ABSTRACT
Objectives
This review aimed to identify the demographic and clinical differences between those older adults admitted directly under neurosurgical care and those that were not, and whether EMS clinicians could use these differences to improve patient triage.
Methods
The authors searched for papers that included older adults who had suffered a TBI and were either admitted directly under neurosurgical care or were not. Titles and abstracts were screened, shortlisting potentially eligible papers before performing a full-text review. The Newcastle-Ottawa Scale was used to assess the risk of bias.
Results
A total of nine studies were eligible for inclusion. A high abbreviated injury score head, Marshall score or subdural hematoma greater than 10 mm were associated with neurosurgical care. There were few differences between those patients who did and did not receive neurosurgical intervention.
Conclusions
Absence of guidelines and clinician bias means that differences between those treated aggressively and conservatively observed in the literature are fraught with bias. Further work is required to understand which patients would benefit from an escalation of care and whether EMS can identify these patients so they are transported directly to a hospital with the appropriate services on-site.
Introduction
It is widely established that older adults contribute a large proportion of the traumatic brain injury (TBI) patient population (Citation1–5). The majority of older adults will present to the emergency department (ED) via emergency medical services (EMS) (Citation1), and so responsibility for the initial assessment, triage and transportation of these patients falls to the EMS provider. Recent findings highlight that an older adult’s clinical presentation following a TBI may not correlate with the severity of their injury (Citation3,Citation4,Citation6–8) and that the triage of older adults suffering traumatic injuries by EMS clinicians is inaccurate, resulting in a suboptimal delivery of care (Citation9).
No two patients with TBI are the same. Their injury’s clinical significance can be influenced by the patient’s age, physiological reserve, mechanism of injury, preexisting morbidities, medications, clinical frailty, or recreational use of drugs and alcohol (Citation2,Citation10,Citation11). These factors likely contribute to masking the severity of a patient’s injury and why EMS are transporting them to hospitals without neurosurgical services on-site, ultimately delaying or denying patients from specialist care (Citation12,Citation13)
There is a growing body of evidence demonstrating that ambulance clinicians transport older adults with significant injuries to non-designated trauma centers, and this discrimination rises with age (Citation12,Citation14–19). When EMS clinicians transport older adults with traumatic injuries to an appropriate hospital, their chances of mortality decreases (Citation20). TBI represents the largest subset of trauma patients in the older trauma literature; identifying which patients require transportation to a hospital with onsite neurosurgical services is among the current challenges facing EMS clinicians caring for older adults. A signal from the literature suggests that transporting patients with TBI, even with mild TBI (mTBI), to these hospitals can improve patient outcomes (Citation21).
Understanding which older patients with TBI require hospitalizing with on-site neurosurgery, and the variables that may predict this could help influence this patient group’s outcomes. The development of older adult trauma triage protocols for EMS and the ED are emerging (Citation22–24), older adults may present however to EMS with mTBI symptoms with a significant injury on head computed tomography (CT) scans (Citation4,Citation25,Citation26). There is still debate about whether an older adult with TBI should be transferred directly to a hospital with onsite neurosurgery services (Citation19,Citation26–28). Others have argued that patients with mTBI can be safely managed at hospitals without these services as long as a remote neurosurgical consultation is available (Citation28,Citation29).
This review aims to compare the differences between those older adults with a TBI admitted under neurosurgery and those that are not and whether these differences can be detected by EMS providers to triage these patients proactively.
Method
Reporting of this review is structured based on the guidelines for Synthesis Without Meta-analysis (SWiM) (Citation30) and was registered with PROSPERO (CRD42020203913).
Search strategy
On the 21st of September 2020, the authors searched the following sources: The Cochrane Library, MEDLINE, CINAHL and PUBMED. A gray literature search was conducted through Google scholar using a simplified version of the search strategy. Following consultation with a health sciences librarian, relevant Medical Subject Headings (MeSH) terms were used to search the databases (see appendices for the search terms used). There were four key components to the search terms identified when developing the search strategy:
The authors chose to use an array of phrases to describe adults in their later years of life due to variations in terms used to describes older adults in literature, with examples such as “older adult” (Citation31), “geriatric”, or “elderly” (Citation2).
TBI is a traumatic injury with evidence of pathology to the brain (Citation32). However, to ensure the search included studies that have patients at risk of a TBI, the authors included “head injuries” into the search terms as expanders.
Neurosurgery is a specialty that cares for patients with an injury to the brain, spinal cord and nervous system and includes TBI management. TBI management can include intracranial pressure monitoring, decompressive craniectomy, burr hole surgery, and evacuation of hematomas (Citation33). Search terms were deliberately broad to capture all care variations patients with TBI may receive.
The authors considered the broader major trauma (and derivatives of trauma) literature. The head is the body region most likely to be injured when an older adult is the victim of trauma (Citation34). Broadening the search terms allowed the authors to potentially capture patients with TBI reported as a subgroup in trauma papers.
The authors limited the range of the search strategy from the 1st of January 2000 to reflect the change in approach to trauma care and designation of trauma networks in the last two decades (Citation35).
Eligibility criteria
Following the literature search, the authors applied the PICO framework to screen studies for inclusion. Titles and abstracts were screened based on the population, intervention, control, and outcome.
