ABSTRACT
Background
Physiological differences between a maturing and matured brain alters how Sports-Related Concussion (SRC) affects different age groups; therefore, a review specific to university-aged student-athletes is needed.
Objectives
Determine time to recovery for symptom burden, neurocognitive and Vestibular-Ocular-Motor (VOM) function and academic impact in university-aged student-athletes.
Methods
Searches were conducted in PubMed, SpringerLink, PsycINFO, Science Direct, Scopus, Cochrane, Web of Science and EMBASE. Articles were included if they contained original data collected within 30 days in university-aged student-athletes, analysed SRC associated symptoms, neurocognitive or VOM function or academic ability and published in English. Two reviewers independently reviewed sources, using the Oxford Classification of Evidence-Based Medicine (CEBM) and the Downs and Black checklist, and independently extracting data before achieving consensus.
Results
58 articles met the inclusion criteria. Recovery of symptoms occurred by 7 and 3–5.3 days for neurocognition. The evidence base did not allow for a conclusion on recovery time for VOM function or academic ability. Few papers investigated recovery times at specified re-assessment time-points and have used vastly differing methodologies.
Conclusions
To fully understand the implication of SRC on the university-aged student-athlete’ studies using a multi-faceted approach at specific re-assessments time points are required.
Systematic review registration number: CRD42019130685.
Introduction
Sports-Related Concussion (SRC) has been defined as ‘a complex pathophysiological process affecting the brain, induced by biomechanical forces with several common features that help define its nature’ (Citation1). An estimated 3.8 million SRC occur each year in the United States of America (Citation2) with an injury rate in collegiate-aged students of 5.56/10,000 sessions (Citation3), though the predicted prevalence may be much higher with 50–85% of SRC going undiagnosed or unreported (4). Student-athletes with concussion may present with varying symptoms of headache, dizziness, impaired concentration, memory loss, sleep disturbance, altered mood, problems concentrating and remembering, and visual disturbances. These factors have been reported to affect academic performance in the absence (Citation4–6) and presence of SRC (Citation7–10).
Dysfunction in one system influences performance of another function (Citation11); therefore, a multifaceted approach to SRC management (Citation8) is required to understand how long recovery from SRC takes. Recovery has been reported to occur within 7–10 days (Citation12), with greater injury severities and longer recovery trajectories reported in younger athletes (under 18 years of age) due to increased vulnerability of the developing brain (Citation13,Citation14), brain structure and reduced musculature (Citation15). It is well documented that high-school students (typically 14–18 year olds) have more severe symptoms than collegiate student-athletes (typically 18–22 years old) (Citation16), more severe and protracted neurocognitive recoveries (Citation16,Citation17), altered levels of eye movement control (Citation18) and greater difficulty returning to learn (Citation19). Development of return to learn protocols has been based on current literature, but the majority of research in this field focuses on high-school aged athletes. It may not be appropriate to apply these return to learn protocols to university-aged student-athletes.
A previous systematic review including papers whose participants were of all ages, establishing symptom and cognitive recovery only, reported recovery occurred by 6 and 5 days, respectively (Citation20). Papers were only included up to December 2013 and included papers that were outside of the acute phase (30 days) when many student-athletes may have already recovered. At present no systematic review has examined acute recovery of symptom, neurocognitive, and VOM dysfunction and academic activity impact in solely university-aged student-athletes following a SRC. Therefore, the purpose of this systematic review is to explore the effects of SRC in the acute phase on associated SRC symptoms, neurocognition and VOM function and the impact on academic activities. The review questions are (Citation1): How long does symptom, neurocognitive, VOM and academic ability take to recovery following an SRC in university-aged student-athletes? (Citation2) What symptom, neurocognitive and VOM impairments are seen within the acute period (initial 30 days) of SRC in university-aged student-athletes?
Materials and Methods
The review protocol was structured according to the PROSPERO database for systematic reviews and was approved for registration (protocol ID: CRD42019130685). The university-aged population was defined as students studying full- or part-time courses who were 18–25 years of age and on either a sport scholarship or competing for a university team. Academic activities were defined as any activity that a student-athlete would be expected to do as part of their studies, including reading, listening, processing information or memorising material. Symptom, neurocognitive and VOM impairment was defined as any alteration in state or function from baseline. The acute period was defined as 30 days or less post injury. Definitions sourced in the literature in a recent systematic review (Citation21) were used to define when recovery occurred as either asymptomatic, returning to baseline, or returning to learn.
Study selection
Articles were retrieved via online database searching and hand searching reference lists. Searches were conducted in PubMed, SpringerLink, PsycINFO, Science Direct, Scopus, Cochrane, Web of Science and EMBASE for articles published in English and from database inception to March 2020. Key search terms were used for concussion, neurocognitive testing, VOM function and post-concussion symptoms. Full search strategies can be found in Supplementary Document 1. The titles and abstracts of articles identified in the searches were screened independently by two reviewers according to the inclusion criteria and exclusion criteria.
Articles were included if (Citation1): the article included original data (Citation2); the article was published in English or had been translated into English (Citation3); participants had been diagnosed with SRC and had been assessed within 30 days (Citation22); included collegiate/university-aged participants (18–25 years of age) (Citation4); participants were assessed for either SRC associated symptoms, neurocognitive function, VOM function or academic impact. Articles were excluded if (Citation1): articles were commentaries, letters, editorials, conference proceedings, case reports, conference abstracts or non-peer reviewed articles (Citation2); studies were of animal or biomechanical models of brain injury (Citation3); studies examined non-sport-related concussions, acquired or non-traumatic brain injury (e.g. Alzheimer’s, AIDS, cancer, PTSD and stroke) (Citation22); articles included participants with brain injuries or pathologies other than SRC (e.g., upper body fractures, lesions) (Citation4); data collection was not within acute period post-concussion (within 30 days) (Citation5); participants were not of collegiate/university age or mixed age cohorts in which data specifically to university-aged participants could not be extracted (Citation6); the article did not contain data on symptoms, neurocognitive, VOM assessment or academic impact.
Data Extraction and Quality Assessment
Agreement was sought between both reviewers on the reason for exclusion or inclusion for all papers. Those papers meeting the inclusion criteria were retrieved and reviewed to ensure inclusion criteria were met. The level of evidence was rated using the Oxford Classification of Evidence-Based Medicine (CEBM) and the quality of the papers was assessed using the Downs and Black checklist (Citation23) (see Supplementary Document 2).
To mitigate risk of bias, both reviewers independently extracted the first author, year of publication, number of participants, percentage of males, re-assessment time points, assessment scale used, test scores or analysis of tests and time for recovery, before achieving consensus on the data extracted. Time to recovery was extracted from papers reporting specifically when recovery occurred.
Data Synthesis and Analysis
Comparison to baseline is more reliable (Citation24–26); therefore, in the first instance, baseline scores were used to calculate score change. Scores from healthy controls were only used if baseline scores were not available. The mean score change post recovery was used to identify when test scores had returned to less than the clinically meaningful change and therefore not significant, or when scores had returned to baseline following SRC.
Results
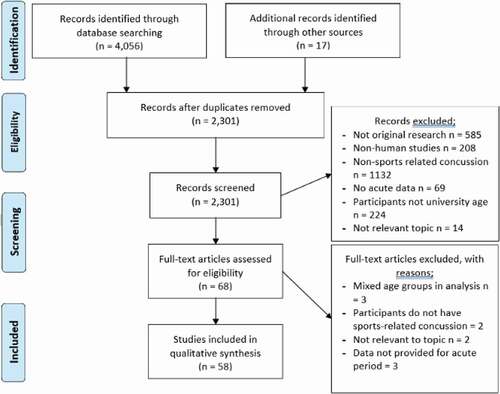
A total of 4056 papers were returned from database searches and 17 additional papers sourced from reference lists. There were 4005 papers that did not fulfil the inclusion criteria and were excluded, with a further 10 papers being excluded after a full review (), resulting in 58 papers being included in the review. There were 27 papers reporting specifically when recovery occurred, 34 reported data on symptom scores, 30 with data on neurocognitive test scores, 19 papers with data on VOM recovery and one paper with data on academic impact. Since the last relevant systematic review (Citation20), an additional 30 papers were sourced from this current search strategy.
Risk of Bias and Paper quality
The level of evidence according to the CEMB grading system ranged from two to four (scoring range of CEMB 1–5) and the Downs and Black quality assessment tool revealed a general trend for poor external validity. All extracted data are presented in Supplementary Document 2, along with the CRMB grade and Down and Black quality assessment score. Many papers recruited an unequal number of males and females (Citation27–32) or participants mainly from one sport (Citation33–38). Internal validity scored poorly, largely due to a lack of randomisation (scoring can be seen in Supplementary Document 2). It is not feasible to control who does and does not suffer a SRC; therefore, it is not feasible to recruit an equal number of females/males, various sports or randomise participants. Therefore, this should be considered when applying quality assessment tools to research in this field.
