ABSTRACT
Background and objective
Adolescents with sports-related concussion (SRC) demonstrate acute and persistent deficits in subtle motor function. However, there is limited research examining related neurological underpinnings. This pilot study examined changes in motor-associated white matter pathways using diffusion tensor imaging (DTI) and their relationship with subtle motor function.
Methods
Twelve adolescents with SRC (12–17 years) within two-weeks post-injury and 13 never-injured neurotypical peers completed DTI scanning. A subset of 6 adolescents with SRC returned for a follow-up visit post-medical clearance from concussion. Subtle motor function was evaluated using the Physical and Neurological Examination of Subtle Signs (PANESS).
Results
Adolescents with SRC showed higher mean diffusivity (MD) of the superior corona radiata and greater subtle motor deficits compared to controls. Across all participants, greater subtle motor deficits were associated with higher (more atypical) MD of the superior corona radiata. Preliminary longitudinal analysis indicated reduction in fractional anisotropy of the corpus callosum but no change in the MD of the superior corona radiata from the initial visit to the follow-up visit post-medical clearance.
Conclusions
These findings support preliminary evidence for a brain–behavior relationship between superior corona radiata microstructure and subtle motor deficits in adolescents with SRC that merits further investigation.
Introduction
Pediatric sports-related concussion (SRC) is a serious public health issue, and the acute and long-term consequences of SRC in adolescents have received increased attention in recent years (Citation1). Concussion, a type of mild traumatic brain injury (mTBI), reflects a complex cascade of events that affect brain physiology, including neural inflammation, diffuse axonal injury, and altered cerebral blood flow (Citation2). Advanced neuroimaging methods such as diffusion tensor imaging (DTI) provide greater potential for identifying abnormalities in SRC than traditional structural neuroimaging methods (Citation3). DTI allows characterization of microscopic damage to specific white matter pathways and has been shown to reliably detect subtle, but clinically relevant, changes in brain microstructure following SRC (Citation3). A growing body of literature suggests that certain DTI parameters are sensitive to inflammation and may serve as a biomarker for neural changes following SRC in both acute and chronic stages (Citation4).
Due to the inflammation in tissue microstructure associated with brain injury, water diffusion along and into white matter fibers may change, with fiber bundles exhibiting highly restricted water diffusion following the injury (Citation5). However, the timing and pattern of these disruptions are not fully understood. DTI can quantify anisotropic (directionally dependent) water diffusion properties of white matter microstructure and fiber tract integrity. Two commonly assessed DTI indices include fractional anisotropy (FA) and mean diffusivity (MD) (Citation6). FA is a measure of the linearity of water movement within axons with values ranging from 0 (disorganized) to 1 (straight/unidirectional), and MD is a measure of the overall diffusion in a tissue averaged over all directions (Citation6). Healthy and organized white matter tracts tend to have higher FA and lower MD values (Citation7). Medium- to long-term effects of mTBI include a decrease in FA combined with an increase in MD and is indicative of decreased integrity of white matter microstructure (Citation7). Conversely, in the context of an acute mTBI, higher FA, and reduced MD values are hypothesized to reflect axonal swelling secondary to water movement into the intracellular axonal space (Citation7).
There are contrasting results about the direction of diffusion changes in the days to months following mTBI (Citation8). The majority of reports in acute mTBI have shown increased FA values and decreased MD values attributed to cytotoxic edema and axonal swelling (Citation8–10). Conversely, some studies have reported increased MD (Citation11,Citation12) while others have shown no changes in DTI indices (Citation13–15). Cross-sectional studies in adolescents have shown altered DTI metrics at 2-months post-SRC (Citation16), at medical clearance, and even one-year post-injury compared to control peers (Citation17). Children with SRC appear to be particularly vulnerable to long-term neuropathology since the immediate and long-term impacts of injury are compounded on a rapidly changing brain. However, there are few longitudinal studies in children with SRC. Compared to controls, high school and collegiate athletes showed a widespread decrease in MD within 24 hours after SRC which became more widespread by 8-days post-SRC (Citation18). Longitudinal examination from the sub-acute (<96 hours) to 3-months post-injury period revealed decreases in FA and increases in MD in adolescents with SRC (Citation19). Taken together, these studies indicate that the brain continues to change during the post-injury recovery period.
The presence of DTI findings post-mTBI has been used to predict post-concussive symptoms and neuropsychological outcomes. Using whole-brain DTI data, increased FA, and decreased MD values were associated with greater impairment on the Sports Concussion Assessment Tool 2 in adolescents with acute SRC (within 2 months) (Citation20). However, several studies have failed to find relationships with neuropsychological outcome measures (Citation21,Citation22), primarily attributing this to the lack of sensitivity of concussion assessments in identifying subtle differences in the acute phase of recovery. It is well established that adolescents with SRC can present with motor deficits during the subacute and acute periods of recovery, most notably in the areas of processing speed and reaction time (Citation23–25). The Consensus Panel on Concussion in Sport recommends evaluation of motor function after SRC as a crucial diagnostic modality and one of the three assessment prongs (Citation26). Emerging evidence highlights that the study of subtle motor performance using the revised Physical and Neurological Examination of Subtle Signs (PANESS) (Citation27) is more sensitive to residual impairment in SRC and mTBI than other standard measures (Citation24,Citation28,Citation29). However, there is a lack of research investigating the underlying neurophysiology of motor impairments in youth with SRC.