Population
Older adults aged 60 years or more who have sustained a TBI regardless of the severity and studies that included young adult patients (≤59 years old) were considered, should older adults form a subgroup and be analyzed separately.
Intervention
Patients with TBI who received a neurosurgical intervention (NSI), admission into a neuro-intensive, critical or intensive care unit, or a secondary transfer. Studies focused on elective surgeries do not fall within the scope of this review.
Control
Patients who have suffered a TBI but do not get admitted under neurosurgery or are deemed not eligible for an NSI admission into a neuro-intensive or critical care unit or are deemed suitable for conservative care only.
Outcome
The primary outcome is to identify the demographic and clinical differences between the intervention and control groups based on the reported data. These data can include patient age, gender, socioeconomic status, and clinical findings such as TBI severity and type, pre-injury comorbidities, pre-injury prescribed medications, level of care provided and reasons for escalation (or not) of care. The reporting of these differences will be descriptive. The authors anticipated an absence of standardization of reporting in this patient population, and studies may differ in their aims and outcomes.
Study selection
The authors managed the search results on a review platform (Mendeley 1.19.4, Elsevier), where title and abstracts were reviewed by JWB and KD independently. JWB and JEG then independently screened shortlisted papers; where there was uncertainty in the study’s eligibility, a third reviewer was consulted to adjudicate and resolve reviewer disagreements.
Data collection
Following inclusion into the review, a data collection tool was used to extract each study’s relevant aspects. These included author, year, title, country, age, severity of the injury, the proportion of patients in the intervention and control groups, injury severity, mechanism of injury (MOI) and comorbidities. Studies were also screened for apparent facilitators and barriers that may have influenced whether a patient received intervention or escalation of care.
Risk of bias assessment
Risk of bias was assessed using the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies (Citation36). As per the NOS reporting guidelines, points are awarded to three areas; selection, comparability, and outcome, divided between eight specific items. Each item can score one point except comparability, which can score two; a paper can achieve a maximum score of nine. A study that scores less than five points suffers from a high risk of bias, and a score of eight or more represents a low risk of bias. JB was the primary reviewer, with JG as the secondary. Again, where there was disagreement, a third reviewer was consulted to adjudicate and resolve reviewer disagreements.
Results
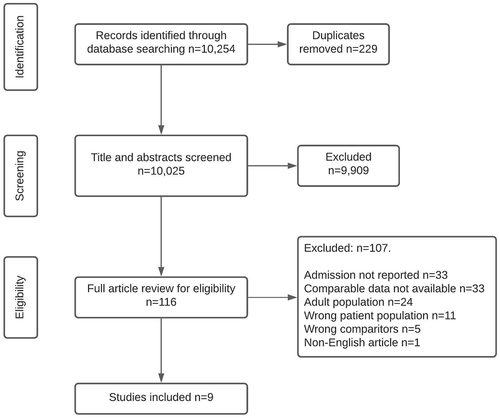
116 articles were reviewed in full, of which the reviewers excluded 107 (). Reasons for exclusion were as follows; the paper did not report neurosurgical or ICU admission (n = 33), there was no control group reported (n = 33), the patient population consisted of young adults with no separate analysis of older adults (n = 24). Five studies were related to other aspects of neurosurgical care, such as adverse events. Finally, the authors excluded a study because the full article was non-English, and a translated document was not available, despite the title and abstract being in English.
A total of nine studies were deemed eligible for inclusion (). Eight studies were full articles and one was an abstract from a conference proceeding (Citation37) for which the full study is unpublished. Contact was made with the corresponding author, and their unpublished manuscript was shared.
Table 1. Summary of included studies. GCS; Glasgow Coma Score, SAH, Subarachnoid Hemorrhage; EDH, Extradural Hematoma; SDH, Subdural Hematoma; aSDH, Acute Subdural hematoma; cSDH, Chronic Subdural Hematoma; NSI, Neurosurgical intervention, I, Intervention; C, Control; AC, Anticoagulant; ICH, Intracranial hemorrhage; AIS, Abbreviated Injury Score.
All papers included in this review were hospital-based retrospective observational cohort studies, with data recorded from hospital registries (Citation37–42) or national registry databases (Citation25,Citation43,Citation44). One study was multicentre (Citation43), six were single center (Citation25,Citation37,Citation39,Citation41,Citation44,Citation45) and two were single center reviews of secondary transfers (Citation38,Citation42). All hospitals were level one trauma centers, with most studies considering ED admissions (n = 5) while the remaining observed neurosurgical units receiving patients with acute TBI (Citation38,Citation39,Citation41,Citation43). Three studies were in North America, four studies in Europe and two in Asia; all from high-income countries. The main objectives of these studies can be broadly characterized into two groups, predicting the need for admission into ICU (n = 3) (Citation37,Citation38,Citation42) or factors associated with neurosurgical intervention (n = 6) (Citation25,Citation39,Citation41,Citation43,Citation44,Citation46).
Risk of bias evaluation
Most studies have a moderate risk of bias based on the NOS () due to their retrospective nature. In some instances, patients selection was from national or multicentre registry databases where particular inclusion criteria were required (Citation25,Citation38,Citation43,Citation44). These databases target particular injury profiles, excluding less severely injured patients.
