ABSTRACT
Objective
Persistent postconcussion symptoms (PPCS) are challenging to diagnose. An improved diagnostic process could consider typical and atypical postconcussion symptoms. This study examined the structure of a modified Rivermead Post-concussion Symptoms Questionnaire (mRPQ) with both symptom types.
Method
298 adult volunteers were randomized into groups: honest responders, mild traumatic brain injury (mTBI) simulators (MS), and biased mTBI simulators (BMS). Both mTBI simulating groups were coached about mTBI and primed about the simulation context (compensation evaluation). The BMS group was also encouraged to bias (exaggerate) symptoms. The participants completed an online battery of tests, including the mRPQ.
Results
An exploratory factor analysis of the mRPQ (full sample) revealed a three-factor solution, including a separate dimension for atypical symptoms (all item loadings >0.45, ~4% of explained variance). The overall and group analyses of the standard RPQ items (typical symptoms) found a one- or two-factor solution, as did the analyses of atypical symptoms.
Conclusions
Consistent with prior RPQ research, a unidimensional or bifactor structure was measurable from standard RPQ symptoms. Whilst this study did not find support for domain-level symptom scores for either typical or atypical symptoms, the findings support the use of an overall atypical symptoms score.
Introduction
Mild TBIs (mTBIs) account for more than 70% of traumatic brain injuries (Citation1). While most injured individuals will recover within 7- to 10-days, a significant minority will experience mTBI symptoms that persist for several months or sometimes years (Citation2,Citation3). This symptom persistence can contribute to ongoing difficulties, affecting people’s daily functioning and quality of life (Citation4).
The presence of persistent mTBI symptoms has been recognized as the Post-Concussion Syndrome (PCS). Formal PCS diagnostic criteria have been described in major diagnostic classification systems (Citation5,Citation6). However, there is significant debate about this syndrome, including its nosology and the defining criteria (Citation2,Citation7,Citation8). Despite this, there is widespread agreement that prolonged symptoms can be reported after a mTBI (Citation9); and that, when this occurs, symptoms are classically described in three functional domains – cognitive (e.g., slowed thinking), somatic (e.g., headaches) and affective (e.g., irritability (Citation10).
There are many issues to consider in the clinical evaluation of PCS symptoms (Citation2,Citation9,Citation11–13). For example, there is no objective marker for many PCS features (Citation14,Citation15). Also, the symptoms themselves (e.g., headache and fatigue) are not specific to mTBI and are commonly reported: a) by people with conditions other than mTBI (Citation16,Citation17), b) in the everyday lives of otherwise “healthy” adults and children (Citation18–21), and; c) by people in circumstances that can co-occur with mTBI (e.g., being involved in litigation (Citation22). Thus, if persistent symptoms are experienced after a mTBI, there are many pre-, peri- and post-injury factors that are likely involved, such as pre-injury mental health, receiving acute medical attention, and the availability of post-injury social support (Citation7,Citation9,Citation23–25).
A key method of assessing PCS symptoms is via standardized questionnaire. There are several such measures in existence including the Rivermead Post-concussion Symptoms Questionnaire (RPQ (Citation26); and the Neurobehavioral Symptom Inventory (NSI (Citation27);. The injured person indicates if a symptom is present and, for the NSI, how much disturbance it causes for daily functioning, or for the RPQ, how much of a problem it is compared to before the mTBI.
Both the RPQ and the NSI include symptoms from the three functional domains thought to be impacted in PCS. This multi-domain structure has been empirically demonstrated for the RPQ (Citation25), although significant variations are documented (see for a summary of the studies pertinent to the RPQ). The demonstration of three underlying dimensions for the RPQ may give assurance that the underlying condition is being adequately assessed and is also important to support and inform the best methods for scoring and RPQ interpretation (i.e. a total score versus domain-specific scores). However, the documented variations mean that significant uncertainty surrounds such critical issues (Citation33,Citation37), and this is compounded by gaps in understanding; such as, if the structure is specific to the clinical group (Citation32,Citation37).
Table 1. Chronologically-ordered Studies of the RPQ Dimensions (Oldest to Newest by Publication [Publ.] Year).
