNIS. The Spearman correlation coefficient was used for the analysis of bivariate correlation between BNIS and GCS, HH, FS, gender, age, GOSE, RLAS, HADS, BI and LiSat-11. In all cases, significance level was set at p < 0.05.
Results
Study population
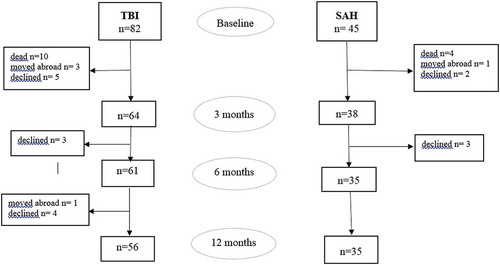
Included were 127 patients, 45 with SAH and 82 with TBI. Of these, 10 patients with SAH and 26 patients with TBI dropped out due to the following reasons. Four patients with SAH and 10 with TBI died before 3 months follow up. Five patients with SAH and 12 with TBI declined further participation in the study. One patient with SAH and 4 with TBI moved abroad. For patients with SAH or TBI who dropped out there was no significant difference in gender (p = 0.095/p = 0.121), age (p = 0.280/p = 0.094) or GSC (p = 0.205/p = 0.073). A flowchart and drop-out of patients with SAH and TBI are presented in .
Demographical and clinical characteristics
Of the 35 patients with SAH, 27 were women and 8 men, mean age was 57.4 ± 9.9 years, 22 patients had severe and 13 patients a moderate brain injury according to the admission GCS score (mean GCS score 7.9 ± 4.2). GCS scores for patients with SAH and TBI respectively were not significantly different (p = 0.144). For patients with SAH, most frequent Hunt & Hess scores at admission were 4 and 3 and all had SAH visible on CT scan (Fisher grades 2–4). Out of 56 patients with TBI, 15 were women and 41 men, mean age was 47.1 ± 16.6 years, 44 patients had a severe brain injury and 12 patients a moderate brain injury according to the admission GCS score (mean GCS score 6.3 ± 2.9). Data are displayed in .
Table 1: Baseline data of patients with subarachnoid haemorrhage and traumatic brain injury.
BNIS
Equally large proportions of patients with SAH and TBI could perform the BNIS at three months [25/35 (71%) of patients with SAH and 41/56 (73%) with TBI, p = 0.853], at six months [28/34 (82%) and 43/54 (80%) p = 0.753] and at 12 months [30/35 (86%) and 46/56 (82%) p = 0.655] and equally large proportions failed pre-screen at 3 months [10/35 (29%) of patients with SAH and 15/56 (27%) with TBI, p = 0.853], 6 months [6/34 (18%) and 11/54 (20%), p = 0.753] and 12 months [5/35 (14%) and 10/56 (18%), p = 0.655]. Numbers and proportions of patients who performed BNIS, of those who did not pass the prescreen and of patients who were not able to be prescreened due to ongoing disorders of consciousness are presented in .
Table 2: BNIS T-points results at 3, 6 and 12 months after SAH and TBI.
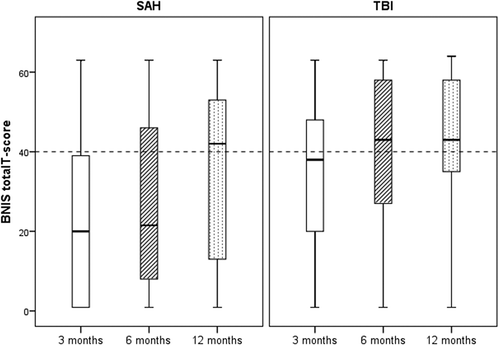
After SAH, 19/25 (76%) of those who performed the test, scored below cut-off for cognitive dysfunction (T-points < 40 or < −1 SD) at three months, 19/27 (70%) at six months and 17/29 (59%) at 12 months. After TBI, 26/41 (63%) of those who performed the test, scored below cut-off for cognitive dysfunction at 3 months, 23/43 (54%) at six months and 15/42 (36%) at 12 months. Differences in proportions of patients with SAH and TBI below the cut-off were not significant at 3 (p = 0.287), 6 (p = 0.160), or 12 (p = 0.057) months post event. The distribution of patients with SAH and TBI above and below the cut-off for cognitive dysfunction are presented in .
BNIS data could be collected at all three time points from 22 patients with SAH and 37 patients with TBI and these data were used for the analyses of change over time.
Change of BNIS over time
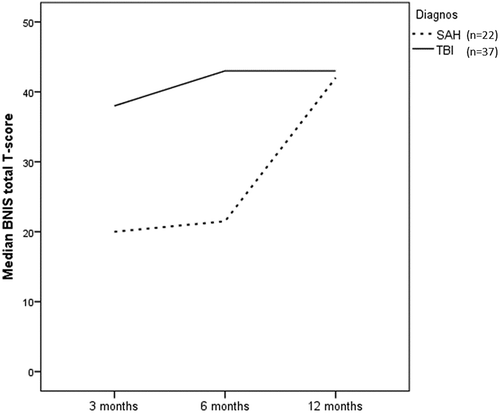
After SAH, median BNIS T-scores improved significantly from 3 months to 6 months (p = 0.017), from 6 months to 12 months (p = 0.010) and from 3 months to 12 months (p = 0.004). After TBI, BNIS T-scores improved significantly from 3 months to 6 months (p = 0.003) and from 3 months to 12 months (p = 0.001), while the improvement from 6 months to 12 months was not significant (p = 0.065). BNIS T-scores at 3, 6 and 12 months after SAH and TBI are presented in .
