ABSTRACT
Background
Following a concussion, approximately 15% of individuals experience persistent symptoms that can lead to functional deficits. However, underlying symptom-clusters that persist beyond 12 months have not been adequately characterized, and their relevance to functional deficits are unclear. The aim of this study was to characterize the underlying clusters of prolonged post-concussive symptoms lasting more than 12 months, and to investigate their association with functional impairments.
Methods
Although hierarchical clustering is ideally suited in evaluating subjective symptom severities, it has not been applied to the Rivermead Post-Concussion Questionnaire (RPQ). The RPQ and functional impairments questions were administered via a smartphone application to 445 individuals who self-reported prolonged post-concussive symptoms. Symptom-clusters were obtained using agglomerative hierarchical clustering, and their association with functional deficits were investigated with sensitivity analyses, and corrected for multiple comparisons.
Results
Five symptom-clusters were identified: headache-related, sensitivity to light and sound, cognitive, mood-related, and sleep-fatigue. Individuals with more severe RPQ symptoms were more likely to report functional deficits (p < 0.0001). Whereas the headache and sensitivity clusters were associated with at most one impairment, at-least-mild sleeping difficulties and fatigue were associated with four, and moderate-to-severe cognitive difficulties with five (all p < 0.01).
Conclusions
Symptom-clusters may be clinically useful for functional outcome stratification for targeted rehabilitation therapies. Further studies are required to replicate these findings in other cohorts and questionnaires, and to ascertain the effects of symptomatic intervention on functional outcomes.
1. Introduction
Concussion is considered a mild subset of traumatic brain injury (TBI) making up around 80% of all TBI (Citation1). The incidence of concussion is estimated to be between 0.1% to over 1% of the general population (Citation1,Citation2), and it is a major cause of morbidity following domestic accidents, high-impact sports (Citation3), and in military veterans (Citation4,Citation5). About half of the one million people with TBI who attend emergency departments in the United Kingdom annually experience post-concussive symptoms (Citation6–8). Concussive symptoms include headaches, dizziness, nausea, impaired attention and memory, changes in sleep, and depression or anxiety (Citation9).
Although most concussive symptoms resolve within 3 months (Citation10), a minority of up to 15% of individuals experience persistent post-concussive symptoms lasting more than 3 months, or even more than 12 months, sometimes without structural imaging correlates (Citation11,Citation12). Persistent post-concussive symptoms are associated with chronic morbidity, lower quality of life (Citation13), and functional deficits including reduced ability to work (Citation14), and loss of social autonomy (Citation15). Therefore, despite progress with modern imaging modalities and biomarkers (Citation16), clinical symptoms remain imperative for patient-centered evaluations in identifying potentially treatable long-term symptoms.
However, the precise role of different post-concussive symptomatology in bringing about different functional deficits has not been adequately investigated, partly due to the use of inappropriate statistical techniques for the evaluation of post-concussive symptom questionnaires (Citation9).
Post-concussion symptoms can be assessed using self-reported questionnaires that evaluate the presence of, or change in, symptoms, their frequency and severity, and associated functional impairments (Citation17). The 16-item Rivermead Post Concussion Symptoms Questionnaire (RPQ-16) measures the severity of symptoms with good test–retest reliability (Citation18). Although functional impairments are not part of the questionnaire, RPQ-16 was originally intended to help assess the degree to which symptoms may contribute to psychosocial dysfunction (Citation18).
Previous studies have investigated the underlying symptom-structure of the RPQ-16 using principal components (PCA) and confirmatory factor analyses (CFA) (Citation9). CFA and PCA make assumptions of linearity that are problematic when applied to self-reported symptom questionnaires such as the RPQ. Despite these methods not being the most suited for ordinal scales (Citation9), their application in symptomatic profiling of TBI has not been restricted to RPQ, having also been applied to the 22 question Post-Concussion Symptom Scale (Citation19). Even though CFA and PCA are distinct methods that often produce similar results when applied to the same dataset (Citation20), their applications to various RPQ-16 datasets have yielded different results, including three and four factor structures (Citation11,Citation21,Citation22), with some suggesting dynamic changes in symptom structures within the first year after injury, while other studies have contradicted this (Citation23,Citation24). Besides differences in cohorts, there may be other explanations for these inconsistent findings, including incongruence amongst, and between, physicians and patients in their interpretations of symptoms. For example, a clinician may interpret “double vision” as diplopia whereas patients may perceive it to adequately describe blurred vision. As another example, dizziness could mean vertigo at rest (vestibular) for one patient, or motion sensitivity from migraine, or benign paroxysmal positional vertigo (BPPV) for another. Such diverging interpretations have implications for symptom-groupings due to errors in clustering different symptoms under single nonspecific terminologies. Additionally, an individual’s interpretation of symptoms may change with time. For example, an individual with acute vertigo from BPPV may report “dizziness” (question 2 of RPQ-16) and although later resolved, may still report “dizziness” to be present due to chronic migraines (Citation25). Unlike PCA and CFA, hierarchical clustering does not assume linearity and does not impose theoretical understandings of underlying factor structures to subjective severity scores. Therefore, compared to PCA and CFA, hierarchical clustering may be a more suitable method to evaluate subjectively scored symptoms (Citation9,Citation26).
The aim of this study was to perform a symptom-centric analysis of prolonged symptoms following a concussion. The primary purpose was to characterize the underlying symptom-structure of prolonged post concussive symptoms that last more than 12 months using agglomerative hierarchical clustering on responses to the RPQ, and to compare the symptom-clusters obtained to the symptom factors obtained from previous methods. Although the majority of post-concussive symptoms usually resolve within the first few months, the persistence of symptoms causes significant morbidity (Citation11,Citation12). Therefore, finding unique clinical profiles in individuals with notably prolonged symptoms could be valuable in stratifying concussion outcomes, supplementing interventions that have been shown to reduce morbidity in randomized control trials (Citation27). Therefore, the secondary aim of this study was to investigate if these symptom-clusters were then associated with unique functional impairments, which were captured along with the RPQ-16 responses using a smartphone application and a bespoke abbreviated functional impairments questionnaire suitable for remote self-reporting.
2. Methods
2.1. Setting: smartphone application
The Mindstep (Mindset4Dementia) application consisted of a 5-minute questionnaire where information on common risk factors for cognitive impairment were gathered, including concussion. The application routinely asked about a history of concussion and, if present, participants were automatically shown the RPQ-16. The application is freely available to iPhone users in the Apple Application Store and has been previously validated (Citation28).
