ABSTRACT
Objectives: To evaluate the frequency of post-concussion symptoms and prevalence and risk factors of post-concussion syndrome (PCS) in the general population, investigate the association between the Rivermead Post-Concussion Symptoms Questionnaire (RPQ) and self-perceived health, and evaluate differences between three European countries.
Methods: A web-based survey including the RPQ and EQ-5D was conducted among representative samples in three European countries.
Results: A total of 11,759 respondents completed the questionnaire. The most frequently reported symptom was fatigue (49.9%). Almost half (45.1%) of the respondents were classified as having PCS considering rating score 2 (three RPQ items with score ≥ 2) as a cut-off. Chronic health complaints were found as a significant risk factor for PCS. All items of the RPQ were positively correlated with the EQ-5D and the strongest positive correlation (0.633, p<0.001) was between RPQ item ‘feeling depressed or tearful’ and EQ-5D domain ‘anxiety/depression’.
Conclusions: We found a high frequency of post-concussion-like symptoms and PCS in the general population, indicating that these symptoms are not specific for patients with traumatic brain injury (TBI), and PCS is not a unique syndrome after TBI. Therefore, the use of post-concussion symptoms and PCS as outcome following mild TBI should be interpreted with caution.
Introduction
Post-concussion symptoms following a traumatic brain injury, and especially mild traumatic brain injury (mTBI), are very common (Citation1). Post-concussion symptoms can be categorized in physical symptoms, cognitive deficits and behavioral/emotional symptoms (Citation2). In general, many patients with mTBI make a full recovery within one year after injury (Citation3), but when several post-concussion symptoms persist over time, patients are considered as having a post-concussion syndrome (PCS). One of the most prominent diagnostic criteria of PCS is the International Classification of Diseases (ICD-10) (Citation4). The Rivermead Post-Concussion Symptoms Questionnaire (RPQ) is a frequently applied instrument to assess the existence and severity of post-concussion symptoms (Citation5),
Over the last decennia, the concept of PCS has been debated in an abundance of studies. The prevalence rates of PCS throughout the literature vary greatly (Citation6) and depend on the definition used (Citation7) as well as the applied classification method (Citation8). Researchers and clinicians who have performed extensive research concerning the etiology of PCS have still not been able to successfully identify the pre- and post-injury-related factors as well as the underlying structure of post-concussion symptoms (Citation9). All controversy (Citation10) and uncertainty leads to a growing concern whether PCS really does exist and if post-concussion symptoms are unique for patients with mTBI. Multiple studies have concluded that the etiology of the post-concussion symptoms and/or syndrome might probably not resort back to the brain damage itself (Citation11–Citation13). Moreover, self-reported symptoms may be non-specific symptoms, which are not exclusively associated with patients with mTBI (Citation14). Post-concussion symptoms can be caused by various factors, and it is complex to interpret which components may be linked specifically to the brain injury and to which extent symptoms already existed before the injury. Additionally, previous studies have shown that post-concussion-like symptoms exist in healthy populations (Citation13,Citation15–Citation19) as well as in patients with a non-head injury trauma (Citation11,Citation14), patients with chronic pain (Citation12) and personal injury claimants (Citation20). However, all previous studies had relatively small sample sizes and samples were not representative for general populations, since the populations studied mainly consisted of university students or patient groups (Citation11–Citation18,Citation21). Furthermore, all studies were only conducted in one country at a time and most research was done in North-America (Citation12,Citation13,Citation16,Citation18–Citation21), with exceptions of China (Citation15), France (Citation14) and Australia (Citation11,Citation17).