Figure 2: BNIS T-scores at 3, 6 and 12 months after SAH (n=22) and TBI (n=37). Dashed line illustrates cut-off level for cognitive dysfunction.
Median BNIS T-scores were lower after SAH than after TBI but the difference was not significant between the groups at 3 months (p = 0.064), 6 months (p = 0.069) or 12 months (p = 0.148) post event. Change of BNIS T-scores over time from 3 to 12 months according to GEE was not significantly different for SAH and TBI (p = 0.591), as illustrated in .
BNIS subscales
In the analyses of BNIS subscales, the age groups 60–69 and 70–87 years were merged due to the low number of BNIS data in the oldest age group (n = 1–2).
Changes between 3 and 6 months: After SAH, in age group 18–59 years, scores improved significantly for language (p = 0.020), memory (p = 0.004), affect (p = 0.034) and awareness (p = 0.046), while no significant changes were observed in the older age group. After TBI, improvements during the same period were observed in the age group 60–87 years for orientation (p = 0.034), but not in the younger age group.
Changes between 6 and 12 months: After TBI, scores improved significantly for memory (p = 0.028) and awareness (p = 0.034) in age group 18–59 years, but not in the older age group or in either age group after SAH.
Changes between 3 and 12 months: After SAH, significant improvement was observed in patients aged 18–59 years for language (p = 0.020), for orientation
(p = 0.038), memory (p = 0.003), and awareness (p = 0.014), while no significant changes were observed in the older age group. In patients with TBI during the same period, awareness (p = 0.011), and language (p = 0.031) improved in the younger age group while no significant changes were observed in the older age group
BNIS by injury severity, gender and years of education
When data at all three time points were merged, BNIS-T-scores were significantly correlated with acute GCS for TBI (r = 0.453, p < 0.001), but not for SAH (r = 0.045, p = 0.720). Similar associations were observed for data at 3 months (r = 0.440, p = 0.006), 6 months (r = 0.447, p = 0.006), and 12 months (r = 0.498, p = 0.002) after TBI but not for SAH (3 months, r = 0.123, p = 0.585; 6 months, r = 0.070, p = 0.758; 12 months, r = - 0.082, p = 0.716).
Patients with severe SAH had a significant improvement in BNIS T-score between 3 and 12 months (p = 0.037), but not between 3 and 6 months (p = 0.110) or between 6 and 12 months (p = 0.065). Patients with moderate SAH had a significant improvement in BNIS T-score between 3 and 6 months (p = 0.046) and 3 and 12 months (p = 0.028), but not between 6 and 12 months (p = 0.068).
Patients with severe TBI had a significant improvement in BNIS T-score between 3 and 12 months (p = 0.008), and between 3 and 6 months (p = 0.019) but not between 6
and 12 months (p = 0.119). Patients with moderate TBI had a significant improvement in BNIS T-score between 3 and 12 months (p = 0.017), but not between 3 and 6 months (p = 0.079) or between 6 and 12 months (p = 0.362).
BNIS-T-scores after SAH were not related to Hunt-Hess scale scores (r = - 0.117, p = 0.35) or Fischer scale scores (r = - 0.149, p = 0.233).
BNIS-T-scores were negative correlated to age (r = - 0.450, p < 0.001) for TBI but not for SAH (r = 0.029, p = 0.817). After TBI, this was observed for BNIS results at 3 (r = - 0.493, p = 0.002), 6 (r = - 0.515, p = 0.001) and 12 months (r = - 0.384, p = 0.019) but not after SAH at 3 (r = 0.142, p = 0.529), 6 (r = 0.046, p = 0.839) or 12 months (r = - 0.139, p = 0.537).
There was no relation between BNIS-T-scores and gender in patients with SAH (p = 0.169) or TBI (p = 0.400).
Patients with more than 12 years of education had higher BNIS T-scores at 3, 6 and 12 months after SAH and after TBI when compared with patients with less than 12 years of education, but the differences were nonsignificant.
RLAS
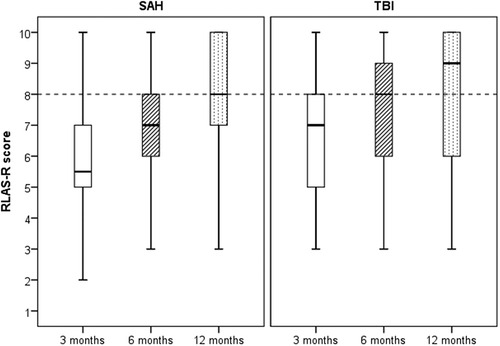
RLAS-R improved significantly after SAH from 3 months to 6 months (p < 0.001), from 6 months to 12 months (p = 0.001) and from 3 months to 12 months (p < 0.001).
RLAS-R improved significantly after TBI from 3 months to 6 months (p < 0.001), from 6 months to 12 months (p < 0.001) and from 3 months to 12 months (p < 0.001).