2.2. Participants
Participants were individuals over the age of 18, who of their own accord, downloaded and self-reported a prior diagnosed concussion within the Mindstep (Mindset4Dementia) application. Eligible participants were not sought out nor paid. Participants gave informed consent for the collection and use of anonymized data for predictive analytics, as described previously (Citation28,Citation29). Aconvenience sample of consecutive data was obtained between the 1st of March to the 14th of April 2021 from individuals who replied in the affirmative to the following question:
“Have you ever had a diagnosed concussion?”
Participants were dichotomized into two groups based on interval since concussion. “Prolonged” symptoms were defined as those persisting for 12 months or more after a concussion, and “early” symptoms as those occurring within 12 months.
The term “prolonged” (≥12 months) was used to distinguish this from the usual definition of “persistent” post-concussion syndrome (>3 months) (Citation10). This longer period of 12 months also ensured that more stable symptoms were captured as post-concussive symptoms may change within the first year (Citation23), and longer symptoms are more likely to give meaningful stable associations with functional outcomes (Citation15).
Although no data was available on the severity of concussion as this was self-reported, the majority of cases are likely to have fallen into the symptomatic (possible) or mild (probable) TBI categories as defined by the Mayo system (Citation30,Citation31).
Participants were therefore a convenience sample with the majority reporting (prolonged) post-concussion symptoms and were likely worried about cognitive repercussions.
2.3. Data
The application routinely collected data on participants’ ages, presence of comorbidities, RPQ-16 symptoms (if the user gave a history of concussion), duration since concussion, and self-reported functional impairments. These data, and other data that were collected as part of the app, such as smoking status and alcohol consumption, are shown for the prolonged group in , and for the “early” group (less than 12 months) in Supplementary Table 1. Data was fully anonymized and stored in secure Amazon web services cloud. The full range of other data that were routinely collected by the application but were not the focus of this study and therefore not analyzed, have been previously described (Citation28,Citation29).
Table 1. Demographics and Comorbidities of Prolonged Symptoms Group.
Table 2. Summary of Self-Reported Functional Impairments.
Table 3. Analysis by Mild Symptom-Clusters in Prolonged Symptoms Group.
Table 4. Analysis by Moderate Symptom-Clusters in Prolonged Symptoms Group.
Table 5: Analysis by Functional Impairments in Prolonged Symptoms Group
2.2.1. RPQ-16 symptom scores
RPQ-16 consists of rating 16 symptoms compared to pre-injury levels using a five-point Likert-like scale from “0” through to “4” (Citation18). The questionnaire asks participants to compare their current symptoms to those before the head injury and rate them as “not experienced at all,” “present but no more of a problem,” “mild problem,” “moderate problem,” or “severe problem.” All 16 questions from the questionnaire were presented, summarized in Supplementary Table 2.
As in previous studies, RPQ scores were corrected by combining the first two response-scales for each question: “not experienced at all” and “present but no more of a problem”; i.e., a score of 1 was reduced to 0 (Citation32). These corrected RPQ-16 scores were used in all further analyses.
Total corrected RPQ-16 scores ≥16 were used as an outcome marker of significant post-concussion symptoms, as previously suggested (Citation33).
2.2.2. Functional impairments
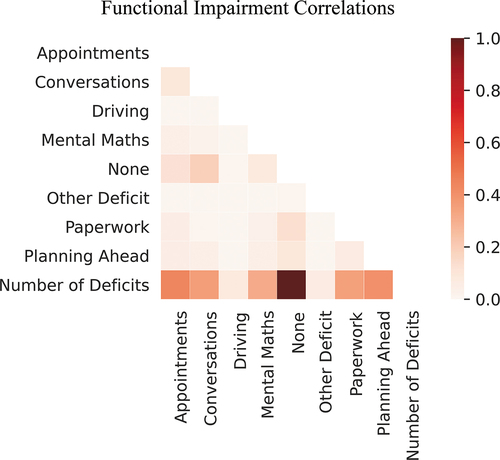
After reviewing the many available questionnaires designed to assess function after TBI (Citation34), an adapted and abbreviated questionnaire suitable for remote self-reporting was used, such that it could be completed in a short period of time in the application. The leading question and list of functional impairments enquired about were “have you had trouble with any of the following?”: “difficulty driving or cycling?,” “difficulty doing paperwork?,” “difficulty remembering appointments?,” “difficulty with mental maths?,” “difficulty with conversations?,” “difficulty planning ahead?,” “other difficulty?” or “None”. Participants could choose one or more functional deficits listed (binarized present/absent) ().
Cramer’s V was used to evaluate correlations between functional deficit categories.
2.4. Hierarchical clustering
PCA and CFA are the most commonly used methods to cluster post-concussive symptoms (Citation9). The reason hierarchical clustering was used instead, is that PCA attempts to identify composite symptoms to explain the variances in RPQ-16 symptom severities, while CFA only tests prior hypotheses on underlying latent factors that could “predict” the data (Citation35). Both PCA and CFA can be problematic when applied to ordinal variables such as the RPQ, because both methods assume variables are linear functions of components/factors (Citation36). Thus, when these methods are applied to the RPQ-16, they assume that symptom severities can be approximated as interval data, which may not always be the case (Citation9). For example, it is unclear whether “moderate” symptoms of irritability are equivalent to “moderate” symptoms of double vision, or whether the interval from “present but no more of a problem” to “mild” is the same as the interval between “moderate” to “severe.” For these reasons, agglomerative hierarchical clustering was used (Citation37).
Agglomerative hierarchical clustering has the advantage that, unlike CFA, it is unsupervised and not biased by the current clinical thinking about the latent structures of post-concussion symptoms, and unlike PCA, does not preserve global variance at the potential cost of losing local similarities (Citation26). Additionally, agglomerative hierarchical clustering on spearman rank correlations does not assume a linear relationship between symptom severities and underlying latent components.
As post-concussion symptoms may change within the first year (Citation23), and persistent symptoms are more likely to be associated with functional deficits (Citation15), the main focus of this study was the clustering of symptoms, and their functional deficits, in the prolonged group.
First, the pairwise Spearman rank correlation matrix between the RPQ-16 symptom severity scores were obtained. Next, the clustermap function from seaborn (version 0.11.1) was used to perform unsupervised agglomerative hierarchical clustering on the correlation matrix using the default Euclidean distance metric. The obtained symptom-clusters were compared with those of somatic, emotional, and cognitive symptoms obtained using previous methods (Citation11,Citation21).