Few papers grouped participants into those with concussion or those without (Citation39,Citation40), or assigned by key features, for example immediate or delayed removal from play (Citation41). Reporting quality was high with most of the papers reviewed (see Supplementary Document 2). Transparency of methodologies was excellent in most papers, with just a few exceptions (Citation42–44).
How long does recovery take following Sports-Related Concussion (SRC)?
Symptom Recovery
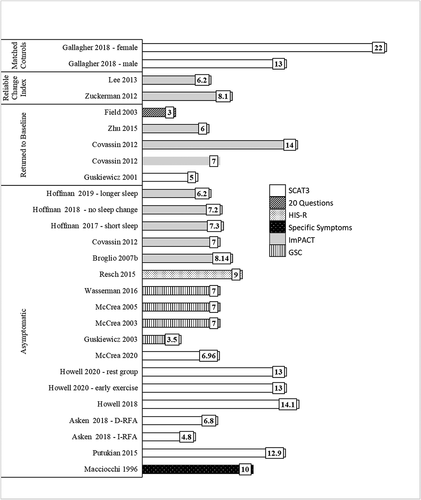
Of the 58 included papers in this systematic review, 34 papers reported either when recovery occurred or baseline scores so that readers were able to see when scores had either resolved, returned to baseline or within the RCI. Four different definitions of recovery were defined in the sourced papers: time until asymptomatic, returned to baseline score, returned to within 80% Reliable Change Index (80% RCI) or when medically cleared. Five symptom scales were used to assess symptom severity post SRC. These were: the Immediate Post Concussion and Cognitive Test (ImPACT) used by 15 studies; Graded Symptom Checklist uses a 17-item list (GSC-17) and was used in five papers; Head Injury Scale (HIS) used by two studies; the Post-Concussion Symptom Scale (PCSS) on a version of the Sport Concussion Assessment Tool (SCAT) was used by 10 studies; and two papers reported specific symptoms.
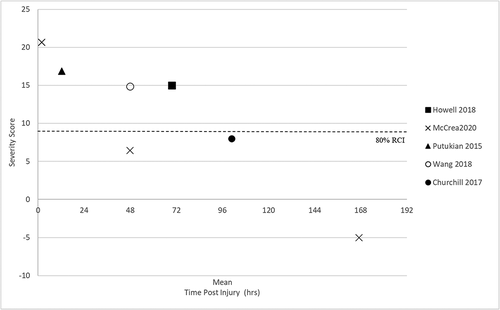
demonstrates that whilst a large range in mean time to recovery was reported (3 (Citation45) to 22 days (Citation35)), the majority of the studies reported symptom recovery occurred by seven days (Citation27,Citation41,Citation45–55). The range of days for recovery to occur may be due to the different criteria the studies used to define recovery. Six papers specifically re-assessed when participants reported when symptoms resolved (Citation27,Citation35,Citation37,Citation50,Citation53,Citation56). Five of the six papers reported the mean time to symptom recovery was 7–8 days, with the exception being Gallagher et al (2018) who reported recovery was at 22 days.
Figure 2. Mean number of days to symptom recovery for papers specifically detailing when symptom recovery occurred (sport concussion assessment tool (SCAT3), the revised head injury symptom scale (HIS-R), immediate post concussion and cognitive test (impact), graded symptom checklist (GSC). method of recovery definition by either being asymptomatic, returning to baseline, medically cleared or within one reliable change index to baseline. Participant groupings; immediate removal from activity (I-RFA), Delayed removal from activity (D-RFA)).
After extracting the data from the other 34 studies, symptom recovery may have occurred sooner than 7–8 days. On ImPACT, an elevated symptom score was reported by all papers using ImPACT post SRC until day 4 (). On the PCSS, SCAT, SCAT2 and SCAT3 symptom, severity scores were within baseline at 4 days () and scores on the HIS were only elevated within 24 hours of SRC (Citation32,Citation37) (26.4 ± 9.9 and 25.7 ± 17.10, respectively). Symptom scores remained elevated on the Graded Symptom Checklist (GSC) at five days post and had returned to baseline by seven days (Citation52).
Figure 3. Symptom score change from baseline on immediate post concussion assessment and cognitive test (impact).
Neurocognitive Recovery
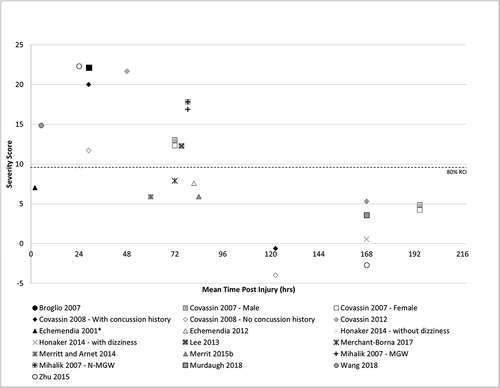
There were 30 papers sourced in this systematic review assessing neurocognition. Two types of neurocognitive tests were used to assess neurocognition; pen and paper assessments were used in 17 papers and computerised neurocognitive tests were used by 22 papers (see Supplementary Document 2). Studies defined neurocognitive recovery by statistical and clinical significance compared to healthy controls (Citation42,Citation45,Citation49,Citation57) or return to mean baseline scores (Citation52,Citation54). Studies meeting the inclusion criteria used four different computerised neurocognitive tests. The Immediate-Post Concussion and Cognitive Test (ImPACT) was used in 16 papers, the Automated Neurocognitive Assessment Metrics (ANAM) was used by three papers and Cogsport and CS Logix were both used in one paper (see Supplementary Document 2). Papers specifically investigating recovery trajectory reported university-aged student-athletes neurocognitive recovery ranged from two to seven days ().
Figure 5. Mean number of days for neurocognitive recovery. (standardised assessment for concussion (sac), immediate post-concussion and cognitive test (impact), vs controls = athletes with concussion compared to controls, no impairment = deemed recovered when test scores had returned to baseline, x axis = hours post sports-related concussion).
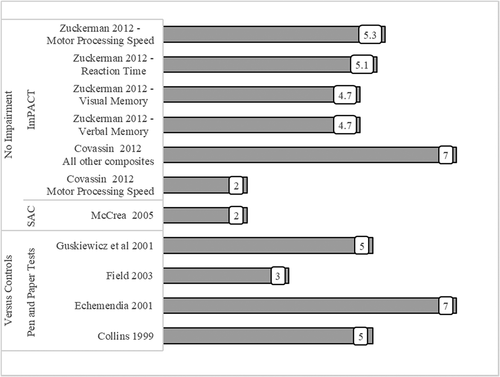
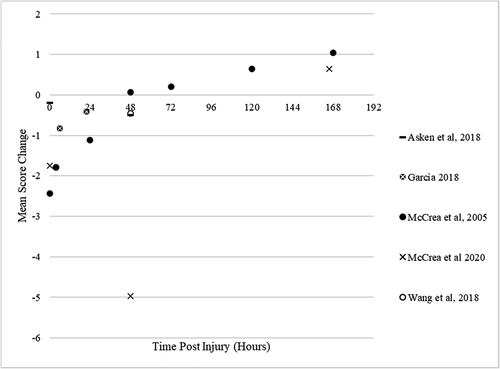
Figure 6. Mean score change post sports related-concussion for standardised assessment for concussion (SAC) (x axis = hours post sports-related concussion).
Studies that published baseline or control data enabled analysis of when recovery had returned to within expected limits or baseline. On pen and paper neurocognitive tests recovery occurred between two (Citation52) to five days (Citation42,Citation49). Comparison of healthy controls to university-aged student-athletes with concussion revealed significant difference in Trail-Making Test A, Paced Auditory Serial Addition Test 3 and 4 and Digit Symbol Substitution Test at 24 hours only (Citation58). The pen and paper Standardised Assessment of Concussion (SAC), advocated by concussion consensus papers (Citation1), demonstrated no significant or clinically meaningful change to baseline or compared to healthy controls at any time point (Citation27,Citation41,Citation52,Citation59–61). Using the 80% RCI to indicate when recovery occurred, impairment was still seen on ImPACT’s verbal memory at one day (Citation46), two days (in males only) (Citation62) and three days post SRC (Citation63) (). Visual memory composite scores were only clinically significant in two papers at one day post SRC (Citation31,Citation46), as were scores on motor processing speed (Citation46,Citation64). Reaction times were clinically significant at one day (Citation31,Citation65), two days (Citation66,Citation67), three days (Citation63,Citation68,Citation69) and in those with previous concussion history at five days post SRC (Citation31).