At 12 months, 21/35 (60%) patients with SAH had ‘inferior cognitive functioning’ and 14/35 (40%) had ‘superior cognitive functioning’; 25/56 (45%) patients with TBI had ‘inferior cognitive functioning’ and 31/56 (55%) ‘superior cognitive functioning’. The proportion of patients with ‘superior cognitive functioning’ at 12 months was not significantly less after SAH than after TBI (p = 0.154). RLAS data are presented in and .
Table 3: Outcome measured with RLAS-R 3, 6 and 12 months post SAH and TBI.
We found strong correlations between BNIS T-scores and RLAS-R at 3 (r = 0.890, p < 0.001), 6 (r = 0.827, p < 0.001), and 12 (r = 0.750, p < 0.001) months after SAH and at 6 (r = 0.752, p < 0.001), and 12(r = 0.716, p < 0.001) months after TBI, and moderate correlation at 3 (r = 0.698, p < 0.001) months after TBI.
HADS
No statistically significant differences were found for HADS depression or anxiety scores between the consecutive time points or between 3 and 12 months after SAH or TBI. The proportion of patients who fulfilled criteria for mild or moderate depression at 12 months was 7/30 (23%) after SAH and 9/41 (22%) after TBI. The proportion of patients who fulfilled criteria for mild, moderate or severe anxiety at 12 months was 6/30 (20%) after SAH group and 12/41 (29%) after TBI.
After TBI, there were weak correlations between BNIS T-scores both HADS scores for depression at 3 (r = - 0.506, p = 0.002) and 12 months (r = - 0.396, p = 0.018), and for anxiety (r = - 0.446, p = 0.006 at 3 months and r = - 0.412, p = 0.014 at 12 months), but not at 6 months. We found no correlations between BNIS T-scores and HADS depression and anxiety at 3, 6 and 12 months after SAH.
Barthel index
Barthel Index improved significantly after SAH from 3 months to 6 months (p < 0.008) and from 3 months to 12 months (p < 0.001), but non-significantly from 6 months to 12 months (p = 0.066). BI improved significantly after TBI from 3 months to 6 months (p = 0.001) and from 6 months to 12 months (p = 0.017) and from 3 months to 12 months (p = 0.001). At 12 months 21/35 (60%) patients with SAH and 33/56 (59%) with TBI are totally independent (p = 0.919).
After SAH, there was a strong correlation between BNIS T-scores and BI at 6 months (r = 0.729, p < 0.001) and moderate at 3 (r = 0.623, p = 0.002) and 12 months (r = 0.678, p = 0.001). After TBI, there was moderate correlation between BNIS T-scores and BI at 12 months (r = 0.565, p < 0.001) and a weak correlation at 3 (r = 0.441, p = 0.006) and 6 months (r = 0.474, p = 0.003).
GOSE
GOSE scores improved significantly from 3 months to 6 months (p < 0.001), from 6 months to 12 months (p = 0.001) and from 3 months to 12 months (p < 0.001) after SAH, and from 3 months to 6 months (p < 0.001), from 6 months to 12 months (p = 0.019) and from 3 months to 12 months (p < 0.001) after TBI.
At 12 months, 13/35 (37%) of patients with SAH had a bad outcome and 22/35 (63%) had a good outcome; 21/56 (38%) of patients with TBI had a bad outcome and 35/56 (62%) had a good outcome. These differences were not significant (p = 0.973).
GOSE data at 3, 6 and 12 months after SAH and TBI are presented in .
Figure 5: GOSE scores at 3, 6 and 12 months after SAH (n=34) and TBI (n=52). Dashed line illustrates lower cut-off level for unfavorable outcome.
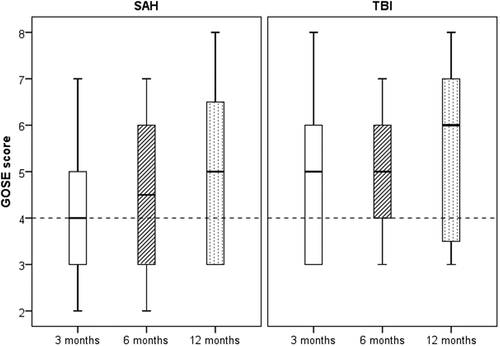
After SAH, there was a strong correlation between BNIS T-scores and GOSE for SAH at 3 (r = 0.771, p < 0.001), 6 (r = 0.846, p < 0.001) and 12 (r = 0.751, p < 0.001) months. After TBI, there was a moderate correlation between BNIS T-scores and GOSE at 3 (r = 0.644, p < 0.001), 6 (r = 0.592, p < 0.001) and 12 (r = 0.694, p < 0.001) months.
LiSat-11
At 3 months, 13/24 (54%) patients with SAH and 14/38 (37%) with TBI were satisfied or very satisfied with life (‘as a whole’), while 11/24 (46%) patients with SAH and 24/38 (63%) with TBI reported different degrees of dissatisfaction. At 6 months 12/28 (43%) patients with SAH and 17/43 (40%) with TBI were satisfied or very satisfied with life, while 16/28 (57%) patients with SAH and 26/43 (60%) with TBI reported different degrees of dissatisfaction.