In agglomerative hierarchical clustering, individual symptoms are paired first (bottom-up) and a symptom-cluster was defined to be formed when all 16 symptoms of the RPQ were paired with at least one other symptom in as few hierarchical levels as possible. In turn, superclusters were defined similarly as the hierarchical clustering of symptom-clusters together (Citation37).
2.5. Analysis of symptom-clusters and functional impairments
Theoretical constructs of questionnaires are only useful insofar as they help with clinically relevant stratification (Citation38). Therefore, in addition to the hierarchical structure of the RPQ-16, functional impairments were also analyzed.
2.5.1. By symptom-cluster membership
The association between functional deficits and symptom-clusters in the prolonged group was investigated. This analysis by symptom-cluster membership categorically assigned each individual to none or more symptom-cluster(s). Membership of a symptom-cluster was defined by requiring a participant’s mean corrected RPQ scores for each symptom-cluster to be 1) at least mild or 2) at least moderate (Citation32). For each of these levels, separate Bonferroni corrections were performed at alpha 0.05 (five clusters, six functional impairments, alpha = 0.05/30 = 0.0017). Two-by-two chi-squared tests (or Fisher’s exact where appropriate) were used to investigate the proportion of reported functional impairments.
2.5.2. By functional impairment
To determine if functional impairments were generally associated with more severe symptoms irrespective of symptom-clusters, the mean RPQ-16 severity scores between individuals with no functional impairment (“none”) and those with any reported functional impairment were compared using a Mann-Whitney U test.
To determine if the severity of reported symptom-clusters varied between given functional deficits, all participants that reported a functional deficit (i.e., excluding “none”) were selected, and Mann-Whitney U tests compared the mean scores for each symptom-cluster. This analysis by functional impairment was a categorical assignment to one or more functional impairment group(s) with a summary mean severity score for each symptom-cluster. In addition to symptom-clusters, the overall RPQ-16 severity scores for each functional deficit was analyzed. The analyses were Bonferroni corrected for multiple comparisons at alpha 0.05 (five symptom-clusters and one overall RPQ-16, six functional impairments, alpha = 0.05/6 × 6 = 0.0014).
Note that in both analyses described above, by symptom-cluster membership (categorical-categorical) and by functional impairments (categorical-ordinal), participants may have belonged to more than one symptom-cluster or more than one functional deficit group, respectively.
2.6. Sensitivity analyses
In the above by symptom-cluster membership analysis, symptom-cluster membership was defined as mean corrected scores at least 1) mild or 2) moderate. Sensitivity analyses were performed for the associations that were significant after Bonferroni correction by changing the criteria for inclusion in a symptom-cluster from “mean score” to either “any single symptom” or “all symptoms” from the symptom-cluster (see Supplementary Methods for further details).
All analyses and figures were produced using python: pandas v 1.1.5, scipy v 1.5.2, matplotlib v 3.3.3, and seaborn v 0.11.1 (Citation39).
2.7. Comparison of RPQ-16 in early vs prolonged groups
In order to better understand the data from this cohort and compare it to previous cohorts, the severity of all reported RPQ-16 symptoms between the two groups were compared using univariate two-by-two chi-squared or Fisher’s exact test as appropriate.
2.8. Data availability
Anonymized analysis-ready data and the statistical analyses are available upon reasonable request at the repository (please contact us to gain access): https://github.com/letsmindstep/ClusteringRPQ
3. Results
3.1. Participants and demographics
2235 people downloaded the application and consented to data collection, of which 523 reported having had at least one concussion, while 1712 responded as not having had a diagnosed concussion. From the 523 participants with at least one concussion, 467 (89.3%) completed the RPQ-16, of whom 22 reported a concussion within the previous 12 months (early), and 445 reported a concussion twelve months or more previously (prolonged).
Of the 445 with prolonged symptoms, 153 (34.4%) had significant post-concussion symptoms (total RPQ-16 score of 16 or more). 10 (45.5%) of 22 participants in the early group had significant symptoms (RPQ-16 score of 16 or more).
There was no significant difference in the age distributions between the early (mean 44.9 years ± 17.4) or prolonged cohorts (47.2 years ± 12.9; Welch’s t-test p = 0.54). Demographics, comorbidities, and numbers of past concussions for the prolonged group are shown in and for the early group in Supplementary Table 1.
3.2. Agglomerative hierarchical clustering
Clustering for prolonged symptoms demonstrated five clinical clusters (). The headache-clusterincluded blurred vision and double vision in addition to the RPQ-3 (headache, dizziness, nausea and/or vomiting).
Figure 1. Agglomerative hierarchical clustering of RPQ-16 symptoms 12 months after concussion (prolonged group, n = 445).
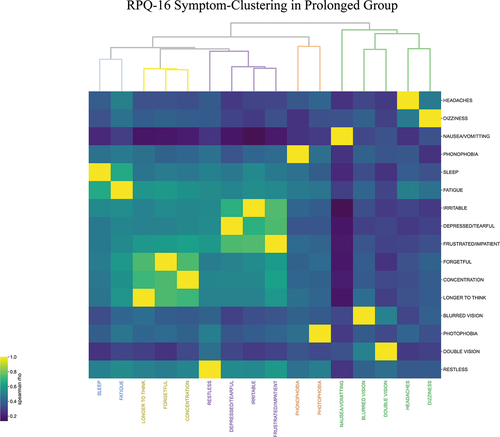
Somatic symptoms of sensitivity to light and sound constituted thesensitivity-cluster.
A sleep-fatigue-cluster, cognitive-cluster (difficulty concentrating, feeling forgetful and taking longer to think); and a mood-cluster (restlessness, depression, irritability and frustration) completed the RPQ-16 symptoms in five clusters. As the height of the dendrogram signifies the difference between clusters, these latter three clusters constituted a supercluster with the most similarity ().
These five clusters represent similarities between the RPQ questions as assessed by similarities in severity perceived by participants, not as intended by the questionnaire or interpreted by the physician. The clusters and their constituent symptoms were obtained unsupervised. The height of each node in the dendrogram in is inversely proportional to the similarity between its constituent symptoms. Therefore, the headache-relatedcluster (in green) and sensitivity-cluster(brown) were associated with the most variance amongst their symptoms, while the three cognitive symptoms (yellow) were the most similar. As migraines are most likely responsible for the symptoms reported in the headache-related and sensitivity clusters, one interpretation of this is that while the severity of the various symptoms that constitute migraines were variable, cognitive symptoms such as forgetfulness, taking longer to think, and lack of concentration were amongst the most semantically equivalent RPQ-16 symptoms.