No impairment was seen in studies using ANAM (Citation43,Citation70,Citation71). Livingston et al. (2012) and Sosnoff et al. (2007) used the Concussion Resolution Index, and reported scores were significantly delayed at day one, three and five compared to baseline (Citation32) but not at day 10, along with significantly slower reaction times compared to controls at 48 hours (Citation72). Reaction time composite on CogSport assessed at 3 days was significantly reduced (Citation34) and on CS Logix (Citation59).
Vestibular-Ocular-Motor (VOM) Recovery
The search strategy sourced 13 papers, using six different tests to assess VOM function (see Supplementary Document 2). No papers focused on investigating when recovery of VOM function occurred. Vestibular impairment, assessed with the Balance Error Scoring System (BESS) revealed university-aged student-athletes with concussion were impaired immediately after concussion but not at 24 hours (Citation52) or within two days (Citation48,Citation61) and the Sensory Organisation Test found no vestibular impairment at any time point post SRC (Citation37,Citation43,Citation70). Significantly lower performances on the King-Devick test indicated vestibulo-ocular impairment immediately post-concussion (Citation73,Citation74), though impairment may recover soon after as no significant difference to baseline was seen within 5 days in a study using Snellen-like characters (Citation75).
Academic Recovery
One paper met the inclusion criteria (Citation28), but the studies design was not focused on when academic ability had recovered. Howell et al. (2020) study observed the majority of student-athletes had returned to class by 2–7 days post SRC. Of those who exercised 92% had returned, compared to 53% of those who had been unable to return to exercise.
What impairments are seen within the acute period (initial 30 days) of SRC in university-aged student-athletes?
Symptom Impairments
Some of the included papers stated specific details about what symptoms were experienced. One study reported university-aged student-athletes who reported dizziness were more likely to have higher symptom severity scores and less sleep (Citation66). One study not reporting scores, but percentages of how many student-athletes reported symptoms, found the reporting of headache, difficulty concentrating, drowsiness, neck pain, dizziness and balance or memory problems were most common (Citation43). Literature suggests symptoms affect an individual’s ability to process visual or verbal information during academic activities (Citation4–6), thus providing specific detail on what symptoms may indicate a student-athlete requires academic alterations following an SRC.
The use of symptom clusters in one study found 30% of the participants had one or more standard deviation’ from the mean in one of the clusters, without a significant change in the symptom severity score (Citation76). Symptom clusters were either cognition, mood, sleep, somatic and cranial (Citation77) or cognitive-sensory, sleep-arousal, vestibular-somatic versus, affective-cognitive, physical and affective-sleep (Citation76,Citation78). Clusters were significantly different to controls when assessed within six days (Citation77) and significantly different to baseline within seven days (Citation78) and 14 days (mean (3.71 ± 2.70 days) in the physical symptom cluster (Citation76). Clustering symptoms may provide further insight to what domain is affected and guide clinical management of the student-athlete.
Neurocognitive Impairments
Of the 30 papers reporting on neurocognitive testing, some papers explored the effects of neurocognition on other facets. Those with previous concussion episodes in Covassin et al. study (2008) and those reporting initial dizziness in Honaker et al. (2014) correlated with impairment on ImPACT’s verbal memory composite at seven and five days, and those with no history of SRC or no dizziness at initial assessment were not impaired at these time points. Reaction time speed was slower in those with previous concussion history (Citation31) and in those reporting dizziness (Citation66). It has been previously reported these factors can delay neurocognitive recovery following SRC (Citation79,Citation80). This suggests student-athletes with history of concussion or reporting dizziness may have more cognitive impairment.
Vestibular-Ocular-Motor (VOM) Impairments
Following a SRC studies revealed VOM impairment is present following a SRC in the form of altered balance (Citation48,Citation52,Citation61),, slower reading times on the King-Devick test immediately post-concussion (Citation73,Citation74), reduced visual acuity within 5 days (Citation75), slower gait at 2.9 1.4 days (Citation75) and at 2–7 days (early exercise groups; 3.8 1.8, rest group 2.8 1.2 days) (Citation28) and significantly greater distance of eye movements on gaze stability tests, more prosaccade errors and more horizontal velocity of eye movements, indicating impaired eye control assessed on Vicon Motion Capture analysis 24–48 hours post SRC (Citation81).
Academic Impairments
No papers described what academic impairments were seen following SRC in university-aged student-athletes.
Discussion
The purpose of this systematic review was to explore the effects of SRC in the acute phase of recovery on associated SRC symptoms, neurocognition and VOM function and the impact on academic activities by answering two questions. Firstly, papers included in this systematic review demonstrated symptoms took 7–8 days to recover (Citation27,Citation37,Citation56) and neurocognitive recovery took two (Citation52) to seven days (Citation57,Citation67). No papers investigated when recovery of VOM control occurred, but of those to include aspects of VOM function recovery may occur within two (Citation48,Citation61) to five days (Citation75). No studies investigated how long it took for academic ability to recover, but most student-athletes were able to return to class by 2–7 days post SRC (Citation28). The second research question sought to establish what impairments were found following a SRC. The most common symptoms following SRC were headache, difficulty concentrating, drowsiness, neck pain, dizziness, and balance or memory problems were most common (Citation43). Symptoms of dizziness was associated with higher symptom severity scores (Citation66), poorer neurocognitive performance (Citation66) and symptom clusters may provide greater insight to what impairments may be present. Balance (Citation48,Citation52,Citation61), altered reading (Citation73,Citation74) and visual acuity (Citation75) and ocular performance (Citation81) was seen on VOM assessments following SRC.
Recovery of Symptoms Following Sports-Related Concussion
Variation of how long symptom recovery took in some studies (3 days (Citation45) to 22 days post SRC (Citation35)) may be a consequence of methodologies not intending to specifically investigating when recovery occurred. Five of the six papers to specifically investigated how long symptom recovery took, reported a mean time to recovery of 7–8 days (Citation27,Citation37,Citation56). The other paper to reassess at specific time points was retrospective and recruited participants from a database by email (Citation35). The response rate was 39% and it is possible those with longer lasting symptoms were more likely to respond, explaining the protracted recovery time. Other studies reporting a longer time to symptom recovery did not describe the frequency of re-assessment (Citation40,Citation82). Returning to learn too soon has been shown to prolong recovery and exacerbate current symptom severity (Citation83); therefore, it is widely accepted that academic alterations following SRC are required (Citation84). The reviewed papers suggest academic adjustments may be required within the initial week post SRC and longer in some cases. Further prospective studies with specific re-assessment time points and transparency of baseline scores are needed to improve understanding of the recovery trajectory of university-aged student-athletes.
Seven different symptom reporting scales were used by the studies included in the systematic review. Of these, the symptom scale on ImPACT was used most frequently. A third of papers reporting on symptoms were retrospective (Citation55,Citation56,Citation66,Citation68,Citation85) using data spanning over a time period where attitudes and behaviour towards symptom reporting, assessment and management has been evolving (Citation86). The methodology enabled a large number of participants to be included, however, variable re-assessment time points narrowed what papers could reliability identify recovery trajectories. Additionally, there were discrepancies in the inclusion and exclusion criteria between studies, such as learning disability (Citation87), mental health disorders (Citation88) and sex (Citation89–91) may have affected the results of included studies. Future studies should consider including participants assessed at specific time points, with minimal variation in individual time to reassessment and post-hoc analysis for groups with risk factors predisposing to a longer recovery time may help to address inconsistency’ in inclusion and exclusion criteria.
Studies reporting symptom scores post SRC indicated mean symptom severity scores increased initially, reduced by day 3 and resolved by day 5 to 7 (). Symptom scores varied vastly between three to five days, which is consistent with pathophysiological studies suggesting recovery between three to five days can vary considerably (Citation92). Methodological differences between the studies may have also resulted in this variation. The mean severity score reported by Merritt et al. (2015b) and Echemendia et al. (2012) was almost half the mean symptom severity score reported by others a similar time point. The large range in days to reassessment post SRC in both Merritt et al. (2015b) and Echemendia et al.’ (2012) studies (within two weeks and 30 days respectively) may have allowed symptoms to resolve for some participants, given other studies have indicated symptoms have recovered by 7 to 10 days (Citation60,Citation93). Whilst this methodology was appropriate for the research question posed by Merritt et al. (2105b) and Echemendia et al. (2012), it may be misleading when determining expected symptom recovery and expected mean symptom severity scores.