At 12 months 13/30 (43%) patients with SAH and 17/44 (39%) with TBI were satisfied or very satisfied with life, while 17/30 (57%) patients with SAH and 27/44 (61%) with TBI reported different degrees of dissatisfaction. Changes were not significant between 3 [SAH median 5, Q1-Q3 (Citation3–Citation6), TBI median 4, Q1-Q3 (Citation4,Citation5)], 6 [SAH median 4, Q1-Q3 (Citation3–Citation5), TBI median 4, Q1-Q3 (Citation4,Citation5)], and 12 months [SAH median 4, Q1-Q3 (Citation4–Citation6), TBI median 4, Q1-Q3 (Citation3–Citation5)] after SAH (p = 0.185) or TBI (p = 0.730), as well as satisfaction in the 10 specific domains. There was no correlation between BNIS T-scores and LiSat 11 for SAH or TBI at 3, 6 or 12 months post event.
EQ-5D
EQ-5D index improved significantly after TBI from 3 (mean 0.623 ± 0.281) months to 12 (mean 0.708 ± 0.250) months (p = 0.034), but not between the other time points after TBI or SAH. EQ-VAS improved significantly after SAH from 3 (mean 64.05 ± 20.1) months to 12 (mean 76.05 ± 15.5) months (p = 0.007), but not between the other time points after TBI or SAH.
Discussion
The main findings of this prospective study are that cognitive function is still impaired in most patients at three months after moderate and severe TBI and SAH but improves in both groups until one year after the event. Improvements were significant in both groups and cognitive or global outcome at 12 months was not significantly different between groups. BNIS data exhibited strong correlations to other measures of cognitive (RLAS) and global function including GOSE. While initial GCS scores, as expected, correlated with BNIS at 12 months after TBI, this was not so for either the initial GCS or the Hunt and Hess scores after SAH.
Change of BNIS T-scores over time
Median BNIS T-scores improved significantly both early and late after SAH while improvements after TBI were significant only during the early (3–6 months) time interval. This indication of differential recovery rates between the diagnostic groups is also supported by the GEE analysis. Stratifying BNIS data by injury severity and considering that the majority of patients in both diagnostic group had a severe injury. This finding seems mainly to reflect differential recovery rates in patients with severe injuries, i.e. that recovery after severe SAH is delayed when compared with severe TBI. However, regarding the small sample sizes, this interpretation must be cautious and need to be confirmed in larger study groups.
The observation that most improvement of cognitive function according to BNIS occurs within the first 6 months after TBI is in agreement with the findings in a study of patients with severe TBI where BNIS scores improved most until 3 months after the injury [41]. However, it should be pointed out that both clinical experience and previous studies (Citation60) show that cognitive improvements may proceed even years after TBI but these may require longer observation periods and/or comprehensive neuropsychological assessment to be captured. However, since BNIS was used in both our diagnostic groups, the observation that recovery after severe SAH is delyed when compared to severe TBI, deserves further attention. Several factors may explain a differential time line of recovery, including different patterns of the primary brain pathology and secondary insults and of related differential induction and character of beneficial neuroplasticity processes. Even though the primary pathology after SAH and TBI overlaps, diffuse axonal injury is a hallmark of TBI, as recently reviewed by van Eijck et al. (Citation63), while cerebral infarction plays a key role for outcome after subarachnoid haemorrhage (Citation20).
Associations between cognitive function and gender, age, or injury severity scores
We did not see an association to gender in either the SAH or the TBI groups. Age correlated significantly to BNIS T scores after TBI but not after SAH.
While initial GCS scores were associated with better cognitive outcome after TBI, this was not the case after SAH, neither was there any association between cognitive recovery and Hunt and Hess scores. These observations indicate that other acute variables may be more relevant for prediction of cognitive outcome after SAH. It should be pointed out that the GCS was introduced as ‘a practical scale’ in 1974 to standardise the recording of levels of impaired consciousness [44]. Since then, the scale has been validated and extensively used in clinical research and practice mainly for patients with TBI (Citation64), but also for other patients including those with SAH, as has also been recommended (Citation65). Further, we found no correlation between Fisher data and BNIS after SAH, which is in accordance with findings in previous study by Wong et al. (Citation12).
Anxiety and depression are potential confounders of cognitive performance. However, in our study sample, HADS anxiety or depression scores did not change significantly between the consecutive time points or over the total observation period from 3 to 12 months after SAH or TBI and we observed no obvious correlations between BNIS T scores and HADS scores. At 12 months, approximately one fifth of patients in both groups fulfilled criteria for depression or anxiety, which is in accordance with previous studies (Citation66–Citation67).
Change of BNIS subscales over time
Most frequent impairments were observed for awareness and orientation. Deviations were less pronounced or frequent for language and memory, which were only observed in younger SAH and TBI patients. Comparison of scores of BNIS subscales at the different time points, showed significant improvements in our study on some subscales from 3 months to 12 months post event. However, frequencies varied by age and diagnostic groups and over time, which hampered conclusions on the relative impact of time on different cognitive domains.
Relation between BNIS t scores and global function over time
There was a strong correlation between BNIS T-scores and GOSE scores after both SAH and TBI. These findings agree with the findings by Wong et al. (Citation12), who reported strong associations between cognition, according to Montreal Cognitive Assessment, and excellent outcome after aneurysmal SAH. In our study, we also found strong or moderate correlations between BNIS T-scores and RLAS-R at 12 months in both diagnostic groups.