Using whole-brain voxel-wise DTI analysis, Borich et al. showed that the corona radiata and the corpus callosum were the two primary areas most affected in adolescents with acute SRC (≤ 2 months prior) (Citation20). The corona radiata and the corpus callosum have been implicated in several concussion studies and are hypothesized to be particularly susceptible to mTBI (Citation7,Citation21,Citation30,Citation31). Moreover, the superior corona radiata and the corpus callosum are large white matter tracts associated with cortical motor regions and have an important role in motor function (Citation32,Citation33). These regions show maturation by childhood (8–12 years) and the FA of these structures remain relatively stable during the adolescent years (13–17 years) in typically developing children (Citation34). Whole-brain analyses of adolescent football players (14–18 years) measured pre- and post-season showed significantly increased FA of the superior corona radiata compared to athletes that did not participate in collision sports (Citation35). Interestingly, increased FA of the superior corona radiata correlated with the number of accumulated hits (Citation35). Although the corticospinal tract (part of the corona radiata) shows altered diffusion in youth after an acute SRC, the motor implications of altered diffusion are lacking (Citation22).
The purpose of this preliminary investigation was to examine white matter diffusivity in motor-associated white matter pathways in adolescents within 2 weeks of a SRC and never-injured neurotypical peers. Given the variability in the literature, we hypothesized that adolescents with SRC would demonstrate changes in the FA and MD measures of the superior corona radiata and corpus callosum compared to never-injured peers. This pilot study also examined the relationship between changes in white matter diffusivity and subtle motor function. We conducted preliminary analysis examining the longitudinal change in white matter diffusivity in a small subset of children following medical clearance to return-to-play after SRC. Identifying brain–behavior relationships of motor function after SRC in a pilot cohort can provide valuable information about promising measures that should be further investigated in larger studies and provides data on effect sizes that can serve for sample size calculations in future studies.
Methods
Participants included 12 children with a SRC (7 males and 5 females), ages 12–17 years, (M (SD) = 15.23 (1.65) years) who were recruited from clinical encounters (e.g., concussion clinic). All participants with SRC were evaluated within two weeks post-injury and were symptomatic at the first visit. A subset of 6 children returned for a follow-up visit following medical clearance as recovered from the SRC. Participants were considered medically cleared after receiving written approval from clinic specialists to return to full participation in high-risk sports and recreational activities without any modifications to the activity. Inclusion criteria were as follows: diagnosis of a concussion, as evidenced by a witnessed trauma to the head or trunk associated with the onset of at least two common post-concussive symptoms. Exclusion criteria were a loss of consciousness of > 30 minutes, post-traumatic amnesia > 24 hours, intracranial findings on clinical imaging, or previous moderate or severe traumatic brain injury.
The control group consisted of 13 never-injured age-matched neurotypical peers (9 males, 4 females; M (SD) = 15.59 years (1.50)). The neurotypical participants were recruited using flyers, radio advertisements, and word-of-mouth. Parent report indicated that participants in both groups did not have a history of seizures, pre-injury mood concerns, behavioral, learning, or educational diagnoses, or other major medical conditions. The Johns Hopkins Medicine Institutional Research Board approved the study protocol. Written informed consent and assent were obtained from a parent/guardian and child participants, respectively.
Procedures
Behavioral and neuroimaging data were acquired from the participants with SRC within two weeks of injury and from a subset of participants (n = 6) within three months of being cleared to return to play. All data were acquired during a single visit for the never-injured neurotypical participants. Clinical scans were obtained and reviewed by a neuroradiologist. There were no injury-related imaging findings. Incidental brain findings included small retrocerebellar arachnoid cysts in one participant with SRC and one control participant; the control also had a dilated perivascular space in the anterior right centrum semiovale. One additional control had an arachnoid cyst centered in the supravermian cistern.
Imaging protocol
Participants underwent a brief mock scan training to reduce anxiety and were trained to lie still. Magnetic resonance images were acquired on a 3.0 T Philips MRI scanner (Best, the Netherlands). DTI scans were obtained using a single-shot echo-planar sequence with sensitivity encoding (TR = 6.356 s, TE = 75 ms, sensitivity encoding acceleration factor 2.5, 2.2 mm axial slices, acquisition matrix of 96 × 96 [FOV = 212 mm], anterior-posterior phase encoding direction). Two DTI scans were collected per participant, with 32 gradient directions at b = 700 s/mm2 and one b0 in each scan. The two scans were concatenated to improve the signal-to-noise ratio (SNR). A double-echo T2 image was collected to correct for susceptibility artifact in the diffusion acquisition with the following parameters: TR = 4.16s, TE = 12/80 ms, flip angle = 90°, matrix 192 x 186, and FOV = 212 mm). A T1-weighted magnetization-prepared rapid gradient recalled echo (MPRAGE) sequence (slice thickness = 1.0 mm; field of view = 26 cm; matrix size = 256 × 256) was also collected.