Wang and colleagues have suggested that the differences in frequency of post-concussion symptoms could be due to cultural differences (Citation15). Additionally, Zakzanis and colleagues (Citation16) have shown that the influence of culture and language should be taken into consideration in PCS research. Consequently, prevalence rates in healthy populations may differ between countries. Apart from culture and language, a linkage between post-concussion symptoms and lower levels of life satisfaction (Citation22) and lower health-related quality of life (HRQoL) (Citation23) have been reported. Nonetheless, the patient populations in both these researches consisted of patients with TBI. A strong link between post-concussion symptoms and HRQoL may suggest that PCS is debilitating. However, a weak association could point out that PCS consists of common symptoms that everyone experiences at some time which do not explicitly have a major effect on HRQoL. Whether this linkage also exists in healthy populations remains to be investigated.
The aims of this paper were to (a) evaluate the frequency of post-concussion symptoms and prevalence of PCS in general healthy populations, (b) assess the risk factors for PCS, (c) compare the RPQ with general HRQoL (EQ-5D), and (d) inspect the differences between three European countries.
Methods
Participants
A web-based survey was conducted among a representative sample in three European countries, namely the United Kingdom (UK), the Netherlands and Italy. The respondents were recruited by Survey Sampling International (SSI), a market research agency, who distributed and launched the questionnaires. Existing large internet panels were used and these samples were designed to be representative of the population aged 18 to 70 in the selected countries with regard to age, gender and education. Data were obtained between June 29th and July 31st 2017. A total of 11,759 respondents filled out the questionnaire, which was comprised of 4,646 respondents in UK, 3,564 respondents in the Netherlands and 3,549 respondents in Italy.
Patient consents
All participants, as members of a web-based panel, had already provided informed consent to participate in online surveys. Informed consent for the present survey was obtained from all those agreeing to complete the survey. Participants were informed on the welcome page that the survey aimed to better understand the consequences of traumatic brain injury, that it would take approximately 20 min to complete, and that all responses were confidential and anonymous. Consent was obtained when respondents clicking the ‘Go to Survey’ button from this page. This study was part of the CENTER-TBI study (EC grant 602150) and ethical approval was obtained from the Leids Universitair Centrum – Commissie Medische Ethiek (approval P14.222/NV/nv).
Measures
Prevalence and severity of post-concussion symptoms were evaluated by the use of the RPQ. A total of 16 different post-concussion symptoms are described in the RPQ, which include headaches, dizziness, nausea/vomiting, noise sensitivity, sleep disturbance, fatigue, being irritable, feeling depressed or tearful, feeling frustrated or impatient, forgetfulness, poor concentration, taking longer to think, blurred vision, light sensitivity, double vision and restlessness. During the questionnaire respondents were asked to assess the severity of the symptoms over the last 24 h on a 5-point Likert scale: 0 (not experienced at all), 1 (no more of a problem), 2 (a mild problem), 3 (a moderate problem) and 4 (a severe problem) (Citation5). The RPQ total score is the sum of all 16 items excluding ratings of 1 (Citation5). During this study, the criteria described in the ICD-10 are mapped onto the RPQ scale and respondents were classified as having PCS when they reported at least three out of the following symptoms: headaches, dizziness, fatigue, irritability, impaired memory, impaired concentration, and insomnia (Citation4). There is not a set standard available in the literature for which severity rating to uses as a cut-off, which resulted in two possible cut-offs; mild or higher (≥ rating score 2) and moderate or higher (≥ rating score 3) () (Citation8). In this study, we looked at both cut-offs separately.
Table 1. Characteristics of the study population.
Table 2. Prevalence of Post-Concussion Syndrome in the general population.
Table 3. Mean EQ-5D utility scores calculated by the Dutch value set for respondents with and without Post-Concussion Syndrome per country.
Table 4. Mean EQ-5D VAS scores for respondents with and without Post-Concussion Syndrome per country.
Table 5. Severity rating cut-offs regarding Post-Concussion Syndrome.