Changes in life satisfaction according to LiSat were not significant over the study period after SAH or TBI, which is in agreement with the findings in a previous study of patients with severe TBI [41], and were not correlated to BNIS. It may be assumed that these findings reflect the complexity of ‘life satisfaction’ according to LiSat 11, which reflects both cognitive, emotional and aspirational factors (Citation60) and is related to sociodemographic, health and physical activity parameters (Citation63).
EQ-5D data improved significantly in both diagnostic groups from 3 to 12 months in both diagnostic groups and EQ-VAS improved significantly from 3–12 months after SAH. These findings are in agreement with previous reports on quality of life after TBI and SAH (Citation69,Citation70).
Study limitations and strengths
This study included patients from only one university hospital managed by the same heath care providers. While this, as well as the sample size, might limit generalisability, it also reduces potential effects of divergent follow-up and rehabilitation interventions. Further, we were not able to apply BNIS earlier than at three months after the event. Even though this is a limitation, the proportion of patients unable to pass the pre-screen would probably have been higher closer to the event. The study protocol did not include comprehensive neuropsychological assessment. This was due both to limited study resources and to minimise the risk for fatigue during extended assessment sessions. BNIS allows rapid and valid assessment of a broad range of higher cognitive functions (Citation34) and enabled us to follow the time course in the whole study sample.
Strengths of this study are the low dropout rate and that all non-acute assessments were performed by the same person (AT) even though the risk for systematic bias of scoring must be considered. However, most assessments are highly standardised and the assessor was aware of this risk, why we consider the risk low.
Conclusions
Significant improvements in cognition occurred from 3 to 12 months after both SAH and TBI. Adjusting for injury severity, relatively more improvement occurred from 6 to 12 months after severe SAH than after severe TBI, but outcomes at 12 months were not significantly different between the groups. We suggest that even though the SAH and TBI brain pathology overlaps, the different recovery rates mainly reflect differences of the primary pathology and neuroplasticity processes. While GCS scores were associated with outcome after TBI, neither GCS or Hunt and Hess scores were associated with 12 months’ outcome after SAH, indicating that other acute severity variables should be used for prediction. Cognitive function according to BNIS correlated with cognitive function according to RLAS and with global outcome according to GOSE.
This exploratory study adds information on cognitive impairments until one year after moderate or severe SAH or TBI and may support rehabilitation planning. Data indicate a need for both early and late interventions to support cognitive recovery. Data may also support further attempts to identify predictor variables for cognitive outcome after SAH.
Competing interests
Authors have no competing interests to declare.
Additional information
Funding
Notes on contributors
Anna Tölli
All authors contributed to the design data collection, analyses and reporting of this study.
References
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. Int Stroke Incidence Collab Stroke. 1997;28(3):491–99. Epub 1997/ 03/01.PubMed PMID: 9056601.
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet (London, England). 2007;369(9558):306–18. Epub 2007/ 01/30. PubMed PMID: 17258671. doi:10.1016/s0140-6736(07)60153-6.
- de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78(12):1365–72. Epub 2007/ 05/02. PubMed PMID: 17470467; PubMed Central PMCID: PMC2095631. doi:10.1136/jnnp.2007.117655.
- Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28(3):660–64. Epub 1997/ 03/01.PubMed PMID: 9056628.
- Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635–42. Epub 2009/ 06/09. PubMed PMID: 19501022. doi:10.1016/s1474-4422(09)70126-7.
- Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10(4):349–56. Epub 2011/03/26. PubMed PMID: 21435599. doi:10.1016/s1474-4422(11)70017-5.
- Mayer SA, Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, Du YE, Connolly ES Jr. Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology. 2002;59(11):1750–58. Epub 2002/12/11.PubMed PMID: 12473764.
- Wong GK, Lam SW, Wong A, Lai M, Siu D, Poon WS, Mok V. MoCA-assessed cognitive function and excellent outcome after aneurysmal subarachnoid hemorrhage at 1 year. Eur J Neurol. 2014;21(5):725–30. Epub 2014/01/30. PubMed PMID: 24471651. doi:10.1111/ene.12363.
- Hackett ML, Anderson CS. Health outcomes 1 year after subarachnoid hemorrhage: an international population-based study. The Australian cooperative research on subarachnoid hemorrhage study group. Neurology. 2000;55(5):658–62. Epub 2000/ 09/12. PubMed PMID: 10980729.
- Haug T, Sorteberg A, Sorteberg W, Lindegaard KF, Lundar T, Finset A. Cognitive outcome after aneurysmal subarachnoid hemorrhage: time course of recovery and relationship to clinical, radiological, and management parameters. Neurosurgery. 2007;60(4):649–56. discussion 56-7. Epub 2007/ 04/07. PubMed PMID: 17415201. doi:10.1227/01.neu.0000255414.70807.a0.
- Haug T, Sorteberg A, Finset A, Lindegaard KF, Lundar T, Sorteberg W. Cognitive functioning and health-related quality of life 1 year after aneurysmal subarachnoid hemorrhage in preoperative comatose patients (Hunt and Hess Grade V patients). Neurosurgery. 2010;66(3):475–84. discussion 84-5. Epub 2010/ 02/04. PubMed PMID: 20124932. doi:10.1227/01.neu.0000365364.87303.ac.