DTI data processing
Diffusion data were preprocessed using FSL FDT version 6.0.1 (FMRIB’s Diffusion Toolbox part of FSL: http://www.fmrib.ox.ac.uk/fsl/fdt/) (Citation36). FSL’s TOPUP was employed to perform susceptibility distortion correction (Citation37,Citation38) using the first b0 image and a structural T2-weighted image with the same geometry as the diffusion data and a different phase-encoding direction. Registration to a non-distorted structural T2-weighted image with similar contrast has been shown to effectively correct susceptibility-induced geometric distortions and results in better anatomical alignment compared to field-map correction (Citation39,Citation40). Between-volume motion, eddy-current induced artifact and outlier slices were corrected using FSL’s Eddy (Citation41) using parameter – “repol,” wherein outlier slices are replaced with estimates by a Gaussian process (Citation42). Magnetic field gradient vectors were rotated to retain accuracy after eddy current and motion correction, and brain masks at each stage were obtained using FSL BET. A tensor model was applied at each voxel using FSL’s DTIFIT to estimate fiber orientation. The diffusion images were warped to a standard template using linear and non-linear registration (Citation36,Citation43,Citation44). To examine motion, framewise displacement (FD) between volumes (Citation45) was estimated and averaged to derive mean FD, using the 6 head motion parameters generated from FSL eddy (Citation41). The distribution of mean FD for each group was visualized in R (Citation46) using package ggplot2. The superior corona radiata and corpus callosum ROIs were generated using the Mori et al. (2008) White Matter Parcellation Map (Citation47) and were back-projected into each participant’s native diffusion space using the inverse non-linear and linear transformations. Regional measures of FA and MD were interrogated for each ROI. For the present study, analyses were focused on white matter tracts known to be affected in SRC and involved in motor function, namely the corpus callosum and superior corona radiata (Citation20,Citation32,Citation33). Corpus callosum values indicate averaged values across the right and left genu, body, and splenium ROIs. Superior corona radiata values indicate averaged values across the right and left regions.
Behavioral measures
Revised Physical and Neurological Examination of Subtle Signs (PANESS)
The Revised PANESS is a standardized neurologic assessment that assesses subtle motor signs in children during balance, gait, and timed motor function tasks (Citation27). Procedures for administering and scoring the PANESS have been published (Citation48). The PANESS consists of two primary subscores, namely the Gaits and Stations and Total Timed. The subscore Gaits and Stations include gait tasks of walking on heels, sides of feet, and toes, tandem gait (forward and backward), and balance tasks of tandem standing with eyes closed, standing on both feet with arms and fingers outstretched and eyes closed and, standing on one foot, and hopping. The Total Timed score consists of the time taken to complete 20 movements of 6 tasks which include foot-tapping, hand patting, finger tapping, heel-toe tapping, hand pronation/supination, and finger apposition (tapping the thumb to each of the four other fingers in a fixed sequence) and 10 movements of moving the tongue side-to-side. The Total PANESS score is a cumulative score of the Gaits and Stations and Total Timed subscores. The Total PANESS score is the primary variable of interest in this study. Higher scores indicate worse motor performance, suggesting the presence of dynamic and static balance issues, subtle signs of overflow and dysrhythmia, and speed/accuracy impairments. The PANESS has been found to have adequate inter-rater and test–retest reliability, internal consistency, and sensitivity to age-related changes in older children (Citation24,Citation48).
Statistical analyses
Using Pearson’s correlations, age was investigated as a potential confounder by checking if it had a large correlation coefficient (greater than .6) with FA and MD measures and PANESS scores (Citation49). Independent sample t-tests were used to examine group differences in mean FD (motion). Univariate analyses of variance (ANOVAs) were used to examine group differences on PANESS scores as well as FA and MD measures of the corpus callosum and superior corona radiata. Effect size is reported using Cohen’s d (.2: small; .5: medium; .8: large) (Citation50). Paired-sample t-tests were used to examine longitudinal changes in white matter diffusivity in a subset of participants with SRC that returned for a follow-up visit post medical clearance. Effect sizes are reported using Hedges’ g (.21: small; .5: medium; .8: large) (Citation51). Partial Pearson’s correlations controlling for age were used to examine the relationship between measures of white matter diffusivity that were significantly different between groups and PANESS scores. To correct for multiple comparisons with 4 regions of interest, the Bonferroni-corrected alpha for the ANOVAs was set at .0125 (.05/4). However, given the exploratory nature of the brain-behavior analyses and the small sample of longitudinal analyses (n = 6), the alpha was set at .05 for the correlation and paired t-tests. Statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS), version 27.0.