Symptom clusters used in some studies (Citation77,Citation94,Citation95) may have more clinical relevance than just total symptom severity scores (Citation94,Citation96). Concussion symptoms vary vastly between people and even between each occurrence in the same person (Citation86), therefore, management of one concussion is never the same as the next (Citation97). An athlete may report many symptoms of low severity, totalling a low symptom severity score that does not reach clinical significance. However, if all these symptoms were within one cluster, authors have indicated an intervention is required for a particular facets (Citation7). Hence, improving the management of the individuals SRC. Future studies should consider the use of symptom cluster analysis, but agreement should be sought on a standardised symptom cluster model to allow comparison between studies. Furthermore, symptom clusters may help to further understand the potential impact of SRC on academic activities and shape assessments to quantify the potential impact.
Recovery of Neurocognition Following Sports-Related Concussion
After applying the inclusion and exclusion criteria, 32 papers detailing aspects of neurocognitive recovery in a collegiate student-athlete population were included in the systematic review. Methodological differences may explain the range in recovery from two (Citation52) to seven days (Citation57,Citation67). Firstly, reassessment time points varied in all but three papers (Citation32,Citation54,Citation64). In some studies, participants were reassessed across different days and then grouped into a single time-point, with the mean day to post SRC re-assessment varying greatly (within three days (Citation33,Citation62), 3.27 ± 5.97 (Citation63), two, seven and fourteen days (Citation54), 1–2 and 5–-7 days (Citation66), within seven days (Citation46), 2.9 ± 2.9 (0–Citation9) days (Citation69), two-four days (Citation27), within seven days (Citation2)). Secondly, two studies utilising pen and paper tests compared healthy controls to student-athletes with concussion and this methodology has since been reported to be unreliable due to learning affects (Citation49,Citation57).
Many studies using pen and paper tests did not detect any significant cognitive decline in participants with concussion on either comparison to controls (Citation42,Citation50,Citation75,Citation77) or baseline (Citation49,Citation98). Inconsistencies between papers on inclusion and exclusion criteria may also have resulted in the wide range of days to recovery. A longer recovery on ImPACT’s verbal memory composite was seen in those with risk factors for prolonged recovery (4 days to recovery (Citation31,Citation66)) than papers not excluding those with risk factors (within 4 days for recovery (Citation46,Citation54,Citation68)). It is important to understand how risk factors affect recovery, and therefore, future research would benefit from secondary analysis of these risk groups rather than excluding them.
Neurocognitive recovery occurred between two to seven days in studies specifically detailing when reported recovery occurred (). All studies repeated tests every other day, except Echemendia et al. (2001) and Covassin et al. (2012) where testing was repeated at two and seven days, and recovery was reported to occur by seven days. It is possible that participants in these studies may have recovered prior to being reassessed. Using data from the other studies in , neurocognitive recovery occurred between 3 and 5.3 days post SRC, which is shorter than recovery times seen on other age groups (Citation16,Citation17,Citation99). Considering all the papers included in this systematic review, only seven reported recovery times. Future studies with clearly defined reassessment time points, inclusion criteria and sub-analysis or higher risk groups and comparison of post SRC test scores to individual baseline may help to provide further clarification on when neurocognitive recovery occurs.
The sensitivity, reliability and validity of the different neurocognitive tests have been questioned in the literature (Citation100) and a recent meta-analysis showed they have poor reliability (Citation101) and are unable to identify those with concussion and without (Citation102). Studies sourced in this systematic review reported impairment on computerised neurocognitive tests, whilst no impairment was seen on the pen and paper tests (Citation27,Citation41,Citation61). Cognitive impairment was also seen despite being asymptomatic (Citation56), whilst another study reported 30% showed no impairment but reported symptoms (Citation71). It is widely accepted that presentation of SRC varies greatly between people and each episode for the same individual (Citation97). It is therefore, unsurprising, there is not a clear consensus on the presence of neurocognitive impairment or projected recovery times, with meta-analysis demonstrating neurocognitive tests are reliable within the initial 24 hours but are not sensitive thereafter (Citation101), hence clinicians are advised to use a multi-faceted approach rather than relying on computerised tests (Citation65).
Recovery of Vestibular-Ocular-Motor (VOM) Function Following Sports-Related Concussion
Methodological variations due to the aims of the studies included resulted in small sample sizes and re-assessment time points after 14 days, so no conclusions can be drawn on when VOM recovery occurs. This does not guide clinicians on an expected recovery trajectory. Many of the papers used methods that would not be feasible in a clinical setting (Citation28,Citation37,Citation43,Citation59,Citation70,Citation81,Citation103,Citation104) and do not provide the clinician with an assessment method that may guide management of student-athletes with concussion. Studies using the King-Devick Test had very low sample sizes of 11 (Citation74) and 9 (Citation73) leaving the study vulnerable to type II error. Advancements in the Vestibular-Ocular-Motor Screening Tool (Citation105–110) may provide an alternative method to assess VOM function. All included studies demonstrated VOM function is impaired after SRC and therefore, it is clear VOM function should be an essential component of assessment when returning student-athletes to academic and sporting activities. Future studies, with larger sample sizes and repeated assessments during the recovery phase, should be conducted to provide further insight to how VOM dysfunction post SRC impacts student-athletes.
Academic Impact of Sports-Related Concussion (SRC)
The search strategy revealed only one paper. Those who’s symptoms allowed physical activity to resume after 48 hours were more likely to have returned to class (Citation28). It has been reported that complete return to learn takes 18.3 ± 7.7 days in a group of collegiate athletes, with mathematical and computer-based studies being most troublesome (Citation111). Self-rated effect of SRC on academic ability has been trialled and found to be a reliable method of assessment in high-school children (Citation3) and shows promise as an objective way of measuring its influence. However, studies are needed in university-aged student-athletes due to the widely accepted premise age alters SRC recovery (Citation14,Citation20,Citation112,Citation113).
Limitations
There was a lack of high-quality papers, specifically investigating recovery times. Much of the analysis and discussion of recovery is based on data sourced from studies investigating other research aims; therefore, the methodologies of the sourced papers differ vastly. Information bias must be considered when interpreting the results of this systematic review, the definition of concussion and recovery differed considerably in the papers presented. The quality of research methods adopted by papers investigating SRS has been reported as variable and with papers lacking appropriate scientific rigor (Citation114). There were many examples of selection bias of the studies included in this review: small sample sizes, use of voluntary or convenient sample sizes, unknown and lack of clarity for attrition and poor matching to control subjects. This review is not exempt from publication bias. The research question focused on three areas of concussion assessment, and it is accepted other areas such as cervicogenic dysfunction, balance, autonomic nervous system function and psychological impact are recommended (Citation115). Only papers published in English were included. There may be further areas of dysfunction unique to student-athletes SRC recovery, and therefore, other papers available that evaluate these domains that have not been included in this systematic review. We were unable to pool data across studies and meta-analyse individual predictors or multiple predictors in combination.
Conclusion
Papers included in this systematic review have called for a multi-faceted approach to assessment post SRC due to concussion affecting individuals differently and concussion episodes being different in the same individual (Citation43,Citation66). Symptom recovery ranged from seven days and neurocognitive recovery occurred between 3 and 5.3 days. Some of the studies looked at the link between symptoms reported and the likelihood of neurocognitive impairment and found a strong correlation between reporting specific symptoms (headache, dizziness and poor sleep) and neurocognitive impairment (Citation27,Citation103,Citation116–118). Recovery of VOM function and academic ability could not be established due to the lack of studies in the evidence base. Given that every concussion can affect the individual differently no SRC is going to be exactly the same and so it may be unreasonable for a consensus on when different aspects of impairment recover post SRC. Comparing test scores on a multi-faceted battery post SRC to the individuals baseline rather than comparing the mean of healthy controls may provide more insight. Thus, providing the clinician with more reliable data and enabling better guidance on decision making for when to return to learn or sport and improving athlete care.
Compliance with Ethics
This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Acknowledgments
No funding was used for the undertaking of this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- McCrory P, Meeuwisse W, Dvořák J, Aubry M, Bailes J, Broglio S, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med [Internet]. 2017 Jun 26 [cited 2019 Nov 12];51(11):838–47. Available from: https://doi.org/10.1136/bjsports-2017-097699
- Murdaugh DL, Ono KE, Morris SO, Burns TG. Effects of developmental age on symptom reporting and neurocognitive performance in youth after sports-related concussion compared to control athletes. J Child Neurol. Internet]. 2018 Jun 18 [cited 2019 Mar 19];33(7):474–81. Available from doi:https://doi.org/10.1177/0883073818766815.