Table 2. Risk of bias assessment of included studies.
Confounders were controlled for by multivariate analysis where variables were associated with outcomes. Only one study did not control for confounders and whose findings were descriptive (Citation39). Patient outcomes and follow-ups were reported consistently, with minimal loss to follow up. However, this is balanced by the weakness in the patient screening process, where studies excluded patient records if they were incomplete (Citation43,Citation44). Therefore, included patients may not be a true reflection of the patient demographic.
Clinical and demographic differences between aggressive and conservatively managed patients
An SDH was the most frequent injury in all studies (22% (Citation41) to 64% (Citation37)) except that reported by Wan et al (Citation41)., where intracerebral and SAH were more common (69% and 41%, respectively) although they recruited solely from patients with a GCS of ≤9.
Overall, there were few clinical or demographic differences between those patients who did or did not receive care in ICU or NSI. In studies that looked at admission for specialist care (transfer to a level 1 trauma center, ICU, NSI), a radiologically significant brain injury influenced admission decision using classification scales such as the head Abbreviated injury score (AIS) (Citation37), or Marshall score (Citation38). Gore et al (Citation37). reported ICU admission was more likely to occur if a patient had a head AIS of 4, and Rajwani et al (Citation38). found that a higher Marshall score was associated with a secondary transfer (OR 2.55 95%CI 1.67–3.91, p < 0.0001). In contrast, Yun et al (Citation42). reported that an SDH greater than 10 mm increased the odds of admission to an ICU (OR 6.28 95%CI 1.24–31.71, p = 0.0263). Yun and colleagues found that warfarin use was associated with a secondary transfer and ICU admission (OR 4.09 95%CI1.64–102.5). However, the same was not seen by Rajwani et al (Citation38). (OR1.05 95%CI 0.33–3.30, p = 0.938) or Gore et al (Citation37). However, Gore et al (Citation37). did note that patients who deteriorated during their stay were more likely to be coagulopathic.
There was an association with age and likelihood of receiving an NSI for SDH, with several studies reporting NSI to occur in the 70–79 age bracket (Citation25,Citation39,Citation43). However, Hawley et al (Citation25). was the only study to find a significant difference in age, observing 279 (44%) patients who presented with an SDH, of which 77 (27.6%) received an NSI. Younger age (77.6 ± 7.7 vs 80 ± 8.9, p = 0.04) and male gender (p < 0.02) were associated with receiving an NSI. Shimoda et al (Citation43). noted that patients who presented with an SDH were likely to have better outcomes if they received treatment within the first four hours of injury (median 3.8 hours). However, the mean time to surgery was 15.1 hours (±46.0 hours).
There were otherwise few differences between those patients that did and did not receive an NSI. Shimoda et al (Citation43). noted that NSI were more likely to occur in patients with a higher GCS and did not suffer a hypoxic or hypotensive episode on admission. Hawley et al (Citation25). noted that younger age (<80 years) was associated with NSI in SDH cases, while Solomon et al (Citation44). observed NSI occurring more frequently in 80+ age groups. Moore, Pasquale and Badellino (Citation45) observed eight commonly associated TBI symptoms in older adults on anticoagulant or antiplatelet therapy (AC/APT). They evaluated whether these could be used as predictors for NSI based on their prevalence. Confusion was the most prevalent symptom (33%), but this was not statistically significant and found not to be a predictor of NSI.
Seven studies reported MOI (Citation25,Citation37–39,Citation41,Citation43,Citation44), the most frequently reported MOI was Falls, ranging from 42% (Citation43) to 94% (Citation44), with RTCs being the second most common MOI. Hawley et al (Citation25). stratified MOI by age. Falls remained the most frequent cause of TBI and increased with age. Falls from height and RTC were the second and third most common causes of TBI, however their prevalence decreased with older age.
There was variability in the reporting of GCS. However, most papers reported that mTBI was the most common presentation. In studies which reported on all severity of GCS (n = 4) the majority of patients were mTBI (GCS 13–15) (59%-85%) (Citation25,Citation38,Citation39,Citation44). In studies that focused solely on mTBI (n = 3) (Citation37,Citation41), most patients were GCS 15 on admission. However, it is unclear in the reported studies how GCS is related to injury patterns or MOI.
Three studies reported on comorbidities (Citation25,Citation39,Citation44), of which cardiovascular disease was the most prevalent. Herou, Romner and Tomasevic (Citation39) reported that 63% of NSI treated patients had cardiovascular disease compared to 45% of conservatively treated patients. In contrast, Hawley et al (Citation25). reported individual disease conditions, noting that hypertension was the most prevalent (22.7%). Solomon et al (Citation44). used the Charlson Comorbidity Index (CCI) to measure the prevalence of preinjury health conditions. The CCI is a prognostic tool to determine mortality in patients based on the number and severity of long-term conditions they have (Citation47). Solomon’s cohort of patients had a median score of 5 (Citation3–13) overall, with older patients (80+) scoring a median of 6 (Citation4–13) in contrast to the younger cohort (70–79-year-old) who scored 5 (Citation3–12). However, they did not explore their relationship with a patient having a clinically significant TBI. Overall, there were between-group similarities in age, sex, MOI, GCS, AC/APT, and comorbidities across the reviewed studies.