Results
Age was not significantly different between the groups, t(23) = .58, p = .57. At the initial symptomatic visit, the mean days since injury was 9.33 days, range: 5–14 days. For the subset of six participants who returned for a follow-up visit post medical clearance, the mean days since injury was 45 days, range: 29–73 days, and the mean days since medical clearance was 34.5 days; range 6–45 days. Means and standard deviations of FA and MD measures and PANESS scores are listed in . Age did not significantly correlate with FA and MD measures of the superior corona radiata or corpus callosum (p’s > .05; See Supplementary file). There were no outliers (> 2SD) except the MD of the superior corona radiata of one control participant; analyses conducted with and without the outlier data showed no difference in the statistical findings, and hence the participant was included. Independent samples t-tests showed that there were no group differences on mean FD between the SRC and control groups (SRC visit 1 vs. controls, p = .48, SRC visit 2 vs. controls, p = .57). See Supplementary file.
Table 1. Means and standard deviations for DTI indices and PANESS scores
Group difference on FA and MD measures
At the initial visit, adolescents with SRC had significantly higher MD of the superior corona radiata compared to controls, F(1,23) = 14.38, p = .001, d = 1.52. There were no group differences on FA of the superior corona radiata, F(1,23) = 2.09, p = .16, d = .58, or FA of the corpus callosum, F(1,23) = 1.02, p = .32, d = .40 or MD of the corpus callosum, F(1,23) = .70, p = .41, d = .34.
Group difference in PANESS scores
In the control group but not in adolescents with SRC, age was significantly associated with total PANESS score (r = −.58, p = .04), such that motor performance improved with increasing age. To control for the effect of age on motor function, age was added as a covariate. At the initial visit, controlling for age, adolescents with SRC had significantly higher (worse) Total PANESS scores than controls, F(1,22) = 20.81, p < .0001, d = 1.79.
Brain–behavior relationships
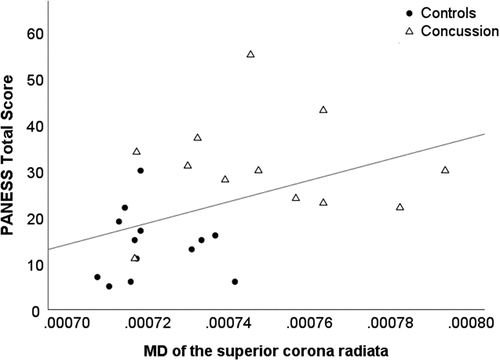
At the initial visit, controlling for age, across all participants, higher MD of the superior corona radiata was significantly associated with poorer motor performance on the Total PANESS score, r = .43, p = .034. See .
Longitudinal changes in white matter diffusivity
Within-group analysis in adolescents with SRC, there was no difference between the initial symptomatic visit and post medical clearance in the FA of the superior corona radiata, t(5) = .96, p = .38, g = .21, nor MD of superior corona radiata, t(5) = .92, p = .40, g = .50. Adolescents with SRC showed significantly lower FA of the corpus callosum post-medical clearance compared to the initial symptomatic visit, t(5) = 2.82, p = .037, g = .21. However, there was no difference between MD of the corpus callosum between visits, t(5) = −.37, p = .72, g = .30.
Discussion
This study examined white matter diffusivity of motor-associated white matter tracts, namely the corpus callosum and superior corona radiata in adolescents with a SRC within two weeks post-injury as compared to never-injured age-matched neurotypical peers. This preliminary investigation provides the first evaluation of the relationship between alterations in white matter diffusivity and subtle motor function in adolescents with SRC. As hypothesized, adolescents with SRC showed significantly higher MD of the superior corona radiata compared to controls. Across all participants, higher MD of the superior corona radiata was associated with greater subtle motor deficits. There were no group differences in the FA of the superior corona radiata and the FA or MD of the corpus callosum. Preliminary longitudinal analysis with a subset of adolescents with SRC showed a significant decrease in FA of the corpus callosum from the initial symptomatic visit (within 2 weeks) to post-medical clearance (mean of 45 days post-injury) with no change in the MD of the superior corona radiata.
Compared to controls, adolescents with a SRC showed significantly higher MD of the superior corona radiata. This is in contrast to some prior studies reporting reduced MD in the acute phase (ranged from 1–21 days post-injury) of SRC/mTBI (Citation30,Citation31,Citation52). However, our findings are consistent with several recent large studies (Citation17,Citation53). In a study of 219 collegiate athletes (82 athletes with concussion, 68 contact-sport controls, and 69 non-contact sport controls), athletes with concussion showed significantly higher MD across several white matter tracts compared to controls at 24 to 48 hours post-injury and MD remained elevated at 6-months post-injury (Citation53). In another recent study of 122 controls and 24 adolescents with SRC, MD of the superior corona radiata was significantly elevated in adolescents with SRC during the initial symptomatic visit (within 1 week after injury) compared to control peers (Citation17). Similarly, symptomatic college athletes with SRC 1-month post-injury showed increased MD in several white matter tracts including the superior corona radiata (Citation11). Higher MD values following brain injury reflect axonal disconnection or damage to myelin sheaths indicative of decreased integrity of white matter microstructure (Citation7). In the current study, FA of the superior corona radiata was lower in adolescents with SRC compared to controls, although this difference did not reach statistical significance (p = .16). This could be attributed to a power issue due to the small sample size.