- Wasserman EB, Bazarian JJ, Mapstone M, Block R, Van Wijngaarden E. Academic dysfunction after a concussion among US high school and college students. Am J Public Health. 2016 Jul 1;106(7):1247–53. https://doi.org/10.2105/AJPH.2016.303154.
- Bigal ME, Bigal JM, Betti M, Bordini CA, Speciali JG. Evaluation of the impact of migraine and episodic tension-type headache on the quality of life and performance of a university student population. Headache. 2001;41(7):710–19.doi:https://doi.org/10.1046/j.1526-4610.2001.041007710.x.
- Desjardins P, Turgeon AF, Tremblay M-H, Lauzier F, Zarychanski R, Boutin A, Moore L, McIntyre LA, English SW, Rigamonti A, et al. Hemoglobin levels and transfusions in neurocritically ill patients: a systematic review of comparative studies. critical Care. 2012;16(2):R54.doi:https://doi.org/10.1186/cc11293.
- Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Medicine Reviews Sleep Med Rev. 2006;10(5):323–37.doi:https://doi.org/10.1016/j.smrv.2005.11.001.
- Ellis M, Krisko C, Selci E, Russell K. Effect of concussion history on symptom burden and recovery following pediatric sports-related concussion. J Neurosurg Pediatr. 2018Apr;214:401–08. Internet]. ;():. Available from https://doi.org/10.3171/2017.9.PEDS17392
- Schneider KJ. Concussion part II: rehabilitation – the need for a multifaceted approach. Musculoskelet Sci Pract [Internet]. 2019 Jul 1 [cited 2020 Feb 4];42:151–61. Available from: https://doi.org/10.1016/j.msksp.2019.01.006
- Halstead ME, McAvoy K, Devore CD, Carl R, Lee MA, Logan KL, Brenner JS, Demorest RA, Halstead ME, Kelly AKW. Returning to Learning Following a Concussion. Pediatrics. Internet]. 2013 Nov 1 [cited 2019 Mar 19];132(5):948–57. Available from ():. doi:https://doi.org/10.1542/peds.2013-2867.
- McGrath N. Supporting the student-athlete’s return to the classroom after a sport-related concussion. J Athl Train [Internet]. 2010 Sep [cited 2019 May 30];45(5):492–98. Available from. https://doi.org/10.4085/1062-6050-45.5.492
- Kenzie ES, Parks EL, Bigler ED, Wright DW, Lim MM, Chesnutt JC, Hawryluk GWJ, Gordon W, Wakeland W, et al. The dynamics of concussion: Mapping pathophysiology, persistence, and recovery with causal-loop diagramming. Front Neurol Internet]. 2018 Apr 4 [cited 2019 May 30];9(APR):1–16. Available from
- Davis GA, Ellenbogen RG, Bailes J, Cantu RC, Johnston KM, Manley GT, Nagahiro S, Sills A, Tator CH, McCrory P, et al. The berlin international consensus meeting on concussion in sport. Neurosurgery. 2018;82(2):232–36.doi:https://doi.org/10.1093/neuros/nyx344.
- Sim A, Terryberry-Spohr L, Wilson KR. Prolonged recovery of memory functioning after mild traumatic brain injury in adolescent athletes. J Neurosurg [Internet]. 2008 Mar [cited 2021 Jan 11];108(3):511–16. Available from. DOI:https://doi.org/10.3171/JNS/2008/108/3/0511.
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport - The 3rd International Conference on concussion in sport, held in Zurich, November 2008. J Clin Neurosci [Internet];16(6):755–63. Available from: https://doi.org/10.1016/j.jocn.2009.02.002
- Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br J Neurosurg. Internet]. 2002 Jan 6 [cited 2020 May 22];16(3):220–42. Available from doi:https://doi.org/10.1080/02688690220148824.
- McClincy MP, Lovell MR, Pardini J, Collins MW, Spore MK. Recovery from sports concussion in high school and collegiate athletes. Brain Inj. Internet]. 2006 Jan 3 [cited 2019 Mar 19];20(1):33–39. Available from doi:https://doi.org/10.1080/02699050500309817.
- Covassin T, Elbin RJ, Nakayama Y. Tracking neurocognitive performance following concussion in high school athletes. Phys Sportsmed [Internet]. 2010 Dec 13;38(4):87–93. Available from: https://doi.org/10.3810/psm.2010.12.1830
- Ventura RE, Balcer LJ, Galetta SL. The neuro-ophthalmology of head trauma. Vol. 13. New York, NY, USA: The Lancet Neurology. Lancet Publishing Group; 2014. p. 1006–16.
- Purcell LK, Davis GA, Gioia GA. What factors must be considered in ‘return to school’ following concussion and what strategies or accommodations should be followed? A systematic review. Br J Sports Med. 2019Feb;534:250–250. Internet. Available fromhttps://doi.org/10.1136/bjsports-2017-097853
- Williams RM, Puetz TW, Giza CC, Broglio SP. Concussion recovery time among high school and collegiate athletes: a systematic review and meta-analysis. Sport Med. Internet]. 2015 Jun 28 [cited 2019 Mar 19];45(6):893–903. Available from https://doi.org/10.1007/s40279-015-0325-8
- Haider MN, Leddy JJ, Pavlesen S, Kluczynski M, Baker JG, Miecznikowski JC, Willer BS, et al. A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med Internet]. 2018 Sep 1 [cited 2020 Dec 9];52(18):1179–90. Available from.https://doi.org/10.1136/bjsports-2016-096551
- Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP, et al. Current and emerging rehabilitation for concussion. A review of the evidence. Clin Sports Med Internet]. 2015 Apr 1 [cited 2019 May 30];34(2):213–31. Available from.https://linkinghub.elsevier.com/retrieve/pii/S0278591914001252
- Downs SH, Black N. Feasibility of creating checklist for assessment of method quality both of randomised and non randomised studies of health care innovations. J Epidemiol Community Health. 1998;52(6):377–84.doi:https://doi.org/10.1136/jech.52.6.377.
- Covassin T, Elbin RJ, Crutcher B, Burkhart S. The management of sport-related concussion: considerations for male and female athletes. Transl Stroke Res [Internet]. 2013 Aug 6 [cited 2019 Mar 19];4(4):420–24. Available from: https://doi.org/10.1007/s12975-012-0228-z
- Louey AG, Cromer JA, Schembri AJ, Darby DG, Maruff P, Makdissi M, Mccrory P, et al. Detecting cognitive impairment after concussion: Sensitivity of change from baseline and normative data methods using the cogsport/axon cognitive test battery Arch Clin Neuropsychol Internet]. 2014 [cited 2020 Jul 9];29(5):432–41.
- Elbin RJ, Schatz P, Lowder HB, Kontos AP. An empirical review of treatment and rehabilitation approaches used in the acute, sub-acute, and chronic phases of recovery following sports-related concussion. Curr Treat Options Neurol. Internet]. 2014 Nov 28 [cited 2019 Mar 19];16(11):320. Available from https://doi.org/10.1007/s11940-014-0320-7
- Hoffman NL, Weber ML, Broglio SP, McCrea M, McAllister TW, Schmidt JD. Influence of postconcussion sleep duration on concussion recovery in collegiate athletes. Clin J Sport Med Off J Can Acad Sport Med [Internet]. 2017 Nov 16 [cited 2019 Mar 19];1. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=29189340&site=ehost-live
- Howell DR, Brilliant AN, Oldham JR, Berkstresser B, Wang F, Meehan WP. Exercise in the first week following concussion among collegiate athletes: Preliminary findings. J Sci Med Sport [Internet]. 2020 Feb 1 [cited 2020 Nov 23];23(2):112–17. Available from: https://doi.org/10.1016/j.jsams.2019.08.294
- Lee H, Sullivan SJ, Schneiders AG. The use of the dual-task paradigm in detecting gait performance deficits following a sports-related concussion: a systematic review and meta-analysis. J Sci Med Sport. 2013;16(1):2–7. Internet]Available from. doi:https://doi.org/10.1016/j.jsams.2012.03.013
- Churchill N, Hutchison MG, Graham SJ, Schweizer TA. Symptom correlates of cerebral blood flow following acute concussion [Internet]. 2017 [cited 2019 Mar 19];16(April):234–39. Available from]. NeuroImage Clin. https://linkinghub.elsevier.com/retrieve/pii/S2213158217301845
- Covassin T, Stearne D, Elbin RJ. Concussion history and postconcussion neurocognitive performance and symptoms in collegiate athletes. J Athl Train [Internet]. 2008 Mar [cited 2019 Mar 19];43(2):119–24. Available from https://doi.org/10.4085/1062-6050-43.2.119.