A recent development in response to the challenges of PCS assessment is the creation of symptom validity indicators (SVIs) for use with the RPQ and NSI (for a discussion see (Citation38)). These PCS SVIs include the Mild Brain Injury Atypical Symptoms scale (mBIAS (Citation39), and the Validity-10 (or Val-10 (Citation40). These SVIs assess symptoms regarded as atypical following a mTBI as initially identified on clinical (mBIAS) or empirical grounds (Val-10). For example, changes in taste and smell was found to be an atypical chronic mTBI symptom (i.e., statistically rare among people several years post injury (Citation40); whereas seeing only in black and white was considered atypical for mTBI on rational (i.e. clinical) grounds (Citation39).
Recently, a suite of rationally derived atypical symptoms items was proposed for use with the RPQ. This modified RPQ (mRPQ) purports to measure atypical symptoms in the three functional domains affected in PCS (Citation41), and it is unique in this regard compared to the other SVIs for PCS measures. The internal consistency for the mRPQ atypical symptom-specific domain scores (i.e., affective, somatic, or cognitive SDS) has been found to be good-to-excellent (all alpha’s ≥ 0.69 (Citation38,Citation41). However, the factor structure of the mRPQ has not been determined. Given that the profile of PCS symptoms is thought to vary over the course of recovery (e.g., somatic>affective early post-recovery versus affective>somatic in the later stages (Citation42), clinical interpretations could be aided by establishing a measure that is capable of charting such trends for both typical and atypical PCS symptoms, and observing if this pattern changes in litigation contexts.
The primary aim of this study was to examine the underlying dimensions of the mRPQ overall, and the atypical PCS symptoms from the mRPQ specifically. This was achieved through a principal component analysis of the mRPQ items, and a separate analysis of the atypical items. A secondary aim was to test the dimensions of the RPQ when administered in the mRPQ format. Although the latter has been extensively examined (see ), there are limited studies in non-clinical (non-mTBI groups) and no previous studies of the RPQ structure in mTBI-simulators, with or without an incentive to bias their reporting.
Method
Participants
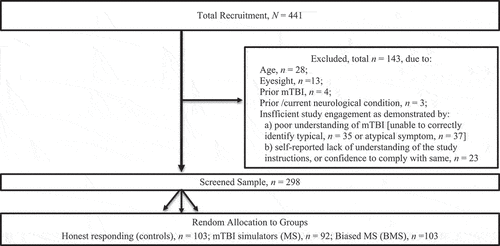
The data were drawn from a larger (parent) study (Citation38). The parent study sought a convenience sample of volunteers recruited from the general community (word of mouth) and a first-year research participation pool at a large metropolitan university. Eligible participants were: (a) aged ≥ 18 years; (b) free from visual impairments (self-reported 20/20 vision, with correction as needed); (c) not recently concussed (in the prior 6-months); (d) free from neurological disorders/conditions (e.g., stroke) and (e) fully engaged with the study (determined via composite: the submission of a complete protocol and the passing of valid response checks, as recommended for online and simulation studies (Citation43).
Materials
The participants completed a demographics and TBI history questionnaire. Standardized measures of postconcussion symptoms – the NSI and the RPQ – were administered with previously published atypical symptom additions. In this report only the RPQ data were used. A post-experimental questionnaire assessed study engagement, and a summary of the relevant information is shown in .
Modified Rivermead Post-concussion Symptoms Questionnaire (mRPQ)
The mRPQ has 16 standard items for assessing postconcussion symptoms (Citation26) and 15 atypical symptom items, originally derived through expert consensus (Citation41). The standard RPQ instructions and response scale was used (ie. 5-point Likert response scale, 0 [“Not experienced at all”] to 4 [“A severe problem”]). The standard and atypical items can be summed or averaged as a subscale score, as can the items that belong to each of the atypical symptom domains (e.g., atypical somatic specific domain symptoms). A higher score indicates greater PCS symptomatology (standard-item subscale) or increased problems due to atypical symptoms (atypical-item subscale).