The Spearman rank correlation matrices for both early and prolonged groups, which were used for hierarchical clustering, are shown in Supplementary Figure 1.
Hierarchical clustering in the early group (Supplementary Figure 2) was different from that of the prolonged group, however this is considered preliminary due to only 22 participants.
3.3. Comparison of RPQ-16 in early vs prolonged groups
For baseline comparisons between these groups, see Supplementary Table 2 and Supplementary Results.
3.4. Functional impairments
From amongst the 467 total participantswho had a concussion and completed the RPQ, 254 individuals’ self-reported functional deficits were available (54.4%), of which 240 were from the prolonged group.
The most common responses were difficulty with conversations (44.9%), completing paperwork (37%) and remembering appointments (34.3%, ). The least common functional impairment reported by only 3.9% was “other deficit(s).” This small percentage of unspecified “other deficit(s),” along with previous results that showed these functional impairments were associated with cognitive measures (Citation28), suggest that the functional deficits experienced after a concussion were adequately captured by the smartphone application. As “other” deficits were nonspecific and only reported by 10 individuals, this was omitted from further analyses.
Due to multiple correlations on Cramer’s V, the “number of deficits” was removed from further analyses ().
3.5. Analysis of symptom-clusters and functional impairments
In the prolonged group, the association between symptom-clusters () and functional impairments were investigated from two perspectives: by symptom-cluster membership (categorical-categorical) and by functional impairments (categorical-ordinal).
3.5.1. By symptom-cluster membership
The results of the at-least-mild severity thresholds are shown in and the results of the moderate-to-severe symptoms are shown in .
3.5.1.1. At Least Mild Severity
Results showed that of the self-reported functional impairments, trouble with appointments and conversations were associated with the sleep-fatigue and cognitive clusters, while trouble with conversations was additionally associated with emotional cluster symptoms (). The largest effect size was for the cognitive-cluster, whereby they were more likely to report difficulties with remembering appointments and following conversations with OR 2.93 (95% CI 1.61–5.34, p < 0.001) and OR 4.41 (2.47–7.88, p < 0.0001), respectively.
Difficulty driving and trouble with executive planning were not significantly reported more frequently in any of the symptom-clusters after correction for multiple comparisons ().
Difficulty with mental arithmetic was reported more frequently in the sensitivity to light and sound cluster with OR 2.98 (1.63–5.45, p < 0.001), and sleep-fatigue cluster with OR 2.64 (1.46–4.78, p < 0.001).
Reports of impairments at filling out paperwork were significantly more frequent in the sleep-fatigue (OR 2.79 [1.61–4.82], p < 0.001) and emotional clusters (OR 2.57 [1.49–4.44], p < 0.001).
Sensitivity analyses, where the membership criteria for symptoms-clusters were changed from mean symptoms to at-least one symptom or all-symptoms satisfying mild severity, showed the findings were robust except for the association between the sleep-fatigue cluster and mental arithmetic on one out of two of the sensitivity tests. Having at least one mild symptom resulted in an insignificant odds ratio ().
In summary, the fewest Bonferroni corrected functional impairments were associated with headache (none) and sensitivity-clusters (one), while the greatest number of functional impairments was associated with the sleep-fatigue-cluster (four). Therefore, of all the symptom-clusters with at least mild severity, the sleep-fatigue cluster was associated with the greatest number of functional impairments. This result was robust to sensitivity analyses and whether results were corrected for multiple comparisons or not ().
3.5.1.2. Moderate-to-severe
A threshold of moderately severe symptoms resulted in both the greatest number of functional impairments and the largest effect sizes to be associated with the cognitive cluster. Participants who reported moderate-or-severe symptoms from the cognitive-cluster (longer to think, difficulty concentrating, being more forgetful) also reported significantly more impairments (five) compared to the sleep-fatigue-cluster (two) and the emotional-cluster (three). The strength of these associations can be found in .
From 10 significant symptom-cluster/functional-impairment associations, seven were robust to both sensitivity analyses, while three from the emotional symptom-cluster were no longer significant when all symptoms from the cluster were moderately severe ().
3.5.2. By functional impairment
Individuals who reported any of the functional deficits generally had RPQ-16 severity scores 14.2 points higher than those who reported none (mean RPQ-16 score with any impairment 21.2 vs mean without any impairment 7.0, MWU statistic = 68931.5, p < 0.0001).
After correction for multiple comparisons, there were only three significant results for the analysis by functional impairment. Participants who reported difficulty with remembering appointments or following conversations had more severe RPQ-16 total symptoms compared to those who did not report these impairments (). Having trouble following conversations was associated with more severe RPQ-16 symptoms (mean score with impairment 23.9 vs 17.8 without impairment, p < 0.0001), and difficulty remembering appointments was also associated with more severe symptoms (mean score with impairment 23.7 vs 19.3 without impairment, p < 0.001). Specifically, those who reported difficulty following conversations also reported more severe cognitive cluster symptoms which included forgetfulness, difficulty concentrating, and taking longer to think (mean cluster score 6.2 with impairment vs 4.4 without impairment,p < 0.0001) ().
4. Discussion
4.1. Overview
This study is the first to use agglomerative hierarchical clustering on post-concussion symptoms as measured by the RPQ. The five unsupervised symptom-clusters obtained from 445 individuals’ responses to the RPQ-16 at least one year after a concussion were: headache-related, sensitivity to light and sound, sleep-fatigue, cognitive, and mood-related.
Of all the symptom-clusters with at least mild severity, the sleep-fatigue cluster was associated with the greatest number of functional impairments, suggesting that mild symptoms of sleep and fatigue may be more disabling than even mild cognitive symptoms. Therefore, even mild trouble sleeping and mild fatigue should be clinical priorities to screen for and treat. However, with increasing severity of symptoms including at least moderate symptoms, the cognitive cluster was the most functionally disabling. The cognitive cluster included symptoms of taking longer to think, forgetfulness, and difficulty concentrating; and at moderate or more severe severities addressing these symptoms should also be prioritized.
These results also established that the presence of any functional impairment was associated with more severe RPQ-16 symptoms, with an average score 14.2 points higher on the RPQ-16 scale than those who reported none.
4.2. Hierarchical RPQ-16 structure
The RPQ-16 questionnaire captures the severity of symptoms after TBI, and a recent review evaluated all the studies that investigated its underlying latent structure using PCA and CFA (Citation9). PCA and CFA often give similar results (Citation20). For example, PCA methods have suggested three components in a study on 200 individuals with TBI: mood and cognition, general somatic, and visual somatic (Citation22); while CFA methods have suggested three similar underlying factors: somatic, emotional, and cognitive (Citation11,Citation21).