Discussion
This systematic review aimed to understand whether there are differences between older adults with TBI admitted under neurosurgical care and those that are not and whether these differences could be aid EMS triage of older patients who may have a clinically significant TBI. This review found that older age, lower injury severity scores and not taking AC/APT medications were associated with not being admitted into ICU or subsequently receiving a secondary transfer (Citation37,Citation38,Citation42).
It is unclear what variables could help EMS clinicians triage older adults with a clinically significant TBI. An SDH was the most common brain injury in older adults, with studies reporting their frequency as high as 64% (Citation37). Notably, a patient with an SDH aged 70–79 receiving an NSI had a better outcome than those SDH patients not treated with an NSI (Citation25,Citation39,Citation43). Patients with a higher GCS and no hypoxic or hypotensive episodes were also more likely to receive an NSI (Citation43). Regarding escalation of care or secondary transfer, injury severity, head AIS (Citation37), Marshall score (Citation38), or an extensive SDH increased the odds of ICU admission or transfer to a hospital with neurosurgical services on site. Only one study reported the predictive value of TBI symptoms and the likelihood of an NSI; while confusion was the most common symptom (33%), none were predictive of an NSI (Citation45).
An unexpected finding of this review was the potential barriers to patients receiving care, categorized as lack of guidelines/protocols and clinician bias. Some authors acknowledged an absence of guidelines or protocols to admit patients to ICU or warrant secondary transfers (Citation37,Citation38,Citation42). Solomon et al (Citation44). and Wan et al (Citation41). referenced the 2006 surgical guidelines for TBI (Citation48), however no other study referenced guidelines or protocols for decision-making regarding the escalation of care or NSI. Two studies reported on the decision-making process and that it was typically the patient’s attending clinician that decided whether a patient may receive a transfer, ICU admission or NSI (Citation39,Citation43). Herou, Romner and Tomasevic (Citation39), noted that decision making is multifactual. Shimoda et al (Citation43). reported that the absence of guidelines meant that those patients with a low GCS (≤8) were deemed to critically unwell and therefore an unsalvageable case in contrast to patients with a High GCS (≥9) who were deemed stable enough not to warrant an NSI.
There is a variation in the management and care of patients with TBI across Europe, arguably the absence of guidelines contributing to the inequality of care in older adults (Citation33). The majority of older patients with TBI present with mTBI, and guidelines for NSI or ICU admission do not exist for this growing patient group (Citation48–50). Volovici et al. (Citation51) conducted a European-wide survey of level one trauma hospitals regarding their ICU admission for TBI. Approximately a third of centers admitted mTBI to their ICU, and that admission is subject to a clinician’s professional judgment and experience. In this review, some studies reported clinical bias toward older age might influence management strategies (Citation41,Citation43). Misconceptions in older adult outcomes and the absence of guidelines for this patient group may contribute to these behaviors.
Discrepancies in the care of older adults suffering from a TBI were highlighted by Skaanser et al (Citation52)., reporting that older adults who received less intensive treatment were likely to have an increased risk of mortality. They propose this has created a self-fulfilling prophecy in the care of this patient group. However, the authors acknowledge that it is unclear whether treatment decisions are due to patient wishes or the clinician’s biases. Kirkman et al (Citation53). noted that older patients were more likely to be reviewed by junior doctors than by senior doctors, who were more likely to review young adults with TBI. The attitudes toward older adults have arguably contributed to a self-fulfilling prophecy where clinicians believe that due to a patient’s older age, their outcomes will be less favorable, choosing to manage their patient conservatively (Citation52). In turn, this approach increases the likelihood of an unfavorable outcome (Citation39,Citation43,Citation52,Citation53), when these patients with TBI may benefit from intensive management (Citation25,Citation43).
In this review, it was unclear what the patient’s baseline status was before their injury, with four studies reporting comorbidities or functional status to describe the patient population rather than their association with TBI injury and patient treatment outcomes (Citation25,Citation39,Citation41,Citation44). Two studies acknowledged that family members expressed a wish for patients to receive conservative care. However, the reasons behind these decisions are not explored (Citation37,Citation41). It is reasonable to expect families to have had consulted with their elders to understand their wishes in a severe illness or injury. It is essential to recognize which patients are likely to respond well to intervention and those that are not. The current picture is murky, and further work is required to tease out these issues.
The variation in TBI practice and clinician bias may suggest it is time to review current practice guidelines to provide support and standardization of patient care. Clinicians rely on guidelines such as the widely used Canadian CT head (CCTH) injury rule (Citation54–56). The CCTH aids the clinician in determining which patients should receive a head CT scan and predicting an NSI. The development of guidelines such as the CCTH and New Orleans CT rule (Citation54,Citation57), which were both developed to aid the screening of adults with head injury presenting to the ED with a GCS of 13–15, note that adults aged over 65 are considered high risk and should receive a head CT scan. However, it is now widely reported that the TBI population’s largest patient cohort is older adults (Citation2). Treating all older adults as high risk may not be practical when some older adults are at a higher risk than others; the challenge is finding variables other than age to differentiate high-risk patients with TBI. The guidelines currently in place may not be suitable for the growing population and require addressing.