Procedure
This study was approved by the QUT’s Human Research Ethics Committee. All data were obtained in compliance with the Helsinki Declaration. The measures were prepared as per precedent (Citation39) – i.e., the item position for the embedded items was determined randomly, in this case, using a random number generator. The items were inserted accordingly. The item lists were then fixed and used in this order for the study duration.
The study was administered online (Qualtrics Version May, 2019; professional license). The participants entered the study by activating a link that they received through e-mail or other study information. Once activated, the participants reviewed the study terms and conditions and supplied their consent to proceed. The demographic and injury history questions were completed. Before completing the measures, the Qualtrics Survey Flow Randomizer allocated each participant to one of three groups. The groups comprized three assessment contexts: honest-responding (control, no mTBI), mTBI simulator (MS), or biased mTBI simulator (BMS) (Citation38). In the control condition, the participants honestly reported their symptoms. In the MS condition, the participants were informed about mTBI and how it can affect people. They were advised to respond as if they had been injured one month ago and were now undertaking an assessment for mTBI compensation. In all conditions, symptoms were reported as experienced in the past month, relative to the prior month (e.g., pre-injury for MS group participants). Further, the MS group was told that they had considered distorting the response to maximize a pay-out; but had decided against it, fearing that – if caught – the payment would be jeopardized. The BMS group were instructed as per the MS group; except, they were told that the decision was to distort the response to maximize the compensation, but to do so carefully in order not to jeopardize the payment (for the full instructions see 31).
Statistical analyses
The data were exported from Qualtrics to IBM Statistical Package for the Social Sciences (SPSS) version 27 for data analysis. Unless otherwise stated, an alpha level of 0.05 was used to determine statistical significance. The data were checked to confirm participant eligibility. Ineligible participants were removed (see ). The remaining data were screened for missing values. There was one missing value (not replaced).
An exploratory factor analysis was performed on the 31 items of the mRPQ (atypical and standard symptoms); first using only the 16 standard items, and then using only the 15 atypical items. Analyses by group were performed separately for the standard and atypical subscale items. Given that this was an item-level analysis of Likert-scaled data, polychoric correlations were used (Citation44); an approach also adopted by others (Citation36). This approach is suitable if the univariate distributions of the items are asymmetric or kurtotic (Citation45,Citation46), as was the case with these data ().
Table 2. Descriptive Statistics for the mRPQ.
The analyses were performed using Factor Release 10.10.02 (Citation47,Citation48). Sweet smoothing was used if the correlation matrix was not positive definite (Citation49). The number of factors was determined from the minimum average partial (MAP (Citation50), and/or Kaiser’s criterion (eigenvalue >1). A varimax rotation with Kaiser normalization was used. Given the sample size (Citation51) and based on precedent (Citation32), the a priori decision was taken to retain items with rotated loadings of greater than 0.45 (i.e., 20% of the variance). The rotation method, and consideration of eigenvalues > 1, was based on precedent (Citation33,Citation35). For retained but cross-loading items, all loadings are shown. The factors were interpreted by considering the majority of the item content, vis a vis their rationally derived categories (somatic, affective, cognitive; e.g. (Citation10)).
Results
Four hundred and forty-one people commenced the study. After eligibility and data screening, ~70% of this group remained (n = 298). The major reason for ineligibility was insufficient study engagement (failing post-experimental checks, n = 95; ). As noted, multiple checks were applied to ensure a sample that demonstrated sufficient effort and task knowledge for valid interpretation. The final screened sample was (>70%) young, adult, white, single, women; two-thirds of whom were current tertiary students (Mage = 22.58 years, SD = 7.70; 72.1% were women; 75.2% identified as Caucasian, 74.8% were single, and 65.1% were tertiary students). There was no difference in the groups (honest responding, n = 103, MS, n = 92; BMS, n = 103) on any of these demographic variables, p > 0.05.
mRPQ PCA
After sweet smoothing for the overall sample, the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy was 0.98 (very good), and Bartlett’s statistic was significant (3290.8, df = 465, p < 0.001). Three components were indicated (MAP test, Kaiser’s criterion), accounting for 71.55%. 4.84%, and 3.70% of the variance, respectively. All 31 items were included in the final solution (component loadings > 0.45). lists the items and their associated component loadings. The third component was comprised exclusively of atypical items. The first component had loadings from almost all standard RPQ items (cognitive, somatic, affective) and some atypical items (primarily cognitive and somatic). The second component had loadings primarily from standard cognitive-somatic items, and one cross-loading atypical item.