HRQoL was measured by the EQ-5D. The EQ-5D constitutes of two parts: the EQ-5D descriptive system and the EQ visual analog scale (EQ VAS). The EQ-5D descriptive system encompasses five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). The EQ-5D-5L was introduced in 2009 and gives respondents the opportunity to score the dimensions on five levels (no problems, slight problems, moderate problems, severe problems and extreme problems). The EQ-VAS consists of a vertical VAS rating scale, where 0 is labeled as “The worst health you can imagine” and 100 as “The best health you can imagine” and documents the respondent’s self-rated health. The EQ-5D utility scores, which are on a scale from 0 (dead) to 1 (full health), for each country were calculated by the use of the Dutch value set (Citation24).
Risk factors
Age, gender, education level, work status, income level, the experience of serious illness in respondents themselves; or their immediate family, whether respondents cared for others, and the experience of chronic health complaints were considered risk factors. This selection was based on the available data in our dataset and by looking at risk factors in previous literature (Citation25–Citation27). The categorizations for the risk factors can be found in Appendix B.
Statistical analysis
Descriptive analyses were performed for demographic data (age, gender, education, work status, annual household income, the experience of serious illness in yourself, immediate family and caring for others, and chronic health complaints). The frequency of post-concussion-like symptoms was assessed by computing the percentages for respondents, and the prevalence of PCS was calculated by identifying the percentage of respondents that complied with our classifications.
Differences in mean EQ-5D utility and EQ-5D VAS scores per country were assessed by the use of the Kruskal Wallis H test, followed by post-hoc analyses where the significance values were adjusted by the Bonferroni correction for multiple tests. Statistical significance was determined by a p-value of p<0.05.
By the use of Mann Whitney U tests, we inspected the difference for respondents with and without PCS in mean EQ-5D utility and mean EQ-5D VAS. To evaluate the correlation between the various EQ-5D dimensions and EQ-5D total score and the RPQ items, which were not normally distributed, the Spearman’s correlation coefficients were administered. Strong, moderate and weak correlations were differentiated between by Cohen’s Set Correlation and Contingency Tables: a coefficient above 0.5 the correlation was considered strong, a coefficient between 0.3 and 0.5 moderate, and when the coefficient was below 0.3 it was considered as weak (Citation28).
The survey was translated from English into Dutch and Italian using translation software and subsequently translated back into English. Bilingual native speakers verified the translations independently.
All analyses were done for the complete database and per country. SPSS version 24 for Windows (IBM SPSS Statistics, SPSS Inc, Chicago, IL) was used to perform all statistical analyses.
Data availability
The data that support the findings of this study are available from the corresponding author, [DV], upon reasonable request. Anonymized data will be shared.
Results
Study population
In total 11,759 respondents were included in this study. The characteristics of our study sample are shown in . The median age of the respondents was 44 years (interquartile range (IQR); 32–57 years) and women and men were evenly represented. The educational level of the respondents can be divided up in 28.3% (low), 47.2% (middle) and 25.3% (high). Approximately 50% was employed and just over a half (52.2%) had experienced serious illness in their immediate family. One in two (50.9%) respondents has reported to have one or more chronic health complaints.
Frequency of post-concussion-like symptoms and prevalence of PCS
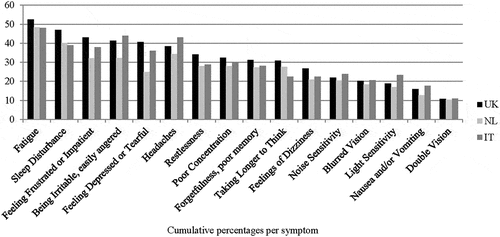
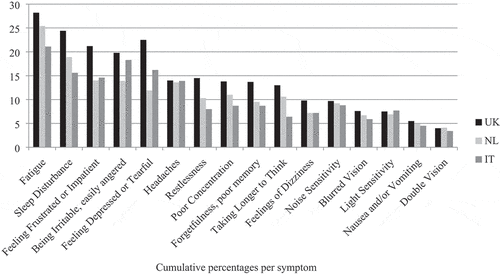
The most frequently reported symptom was fatigue (49.9%) followed by sleep disturbance (42.4%) (). The least reported symptom was double vision (10.7%). The patterns for the reported post-concussion symptoms in the individual countries were quite similar. Fatigue was also the most frequently reported symptom in each country (UK: 52.6%, the Netherlands: 48.4% and Italy: 48.1%), followed by sleep disturbance (UK: 47.0%, the Netherlands: 40.1%), except for Italy where being irritable was the second most reported symptom (Italy: 44.0%). When using rating score 3 as a cut-off the same pattern is detected (Appendix A).