Recent systematic reviews focusing on pediatric SRC have noted the heterogeneity in DTI findings (Citation54,Citation55). In addition to differences in study methodology and participant characteristics, this variability is partly attributed to SRC being one of the mildest forms of brain injury with the greatest fluctuations of symptom presentations and mechanisms of injury (Citation56). There is some evidence suggesting that due to repetitive head impacts, even participation in contact sports may negatively impact white matter integrity (Citation57,Citation58). Additionally, depending on the brain region measured, the direction of change can be inconsistent for a specific DTI metric within individual athletes (Citation59). This illustrates the currently incomplete understanding of the precise neurobiological sequelae following SRC. While the current preliminary study adds to the growing body of literature demonstrating DTI as a sensitive tool in the evaluation of SRC, additional research is necessary to further examine the patterns of DTI findings and their clinical implications.
Consistent with prior research, adolescents with SRC demonstrated significantly greater subtle motor deficits as measured by the PANESS (Citation24,Citation25,Citation29). Higher MD of the superior corona radiata was associated with greater subtle motor deficits. A recent study showed that elevated MD of white matter tracts following acute SRC (24–48 hours post-injury) was associated with prolonged recovery time and worse clinical outcomes as measured by the Brief Symptom Inventory scores and symptom severity scores (Citation53). The direct brain-behavior relationship between elevated MD and motor function suggests that the PANESS may be a promising marker for structural signs of injury post-SRC. In a longitudinal study of motor recovery, even after apparent clinical recovery, adolescents with SRC showed persistent motor deficits as measured by the PANESS as compared to controls (Citation24,Citation29). Further longitudinal research is warranted to examine how subtle motor deficits and alterations in white matter diffusivity recover over time.
Few studies have examined the association between DTI metrics and motor measures in children with brain injury. In children with mild to moderate brain injury assessed 6-months post-injury, lower FA of the corpus callosum was associated with slower motor speed (Citation60). In children with varying degrees of severity of brain injury in the chronic stage of recovery (> 6 months post-injury), reduced FA of the corticospinal tract as compared to controls was associated with balance and motor coordination deficits (Citation61,Citation62). The superior corona radiata which includes the corticospinal tract has an important role in motor function (Citation33). These results show that DTI metrics can link the microstructure of motor-associated white matter pathways to subtle motor function in adolescents with acute SRC. However, further research is required to identify if injury to specific white matter pathways and regions is responsible for motor deficits observed in SRC. Given the minimal studies examining brain–behavior relations with sensitive assessments in adolescents with SRC, the present study findings may be of particular importance in identifying promising measures that should be further explored in larger studies.
In our current study, there were no group differences on the FA or MD of the corpus callosum at the initial symptomatic visit. However, the preliminary longitudinal analysis showed a reduction in the FA of the corpus callosum between the initial symptomatic visit and the visit post-medical clearance. This pattern of reduction is consistent with longitudinal DTI studies showing reductions in FA of the corpus callosum from the subacute to the acute stages of recovery (Citation19,Citation63). A recent study in 24 adolescents with SRC showed no group difference in FA or MD measures of the corpus callosum compared to an orthopedically injured control group at 96 hours post-injury but found significant reductions in the FA and increases in the MD of the corpus callosum in the same participants at 3-months post-injury (Citation19). The authors suggested that the longitudinal decrease in the FA of the corpus callosum in participants with SRC group may indicate that microstructural sequelae following SRC may progressively develop (Citation19). In another large longitudinal study, collegiate athletes with SRC showed persistently elevated MD of the corpus callosum at 6 months post-injury (Citation53). However, others have failed to find differences in the FA or MD of the corpus callosum in children (8–17 years) with persistent post-concussive symptoms (Citation64). In the current study, there was no difference in the MD of the superior corona radiata between the initial symptomatic and post-medical clearance visit, suggesting that elevated MD of the superior corona radiata may be a persistent effect. A recent longitudinal study showed that while other white matter tracts such as the posterior corona radiata demonstrated reductions in FA from the acute symptomatic visit to the asymptomatic visit after return-to-play, significant elevations of MD of the superior corona radiata persisted at the later return-to-play visit, and the 1-year follow-up visit, suggesting long-term impact (Citation17). However, further research in a larger cohort is required to validate this finding.