- Wong GK, Lam SW, Wong A, Mok V, Siu D, Ngai K, Poon WS. Early MoCA-assessed cognitive impairment after aneurysmal subarachnoid hemorrhage and relationship to 1-year functional outcome. Transl Stroke Res. 2014;5(2):286–91. Epub 2013/ 12/11. PubMed PMID: 24323708. doi:10.1007/s12975-013-0284-z.
- Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–36. Epub 2010/07/03. PubMed PMID: 20595669. doi:10.1161/strokeaha.110.581975.
- Jaja BN, Cusimano MD, Etminan N, Hanggi D, Hasan D, Ilodigwe D, Lantigua H, Le Roux P, Lo B, Louffat-Olivares A, et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: a systematic review. Neurocrit Care. 2013;18(1):143–53. Epub 2012/ 11/10. PubMed PMID: 23138544. doi:10.1007/s12028-012-9792-z.
- Lo BW, Fukuda H, Nishimura Y, Farrokhyar F, Thabane L, Levine MA. Systematic review of clinical prediction tools and prognostic factors in aneurysmal subarachnoid hemorrhage. Surg Neurol Int. 2015;6:135. Epub 2015/09/01. PubMed PMID: 26322245; PubMed Central PMCID: PMC4544120. doi:10.4103/2152-7806.162676.
- Frazer D, Ahuja A, Watkins L, Cipolotti L. Coiling versus clipping for the treatment of aneurysmal subarachnoid hemorrhage: a longitudinal investigation into cognitive outcome. Neurosurgery. 2007;60(3):434–41. discussion 41-2. Epub 2007/ 03/01. PubMed PMID: 17327787. doi:10.1227/01.neu.0000255335.72662.25.
- Li ZQ, Wang QH, Chen G, Quan Z. Outcomes of endovascular coiling versus surgical clipping in the treatment of ruptured intracranial aneurysms. J Int Med Res. 2012;40(6):2145–51. Epub 2013/ 01/17. PubMed PMID: 23321171. doi:10.1177/030006051204000612.
- Haley EC Jr., Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86(3):467–74. Epub 1997/ 03/01. PubMed PMID: 9046304. doi:10.3171/jns.1997.86.3.0467.
- Longstreth WT Jr., Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43(4):712–18. Epub 1993/ 04/01.PubMed PMID: 8469328.
- Kumar A, Brown R, Dhar R, Sampson T, Derdeyn CP, Moran CJ, Diringer MN. Early vs. delayed cerebral infarction after aneurysm repair after subarachnoid hemorrhage. Neurosurgery. 2013;73(4):617–23. discussion 23. Epub 2013/06/22. PubMed PMID: 23787882. doi:10.1227/neu.0000000000000057.
- Naidech AM, Bendok BR, Bassin SL, Bernstein RA, Batjer HH, Bleck TP. Classification of cerebral infarction after subarachnoid hemorrhage impacts outcome. Neurosurgery. 2009;64(6):1052–57. discussion 7-8. Epub 2009/06/03. PubMed PMID: 19487883. doi:10.1227/01.neu.0000343543.43180.9c.
- Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34(3):617–23. quiz 24. Epub 2006/03/08. PubMed PMID: 16521258.
- Wolters AE, Slooter AJ, van der Kooi AW, van Dijk D. Cognitive impairment after intensive care unit admission: a systematic review. Intensive Care Med. 2013;39(3):376–86. Epub 2013/ 01/19. PubMed PMID: 23328935. doi:10.1007/s00134-012-2784-9.
- Nedergaard HK, Jensen HI, Toft P. Interventions to reduce cognitive impairments following critical illness: a topical systematic review. Acta Anaesthesiol Scand. 2017;61(2):135–48. Epub 2016/ 11/24. PubMed PMID: 27878815. doi:10.1111/aas.12832.
- Cristofori I, Levin HS. Traumatic brain injury and cognition. Handb Clin Neurol. 2015;128:579–611. Epub 2015/02/24. PubMed PMID: 25701909. doi:10.1016/b978-0-444-63521-1.00037-6.
- Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien). 2006;148(3):255–68. discussion 68. Epub 2005/ 11/29. PubMed PMID: 16311842. doi:10.1007/s00701-005-0651-y.
- Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, Maas AI. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015;157(10):1683–96. Epub 2015/08/14. PubMed PMID: 26269030; PubMed Central PMCID: PMC4569652. doi:10.1007/s00701-015-2512-7.
- Carroll LJ, Cassidy JD, Cancelliere C, Cote P, Hincapie CA, Kristman VL, Holm LW, Borg J, Nygren-de Boussard C, Hartvigsen J. Systematic review of the prognosis after mild traumatic brain injury in adults: cognitive, psychiatric, and mortality outcomes: results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. 2014;95(3 Suppl):S152–73. Epub 2014/ 03/04. PubMed PMID: 24581903. doi:10.1016/j.apmr.2013.08.300.
- Maas AI, Lingsma HF, Roozenbeek B. Predicting outcome after traumatic brain injury. Handb Clin Neurol. 2015;128:455–74. Epub 2015/02/24. PubMed PMID: 25701901. doi:10.1016/b978-0-444-63521-1.00029-7.