Almost half (45.1%) of the respondents were classified as having PCS considering rating score 2 (three RPQ items with score ≥ 2) as a cut-off (). When using rating score 3 (three RPQ items with score ≥ 3) as a cut-off, this prevalence rate dropped substantially to 17.5%. When we inspected all respondents with chronic health complaints, higher PCS prevalence rates were found for every single complaint compared to the sample as a whole. Furthermore respondents with memory problems due to a neurological disease/dementia had the highest percentage of PCS prevalence for rating score 2 (81.9%) and rating score 3 (53.4%). The prevalence of PCS differed per country with the UK (47.8%) having the highest prevalence rates. When using rating score 3 as a cut-off, the biggest drop in prevalence rate is seen in Italy, which implies that Italians report less frequently moderate problems.
Risk factors
Lower age, female gender, low education, unable to work, low-income level and when respondents indicated they experienced serious illness in respondents themselves, their immediate family, and when they cared for others, and chronic health complaints are all significantly associated with PCS (Appendix B). The most pronounced effects on PCS are “being a student” or “retired” compared to being “unable to work” and chronic health complaints. Multivariable prediction models explained 26% (rating score 2) and 24% (rating score 3) (Nagelkerke R2) of the variance in PCS.
EQ-5D utility
The mean EQ-5D utility score was 0.81. The lowest utility measured in this sample was −0.45% and 33.5% of the respondents reported no problems on any of the EQ-5D domains. As expected, the mean utility score was significantly lower for respondents with PCS compared to respondents without PCS (0.70 vs. 0.90; p<0.001) (). The mean EQ-5D VAS score was 74.7 () and was also found to significantly differ between respondents with and without PCS (66.8 vs. 81.2; p<0.001).
The highest mean utility score was found for Italian respondents (μ = 0.86, SD = 0.16), followed by Dutch respondents (μ = 0.83, SD = 0.21) and lastly British respondents (μ = 0.77, SD = 0.28). The lowest mean utility score was found for respondents from the UK with PCS according to rating score 3. There were statistically significant differences in EQ-5D utility and total scores between countries (p<0.05), except for the utility between the Netherlands and Italy (p=0.051). and also shows the mean utility scores for respondents with and without PCS according to the two cut-offs and per country. The biggest difference in utility was determined for British respondents without PCS and with PCS according to rating score 3.
For the EQ-5D VAS scores, the same order was found as for the mean utility score, which means Italian respondents rate their own health the highest and British respondents the lowest, with the Dutch respondents in between both of them. The EQ-5D-VAS was determined to be significantly different for respondents with and without PCS in all countries (p<0 .001).
RPQ and EQ-5D
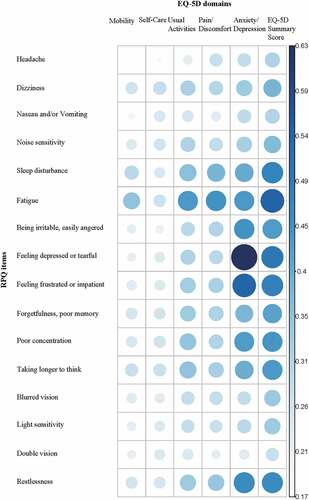
shows Spearman’s correlation coefficients between RPQ items and EQ-5D dimensions indicating that all items of the RPQ are positively correlated with the EQ-5D dimensions. The strongest positive correlation (0.633, p< .001) was found between ‘feeling depressed or tearful’ and the anxiety/depression dimension. The weakest correlation was between ‘headache’ and the mobility dimension. Fatigue has a moderate correlation with all EQ-5D dimensions, with the exception of the self-care dimension. All correlations were statistically significant on a p<0.001 level. Lastly, when looking at correlations between the EQ-5D total score and all RPQ items separately, fatigue (0.546, p<0.001) was determined as the strongest positive correlation and double vision (0.278, p<0.001) showed the weakest correlation with the EQ-5D total score.