There has been work guiding clinicians on identifying patients with mTBI who do not require admission under neurosurgery. The Brain Injury Guidelines (BIG) have been developed by Joseph et al (Citation58). to rule out patients with a non-significant TBI. They suggest that an SDH smaller than 4 mm does not need neurosurgical consultation, which could increase resource utilization. However, they have received criticism because of excluding patients taking AC/APT medications (Citation59), limiting their applicability to older adults.
Lessard et al (Citation59). built on BIG and included patients on preinjury AC/APT. The inclusion of AC/APT did not influence patient management. Nevertheless, a bleed of 4 mm or greater was considered clinically significant. In this review, Yun et al (Citation42). reported an SDH less than 10 mm was unlikely to result in a secondary transfer. Whereas Gore et al (Citation37). and Rajwani et al (Citation38). reported that head AIS of 4 or more or a Marshall Score of 5 or 6 would escalate care. The reality of current practice is that there is a higher threshold to escalate care in patients with TBI. However, it is unclear whether the theoretical approach would improve patient outcomes over current practice or unnecessarily increase demand for a specialist resource.
Limitations
Several limitations of this review should be addressed. Firstly, this review aimed to ascertain whether differences in patient cohorts that have been accepted by neurosurgery could identify variables that EMS clinicians could use to aid the triage of older adult patients with TBI. The study sites included in this review were the ED or neurosurgical wards with no reference to the patient in the prehospital phase. Therefore, it is unclear whether the hyper-acute phase of the injury seen by the EMS clinician is consistent with the acute phase of the injury observed in the ED or once admitted under neurosurgery. Secondly, while some studies reported that the decision to treat a patient was a clinician decision, the absence of context surrounding these decisions limits this finding and the underpinning reasoning. The variation in practice means that the differences reported could reflect current decision-making rather than the basis for future practice. Finally, because of the variation of practice and the retrospective nature of the included studies there is a risk of selection bias.
Conclusion
Older adults represent a large proportion of the TBI population, and EMS are responsible for transporting most of them to a hospital. The evidence is emerging that the hospital destination is crucial to optimizing an older trauma victim’s care. Identification of a clinically significant TBI by EMS could be improved, and understanding the difference between those older adults that are accepted under the care of neurosurgeons and those that are not. A large proportion of older adults present with an SDH with a high GCS following their injury. Clinician bias and the absence of appropriate guidelines means that the differences observed in the literature are fraught with uncertainty. Further work is required to understand which patients would benefit from direct admission to neurosurgical services and whether EMS can identify these patients to ensure they are transported directly to a hospital with the appropriate services on-site.
Acknowledgments
The authors would like to express their sincere thanks to Kevin Drury who was the second reviewer for the title and abstract search. Thanks also to the University of Surrey Postgraduate Researcher Writing group, a peer support group that reviewed and provided helpful feedback on the original manuscript. Finally, the authors would like to thank the journal Reviewers for taking the time and effort necessary to review the manuscript. The authors sincerely appreciate all valuable comments and suggestions, which helped to improve the quality of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lawrence T, Helmy A, Bouamra O, Woodford M, Lecky F, Hutchinson PJ. Traumatic brain injury in England and wales: prospective audit of epidemiology, complications and standardised mortality. BMJ Open. 2016 Nov;6(11):e012197. doi:10.1136/bmjopen-2016-012197.
- Gardner RC, Dams-O’Connor K, Morrissey MR, Manley GT. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma. Internet]. 2018 Apr 1 [cited 2020 Jun 1];35(7):889–906. doi:10.1089/neu.2017.5371.
- Salottolo K, Carrick M, Stewart Levy A, Morgan B, Slone D, and Bar-Or D. The epidemiology, prognosis, and trends of severe traumatic brain injury with presenting Glasgow coma scale of 3. J Crit Care. Internet]. 2017;38:197–201. [Accessed 31st July 2021]. Available from. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=ovfts&NEWS=N&AN=00009772-201704000-00035
- Barrett JW. A retrospective review of patients with significant traumatic brain injury transported by emergency medical services within the south east of England. Br Paramed J. 2019;3(4):1–7.doi:10.29045/14784726.2019.03.3.4.1.
- Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–36.doi:10.1038/nrneurol.2013.22.
- Kehoe A, Rennie S, Smith JE. Glasgow coma scale is unreliable for the prediction of severe head injury in elderly trauma patients. Emergency Medicine Journal: EMJ. 2015;32(8):613–15.doi:10.1136/emermed-2013-203488.
- Kehoe A, Smith J, Bouamra O, Edwards A, Yates D, and Lecky F. Older patients with traumatic brain injury present with a higher GCS score than younger patients for a given severity of injury. Emerg Med J Internet]. 2016;33(6):381–85. Accessed 31st July 2021. doi:10.1136/emermed-2015-205180.
- Ibañez Pérez De La Blanca MA, Fernández Mondéjar E, Gómez Jimènez FJ, Alonso Morales JM, Lombardo MDQ, Viso Rodriguez JL. Risk factors for intracranial lesions and mortality in older patients with mild traumatic brain injuries. Brain Inj. 2018Jan;32(1):99–104. Internet]. doi:10.1080/02699052.2017.1382716.