This study's hierarchical clustering results are most consistent with this three-factor model: Cognitive: forgetfulness, poor concentration, taking longer to think. Emotional: irritable, depressed or tearful, frustrated, restless. Somatic: headache, dizziness, nausea or vomiting, sensitivity to noise or sound, sleep disturbance, fatigue, blurred vision and double vision (Citation21). The major difference is that instead of sleep-fatigue being within a “somatic” group, hierarchical clustering suggested that they are a separate cluster, and, for at-least mild severities, associated with the most functional impairments. A further difference is that sensitivity symptoms were also clustered separately.
The results of applying PCA and CFA to RPQ-16 have not been homogenous, with some identifying four factors instead of three, e.g., in 181 military veterans (Citation4), and some suggesting dynamic changes in factor structures within the first year after TBI (Citation23), while others have contradicted this (Citation24). Another study assumed six symptomatic RPQ-16 groups during the early post-TBI stage, including “cognitive-fatigue”, “vestibular”, “oculomotor”, “anxiety/mood”, “migraine”, and “cervical”. However, this particular study used a modified RPQ-16, including symptoms that do not feature in the standard RPQ-16 (Citation40). Such studies assume homogeneity of interpretation of symptom descriptions between the clinician and patients completing the questionnaire. The unsupervised clustering method used in this study does not make this assumption. Furthermore, they only profiled early TBI symptoms, during which symptom interpretations may vary from those of chronic symptoms.
Some have suggested using the first three (RPQ-3) and last 13 questions (RPQ-13) as separate measures for early and prolonged symptoms, respectively (Citation41). The RPQ-3 contains questions from the headache cluster, and as these were common, it is unsurprising that RPQ-3 and RPQ-13 have been shown to be good constructs for people with head injury with good retest reliability and external construct validity (Citation41). In other words, hierarchical clustering suggests RPQ-3 may be a special subset of the headache symptom-cluster. As preexisting comorbidities, including migraine, may be associated with higher risks of persistent post-concussion symptoms (Citation42), their treatment should be made a clinical priority. The sensitivity-cluster straddled between the headache-cluster and the supercluster which included mood symptoms, suggesting possible similarities between them. This is in line with previous migraine studies that have found bidirectional associations not just between depressive symptoms and migraine (Citation43), but also specifically with photophobia (Citation44).
This study is the first to use agglomerative hierarchical clustering on RPQ-16. This clustering method groups symptoms based on how well their severity co-varies with all other symptoms on the RPQ-16. While there may not be a strong correlation between two symptom severities, they may still be clustered together due to a strong correlation of their two-by-two spearman correlations with all other symptoms (similarity). This overcomes the incongruence between physicians and patients in the interpretation of symptoms by using a patient-reported measure that is not dependent on the provider’s interpretation of terminologies to co-cluster similar symptoms, as is the case with CFA. Therefore, hierarchical clustering is a more analytically sound approach to define post-concussion RPQ-16 symptom-clusters (Citation45).
4.3. Symptom-clusters may stratify functional impairments
Studies have shown no significant differences between RPQ-16 scores in patients with normal or abnormal MRI brain imaging, whether evaluated with a binary threshold (>19 or >35) or using non-parametric tests (Citation46). This highlights the importance of our study in directly evaluating the relationship between clinical symptom-clusters and functional impairments.
From all five symptom-clusters with at least mild severity, sleep-fatigue was associated with the greatest number of functional impairments, highlighting the role mild-to-severe sleep disturbance and fatigue can have in functional impairments. Conversely, moderate-to-severe cognitive symptoms were more functionally disabling than other symptoms. These results therefore suggest that mild cognitive symptoms may be associated with fewer disabling impairments than mild sleep disturbances or fatigue, while more severe cognitive symptoms may be associated with more functional impairments. Symptom-clustering may therefore have clinical value in stratifying functional impairments post-TBI.
Previous studies investigating the association between RPQ scores and outcomes have used the Glasgow Outcome Scale Extended (GOSE), finding that 27.1% (198/731) of participants at 6-months had post-concussion functional impairment when using a GOSE cutoff score less than or equal to 6 (Citation21).It is acknowledged that dichotomization of GOSE can reduce sensitivity (Citation34), therefore, this study used individual functional impairments without an overall functional score. Their study found 6 or more symptoms from the RPQ-16 with a score of at least moderate severity was associated with the highest percentage of dichotomized functional impairments, more than use of the three-factor model. Six or more at-least moderate symptoms from amongst the RPQ-16 is however arbitrary, and their analysis investigating RPQ functional outcomes is categorical-categorical; our current study, however, used both categorical-categorical and the underlying ordinal data to ascertain the presence of correlations – the latter more in line with recommendations (Citation47). Additionally, they assigned individuals to the three-factor model if any of the items of the factors had at least moderate severity, whereas our study used the mean of all the items of a cluster as more representative of an RPQ-cluster, in addition to further sensitivity analyses. Nevertheless, despite their use of different cutoffs for the RPQ and use of the GOSE questionnaire, their functional impairment results are in line with this study’s results which found that more severe RPQ symptoms are associated with more functional impairments (Citation21).
The list of functional impairments used in this study mostly relate to deficits in attention, memory, or executive function, including difficulty tracking conversations, completing paperwork, remembering appointments, and planning ahead (). These activities require constant attention, which has previously been shown to objectively be correlated with self-reported cognitive symptoms (Citation48). This explains why moderate-to-severe cognitive symptoms were found in this study to be associated with nearly all of the functional impairments (). Dysexecutive symptoms have also previously been shown to be correlated with cognitive and emotional symptoms (Citation48), similar to the associations found here between symptom-clusters and functional impairments, where increasing severity of symptoms in the cognitive and emotional clusters were associated with more functional impairments ( vs ).
4.4. Sleep-fatigue cluster: potential clinical target?
Although the RPQ does not differentiate between specific sleep complaints, minor TBIs are known to contribute to insomnia, specifically delayed sleep phase and irregular sleep-wake patterns (Citation49). The prevalence of insomnia one year after a concussion is three times the rate found in the general population (Citation50).
Sleep is known to have a neuroprotective role, and its disturbance is associated with mood disturbances, fatigue, and cognitive impairments (Citation51,Citation52), similar to the supercluster and findings in this study that associate them with functional impairments. There is potentially a single pathophysiological mechanism underlying this post-concussion symptom-supercluster, that of the functional disruption of the network between the cortex and dentate nucleus of the hippocampus (Citation53). This results in dysregulated slow-wave sleep and trouble retrieving memories (Citation52,Citation53), which is the archetypal cognitive symptom at the center of the cognitive-cluster (“forgetfulness” in ).