- Livingston SC, Goodkin HP, Hertel JN, Saliba EN, Barth JT, Ingersoll CD. Differential rates of recovery after acute sport-related concussion: electrophysiologic, symptomatic, and neurocognitive indices. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc [Internet]. 2012 Feb [cited 2019 Mar 19];29(1):23–32. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=22353982&site=ehost-live
- Broglio SP, Macciocchi SN, Ferrara MS. Neurocognitive performance of concussed athletes when symptom free. J Athl Train. 2007;42(4):504–08.
- Eckner JT, Kutcher JS, Richardson JK. Effect of concussion on clinically measured reaction time in 9 NCAA division I collegiate athletes: a preliminary study. PM R J Inj Funct Rehabil [Internet]. 2011 Mar [cited 2019 Mar 19];3(3):212–18. Available from
- Gallagher V, Kramer N, Abbott K, Alexander J, Breiter H, Herrold A, Lindley T, Mjaanes J, Reilly J, et al. The effects of sex differences and hormonal contraception on outcomes after collegiate sports-related concussion. J Neurotrauma. 2018Jun [cited 2019 Mar 20];3511:1242–47. Internet. Available from
- Merritt VC, Rabinowitz AR, Arnett PA. Injury-related predictors of symptom severity following sports-related concussion. J Clin Exp Neuropsychol. Internet]. 2015 Mar 16 [cited 2019 Mar 19];37(3):265–75. Available from
- Resch JE, Brown CN, Macciocchi SN, Cullum CM, Blueitt D, Ferrara MS. A preliminary formula to predict timing of symptom resolution for collegiate athletes diagnosed with sport concussion. J Athl Train [Internet]. 2015 Dec [cited 2019 Mar 19];50(12):1292–98. Available from
- Hill K. Concussion symptoms and return to play time in youth, high school, and college american football athletes: Kerr ZY, Zuckerman SL, Wasserman EB, et al. JAMA Pediatrics. 2016;170:647–53. Accessed 19 March 2019. J Emerg Med [Internet]. 51(5). Available from: http://www.sciencedirect.com/science/article/pii/S0736467916308216
- Howell DR, Meehan WP, Barber Foss KD, Reches A, Weiss M, Myer GD, et al. Reduced dual-task gait speed is associated with visual go/no-go brain network activation in children and adolescents with concussion. BRAIN Inj. 2018;32(9):1129–34. Internet Available from. doi:https://doi.org/10.1080/02699052.2018.1482424
- Putukian M, Echemendia R, Dettwiler-Danspeckgruber A, Duliba T, Bruce J, Furtado JL, Murugavel M, et al. Prospective clinical assessment using sideline concussion assessment tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med. 2015Jan [cited 2019 Mar 19];251:36–42. Internet]. Available from http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00042752-201501000-00005
- Asken B, Bauer RM, Guskiewicz KM, McCrea M, Schmidt JD, Giza CC, Snyder AR, Houck ZM, Kontos AP, McAllister TW, et al. Immediate removal from activity after sport-related concussion is associated with shorter clinical recovery and less severe symptoms in collegiate student-athletes. Am J Sports Med Internet]. 2018 May 20 [cited 2019 Mar 20];46(6):1465–74. Available from.
- Collins MW, Grindel SH, Lovell MR, Dede DE, Moser DJ, Phalin BR, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA Internet]. 1999 Sep 8 [cited 2019 Mar 19];282(10):964–70. Available from.http://www.ncbi.nlm.nih.gov/pubmed/10485682
- Guskiewicz KM, Register-Mihalik JK. Postconcussive impairment differences across a multifaceted concussion assessment protocol. Pm&r. Internet]. 2011 Oct [cited 2019 Mar 19];3(10 SUPPL. 2):S445–51. Available from. https://doi.org/10.1016/j.pmrj.2011.08.009
- Murdaugh DL, King TZ, Sun B, Jones RA, Ono KE, Reisner A, Burns TG, et al. Longitudinal changes in resting state connectivity and white matter integrity in adolescents with sports-related concussion. Erratum J Int Neuropsychol Soc Internet]. 2018 Sep 3 [cited 2019 Mar 19];24(8):890. Available from.https://www.cambridge.org/core/product/identifier/S1355617718000875/type/journal_article
- Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. Internet]. 2003 May 1 [cited 2019 Mar 20];142(5):546–53. Available fromhttp://linkinghub.elsevier.com/retrieve/pii/S0022347603001161
- Zhu DC, Covassin T, Nogle S, Doyle S, Russell D, Pearson RL, et al. A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over thirty days. J Neurotrauma Internet]. 2015 Mar 1 [cited 2019 Mar 19];32(5):327–41. Available from. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2015-11190-005&site=ehost-live
- Wasserman EB, Kerr ZY, Zuckerman S, Covassin T. Epidemiology of sports-related concussions in national collegiate athletic association athletes from 2009-2010 to 2013-2014. Am J Sports Med. 2016;44(1):226–33.doi:https://doi.org/10.1177/0363546515610537.
- McCrea M, Broglio SP, McAllister TW, Gill J, Giza CC, Huber DL, et al. Association of blood biomarkers with acute sport-related concussion in collegiate athletes: findings from the ncaa and department of defense care consortium. JAMA Netw open 2020;3:e1919771.
- Elbin RJ, Schatz P, Covassin T. One-year test-retest reliability of the online version of Impact in high school athletes. Am J Sports Med [Internet]. 2011 Nov [cited 2021 Jan 13];39(11):2319–24. Available from http://myaccess.library.utoronto.ca/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=106900629&site=ehost-live%0Ahttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medp&NEWS=N&AN=12937495
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP, et al.Acute effects and recovery time following concussion in collegiate football players: the ncaa concussion study. J Am Med Assoc. 2003 Nov 19;290(19):2556–63.
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP, et al. Cumulative effects associated with recurrent concussion in collegiate football players - the NCAA concussion study. JAMA-JOURNAL Am Med Assoc Internet 2003 Nov 19 [cited 2019 Mar 20];290(19):2549–55. Available from.
- McCrea M, Barr WB, Guskiewicz K, Randolph C, Marshall SW, Cantu R, Onate JA, Kelly JP, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc Internet 2005 Jan 28 [cited 2019 Mar 20];11(1):58–69. Available from http://www.journals.cambridge.org/abstract_S1355617705050083
- Zuckerman S, Lee YM, Odom MJ, Solomon GS, Sills AK, Forbes JA. Recovery from sports-related concussion: days to return to neurocognitive baseline in adolescents versus young adults. Surg Neurol Int Internet. 2012 [cited 2019 Mar 19];3(1):130. Available from. http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=23227435&site=ehost-live.
- Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. Internet]. 2012 Jun 26 [cited 2019 Mar 19];40(6):1303–12. Available from
- Lee Y, Odom M, Zuckerman S, Solomon G, Sills A. Does age affect symptom recovery after sports-related concussion? A study of high school and college athletes. J Neurosurg Pediatr. 2013Dec;126:537–44. Internet Available fromhttps://thejns.org/view/journals/j-neurosurg-pediatr/12/6/article-p537.xml
- Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007 Jun;60(6):1050–57. doi:https://doi.org/10.1227/01.NEU.0000255479.90999.C0.
- Echemendia RJ, Putukian M, Mackin RS, Julian L, Shoss N. Neuropsychological test performance prior to and following sports-related mild traumatic brain injury. Clin J Sport Med [Internet. 2001 Jan [cited 2019 Mar 20];11(1):23–31. Available from http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00042752-200101000-00005
- Macciocchi SN, Barth JT, Alves W, Rimel RW, Jane JA. Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery. 1996 Sep;39(3):510–14. doi:https://doi.org/10.1227/00006123-199609000-00014.