A limitation of this study includes the small sample size, which may have limited our ability to detect significant relationships with smaller effects; nevertheless, the findings from this pilot cohort can be used to inform hypothesis generation, measure selection, and sample sizes for future studies. Further, the large effect sizes that were detected and corroborations of findings from recent large studies suggest that though our sample size is limited, the findings may be clinically relevant. The preliminary longitudinal analyses consisted of a small sample size and these results must be interpreted with caution due to the lack of correction for multiple comparisons. Given the small sample size, the participants with and without SRC were not matched on level of sports participation. However, level of participation must be considered as a potential confounding variable in future studies. Prior work has shown that SRC-associated differences in DTI metrics are subtler when compared to an orthopedically injured control group versus a typically developing control group (Citation19), and this should be considered when designing future studies. Given the small sample size of the current study, brain-behavior correlations were conducted across the entire sample; future studies must explore whether the relationship between DTI measures and motor performance differs among controls and children with an SRC. Another potential limitation includes the use of a less advanced, single shell, low b-value DTI sequence and using a registration-based approach with a structural T2-weighted acquisition rather than two or more non-distorted images with opposing phase-encoding for the correction of susceptibility artifacts. However, prior studies using similar DTI acquisition parameters (Citation62) as well as studies using more modern DTI parameters (Citation17,Citation53) have shown similar findings of white matter alterations in adolescents with SRC, providing support for the sensitivity of this acquisition to detect meaningful and expected white matter alterations in this population. Given the continuous advancements in neuroimaging, novel DWI sequences and methods for correcting susceptibility-induced distortions are being implemented to improve acquisition accuracy.
Currently, there are no clear, objective neurobiological biomarkers that can assist in predicting recovery from SRC despite the significant implications of a pediatric brain injury. This may lead to early or late medical clearance to return-to-play, both of which can have negative consequences. Subtle motor function appears to be a sensitive marker of motor impairment in youth with mTBI, such as SRC, in the acute and subacute stages. The current study findings demonstrate that this cohort of adolescents with SRC showed white matter alterations in the superior corona radiata which correlated with subtle motor signs. Given the important role of the superior corona radiata in motor function, these results suggest that observed subtle motor deficits in adolescents with SRC may be a clinically relevant marker for white matter injury. The use of sensitive biomarkers is expected to improve the prediction of outcomes after SRC. This pilot cohort demonstrates the potential of DTI and subtle motor function as markers of injury that merit further investigation.
Supplemental Material
Download MS Word (209.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Please contact the corresponding author to get the dataset associated with this study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Wiebe DJ, Comstock RD, Nance ML. Concussion research: a public health priority. Inj Prev. 2011;17(1):69–70. doi:10.1136/ip.2010.031211.
- Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24–33. doi:10.1227/NEU.0000000000000505.
- Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, Schofield P, Levi C, and Jones DK. A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma. 2012;29(16):2521–38. doi:10.1089/neu.2012.2628.
- Wilde EA, Goodrich-Hunsaker NJ, Ware AL, Taylor BA, Biekman BD, Hunter JV, Newman-Norlund R, Scarneo S, Casa DJ, Levin HS, et al. DTI indicators of white matter injury are correlated with multimodal EEG-based biomarker in slow recovering, concussed collegiate athletes. J Neurotrauma. 2020;37(19):2093–101. doi:10.1089/neu.2018.6365.
- Roberts RM, Mathias JL, Rose SE. Diffusion tensor imaging (DTI) findings following pediatric non-penetrating TBI: a meta-analysis. Dev Neuropsychol. 2014;39(8):600–37. doi:10.1080/87565641.2014.973958.
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–29. doi:10.1016/j.nurt.2007.05.011.
- Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25(4):241–55. doi:10.1097/HTR.0b013e3181e52c2a.
- Borja MJ, Chung S, Lui YW. Diffusion MR imaging in mild traumatic brain injury. Neuroimaging Clin N Am. 2018;28(1):117–26. doi:10.1016/j.nic.2017.09.009.
- Mainwaring L, Ferdinand Pennock KM, Mylabathula S, Alavie BZ. Subconcussive head impacts in sport: a systematic review of the evidence. Int J Psychophysiol. 2018;132:39–54. doi:10.1016/j.ijpsycho.2018.01.007.
- Narayana PA. White matter changes in patients with mild traumatic brain injury: MRI perspective. Concussion. 2017;2(2):CNC35. doi:10.2217/cnc-2016-0028.
- Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2010;28(2):189–201. doi:10.1089/neu.2010.1430.
- Smits M, Houston GC, Dippel DWJ, Wielopolski PA, Vernooij MW, Koudstaal PJ, Hunink MGM, and van der Lugt A Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2011;53(8):553–63. doi:10.1007/s00234-010-0774-6.
- Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28–37. doi:10.1542/peds.2011-2083.
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res. 2010;204(1):57–70. doi:10.1007/s00221-010-2294-3.
- Satchell EK, Friedman SD, Bompadre V, Poliakov A, Oron A, Jinguji TM. Use of diffusion tension imaging in the evaluation of pediatric concussions. Musculoskeletal Sci Pract. 2019;42:162–65. doi:10.1016/j.msksp.2019.05.002.