- Staudenmayer KL, Hsia RY, Mann NC, Spain DA, Newgard CD. Triage of elderly trauma patients: a population-based perspective. J Am Coll Surg. 2013;217(4):569–76.doi:10.1016/j.jamcollsurg.2013.06.017.
- Karibe H, Hayashi T, Narisawa A, Kameyama M, Nakagawa A, Tominaga T. Clinical characteristics and outcome in elderly patients with traumatic brain injury: for establishment of management strategy. Neurol Med Chir (Tokyo). Internet]. 2017/July/05. 2017 Aug 15 [cited 2020 Jun 1];57(8):418–25. doi:10.2176/nmc.st.2017-0058.
- Ruge T, Carlsson AC, Hellstrom M, Wihlborg P, Undén J. Is medical urgency of elderly patients with traumatic brain injury underestimated by emergency department triage? Ups J Med Sci. 2020Feb;125(1):58–63. Internet]. doi:10.1080/03009734.2019.1706674.
- Chang DC. Undertriage of elderly trauma patients to state-designated trauma centers. Arch Surg. Internet]. 2008 Aug 18;143(8):776–81. doi:10.1001/archsurg.143.8.776.
- Garwe T, Stewart K, Stoner J, Newgard CD, Scott M, Zhang Y, Cathey T, Sacra J, and Albrecht RM . Out-of-hospital and inter-hospital under-triage to designated tertiary trauma centers among injured older adults: a 10-year statewide geospatial-adjusted analysis. Prehospital Emerg Care. 2017;21(6):734–43.doi:10.1080/10903127.2017.1332123.
- Vassar MJ, Holcroft JJ, Knudson MM, Kizer KW. Fractures in access to and assessment of trauma systems. J Am Coll Surg. 2003;197(5):717–25.doi:10.1016/S1072-7515(03)00749-X.
- Hsia RY, Wang E, Torres H, Saynina O, Wise PH. Disparities in trauma center access despite increasing utilization: data from California, 1999 to 2006. J Trauma - Inj Infect Crit Care. 2010;68(1):217–24.doi:10.1097/TA.0b013e3181a0e66d.
- Nakamura Y, Daya M, Bulger EM, Schreiber M, Mackersie R, Hsia RY, Mann NC, Holmes JF, Staudenmayer K, Sturges Z, et al. Evaluating age in the field triage of injured persons. Ann Emerg Med. [Internet]. 2012;60(3):335–45. Available from. doi:10.1016/j.annemergmed.2012.04.006.
- Newgard CD, Richardson D, Holmes JF, Rea TD, Hsia RY, Mann NC, Staudenmayer K, Barton ED, Bulger EM, Haukoos JS, et al. Physiologic field triage criteria for identifying seriously injured older adults. Prehospital Emerg Care. 2014;18(4):461–70.doi:10.3109/10903127.2014.912707.
- Amoako J, Evans S, Brown NV, Khaliqdina S, Caterino JM. Identifying predictors of undertriage in injured older adults after implementation of statewide geriatric trauma triage criteria. Acad Emerg Med. Internet]. 2019 Jun 1;26(6):648–56. Available from; ():. doi:10.1111/acem.13695.
- Kaufman EJ, Ertefaie A, Small DS, Holena DN, Delgado MK. Comparative effectiveness of initial treatment at trauma center vs neurosurgery-capable non-trauma center for severe, Isolated head injury. J Am Coll Surg. 2018Mar;226(3):N.PAGN.PAG. Internet].):N.PAGN.PAG. Internet]. doi:10.1016/j.jamcollsurg.2018.01.055.
- Carr BW, Hammer PM, Timsina L, Rozycki G, Feliciano DV, and Coleman JJ. Increased trauma activation is not equally beneficial for all elderly trauma patients. J Trauma Acute Care Surg Internet]. 2018;85(3):598–602. Accessed 1st August 2021. doi:10.1097/TA.0000000000001986.
- Velez AM, Frangos SG, DiMaggio CJ, Berry CD, Avraham JB, and Bukur M. Trauma center transfer of elderly patients with mild Traumatic Brain Injury improves outcomes. Am J Surg Internet]. 2020;219(4):665–69. Accessed 1st August 2021. doi:10.1016/j.amjsurg.2019.06.008.
- Nishijima DK, Gaona S, Waechter T, Maloney R, Bair T, and Blitz A, et al. Do EMS providers accurately ascertain anticoagulant and antiplatelet use in older adults with head Trauma? Prehospital Emerg Care off J Natl Assoc EMS Physicians Natl Assoc State EMS Dir 2017;21:209–15. Accessed 1st July 2021.
- Newgard CD, Holmes JF, Haukoos JS, Bulger EM, Staudenmayer K, Wittwer L, Stecker E, Dai M, and Hsia RY, et al. Improving early identification of the high-risk elderly trauma patient by emergency medical services. Injury. 2016;47(1):19–25. Accessed 1st July 2021. doi:10.1016/j.injury.2015.09.010.
- Wasserman EB, Shah MN, Jones CMC, Cushman JT, Caterino JM, Bazarian JJ, Caterino JM, Bazarian JJ, Gillespie SM, Cheng JD, et al. Identification of a neurologic scale that optimizes EMS detection of older adult traumatic brain injury patients who require transport to a trauma center. Prehospital Emerg Care. Internet]. 2015 [cited 2019 Feb 8];19(2):202–12. doi:10.3109/10903127.2014.959225.