Discussion
This study provides the first examination of the frequency of post-concussion-like symptoms and the prevalence of PCS in a large and representative sample of the general population, and within and across three European countries. We found a high base rate of post-concussion-like symptoms and respondents with memory problems due to a neurological disease/dementia had the highest prevalence rate for PCS. The use of post-concussion symptoms and PCS as outcome following mTBI should be interpreted with caution.
Our findings correspond to those of preceding studies. Wang and colleagues investigated a group of university students, in which they found fatigue as the highest reported symptom with a frequency of 38.1% (Citation15). During this study, we also determined fatigue (49.9%) as the highest reported symptom for all respondents in the database. The prevalence rate of PCS was 45.1% considering rating score 2 as a cut-off, however; when using rating score 3 the prevalence for PCS decreased to 17.5%, which is comparable to prevalence rates found by Lagarde and colleagues in patients with head injuries (28.7%) and patients with non-head injuries (22.9%) (Citation14).
The following risk factors were all significantly associated with PCS: lower age, female gender, low education, work status, low-income level, chronic health complaints, and when respondents experienced serious illness in themselves, their immediate family, and when they cared for others, and chronic health complaints. These findings are in line with previous studies (Citation25–Citation27). Being a “student” or “retired” compared to being “unable to work” and chronic health complaints had the most noticeable effect on PCS. Statistically significant differences in EQ-5D utility, total scores, and EQ-5D VAS scores were found for patients with and without PCS. This indicates that being classified with PCS had a strong impact on the respondent’s HRQoL. In addition, correlations between all RPQ items and EQ-5D dimensions were high.
The current study is unique compared to previous studies, because none of them have looked at large samples such as in this study nor did they compare three different countries at the same time. Additionally, the database used is also representative for the general population with regards to age, gender, and educational level, where in previous studies mostly healthy university students were used (Citation13,Citation15–Citation19).
Limitations include that for the calculation of the utility scores of the EQ-5D, Dutch value sets were used for all countries included in the analysis, mainly because there is no value set available yet for Italy. Using the same tariff for each country could potentially limit the representativeness of these scores in the separate countries, as the relative value of dimensions and levels may differ from those in the Netherlands. However, it does substantiate the comparability across the three countries. When comparing the population norms with the mean EQ-5D utility and VAS scores, the reported mean scores were comparable for the Netherlands and Italy. However, the mean UK scores, 0.77 and 71.3, respectively, are lower than the population norms; 0.86 and 82.8 (Citation29).
Our study was conducted by the use of a web-based checklist, which might have led to ‘over’ reporting of symptoms, because according to Edmed & Sullivan, the method used to assess PCS symptoms influences the number and type of symptoms reported (Citation17). On the other hand, the RPQ is the most frequently applied instrument to classify PCS. By also incorporating this method in our study, our prevalence rates are comparable with previous mTBI studies. Another limitation is based on the fact that there were no questions asked if respondents had experienced a concussion, TBI or brain injury in their life or trauma’s in general. However, the expected TBI prevalence is 639.2 (UK), 278.6 (the Netherlands) and 214.5 (Italy), extrapolated from reported country-specific age-adjusted hospital discharge rates per 100.000 due to TBI by Majdan and colleagues (Citation30). This is considerably lower than the found prevalence rates for PCS in this population. Nevertheless, the found pattern is similar to PCS distribution, where the UK was the highest and IT the lowest. Additionally, previous literature has determined that respondents suffering from depression and/or burn-out or PTSD, or being involved in a litigation at the time of the questionnaire assessment are factors that could be associated with PCS. However, in the current study, there is no information representing these aspects (Citation1,Citation13). There is also no information available if respondents are enduring intolerance of stress, emotion or alcohol, which is the last criterion described in the ICD-10 criteria (Citation4). Furthermore, we do not know to what extent our samples are representative for the population in the three countries with regards to characteristics other than age, gender, and educational level. Additionally, the people who partake in a market research panel might not be illustrative of the general population.