- Virji-Babul N, Borich MR, Makan N, Moore T, Frew K, Emery CA, and Boyd LA. Diffusion tensor imaging of sports-related concussion in adolescents. Pediatr Neurol. 2013;48(1):24–29. doi:10.1016/j.pediatrneurol.2012.09.005.
- Churchill NW, Hutchison MG, Graham SJ, Schweizer TA. Mapping brain recovery after concussion: from acute injury to 1 year after medical clearance. Neurology. 2019;93(21):e1980–92. doi:10.1212/WNL.0000000000008523.
- Lancaster MA, Olson DV, McCrea MA, Nelson LD, LaRoche AA, Muftuler LT. Acute white matter changes following sport-related concussion: a serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum Brain Mapp. 2016;37(11):3821–34. doi:10.1002/hbm.23278.
- Wu T, Merkley TL, Wilde EA, Barnes A, Li X, and Chu ZD. A preliminary report of cerebral white matter microstructural changes associated with adolescent sports concussion acutely and subacutely using diffusion tensor imaging. Brain Imaging and Behavior 2018; 12:962–73.
- Borich M, Makan N, Boyd L, Virji-Babul N. Combining whole-brain voxel-wise analysis with in vivo tractography of diffusion behavior after sports-related concussion in adolescents: a preliminary report. J Neurotrauma. 2013;30(14):1243–49. doi:10.1089/neu.2012.2818.
- Babcock L, Yuan W, Leach J, Nash T, Wade S, Suskauer S. White matter alterations in youth with acute mild traumatic brain injury. J Pediatr Rehabil Med. 2015;8(4):285–96. doi:10.3233/PRM-150347.
- Murdaugh DL, King TZ, Sun B, Jones RA, Ono KE, Reisner A, and Burns TG. Longitudinal changes in resting state connectivity and white matter integrity in adolescents with sports-related concussion. J Int Neuropsychol Soc. 2018;24(8):781–92. doi:10.1017/S1355617718000413.
- Kirkwood MW, Yeates KO, Taylor HG, Randolph C, McCrea M, Anderson VA. Management of pediatric mild traumatic brain injury: a neuropsychological review from injury through recovery. Clin Neuropsychol. 2008;22(5):769–800. doi:10.1080/13854040701543700.
- Stephens JA, Denckla MB, McCambridge T, Slomine BS, Mahone EM, Suskauer SJ. Preliminary use of the physical and neurological examination of subtle signs for detecting subtle motor signs in adolescents with sport-related concussion. Am J Phys Med Rehabil. 2018;97(6):456. doi:10.1097/PHM.0000000000000906.
- Stephens JA, Davies PL, Gavin WJ, Mostofsky SH, Slomine BS, Suskauer SJ. Evaluating motor control improves discrimination of adolescents with and without sports related concussion. J Mot Behav. 2020;52(1):13–21. doi:10.1080/00222895.2019.1570908.
- McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–47. doi:10.1136/bjsports-2017-097699.
- Denckla MB. Revised neurological examination and subtle signs. Psychopharmacol Bull. 1985;21(4):773–79.
- Stephens JA, Salorio C, Denckla M, Mostofsky S, Suskauer S. Subtle motor findings during recovery from pediatric traumatic brain injury: a preliminary report. J Mot Behav. 2017;49(1):20–26. doi:10.1080/00222895.2016.1204267.
- Crasta JE, Raja AE, Caffo BS, Hluchan CM, and Suskauer SJ. The effect of age and competition level on subtle motor performance in adolescents medically-cleared post-concussion: preliminary findings. Am J Phys Med Rehabil. 2021;100(6): 563–569. doi:10.1097/PHM.0000000000001589.
- Mayer AR, Ling JM, Yang Z, Pena A, Yeo RA, Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. J Neurosci. 2012;32(50):17961–69. doi:10.1523/JNEUROSCI.3379-12.2012.
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–55. doi:10.1212/01.wnl.0000305961.68029.54.
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, and Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27(45):12132–38. doi:10.1523/JNEUROSCI.2320-07.2007.
- Kim JS, Pope A. Somatotopically located motor fibers in Corona radiata: evidence from subcortical small infarcts. Neurology. 2005;64(8):1438. doi:10.1212/01.WNL.0000158656.09335.E7.
- Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage. 2014;92:356–68. doi:10.1016/j.neuroimage.2013.12.044.
- Chun IY, Mao X, Breedlove EL, Leverenz LJ, Nauman EA, Talavage TM. DTI detection of longitudinal WM abnormalities due to accumulated head impacts. Dev Neuropsychol. 2015;40(2):92–97. doi:10.1080/87565641.2015.1020945.
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782–90. doi:10.1016/j.neuroimage.2011.09.015.
- Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20(2):870–88. doi:10.1016/S1053-8119(03)00336-7.
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19.