Screening for sleep difficulties could aide recovery (Citation50), and a recent study has reviewed pharmacological and psychological interventions for sleep disturbances after TBI (Citation54). In fact, reconsolidation of the 24-hour sleep-wake cycle after TBI has been purported to predict emergence from posttraumatic amnesia as well as cognitive impairment in rehabilitation settings (Citation55).
The data and results from this study are therefore important in shaping understanding of symptomatology and potential recovery in those who have notably prolonged symptoms for over 12 months.
The sleep-fatigue cluster results should be taken in the context of the spectrum of TBI and symptom severities. Results suggest that at least mild sleep and fatigue disturbances after mild TBI are associated with significant impairments in daily function, even more than cognitive symptoms. However, at the mild end of TBI, prior studies have shown that difficulty sleeping in 346 individuals was associated with worse post-concussive symptoms including cognition (Citation50). At the moderate-to-severe TBI spectrum in hospitalized patients, previous studies have also shown that sleep quality was linearly associated with consciousness and cognitive function (Citation55). While they measured sleep quality objectively using continuous wrist actigraphy and measures of sleep-wake consolidation, sleep duration and sleep fragmentation index; the RPQ-16 utilized in this present hierarchical clustering study used subjective single scale measures for sleep, fatigue and the three cognitive symptoms. It would therefore be ideal in future to link the subjective RPQ-16 symptoms of difficulty sleeping and cognitive difficulties with objective measures of sleep quality and cognition, to assess the degree of correlation between subjective and objective measures.
5.5. Limitations
Although the value of the term “concussion” is disputed by some authors as it has no verifiable ground-truth (Citation56), and is heterogeneously defined by some to be as mild as “any blow to the head” (Citation11), nevertheless the question used in the application mentioned “diagnosed concussion” to identify suitable participants. This is likely to signify symptomatic mild-TBI as per guidelines (Citation57), and is more suited for use in the remote collection of self-reported symptoms as it is easier to understand. Additionally, although self-reported data may be susceptible to selection and recall biases, a prior study using this same application’s data has demonstrated epidemiological validity (Citation28).
Nearly a quarter of the users of the application from which data was collected reported having had a diagnosed concussion (523/2235). As the Mindstep application (Mindset4Dementia) was designed as a screening tool for cognitive impairment, the sample therefore comprised of individuals with mild TBI who were worried about cognitive repercussions, for example due to prolonged symptoms, and may not be representative of all TBI cases with prolonged symptoms. Nevertheless, this study replicated some previous results, indirectly validating our participant cohort (see Supplementary Results comparing the two early and prolonged groups).
RPQ-16 asks about relative symptoms compared to before a concussion, but it is conceivable that some participants would report the absolute severity of their current symptoms, compounded by the high prevalence of RPQ-16 symptoms in individuals without TBI (Citation17,Citation58). Symptoms developed and reported as post-concussive may not have been conclusively caused by a concussion, as the concussion could have been incidental, and coincidental comorbidities could result in similar symptoms (Citation12). Therefore, symptoms may be incorrectly attributed to the concussion, however, this is a known limitation in all RPQ studies (Citation59). As a result, the symptom-clusters identified in all RPQ studies, irrespective of methodology, may not be specific to post-concussion syndrome.
Although a bespoke list of functional impairments were carefully curated, this list has not been previously validated. Nevertheless, there was an “other deficit(s)” category which was the least selected at 3.9%, suggesting this list of impairments captured most functional impairments.
Another point to highlight is that a cutoff of 12 months was used to define more prolonged symptoms, whereas persistent post-concussion syndrome is usually defined as over 3 months (Citation10). 12.8% of individuals with prolonged symptoms in this study reported having had COVID-19 (), which may impact the reporting of symptoms.
This study was observational so causality cannot be inferred, only a prospective design can establish whether the identified symptom-clusters may be causally associated with the development of specific functional impairments. Further investigation of sleep-related symptoms would require use of objective sleep measures. Future studies assessing post-concussion symptoms should also ask about preexisting comorbidities such as migraines and sleep disturbances prior to TBI.
6. Conclusions
The strength of hierarchical clustering, unlike previous methods, is that results are not biased by how clinicians intended for the questions to be interpreted and may be especially useful in self-administered questionnaires on subjective experiences of symptoms. Using this method, our findings suggest we should rethink RPQ-16 symptoms in individuals 12 months after a concussion as having five symptom-clusters, where the severity of symptom-clusters are associated with functional impairments. Specifically, at least mild difficulty sleeping and fatigue, and moderate-to-severe cognitive symptoms, were more functionally disabling than other similarly rated symptoms.
Our results are important in shaping understanding of symptomatology and recovery from concussion in those who have notably prolonged symptoms and may help with rehabilitation strategies targeting sleep and fatigue. Our results suggest that focused clinical screening should be performed routinely, and are supportive of the increased involvement of neurologists in the care of patients with concussion to help diagnose and treat associated underdiagnosed and undertreated conditions, as previously proposed (Citation56).
Further studies are required to replicate our findings using agglomerative hierarchical clustering in other cohorts and questionnaires, establish the causal direction of sleep and fatigue problems with functional impairments, and the effects of intervention.
Ethics statement
On using the application users agreed to transparent terms and conditions which included having their data stored and anonymously used for further research. We were retrospectively granted favourable ethical opinion for the use of this data for research by West Midlands - Solihull Research Ethics Committee, REC Reference 21/WM/0202. All data were stored in line with GDPR regulations.
Disclosure statement
Funding for this paper was provided by Mindset Technologies Ltd. All authors are, or were, employees and/or shareholders of Mindset Technologies Ltd. The funder designed the application setting in which the data was collected, but otherwise had no role in the design, analysis, write-up or decision to submit.
Additional information
Funding
References
- Cassidy JD, Carroll L, Peloso P, Borg J, von Holst H, Holm L, Kraus J, Coronado V. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;36:28–60. doi:10.1080/16501960410023732.
- Langer L, Levy C, Bayley M. Increasing incidence of concussion: true epidemic or better recognition? J Head Trauma Rehabil. 2020;35(1):E60–E66. doi:10.1097/HTR.0000000000000503.
- James SL, Theadom A, Ellenbogen RG, Bannick MS, Montjoy-Venning W, Lucchesi LR, Abbasi N, Abdulkader R, Abraha HN, Adsuar JC, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. doi:10.1016/S1474-4422(18)30415-0.