- Howell DR, Osternig LR, Chou L-S-S. Detection of acute and long-term effects of concussion: dual-task gait balance control versus computerized neurocognitive test. Arch Phys Med Rehabil [Internet]. 2018 Jul [cited 2019 Mar 19];99(7):1318–24. Available from http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=130301574&site=ehost-live
- Mccrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP, et al. Acute effects and recovery time following concussion in collegiate football players - The NCAA Concussion Study. JAMA-JOURNAL Am Med Assoc [Internet]. 2003 Nov 19 [cited 2021 Jan 11];290(19):2556–63. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed6&NEWS=N&AN=2003477981
- Wang Y, Nencka AS, Meier TB, Guskiewicz K, Mihalik JP, Alison Brooks M, Saykin AJ, Koch KM, Wu Y-C, Nelson LD, et al. Cerebral blood flow in acute concussion: preliminary ASL findings from the NCAA-DoD CARE consortium. Brain Imaging Behav Internet]. 2018 Oct 29 [cited 2018 Mar 19];13(5):1375–85. Available from.https://doi.org/10.1007/s11682-018-9946-5
- Covassin T, Schatz P, Swanik CB. Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes. Neurosurgery. Internet]. 2007 Aug 1 [cited 2019 Mar 20];61(2):345–50. Available from https://academic.oup.com/neurosurgery/article/61/2/345/2556370
- Mihalik JP, McCaffrey MA, Rivera EM, Pardini JE, Guskiewicz KM, Collins MW, Lovell MR, et al. Effectiveness of mouthguards in reducing neurocognitive deficits following sports-related cerebral concussion. Dent Traumatol. 2007Feb [cited 2019 Mar 19];231:14–20. Internet Available from http://www.ncbi.nlm.nih.gov/pubmed/17227375
- Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: an update of the national collegiate athletic association injury surveillance program from 2004-2005 through 2008-2009 [Internet]. Vol. 51, Journal of Athletic Training. 2016 [cited 2019 Nov 29]. p. 189–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26950073
- Broglio SP, Ferrara MS, Macciocchi SN, Baumgartner TA, E R, Baumgartner TA, et al. Test-retest reliability of computerized concussion assessment programs. J Athl Train 2007;42:509–14.
- Honaker JA, Lester HF, Patterson JN, Jones SM. Examining postconcussion symptoms of dizziness and imbalance on neurocognitive performance in collegiate football players. Otol Neurotol off Publ Am Otol Soc Am Neurotol Soc [And] Eur Acad Otol Neurotol [Internet]. 2014 Jul [cited 2019 Mar 19];35(6):1111–17. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00129492-201407000-00031
- Covassin T, Elbin RJ, Larson E, Kontos AP, Larson E, K AP. Sex and age differences in depression and baseline sport-related concussion neurocognitive performance and symptoms. Clin J Sport Med [Internet]. 2012 Mar [cited 2019 Mar 19];22(2):98–104. Available from https://insights.ovid.com/crossref?an=00042752-201203000-00005
- Echemendia RJ, Bruce JM, Bailey CM, Sanders JF, Arnett P, Vargas G. The utility of post-concussion neuropsychological data in identifying cognitive change following sports-related mtbi in the absence of baseline data. Clin Neuropsychol [Internet]. 2012 Oct 1 [cited 2019 Mar 19];26(7):1077–91. Available from:
- Asken B, Clugston JR, Snyder AR, Bauer RM. Baseline neurocognitive performance and clearance for athletes to return to contact. J Athl Train [Internet]. 2017 Jan [cited 2019 Mar 19];52(1):51–57.
- Register-Mihalik JK, Guskiewicz KM, Mihalik JP, Schmidt JD, Kerr ZY, McCrea MA. Reliable change, sensitivity, and specificity of a multidimensional concussion assessment battery: implications for caution in clinical practice. J Head Trauma Rehabil [Internet]. 2013 Jul [cited 2019 Mar 19];28(4):274–83. Available from http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=89444542&site=ehost-live
- Schmidt JD, Register-Mihalik JK, Mihalik JP, Kerr ZY, Guskiewicz KM. Identifying impairments after concussion: Normative data versus individualized baselines. Med Sci Sports Exerc [Internet]. 2012 Sep [cited 2020 Apr 30];44(9):1621–28. Available from http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=22525765&site=ehost-live
- Sosnoff JJ, Broglio SP, Hillman CH, Ferrara MS. Concussion does not impact intraindividual response time variability. Neuropsychology. 2007 Nov;21(6):796–802. doi:https://doi.org/10.1037/0894-4105.21.6.796.
- Galetta KM, Brandes LE, Maki K, Dziemianowicz MS, Laudano E, Allen M, Lawler K, Sennett B, Wiebe D, Devick S, et al. The king-devick test and sports-related concussion: study of a rapid visual screening tool in a collegiate cohort. J Neurol Sci. 2011 Oct; 3091–2: 34–39. InternetAvailable from. https://doi.org/10.1016/j.jns.2011.07.039
- Leong DF, Balcer LJ, Galetta SL, Evans G, Gimre M, Watt D. The king-devick test for sideline concussion screening in collegiate football. J Optom [Internet]. 2015 Apr [cited 2019 Mar 19];8(2):131–39. Available fromhttps://linkinghub.elsevier.com/retrieve/pii/S1888429614001162
- Howell DR, Stillman A, Buckley TA, Berkstresser B, Wang F, Meehan W. The utility of instrumented dual-task gait and tablet-based neurocognitive measurements after concussion. J Sci Med Sport [Internet]. 2018 Apr [cited 2019 Mar 19];21(4):358–62. Available from https://linkinghub.elsevier.com/retrieve/pii/S1440244017309921
- Merritt VC, Meyer JE, Arnett PA. A novel approach to classifying postconcussion symptoms: The application of a new framework to the post-concussion symptom scale. J Clin Exp Neuropsychol [Internet]. 2015 Aug 9 [cited 2019 Mar 19];37(7):764–75. Available from: doi: https://doi.org/10.1080/13803395.2015.1060950
- Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J Neurotrauma. 2010 Jan;27(1):65–76. doi:https://doi.org/10.1089/neu.2009.0962.
- Kontos REPSTCLHJP A, Kontos AP, Elbin RJ, Schatz P, Covassin T, Henry L, Collins MW, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med Internet]. 2012 Oct 16 [cited 2019 Mar 20];40(10):2375–84. Available from.
- Iverson GL, Gaetz M, Lovell MR, Collins MW. Relation between subjective fogginess and neuropsychological testing following concussion. J Int Neuropsychol Soc. Internet]. 2004 Oct 1 [cited 2019 Mar 19];10(6):904–06. Available from http://www.journals.cambridge.org/abstract_S1355617704106139
- Moser RS, Schatz P, Jordan BD. Prolonged effects of concussion in high school athletes. Neurosurgery. 2005 Aug;57(2):300–06. doi:https://doi.org/10.1227/01.NEU.0000166663.98616.E4.
- Murray NG, D’Amico NR, Powell DW, Mormile ME, Grimes KE, Munkasy BA, et al. ASB clinical biomechanics award winner 2016: assessment of gaze stability within 24-48 hours post-concussion. clin biomech (bristol, avon) [Internet]. 2017 May [cited 2019 Mar 19];44:21–27. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0268003317300608
- Howell DR, Taylor JA, Tan CO, Orr R, Meehan WP. The role of aerobic exercise in reducing persistent sport-related concussion symptoms [Internet]. 2019 [cited 2020 Feb 4];51(4):647–52. Available from]. Med Sci Sports Exerc
- Baker JG, Leddy JJ, Darling SR, Rieger BP, Mashtare TL, Sharma T, Willer BS, et al. Factors associated with problems for adolescents returning to the classroom after sport-related concussion. Clin Pediatr (Phila) Internet]. 2015 Sep 17 [cited 2019 Mar 19];54(10):961–68. Available from.
- Hall EE, Ketcham CJ, Crenshaw CR, Baker MH, McConnell JM, Patel K. Concussion management in collegiate student-athletes: return-to-academics recommendations. Clin J Sport Med. 2015;25(3):291–96.doi:https://doi.org/10.1097/JSM.0000000000000133.
- Merritt VC, Arnett PA. Premorbid predictors of postconcussion symptoms in collegiate athletes. J Clin Exp Neuropsychol. Internet]. 2014 Nov 26 [cited 2019 Mar 19];36(10):1098–111. Available from.
- McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvořák JJ, Echemendia RJ, et al. Consensus statement on concussion in sport: The 4th international conference on concussion in sport, Zurich, November 2012. J Athl Train [Internet]. [cited 2019 May 30];48(4):554–75. Available from:
- Iaccarino MA, Fitzgerald M, Pulli A, Woodworth KY, Spencer TJ, Zafonte R, Biederman J, et al. Sport concussion and attention deficit hyperactivity disorder in student athletes: a cohort study. Neurol Pract. 2018 Oct;8(5):403–11. doi:https://doi.org/10.1212/CPJ.0000000000000525.
- Chamelian L, Feinstein A. The effect of major depression on subjective and objective cognitive deficits in mild to moderate traumatic brain injury neurosci. 18. J Neuropsychiatr. 2006;18(1):33–38.doi:https://doi.org/10.1176/jnp.18.1.33.
- Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT. Sex differences in outcome following sports-related concussion. J Neurosurg. 2005May;1025:856–63. Internet Available fromhttp://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=15926710&site=ehost-live
- Baker JG, Leddy JJ, Darling SR, Shucard J, Makdissi M, Willer BS. Gender differences in recovery from sports-related concussion in adolescents. Clin Pediatr (Phila) Internet]. 2016;55(8):771–75. Acessed 19 March 2019. Available from.
- Stone S, Lee B, Garrison JC, Blueitt D, Creed K. Sex differences in time to return-to-play progression after sport-related concussion. Sports Health. 2017;9(1):41–44.doi:https://doi.org/10.1177/1941738116672184.
- Giza CC, Hovda DA. The pathophysiology of traumatic brain injury. In: Lovell M, Echemendia R, Barth J, Collins M, editors. Traumatic Brain Injury in Sports: an International Neuropsychological Perspective. Lisse, The Netherlands: Swets & Zeitlinger Pub; 2004. p. 45–70.
- Iverson GL, Lange RT, Wäljas M, Liimatainen S, Dastidar P, Hartikainen KM, et al. Outcome from complicated versus uncomplicated mild traumatic brain injury.Rehabil Res Pract. 2012. Internet]. [cited 2019 Mar 19];2012:415740. Available from http://www.hindawi.com/journals/rerp/2012/415740/
- Piland SG, Motl R, Guskiewicz KM, Mccrea M, Ferrara MS. Structural validity of a self-report concussion symptom scale. Med Sci Sports Exerc. 2006;38:27–32.doi:https://doi.org/10.1249/01.mss.0000183186.98212.d5.
- McLeod TC V, Leach C, McLeod TCV, Leach C. Psychometric properties of self-report concussion scales and checklists. J Athl Train. 2012;47(2):221–23.
- McLeod TC V, Leach C. Psychometric properties of self-report concussion scales and checklists. J Athl Train. 2012;47(2):221–23.
- Gagnon I, Galli C, Friedman D, Grilli L, Iverson GL. Active rehabilitation for children who are slow to recover following sport-related concussion. Brain Inj Internet]. 2009 [cited 2020 Jun 17];23(12):956–64. Available from..
- Bailey CM, Echemendia RJ, Arnett PA, B CM, E RJ, A PA, et al. The impact of motivation on neuropsychological performance in sports-related mild traumatic brain injury. J Int Neuropsychol Soc Internet]. 2006 Jul 27 [cited 2019 Mar 19];12(4):475–84. Available from.http://www.journals.cambridge.org/abstract_S1355617706060619
- Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. BRAIN Inj. 2006 Mar;20(3):245–52. doi:https://doi.org/10.1080/02699050500487910.
- Kontos AP, Sufrinko A, Womble M, Kegel N. Neuropsychological assessment following concussion: an evidence‐based review of the role of neuropsychological assessment pre- and post-concussion. Curr Pain Headache Rep [Internet]. 2016 Jun 20 [cited 2019 Mar 19];20(6):38. Available from: https://doi.org/10.1007/s11916-016-0571-y
- Farnsworth JL 2nd, Dargo L, Ragan BG, Kang M, Farnsworth James L2, Dargo L, et al. Reliability of computerized neurocognitive tests for concussion assessment: a meta-analysis. J Athl Train Internet]. 2017 Sep 1 [cited 2019 Mar 19];52(9):826–33. Available from. https://pubmed.ncbi.nlm.nih.gov/28771032/
- Nelson LD, LaRoche AA, Pfaller AY, Lerner EB, Hammeke TA, Randolph C, Barr WB, Guskiewicz K, McCrea MA, et al. Prospective, head-to-head study of three computerized neurocognitive assessment tools (cnts): reliability and validity for the assessment of sport-related concussion. J Int Neuropsychol Soc Internet]. 2016 Jan 30 [cited 2019 Mar 19];22(1):24–37. Available from.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4882608/
- Broglio SP, Sosnoff JJ, Ferrara MS. The relationship of athlete-reported concussion symptoms and objective measures of neurocognitive function and postural control. Clin J Sport Med [Internet]. 2009 Sep [cited 2019 Mar 19];19(5):377–82. Available from http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00042752-200909000-00005
- Garner DP, Goodwin JS, Tsai NT, Kothera RT, Semler ME, Wolf BJ. Blink reflex parameters in baseline, active, and head-impact Division I athletes. COGENT Eng. 2018 Feb;5(1). doi:https://doi.org/10.1080/23311916.2018.1429110.
- Kontos AP, Sufrinko A, Elbin RJ, Puskar A, Collins MW. Reliability and associated risk factors for performance on the vestibular/ocular motor screening (VOMS) tool in healthy collegiate athletes. Am J Sports Med. Internet]. 2016 Jun 15 [cited 2020 Jun 4];44(6):1400–06. Available from ():. doi:https://doi.org/10.1177/0363546516632754
- Petit KM, Anderson M, Bretzin AC, Tomczyk CP, Savage JL, Covassin T. Association between vestibular/ocular motor screening (VOMS) and Concussion Recovery Time in Collegiate Participants. Arch Clin Neuropsychol [Internet]. 2019 Jul 26;34(5):744–744. Available from: https://doi.org/10.1093/arclin/acz026.14
- Anzalone DBTCTMKPJG AJ, Anzalone AJ, Blueitt D, Case T, McGuffin T, Pollard K, Jones MT, Pavur R, Turner S, Oliver JM, et al. A positive vestibular/ocular motor screening (voms) is associated with increased recovery time after sports-related concussion in youth and adolescent athletes. Am J Sports Med Internet]. 2017 Feb 1 [cited 2021 Jan 13];45(2):474–79. Available from http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=121164422&site=ehost-live
- Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, Dewolf R, Marchetti G, Kontos AP, et al. Brief VOMS assessment to eval concussions. Am J Sports Med. 2014;42(10):2479–86.doi:https://doi.org/10.1177/0363546514543775.
- Moran RN, Covassin T, Elbin RJ, Gould D, Nogle S. Reliability and normative reference values for the vestibular/ocular motor screening (voms) tool in youth athletes. Am J Sports Med. 2018May;466:1475–80. Internet Available from https://doi.org/10.1177/0363546518756979
- Worts PR, Schatz P, Burkhart SO, Devick S, Worts PR, Schatz P, et al. Test performance and test-retest reliability of the vestibular/ocular motor screening and king-devick test in adolescent athletes during a competitive sport season. Am J Sports Med Internet. 2018 Jul 9 [cited 2019 Mar 20];46(8):2004–10. Available from.
- Bevilacqua ZW, Kerby ME, Fletcher D, Chen Z, Merritt B, Huibregtse ME, et al. Preliminary evidence-based recommendations for return to learn: a novel pilot study tracking concussed college students. Concussion (London, England) [Internet]. 2019 Sep 20 [cited 2020 May 21];4(2):CNC63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31608152
- Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006 Apr;117(4):1359–71. doi:https://doi.org/10.1542/peds.2005-0994.
- Zuckerman S, Lee YM, Odom MJ, Solomon GS, Sills AK, Forbes JA. Recovery from sports-related concussion: days to return to neurocognitive baseline in adolescents versus young adults. Surgical Neurology International Internet. 2012;3(1):130. Accessed 19 March 2019. Available from
- Cancelliere C, Hincapié CA, Keightley M, Godbolt AK, Côté P, Kristman VL, Stålnacke B-M, Carroll LJ, Hung R, Borg J, et al. Systematic review of prognosis and return to play after sport concussion: results of the International collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. 2014Mar [cited 2019 Mar 26];953 Suppl:S210–29. Internet Available from https://doi.org/10.1016/j.apmr.2013.06.035
- Ellis MJ, Leddy JJ, Cordingley D, Willer B. A physiological approach to assessment and rehabilitation of acute concussion in collegiate and professional athletes. Front Neurol. Internet. 2018 Dec 20 [cited 2019 May 30];9(1):1–14. Available from doi:https://doi.org/10.3389/fneur.2018.01115.
- Guty E, Arnett P. Post-concussion symptom factors and neuropsychological outcomes in collegiate athletes. J Int Neuropsychol Soc. 2018 Aug;24(7):684–92. doi:https://doi.org/10.1017/S135561771800036X.
- Pedersen HA, Ferraro FR, Himle M, Schultz C, Poolman M. Neuropsychological Factors Related to College Ice Hockey Concussions. Am J Alzheimer’s Dis Other Dementiasr [Internet]. 2014 May 26 [cited 2019 Mar 19];29(3):201–04. Available from:
- M D, L A, T J, A DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLOS Med. 2009;6(6):e1000097.doi:https://doi.org/10.1371/journal.pmed.1000097.