- Wu M, Chang L-C, Walker L, Lemaitre H, Barnett AS, and Marenco S. Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. In: Metaxas D, Axel L, Fichtinger G, and Székely G, editors. Medical image computing and computer-assisted intervention – MICCAI 2008. Berlin, Heidelberg: Springer; 2008. p. 321–29. (Lecture Notes in Computer Science).
- Tao R, Fletcher PT, Gerber S, Whitaker RT. A variational image-based approach to the correction of susceptibility artifacts in the alignment of diffusion weighted and structural MRI. In: Prince JL, Pham DL, Myers KJ, editors. Information processing in medical imaging Berlin. Heidelberg: Springer; 2009. p. 664–75. (Lecture Notes in Computer Science).
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063–78. doi:10.1016/j.neuroimage.2015.10.019.
- Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage. 2016;141:556–72. doi:10.1016/j.neuroimage.2016.06.058.
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56. doi:10.1016/S1361-8415(01)00036-6.
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–41. doi:10.1006/nimg.2002.1132.
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–54. doi:10.1016/j.neuroimage.2011.10.018.
- R Core Team. R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available from: http://www.R-project.org
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40(2):570–82. doi:10.1016/j.neuroimage.2007.12.035.
- Gidley Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB, Mahone EM. Effects of gender and age on motor exam in typically developing children. Dev Neuropsychol. 2007;32(1):543–62. doi:10.1080/87565640701361013.
- Feldt LS. A comparison of the precision of three experimental designs employing a concomitant variable. Psychometrika. 1958;23(4):335–53. doi:10.1007/BF02289783.
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi:10.3389/fpsyg.2013.00863.
- Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. [Internet]. J Exp Psychol Gen. 2020;141(1):2. doi:10.1037/a0024338. US: American Psychological Association; 20110808
- Chu Z, Wilde EA, Hunter JV, McCauley SR, Bigler ED, Troyanskaya M, Yallampalli R, Chia JM, and Levin HS. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol. 2010;31(2):340–46. doi:10.3174/ajnr.A1806.
- Wu Y-C, Harezlak J, Elsaid NMH, Lin Z, Wen Q, Mustafi SM, Riggen LD, Koch KM, Nencka AS, Meier TB, et al. Longitudinal white-matter abnormalities in sports-related concussion: a diffusion MRI study. Neurology. 2020;95(7):e781–e792. doi:10.1212/WNL.0000000000009930.
- Chamard E, Lichtenstein JD. A systematic review of neuroimaging findings in children and adolescents with sports-related concussion. Brain Inj. 2018;32(7):816–31. doi:10.1080/02699052.2018.1463106.
- Narayana S, Charles C, Collins K, Tsao JW, Stanfill AG, Baughman B. Neuroimaging and neuropsychological studies in sports-related concussions in adolescents: current state and future directions. Front Neurol. 2019;10. doi:10.3389/fneur.2019.00538.
- Murugavel M, Cubon V, Putukian M, Echemendia R, Cabrera J, Osherson D, and Dettwiler A A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. J Neurotrauma. 2014;31(22):1860–71. doi:10.1089/neu.2014.3368.
- Myer GD, Yuan W, Barber Foss KD, Thomas S, Smith D, Leach J, Kiefer AW, Dicesare C, Adams J, Gubanich PJ, et al. Analysis of head impact exposure and brain microstructure response in a season-long application of a jugular vein compression collar: a prospective, neuroimaging investigation in American football. Br J Sports Med. 2016;50(20):1276–85. doi:10.1136/bjsports-2016-096134.
- Davenport EM, Whitlow CT, Urban JE, Espeland MA, Jung Y, Rosenbaum DA, Gioia GA, Powers AK, Stitzel JD, Maldjian JA, et al. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J Neurotrauma. 2014;31(19):1617–24. doi:10.1089/neu.2013.3233.
- Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging. 2012;30(2):171–80. doi:10.1016/j.mri.2011.10.001.
- Wozniak J, Krach L, Ward E, Mueller B, Muetzel R, Schnoebelen S, Kiragu A, and LIM K. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22(5):555–68. doi:10.1016/j.acn.2007.03.004.
- Caeyenberghs K, Leemans A, Geurts M, Taymans T, Vander Linden C, Smits-Engelsman BCM, Sunaert S, and Swinnen SP. Brain-Behavior relationships in young traumatic brain injury patients: fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia. 2010;48(5):1472–82. doi:10.1016/j.neuropsychologia.2010.01.017.
- Caeyenberghs K, Leemans A, Geurts M, Linden CV, Smits-Engelsman lBCM, Sunaert S, and Swinnen SP. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil Neural Repair. 2011;25(6):492–502. doi:10.1177/1545968310394870.
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, Reichard R, Yeo RA, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74(8):643–50. doi:10.1212/WNL.0b013e3181d0ccdd.
- Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, and Ashwal S. Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. J Neurotrauma. 2014;31(17):1497–506. doi:10.1089/neu.2013.3213.