Table 3. mRPQ Atypical Symptoms (with rationally derived coding [bracketed text]; upper panel, leftmost column) and Standard Symptoms (RPQ with rationally derived coding [bracketed text], lower panel, leftmost column) and Component Loadings and Variance Explained (%) for the Overall mRPQ (31-item PCA; upper and lower panels, columns 2 − 4), Atypical Symptoms (15-item PCA; upper panel, columns 5–8) and Standard Symptoms/RPQ (16-item PCA; lower panel, columns 5–7).
Atypical items PCA
For the overall sample and two of the three subgroup analyses (MS, BMS), the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy was > 0.91 (very good) and Bartlett’s statistic was significant (p < 0.001). The exception was for the honest-responding group: these data were not factorable. A one- (MS) or two-component solution (Overall, BMS) emerged. The one component solution included all atypical items and accounted for 63.25% of the variance (Sweet smoothed correlations). For the overall sample, the two atypical symptom components were predominantly: (a) affective-somatic (75.98% of the variance); and (b) affective-cognitive-somatic (5.8% of the variance). For the BMS group, the two atypical symptom components were predominantly: (a) cognitive-affective-somatic (69.78% of the variance); and (b) affective-somatic (7.77% of the variance; see ).
Standard item PCA
For the overall sample and the subgroup analyses, the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy was 0.74 (fair; MS) to 0.96 (very good; Overall). Bartlett’s statistic for all PCA’s was significant (p’s <0.001). Based on the MAP test a one component solution was indicated for the honest-responding and MS groups, and two components were indicated for the overall and BMS groups. In the one component solution all variables were retained (loadings >0.45) and accounted for a cumulative 53.6% (honest responding) to 68.27% (MS) of the variance. For the overall sample, the two symptom components were predominantly: (a) affective-cognitive (76.12% of variance); and (b) cognitive-somatic (5.97% of variance). For the BMS group, the two symptom components were predominantly: (a) cognitive-somatic (74.05% of variance); and (b) affective-cognitive (7.19% of variance; see ).
Discussion
This study investigated the underlying structure of the mRPQ; a measure of standard and atypical postconcussion symptoms. To our knowledge, this is the first study to examine the underlying structure of an embedded PCS scale (atypical symptoms added). Although other embedded PCS scales have been described (e.g., mBIAS), investigations of the structure of these modified measures are relatively rare (for an exception see 33). Further, by performing the first investigation of the RPQ’s underlying structure in a mTBI simulator sample, this study adds to our understanding of the RPQ.
This study analyzed all mRPQ symptoms to determine if standard and atypical symptoms might form distinct components. Some support for this position was found: a) the third and smallest contributing dimension was comprised solely of atypical symptoms; b) the second component, included standard symptoms and only one evenly cross-loading atypical affective symptom (apocalyptic worry), and; c) the first component, whilst comprised of both atypical and standard symptoms, included only one affective atypical symptom. Together, these results suggest that atypical symptoms can occupy a dimension that is distinct from standard RPQ symptoms. This shows that if the scale is further developed and researched (including in other samples) it may be possible to derive a distinct, atypical symptom summary score from the mRPQ.
Contrary to expectations, this study did not find support for a three-component model of atypical postconcussion symptoms. Thus far, studies of atypical postconcussion symptoms have largely focused on somatic symptoms, including in novel applications for the prediction of longer term postinjury function (Citation39,Citation52,Citation53). However, the mRPQ items were rationally derived to capture atypical PCS symptoms from multiple domains, including somatic, cognitive, and affective functioning. Despite this intention, this study does not indicate that the proposed domain-level scores for atypical symptoms can be meaningfully interpreted.