- Franke LM, Czarnota JN, Ketchum JM, Walker WC. Factor analysis of persistent post-concussive symptoms within a military sample with blast exposure. J Head Trauma Rehabil. 2015;30(1):E34. doi:10.1097/HTR.0000000000000042.
- Marvasti JA. WAR TRAUMA IN VETERANS AND THEIR FAMILIES: diagnosis and management of PTSD, TBI and comorbidities of combat trauma-from pharmacotherapy to a 12-step self-help program for combat veterans. Springfield, Illinois, USA: Charles C Thomas Publisher; 2012.
- HJ Haboubi JL, Koshy M, AB Ward N. Short-term sequelae of minor head injury (6 years experience of minor head injury clinic). Disabil Rehabil. 2001;23(14):635–38. doi:10.1080/09638280110038966.
- Swann I, Walker A. Who cares for the patient with head injury now? Emerg Med. 2001;18(5):352–57.doi:10.1136/emj.18.5.352.
- Kay A, Teasdale G. Head injury in the United Kingdom. World J Surg. 2001;25(9):1210–20. doi:10.1007/s00268-001-0084-6.
- Chandran A, Kay MC, Nedimyer AK, Morris SN, Kerr ZY, Register-Mihalik JK. A review of analytical methods used for evaluating clustering in concussion-related symptoms. Curr Epidemiol Rep. 2020;7(4):315–26.
- Permenter CM, Fernández-de Thomas RJ, Sherman AI. Postconcussive syndrome. [Updated 2022 Aug 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534786/
- Potter S, Leigh E, Wade D, Fleminger S. The rivermead post concussion symptoms questionnaire. J Neurol. 2006;253(12):1603–14. doi:10.1007/s00415-006-0275-z.
- Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry. 2005;18(3):301–17. doi:10.1097/01.yco.0000165601.29047.ae.
- Karr JE, Iverson GL, Berghem K, Kotilainen A-K, Terry DP, Luoto TM. Complicated mild traumatic brain injury in older adults: post-concussion symptoms and functional outcome at one week post injury. Brain Inj. 2020;34(1):26–33. doi:10.1080/02699052.2019.1669825.
- Chu S-Y, Tsai Y-H, Xiao S-H, Huang S-J, Yang -C-C. Quality of return to work in patients with mild traumatic brain injury: a prospective investigation of associations among post-concussion symptoms, neuropsychological functions, working status and stability. Brain Inj. 2017;31(12):1674–82. doi:10.1080/02699052.2017.1332783.
- Mazaux J-M, Masson F, Levin HS, Alaoui P, Maurette P, Barat M. Long-term neuropsychological outcome and loss of social autonomy after traumatic brain injury. Arch Phys Med Rehabil. 1997;78(12):1316–20. doi:10.1016/S0003-9993(97)90303-8.
- Graham NS, Sharp DJ. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry. 2019;90(11):1221–33. doi:10.1136/jnnp-2017-317557.
- Gouvier WD, Cubic B, Jones G, Brantley P, Cutlip Q. Postconcussion symptoms and daily stress in normal and head-injured college populations. Arch Clin Neuropsychol. 1992;7(3):193–211. doi:10.1093/arclin/7.3.193.
- King N, Crawford S, Wenden F, Moss N, Wade D. The rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–92. doi:10.1007/BF00868811.
- Kontos AP, Elbin R, Schatz P, Covassin T, Henry L, Pardini J, Collins MW. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375–84. doi:10.1177/0363546512455400.
- Joliffe IT, Morgan B. Principal component analysis and exploratory factor analysis. Stat Methods Med Res. 1992;1(1):69–95. doi:10.1177/096228029200100105.
- Voormolen DC, Cnossen MC, Polinder S, Von Steinbuechel N, Vos PE, Haagsma JA. Divergent classification methods of post-concussion syndrome after mild traumatic brain injury: prevalence rates, risk factors, and functional outcome. J Neurotrauma. 2018;35(11):1233–41. doi:10.1089/neu.2017.5257.
- Herrmann N, Rapoport MJ, Rajaram RD, Chan F, Kiss A, Ma AK, Feinstein A, M C Cullagh S, Lanctôt KL. Factor analysis of the rivermead post-concussion symptoms questionnaire in mild-to-moderate traumatic brain injury patients. J Neuropsychiatry Clin Neurosci. 2009;21(2):181–88. doi:10.1176/jnp.2009.21.2.181.
- Barker-Collo S, Theadom A, Starkey N, Kahan M, Jones K, Feigin V. Factor structure of the rivermead post-concussion symptoms questionnaire over the first year following mild traumatic brain injury. Brain Inj. 2018;32(4):453–58. doi:10.1080/02699052.2018.1429659.
- Barker-Collo S, Theadom A, Starkey NJ, Kahan M, Jones K, Feigin V. Long-term factor structure of the Rivermead Post-Concussion Symptom Questionnaire in mild traumatic brain injury and normative sample. Brain Inj. 2019;33(5):618–22. doi:10.1080/02699052.2019.1570339.
- Bronstein A, Lempert T, Seemungal B. Chronic dizziness: a practical approach. Pract Neurol. 2010;10(3):129–39. doi:10.1136/jnnp.2010.211607.
- Jafarzadegan M, Safi-Esfahani F, Beheshti Z. Combining hierarchical clustering approaches using the PCA method. Expert Syst Appl. 2019;137:1–10. doi:10.1016/j.eswa.2019.06.064.
- Wade D, King N, Wenden F, Crawford S, Caldwell F. Routine follow up after head injury: a second randomised controlled trial. J Neurol Neurosurg Psychiatry. 1998;65(2):177–83. doi:10.1136/jnnp.65.2.177.
- Rifkin-Zybutz R, Selim H, Johal M, Kuleindiren N, Palmon I, Lin A, Yu Y, Mahmud M. Preliminary validation study of the mindset4dementia application: assessing remote collection of dementia risk factors and cognitive performance. BMJ Innov. 2021;7(4):bmjinnov-2021–000780.
- Kuleindiren N, Rifkin-Zybutz RP, Johal M, Selim H, Palmon I, Lin A, Yu Y, Alim-Marvasti A, Mahmud M. Optimizing existing mental health screening methods in a dementia screening and risk factor app: observational machine learning study. MIR Form Res. 2022;6(3):e31209. doi:10.2196/31209.
- Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, Perkins PK. The mayo classification system for traumatic brain injury severity. J Neurotrauma. 2007;24(9):1417–24. doi:10.1089/neu.2006.0245.