We were able to look at the representativeness of the sample with regards to HRQoL by comparing our scores with the population norms. However, it could be that our sample is not representative with regards to other factors and characteristics that impact the likelihood of developing PCS, and which should be taken into account when pooling representative samples. Moreover, the maximum age in our study sample was 70, whereas the TBI epidemiology is changing with a greater deal of patients aged 70 and older (Citation31).
More research is needed into which cut-off point is sufficient for PCS research, because the current literature is inconclusive concerning the severity rating score that should be used as a cut-off when the RPQ is applied to classify PCS. As shown during this study, and previous studies, the results change considerably depending on the cut-off (Citation8). Rating score 2 seems to be less discriminating as healthy adults are also being diagnosed with PCS, which points towards a high percentage of false-positives. Additionally, to correctly diagnose people with PCS, a clinical examination should take place rather than basing it on self-report of symptoms by the patient. Clinicians should be aware of the high post-concussion-like symptom endorsement and prevalence of PCS in the healthy population and the possible contributing risk factors in a specific country, and take this into consideration during their clinical examination (Citation13). Considering the issues with current PCS assessment tools, more and more research is being done into new methods that may be better suited in the assessment of PCS (e.g. ocular motor assessment (Citation32) and robotic technology (Citation33)). It is very clear that a high base rate of PCS symptoms is present in the general population, so when looking at patients with TBI, one should wonder which part of the reported symptoms are actually due to the injury. There is a plethora of research being performed in the field of PCS, however, this study shows that there is no clear view on what is really being researched. Furthermore, this is supported by the fact that the prevalence rates of PCS halved when we looked at the respondents without any chronic health complaints and that prevalence depended substantially on the distribution of risk factors in a population that are not specific for TBI. The terminologies post-concussion symptoms and PCS should be modified as they are deceptive, since they incorrectly assume that the underlying principle of the symptoms and/or syndrome is a brain injury (Citation11).
Conclusions
This study showed that post-concussion-like symptoms are frequently reported, and the prevalence of PCS is prominent in the general population, indicating that post-concussion-like symptoms are not specific for patients with TBI, and PCS is not a unique syndrome after TBI. Post-concussion-like symptoms are highly correlated with EQ-5D dimensions. This suggests that post-concussion-like symptoms are debilitating and that also in the healthy population these symptoms have a major effect on HRQoL.
Authors’ contribution
DV developed the study design, performed statistical analysis and interpretation, and wrote the manuscript. BG performed statistical analysis and interpretation. JH developed the study design, interpreted the data and provided study supervision. All authors critically revised the paper. All authors read and approved the final manuscript.
Disclosure Statement
The authors have declared that no competing interests exist.
Additional information
Funding
Notes on contributors
Daphne C. Voormolen
Daphne C. Voormolen is a PhD student for the CENTER-TBI project with a focus on measuring Quality of Life and predicting recovery patterns for patients with Traumatic Brain Injury.
Maryse C. Cnossen
Maryse C. Cnossen works as a lecturer at the University of Applied Sciences Rotterdam and was a PhD student and Postdoc researcher for the CENTER-TBI project with a focus on outcome research, provider profiling and comparative effectiveness research.
Suzanne Polinder
Suzanne Polinder is an associate professor at the department of Public Health at the Erasmus University Medical Centre Rotterdam with the main focus on health outcomes, economic evaluations and health technology assessment.