- Perel P, Arango M, Clayton T, Edwards P, Komolafe E, Poccock S, Roberts I, Shakur H, Steyerberg E, Yutthakasemsunt S. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ (Clinical Research Ed). 2008;336(7641):425–29. Epub 2008/02/14. PubMed PMID: 18270239; PubMed Central PMCID: PMC2249681. doi:10.1136/bmj.39461.643438.25.
- Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165. discussion e. Epub 2008/ 08/08. PubMed PMID: 18684008; PubMed Central PMCID: PMC2494563. doi:10.1371/journal.pmed.0050165.
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85. Epub 1998/ 09/03.PubMed PMID: 9726257. doi:10.1089/neu.1998.15.573.
- Prigatano GP. Screen for higher cerebral functions: rationale and initial validation. BNI Quarterly. 7(1):2–9, 1991.
- Prigatano GPAK, Rosenstein LD. Validity studies on the BNI screen for higher cerbral functions. BNI Quarterly. 1993;9(1):2–9.
- Denvall V, Elmstahl S, Prigatano GP. Replication and construct validation of the barrow neurological institute screen for higher cerebral function with a Swedish population. J Rehabil Med. 2002;34(4):153–57. Epub 2002/08/31.PubMed PMID: 12201609.
- Hofgren C, Esbjornsson E, Aniansson H, Sunnerhagen KS. Application and validation of the barrow neurological institute screen for higher cerebral functions in a control population and in patient groups commonly seen in neurorehabilitation. J Rehabil Med. 2007;39(7):547–53. Epub 2007/08/29. PubMed PMID: 17724554. doi:10.2340/16501977-0085.
- Borgaro SR, Prigatano GP. Early cognitive and affective sequelae of traumatic brain injury: a study using the BNI screen for higher cerebral functions. J Head Trauma Rehabil. 2002;17(6):526–34. Epub 2003/ 06/13.PubMed PMID: 12802243.
- Borgaro SR, Kwasnica C, Cutter N, Alcott S. The use of the BNI screen for higher cerebral functions in assessing disorientation after traumatic brain injury. J Head Trauma Rehabil. 2003;18(3):284–91. Epub 2003/ 06/13.PubMed PMID: 12802170.
- Boosman H, Visser-Meily JM, Post MW, Duits A, van Heugten CM. Validity of the Barrow Neurological Institute (BNI) screen for higher cerebral functions in stroke patients with good functional outcome. Clin Neuropsychol. 2013;27(4):667–80. Epub 2013/ 03/12. PubMed PMID: 23472712. doi:10.1080/13854046.2013.777787.
- Redfors P, Hofgren C, Eriksson I, Holmegaard L, Samuelsson H, Jood K. The Barrow Neurological Institute screen for higher cerebral functions in cognitive screening after stroke. J Stroke Cerebrovasc Dis. 2014;23(2):349–55. Epub 2013/ 06/01. PubMed PMID: 23721621. doi:10.1016/j.jstrokecerebrovasdis.2013.04.026.
- Stenberg M, Godbolt AK, Nygren de Boussard C, Levi R, Stalnacke BM. Cognitive impairment after severe traumatic brain injury, clinical course and impact on outcome: a Swedish-Icelandic study. Behav Neurol. 2015;2015:680308. Epub 2016/ 01/20. PubMed PMID: 26783381; PubMed Central PMCID: PMC4689900. doi:10.1155/2015/680308.
- Tölli A, Borg J, Bellander B-M, Höybye C. Pituitary function in the acute phase of traumatic brain injury and subarachnoid hemorrhage. Int J Clin Med. 2015;06(06):411–22. doi:10.4236/ijcm.2015.66054.
- Tolli A, Borg J, Bellander BM, Johansson F, Hoybye C. Pituitary function within the first year after traumatic brain injury or subarachnoid haemorrhage. J Endocrinol Invest. 2017;40(2):193–205. Epub 2016/ 09/28. 10.1007/s40618-016-0546-1. PubMed PMID: 27671168.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England). 1974;2(7872):81–84. Epub 1974/ 07/13. PubMed PMID: 4136544.
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20. Epub 1968/01/01. PubMed PMID: 5635959. doi:10.3171/jns.1968.28.1.0014.
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9. Epub 1980/01/01.PubMed PMID: 7354892.
- Edwards P, Farrell B, Lomas G, Mashru R, Ritchie N, Roberts I, Sandercock P, Wasserberg J, Yates D. The MRC CRASH Trial: study design, baseline data, and outcome in 1000 randomised patients in the pilot phase. Emerg Med J. 2002;19(6):510–14. Epub 2002/ 11/08.PubMed PMID: 12421773; PubMed Central PMCID: PMC1756291.
- Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020–29. Epub 2004/ 12/18.PubMed PMID: 15605342.
- Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Reviews Neurol. 2014;10(2):99–114. Epub 2014/ 01/29. PubMed PMID: 24468878. doi:10.1038/nrneurol.2013.279.
- Prigatano GP, Amin K, Rosenstein LD. Administration and scoring manual for the BNI Screen for higher cerebral functions. Registration No. TXu 628 487. Phoenix, Arizona: Barrow Neurological Institute; 1995.