- Kazl C, Torres A. Definition, classification, and epidemiology of concussion. Paper presented at: Seminars in pediatric neurology 2019.
- Sveen U, Bautz-Holter E, Sandvik L, Alvsåker K, Røe C. Relationship between competency in activities, injury severity, and post-concussion symptoms after traumatic brain injury. Scand J Occup Ther. 2010;17(3):225–32. doi:10.3109/11038120903171295.
- Thompson C, Davies P, Herrmann L, Summers M, Potter S. Approaches to establishing validated cut-off scores on the rivermead post-concussion symptoms questionnaire (RPQ). Paper presented at: Brain Injury 2016.
- Nichol AD, Higgins A, Gabbe B, Murray L, Cooper D, Cameron P. Measuring functional and quality of life outcomes following major head injury: common scales and checklists. Injury. 2011;42(3):281–87. doi:10.1016/j.injury.2010.11.047.
- Flora DB, Curran PJ. An empirical evaluation of alternative methods of estimation for confirmatory factor analysis with ordinal data. Psychol Methods. 2004;9(4):466. doi:10.1037/1082-989X.9.4.466.
- Grimm LG, Yarnold PR. Reading and understanding multivariate statistics. Washington, DC, USA: American Psychological Association; 1995.
- Klimberg R, and McCullough BD. Chapter 9 in fundamentals of predictive analytics with JMP. Cary, NC, USA: SAS Institute; 2016.
- Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nature Medicine. 2019;25(1):44–56.
- Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cnapeau D, Burovski E, Peterson P, Weckesser W, Bright J, Van Der Walt SJ. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods. 2020;17(3):1–12.
- Maruta J, Lumba-Brown A, Ghajar J. Concussion subtype identification with the rivermead post-concussion symptoms questionnaire. Front Neurol. 2018;9:1034. doi:10.3389/fneur.2018.01034.
- Eyres S, Carey A, Gilworth G, Neumann V, Tennant A. Construct validity and reliability of the Rivermead post-concussion symptoms questionnaire. Clin Rehabil. 2005;19(8):878–87. doi:10.1191/0269215505cr905oa.
- Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(2):217–23. doi:10.1136/jnnp-2011-300767.
- Breslau N, Lipton R, Stewart W, Schultz L, Welch K. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology. 2003;60(8):1308–12. doi:10.1212/01.WNL.0000058907.41080.54.
- Llop SM, Frandsen JE, Digre KB, Katz BJ, Crum AV, Zhang C, Warner JEA. Increased prevalence of depression and anxiety in patients with migraine and interictal photophobia. J Headache Pain. 2016;17(1):1–7. doi:10.1186/s10194-016-0629-6.
- Shlens J. A tutorial on principal component analysis. arXiv preprint arXiv:14041100. 2014.
- Whitehouse D, Richter S, Winzeck S, Kornaropoulos EN, Das T, Vyvere TV, Verheyden J, Williams GB, Correia MM, Menon DK, et al. 361 The relationship between intracranial MRI abnormalities and post-concussive symptoms in ED patients with a normal CT: as demonstrated on the rivermead post concussion symptom questionnaire (RPQ). Emerg Med. 2020;37(12):842. 2020. doi:10.1136/emj-2020-rcemabstracts.35.
- Maas AI, Steyerberg EW, Marmarou A, McHugh GS, Lingsma HF, Butcher I, Lu J, Weir J, Roozenbeek B, Murray GD, et al. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics. 2010;7(1):127–34. doi:10.1016/j.nurt.2009.10.020.
- Stillman AM, Madigan N, Torres K, Swan N, Alexander MP. Subjective cognitive complaints in concussion. J Neurotrauma. 2020;37(2):305–11. doi:10.1089/neu.2018.5925.
- Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007;68(14):1136–40. doi:10.1212/01.wnl.0000258672.52836.30.
- Theadom A, Cropley M, Parmar P, Barker-Collo S, Starkey N, Jones K, Feigin VL, and BIONIC Research Group. Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 2015;16(8):926–32. doi:10.1016/j.sleep.2015.04.013.
- Wickwire EM, Williams SG, Roth T, Capaldi VF, Jaffe M, Moline M, Motamedi GK, Morgan GW, Mysliwiec V, Germain A, et al. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a national working group. Neurotherapeutics. 2016;13(2):403–17. doi:10.1007/s13311-016-0429-3.
- Markovic SJ, Fitzgerald M, Peiffer JJ, Scott BR, Rainey-Smith SR, Sohrabi HR, Brown BM. The impact of exercise, sleep, and diet on neurocognitive recovery from mild traumatic brain injury in older adults: a narrative review. Ageing Res Rev. 2021;68:101322.
- Krone LB, Yamagata T, Blanco-Duque C, Guillaumin MC, Kahn MC, van der Vinne V, McKillop LE, Tam SK, Peirson SN, Akerman CJ, Hoerder-Suabedissen A. A role for the cortex in sleep–wake regulation. Nat Neurosci. 2021;24(1):1–6. doi:10.1038/s41593-020-00769-2.
- Stewart K, Shakarishvili N, Michalak A, Maschauer EL, Jenkins N, Riha RL. Treating sleep disorders following traumatic brain injury in adults: time for renewed effort? Sleep Med Rev. 2022;63:101631. doi:10.1016/j.smrv.2022.101631.
- Duclos C, Dumont M, Arbour C, Paquet J, Blais H, Menon DK, De Beaumont L, Bernard F, Gosselin N. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology. 2017;88(3):268–75. doi:10.1212/WNL.0000000000003508.
- Sharp DJ, Jenkins PO. Concussion is confusing us all. Pract Neurol. 2015;15(3):172–86. doi:10.1136/practneurol-2015-001087.
- Marshall S, Bayley M, McCullagh S, Velikonja D, Berrigan L, Ouchterlony D, Weegar K. Updated clinical practice guidelines for concussion/mild traumatic brain injury and persistent symptoms. Brain Inj. 2015;29(6):688–700. doi:10.3109/02699052.2015.1004755.
- Smith-Seemiller L, Fow NR, Kant R, Franzen MD. Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Inj. 2003;17(3):199–206. doi:10.1080/0269905021000030823.
- Gunstad J, Suhr JA. “Expectation as etiology” versus “the good old days”: postconcussion syndrome symptom reporting in athletes, headache sufferers, and depressed individuals. J Int Neuropsychol Soc. 2001;7(3):323–33. doi:10.1017/S1355617701733061.