Benjamin Y. Gravesteijn
Benjamin Y. Gravesteijn is a medical master student and research associate at Erasmus University Medical Centre Rotterdam studying effectiveness of treatment in acute care through modern statistical techniques (machine learning, instrumental variable analysis) and prediction.
Nicole Von Steinbuechel
Nicole von Steinbuechel is the head of department at the institute of Medical Psychology and Medical Sociology at the Georg-August-University with a focus on health outcomes, training and health-related quality of life.
Ruben G.L. Real
Ruben G.L. Real is a researcher at the institute of Medical Psychology and Medical Sociology at the Georg-August-University mainly focussing on neuropsychology, patient reported outcomes and psychometric analysis.
Juanita A. Haagsma
Juanita A. Haagsma is an assistant professor the department of Public Health at the Erasmus University Medical Centre Rotterdam involved with burden of diseases estimates of injury and quantifying long-term consequences of injury in particular.
References
- Evans RW. The postconcussion syndrome and the sequelae of mild head injury. Neurol Clin. 1992;10(4):815–47.
- American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86–87.
- Losoi H, Silverberg ND, Waljas M, Turunen S, Rosti-Otajarvi E, Helminen M, Luoto TM, Julkunen J, Ohman J, Iverson GL. Recovery from mild traumatic brain injury in previously healthy adults. J Neurotrauma. 2016;33(8):766– 76. doi:10.1089/neu.2015.4070.
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: WHO; 1993.
- Ns K, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–92.
- Hiploylee C, Dufort PA, Davis HS, Wennberg RA, Tartaglia MC, Mikulis D, Hazrati LN, Tator CH. Longitudinal study of postconcussion syndrome: not everyone recovers. J Neurotrauma. 2017;34(8):1511–23.
- Laborey M, Masson F, Ribereau-Gayon R, Zongo D, Salmi LR, Lagarde E. Specificity of postconcussion symptoms at 3 months after mild traumatic brain injury: results from a comparative cohort study. J Head Trauma Rehabil. 2014;29(1):E28–36. doi:10.1097/HTR.0b013e318280f896.
- Voormolen DC, Cnossen MC, Polinder S, von Steinbuechel N, Vos PE, Haagsma JA. Divergent classification methods of post-concussion syndrome after mild traumatic brain injury: prevalence rates, risk factors and functional outcome. J Neurotrauma. 2018;35:1233–41. doi:10.1089/neu.2017.5257.
- Mittenberg W, DiGiulio DV, Perrin S, Bass AE. Symptoms following mild head injury: expectation as aetiology. J Neurol Neurosurg Psychiatry. 1992;55(3):200–04.
- Evans RW. The postconcussion syndrome: 130 years of controversy. Semin Neurol. 1994;14(1):32–39. doi:10.1055/s-2008-1041056.
- Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, Chapman J, Gurka J, Dawson K, Capon L, et al. Mild traumatic brain injury does not predict acute postconcussion syndrome. J Neurol Neurosurg Psychiatry. 2008;79(3):300–06. doi:10.1136/jnnp.2007.126565.
- Iverson GL, McCracken LM. ‘Postconcussive‘ symptoms in persons with chronic pain. Brain Inj. 1997;11(11):783–90.
- Iverson GL, Lange RT. Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003;10(3):137–44. doi:10.1207/S15324826AN1003_02.
- Lagarde E, Salmi LR, Holm LW, Contrand B, Masson F, Ribereau-Gayon R, Laborey M, Cassidy JD. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs. postconcussion syndrome. JAMA Psychiatry. 2014;71(9):1032–40. doi:10.1001/jamapsychiatry.2014.666.
- Wang Y, Chan RC, Deng Y. Examination of postconcussion-like symptoms in healthy university students: relationships to subjective and objective neuropsychological function performance. Arch Clin Neuropsychol. 2006;21(4):339–47. doi:10.1016/j.acn.2006.03.006.