- Neukrug ES, Fawcett RC. Essentials of Testing and Assessment: A Practical Guide for Counselors, Social Workers, and Psychologists. 3rd ed. Stamford, CT, USA: Cengage Learning 2014. 368 p
- Hagen C, Malkmus D, Durham P. Levels of cognitive functioning. In: Professional Staff Association of Rancho Los Amigos Hospital, editors. Rehabilitation of the head injured adult: comprehensive physical management. Downey, CA: Rancho Los Amigos Hospital Inc; 1987.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. Epub 1983/06/01.PubMed PMID: 6880820.
- Hung CI, Liu CY, Wang SJ, Yao YC, Yang CH. The cut-off points of the depression and somatic symptoms scale and the hospital anxiety and depression scale in detecting non-full remission and a current major depressive episode. Int J Psychiatry Clin Pract. 2012;16(1):33–40. Epub 2011/ 11/30. PubMed PMID: 22122659. doi:10.3109/13651501.2011.617456.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. Epub 1965/ 02/01.PubMed PMID: 14258950.
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet (London, England). 1975;1(7905):480–84. Epub 1975/ 03/01. PubMed PMID: 46957.
- Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285–93. Epub 1981/ 04/01.PubMed PMID: 6453957; PubMed Central PMCID: PMC490949.
- Levin HS, Boake C, Song J, McCauley S, Contant C, Diaz-Marchan P, Brundage S, Goodman H, Kotrla KJ. Validity and sensitivity to change of the extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J Neurotrauma. 2001;18(6):575–84. Epub 2001/07/05. PubMed PMID: 11437080. doi:10.1089/089771501750291819.
- Group TE. EuroQol–a new facility for the measurement of health-related quality of life. Health policy (Amsterdam, Netherlands). 1990;16(3):199-208. Epub 1990/11/05. PubMed PMID: 10109801. Authors info: Group TE: The EuroQol Group (Centre for Health Economics, University of York, York Y01 5DD, United Kingdom)
- Fugl-Meyer AR, Melin R, Fugl-Meyer KS. Life satisfaction in 18- to 64-year-old Swedes: in relation to gender, age, partner and immigrant status. J Rehabil Med. 2002;34(5):239–46. Epub 2002/ 10/24.PubMed PMID: 12392240.
- Melin R, Fugl-Meyer KS, Fugl-Meyer AR. Life satisfaction in 18- to 64-year-old Swedes: in relation to education, employment situation, health and physical activity. J Rehabil Med. 2003;35(2):84–90. Epub 2003/ 04/15.PubMed PMID: 12691338.
- Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM Jr., Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16(4):343–55. Epub 2001/07/20.PubMed PMID: 11461657.
- van Eijck MM, Schoonman GG, van der Naalt J, de Vries J, Roks G. Diffuse axonal injury after traumatic brain injury is a prognostic factor for functional outcome: a systematic review and meta-analysis. Brain Inj. 2018;32(4):395–402. Epub 2018/ 01/31. PubMed PMID: 29381396. doi:10.1080/02699052.2018.1429018.
- Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844–54. Epub 2014/ 07/18. PubMed PMID: 25030516. doi:10.1016/s1474-4422(14)70120-6.
- Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, de Villiers JC. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51(11):1457. Epub 1988/ 11/01.PubMed PMID: 3236024; PubMed Central PMCID: PMC1032822. doi:10.1136/jnnp.51.11.1457.
- Lavoie S, Sechrist S, Quach N, Ehsanian R, Duong T, Gotlib IH, Isaac L. Depression in men and women one year following Traumatic Brain Injury (TBI): a TBI model systems study. Front Psychol. 2017;8:634. Epub 2017/ 05/23. PubMed PMID: 28529492; PubMed Central PMCID: PMC5418333. doi:10.3389/fpsyg.2017.00634.
- Juengst SB, Kumar RG, Wagner AK. A narrative literature review of depression following traumatic brain injury: prevalence, impact, and management challenges. Psychol Res Behav Manag. 2017;10:175–86. Epub 2017/ 06/28. PubMed PMID: 28652833; PubMed Central PMCID: PMC5476717. doi:10.2147/prbm.s113264.
- Ackermark PY, Schepers VP, Post MW, Rinkel GJ, Passier PE, Visser-Meily JM. Longitudinal course of depressive symptoms and anxiety after aneurysmal subarachnoid hemorrhage. Eur J Phys Rehabil Med. 2017;53(1):98–104. Epub 2016/ 07/15. PubMed PMID: 27412071. doi:10.23736/s1973-9087.16.04202-7.
- Gross T, Schuepp M, Attenberger C, Pargger H, Amsler F. Outcome in polytraumatized patients with and without brain injury. Acta Anaesthesiol Scand. 2012;56(9):1163–74. Epub 2012/06/28. PubMed PMID: 22735047. doi:10.1111/j.1399-6576.2012.02724.x.
- Meyer B, Ringel F, Winter Y, Spottke A, Gharevi N, Dams J, Balzer-Geldsetzer M, Mueller IK, Klockgether T, Schramm J, et al. Health-related quality of life in patients with subarachnoid haemorrhage. Cerebrovasc Dis. 2010;30(4):423–31. Epub 2010/ 08/20. PubMed PMID: 20720412. doi:10.1159/000317078.