- Hawley C, Sakr M, Scapinello S, Salvo J, and Wrenn P. Traumatic brain injuries in older adults - 6 years of data for one UK trauma centre: retrospective analysis of prospectively collected data. Emerg Med J Internet]. 2017;34(8):509–16. Accessed 25th September 2021. doi:10.1136/emermed-2016-206506.
- Lawrence T, Helmy A, Bouamra O, Woodford M, Lecky F, and Hutchinson PJ. Traumatic brain injury in England and wales: prospective audit of epidemiology, complications and standardised mortality, Vol. 6. BMJ Open. BMJ Publishing Group, 2016. Accessed 25th June 2021.
- De Vloo P, Nijs S, Verelst S, van Loon J, Depreitere B. Prehospital and intrahospital temporal intervals in patients requiring emergent trauma craniotomy. A 6-year observational study in a level 1 Trauma center. World Neurosurg. 2018 Jun;114:e546–58. doi:10.1016/j.wneu.2018.03.032.
- Nishijima DK, Gaona SD, Faul M, Tancredi DJ, Waechter T, Maloney R, Bair T, Blitz A, Elms AR, Farrales RD, et al. The association of trauma center transport and long-term functional outcomes in Head-injured older adults transported by emergency medical services. Acad Emerg Med. 2020 Mar;27(3):207–16. doi:10.1111/acem.13915.
- Orlando A, Levy AS, Carrick MM, Tanner A, Mains CW, Bar-Or D. Epidemiology of mild traumatic brain injury with intracranial hemorrhage: focusing predictive models for neurosurgical intervention. World Neurosurg. 2017 Nov 1;107:94–102. doi:10.1016/j.wneu.2017.07.130
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. Internet]. 2020 Jan 16;368:l6890. doi: 10.1136/bmj.l6890.
- Techar K, Nguyen A, Lorenzo RM, Yang S, Thielen B, Cain-Nielsen A, Hemmila MR, and Tignanelli CJ . Early imaging associated with improved survival in older patients with mild traumatic brain injuries. J Surg Res. 2019 Oct;242:4–10. doi:10.1016/j.jss.2019.04.006.
- Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010 Nov;91(11):1637–40. doi:10.1016/j.apmr.2010.05.017.
- van Essen TA, den Boogert HF, Cnossen MC, de Ruiter GCW, Haitsma I, Polinder S, Steyerberg EW, Menon D, Maas AIR, Lingsma HF, et al. Variation in neurosurgical management of traumatic brain injury: a survey in 68 centers participating in the CENTER-TBI study. Acta Neurochir (Wien). 2019 Mar;161(3):435–49. doi:10.1007/s00701-018-3761-z.
- TARN. Major trauma in older people - England and wales. Trauma Audit and Research Network. 2017. Accessed 1st March 2021.
- Kehoe A, Smith JE, Edwards A, Yates D, Lecky F. The changing face of major trauma in the UK. Emerg Med J. 2015;32(12):911–15.doi:10.1136/emermed-2015-205265.
- Wells G, Shea A, O’Connell B, Peterson D, Welch J, Losos V, Tugwell M P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysestle [Internet]. 2000 [cited 2020 Jul 31]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Gore A, Mau CY, Prestigiacomo CJ, Sifri ZC. Mild traumatic brain injury in elderly patients: is routine ICU admission necessary? J Am Coll Surg. Internet]. 2015 Oct 2;221(4):S82–3. doi:10.1016/j.jamcollsurg.2015.07.188.
- Rajwani KM, Lavrador JP, Ansaripour A, Christos M. Which factors influence the decision to transfer patients with traumatic brain injury to a neurosurgery unit in a major trauma network ? Br J Neurosurg. Internet]. 2020;34(3):271–75. Available from. doi:10.1080/02688697.2020.1742289.
- Herou E, Romner B, Tomasevic G. Acute traumatic brain injury: mortality in the elderly. World Neurosurg. 2015 Jun 1;83(6):996–1001. doi:10.1016/j.wneu.2015.02.023.
- Moore MM, Pasquale MD, Badellino M. Impact of age and anticoagulation: need for neurosurgical intervention in trauma patients with mild traumatic brain injury. J Trauma Acute Care Surg [Internet]. 2012 Jul;73(1):126–30. Available from]. http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=22710784&site=ehost-live.
- Wan X, Liu S, Wang S, Zhang S, Yang H, Ou Y, Zhao M, James L, Shu K, Chen J, et al. Elderly patients with severe traumatic brain injury could benefit from surgical treatment. World Neurosurg. Internet]. 2016 May 1 [cited 2020 Jun 1];89:147–52. doi:10.1016/j.wneu.2016.01.084.
- Yun BJ, White BA, Benjamin Harvey H, Prabhakar AM, Sonis JD, Glover M, Vallillo E, Choi S, Borczuk P, Raja AS, et al. Opportunity to reduce transfer of patients with mild traumatic brain injury and intracranial hemorrhage to a Level 1 trauma center. Am J Emerg Med. 2017 Sep;35(9):1281–84. doi:10.1016/j.ajem.2017.03.071.