- Zakzanis KK, Yeung E. Base rates of post-concussive symptoms in a nonconcussed multicultural sample. Arch Clin Neuropsychol. 2011;26(5):461–65. doi:10.1093/arclin/acr021.
- Edmed SL, Sullivan KA. Method of symptom assessment influences cognitive, affective and somatic post-concussion-like symptom base rates. Brain Injury. 2014;28(10):1277–82. doi:10.3109/02699052.2014.915988.
- Wong JL, Regennitter RP, Barrios F. Base rate and simulated symptoms of mild head injury among normals. Arch Clin Neuropsychol. 1994;9(5):411–25.
- Gouvier WD, Uddo-Crane M, Brown LM. Base rates of post-concussional symptoms. Arch Clin Neuropsychol. 1988;3(3):273–78.
- Lees-Haley PR, Brown RS. Neuropsychological complaint base rates of 170 personal injury claimants. Arch Clin Neuropsychol. 1993;8(3):203–09.
- Gouvier WD, Cubic B, Jones G, Brantley P, Cutlip Q. Postconcussion symptoms and daily stress in normal and head-injured college populations. Arch Clin Neuropsychol. 1992;7(3):193–211.
- Stalnacke BM. Community integration, social support and life satisfaction in relation to symptoms 3 years after mild traumatic brain injury. Brain Inj. 2007;21(9):933–42. doi:10.1080/02699050701553189.
- Emanuelson I, Andersson Holmkvist E, Bjorklund R, Stalhammar D. Quality of life and post-concussion symptoms in adults after mild traumatic brain injury: a population-based study in western Sweden. Acta Neurol Scand. 2003;108(5):332–38.
- Versteegh MM, Vermeulen KM, Evers SM, de Wit GA, Prenger R, Stolk EA. Dutch Tariff for the Five-Level Version of EQ-5D. Value Health. 2016;19(4):343–52. doi:10.1016/j.jval.2016.01.003.
- Cnossen MC, Winkler EA, Yue JK, Okonkwo DO, Valadka A, Steyerberg EW, Lingsma H, Manley GTMDPD. Development of a Prediction Model for Post-Concussive Symptoms following Mild Traumatic Brain Injury: A TRACK-TBI Pilot Study. J Neurotrauma. 2017;34:2396–409. doi:10.1089/neu.2016.4819.
- Silverberg ND, Gardner AJ, Brubacher JR, Panenka WJ, Li JJ, Iverson GL. Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma. 2015;32(8):517–26. doi:10.1089/neu.2014.3600.
- Cassidy JD, Cancelliere C, Carroll LJ, Cote P, Hincapie CA, Holm LW, Hartvigsen J, Donovan J, Nygren-de Boussard C, Kristman VL, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95(3 Suppl):S132–51. doi:10.1016/j.apmr.2013.08.299.
- Cohen J. Set Correlation and Contingency Tables. Appl Psychol Meas. 1988;12(4):425–34. doi:10.1177/014662168801200410.
- Janssen B, Szende A. Population Norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: an International Perspective based on EQ-5D. Dordrecht: Springer Netherlands; 2014. p. 19–30.
- Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, Maas A. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. 2016;1(2):e76–e83. doi:10.1016/S2468-2667(16)30017-2.
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Buki A, Chesnut RM, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi:10.1016/S1474-4422(17)30371-X.
- Clough M, Mutimer S, Wright DK, Tsang A, Costello DM, Gardner AJ, Stanwell P, Mychasiuk R, Sun M, Brady RD, et al. Oculomotor Cognitive Control Abnormalities in Australian Rules Football Players with a History of Concussion. J Neurotrauma. 2018;35(5):730–38. doi:10.1089/neu.2017.5204.
- Subbian V, Meunier JM, Korfhagen JJ, Ratcliff JJ, Shaw GJ, Beyette FR Jr. Quantitative assessment of post-concussion syndrome following mild traumatic brain injury using robotic technology. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5353–56. doi:10.1109/EMBC.2014.6944835.