- Shimoda K, Maeda T, Tado M, Yoshino A, Katayama Y, Bullock MR. Outcome and surgical management for geriatric traumatic brain injury: analysis of 888 cases registered in the Japan neurotrauma data bank. World Neurosurg. 2014Dec;82(6):1300–06. Internet]. doi:10.1016/j.wneu.2014.08.014.
- Solomon D, Kaminski O, Schrier I, Kashtan H, Stein M. Isolated traumatic brain injury in the very old. Isr Med Assoc J [Internet]. 2019 Dec;12(21):779–84. Available from]. http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=31814339&site=ehost-live.
- Moore MM, Pasquale MD, Badellino M. Impact of age and anticoagulation: need for neurosurgical intervention in trauma patients with mild traumatic brain injury. J Trauma Acute Care Surg [Internet]. 2012 Jul [cited 2019 Feb 5];73(1):126–30. DOI:10.1097/TA.0b013e31824b01af.
- Moore MM, Pasquale MD, Badellino M. Impact of age and anticoagulation: need for neurosurgical intervention in trauma patients with mild traumatic brain injury. J Trauma Acute Care Surg. Internet]. 2012 [cited 2019 Feb 5];73(1):126–30. doi:10.1097/TA.0b013e31824b01af.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.doi:10.1016/0021-9681(87)90171-8.
- Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, and Wilberger JE, et al. Guidelines for the surgical management of traumatic brain injury author group. Neurosurgery Internet]. 2006 Mar 1;58(3):S2-vi-S2–vi. Accessed 3rd November 2021. doi:10.1097/00006123-200603001-00006
- Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, et al. Guidelines for the management of severe traumatic brain injury. Fourth Edition Neurosurgery. 2017 Jan;80(1):6–15. doi:10.1227/NEU.0000000000001432.
- Barbosa RR, Jawa R, Watters JM, Knight JC, Kerwin AJ, Winston ES, Barraco RD, Tucker B, Bardes JM, Rowell SE, et al. Evaluation and management of mild traumatic brain injury: an Eastern Association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012 Nov;73(5 Suppl 4):S307–14. doi:10.1097/TA.0b013e3182701885.
- Volovici V, Ercole A, Citerio G, Stocchetti N, Haitsma IK, Huijben JA, Dirven CMF, van der Jagt M, Steyerberg EW, Nelson D, et al. Intensive care admission criteria for traumatic brain injury patients across Europe. J Crit Care. 2019 Feb;49:158–61. doi:10.1016/j.jcrc.2018.11.002.
- Skaansar O, Tverdal C, Rønning PA, Skogen K, Brommeland T, Røise O, Aarhus, M, Andelic, N, and Helseth, E . Traumatic brain injury-the effects of patient age on treatment intensity and mortality. BMC Neurol. 2020 Oct;20(1):376. doi:10.1186/s12883-020-01943-6.
- Kirkman MA, Jenks T, Bouamra O, Edwards A, Yates D, Wilson MH. Increased mortality associated with cerebral contusions following trauma in the elderly: bad patients or bad management? J Neurotrauma. 2013 Aug;30(16):1385–90. doi:10.1089/neu.2013.2881.
- Stiell IG, Wells GA, Vandemheen K, Clement C, Lesiuk H, Laupacis A, McKnight RD, Verbeek R, Brison R, Cass D, et al. The Canadian CT head rule for patients with minor head injury. Lancet. 2001 May 5;357(North9266):1391–96. Internet].; doi:10.1016/S0140-6736(00)04561-X
- Stiell IG, Clement, C M, Rowe, B H, Schull, M J, Brison, R, Cass, D, Eisenhauer, M A, McKnight , R G, Bandiera , G, Holroyd, B, Lee, J S, Dreyer, J, Worthington, J R, Reardon, M, Greenberg, G, Lesiuk, H, MacPhail, I, and Wells, G A, et al. Comparison of the Canadian CT head rule and the new orleans criteria in patients with minor head injury. JAMA. 2005 Sep;294(12):1511–18. doi:10.1001/jama.294.12.1511.
- Harnan SE, Pickering A, Pandor A, and Goodacre SW. Clinical decision rules for adults with minor head injury: a systematic review. J Trauma Acute Care Surg Internet]. 2011;71(1):245–51. Accessed 31st July 2021. doi:10.1097/TA.0b013e31820d090f.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000 Jul;343(2):100–05. doi:10.1056/NEJM200007133430204.
- Joseph B, Aziz H, Pandit V, Kulvatunyou N, Sadoun M, Tang A, O’Keeffe T, Gries L, Green DJ, Friese RS, et al. Prospective validation of the brain injury guidelines: managing traumatic brain injury without neurosurgical consultation. J Trauma Acute Care Surg. 2014Dec [cited 2019 Feb 5];77(6):984–88. Internet]. doi:10.1097/TA.0000000000000428.
- Lessard J, Cournoyer A, Chauny J-M, Piette É, Paquet J, Daoust R. Can the “important brain injury criteria” predict neurosurgical intervention in mild traumatic brain injury? A validation study. Am J Emerg Med. 2020Mar;38(3):521–25. Internet]. doi:10.1016/j.ajem.2019.05.043.