A possible reason why the atypical symptoms did not coalesce as expected is that the rationally derived categories oversimplified a complex input (the symptom experience). For example, a symptom such as seeing only in black and white can be classified as somatic, but if sensory systems are intact, it signals cognitive dysfunction. The item wording for the mRPQ atypical symptoms might also have influenced their interpretation along such dimensions. For example, “Feeling that [a body part] is … . missing” sets it up as an emotionally based symptom but if ‘feeling’ is replaced with ‘thinking’ it potentially becomes a cognitively based symptom experience. Similarly, the item about apocalyptic worry was originally categorized as an affective symptom but if the worries are identified as thoughts rather than emotions, it could load onto either factor. Similar points might be made about standard PCS symptoms, since in factor analyses they do not consistently align with their rationally derived categories, possibly due to differing interpretations ((Citation32,Citation33) but see (Citation34)).Footnote1
Another possible reason for the absence of the expected multidimensional structure for the atypical symptoms is the simulation timeframe (1-month post-injury). This timeframe might have constrained the variation in reporting of symptoms from different domains (Citation54), especially as it is known that the dimensionality of the standard RPQ symptoms is sensitive to time-since-injury (Citation32,Citation33). This could be further investigated in a longitudinal study of the underlying structure of the mRPQ. In any case, this study has shown that despite their psychometric reliability (Citation38), the atypical subscale scores (cognitive, affective, somatic) from the mRPQ do not assess unique constructs as currently framed, and only the AS subscale score is interpretable as a summary index in selected contexts.
A second aim of the present study was to examine the component structure of the RPQ when administered in the form of the mRPQ (with interspersed atypical symptoms) and in a simulation study. If the mRPQ is adopted, the modification should not invalidate the standard measure. Consistent with other studies, our study found support for a unidimensional structure in two groups (honest responding and MS (e.g., 29), and a bicomponent solution in the BMS group and the overall sample (e.g., 46). The bicomponent structures for the RPQ showed a “mixed” item composition vis a vis the rationally derived item categories, but such “mixed” patterns (e.g. a “visual-somatic” pattern) are not uncommon (Citation29,Citation32). It must also be acknowledged that the present results do not agree with the reports of a three- (Citation25,Citation29,Citation31,Citation34) or even four-dimensional RPQ structure (Citation33). There are many possible reasons for this including that the composition of these structures themselves are quite varied and can be highly interrelated (Citation34), and the differences in the analytical methods. Despite this, we concur with the recommendation from the TRACK-TBIFootnote2 group and others, that a single total RPQ score can be used to represent overall mTBI symptom burden (Citation36,Citation37). Unique to this study our recommendation applies if the RPQ is administered in the mRPQ format. Our honest responder findings also showed that this group responded similarly to our mTBI simulators; a finding that is consistent with the demonstration from non-simulation studies that the dimensions measured by the RPQ are similar for people with and without mTBI (Citation35). Taken together, the notion of completely separable and conceptually aligned RPQ symptom-domain scores (i.e., affective versus somatic versus cognitive scores) is not supported by this or most other RPQ studies, and by extension, that this concept may not be useful for the framing of atypical symptoms.
This study has several limitations. First, this study used a simulation design without a clinical sample. The sample was largely young female undergraduate students. The absence of a clinical group means that it is difficult to fully evaluate the simulation (i.e., if a clinical presentation was effectively mimicked). Simulation studies have strong internal, but low external validity (Citation43); therefore, further research is needed to determine the mRPQ dimensions in a mTBI group. Nevertheless, this simulation design enabled the structure of this new tool to be explored in various, often difficult-to-isolate contexts. A second limitation is that this study did not use an independent sample to test the invariance of the components. As such, the resulting solutions cannot be interpreted as independent from each other. Third, this study used exploratory rather than confirmatory methods for identifying the latent structure and, the sample was too small to be split to perform both analyses. This atheoretical approach was used because the analysis of the atypical symptoms was the first of its type (Citation55), and although the RPQ (standard items) have been investigated previously, the source items were not drawn from the mRPQ nor examined in a simulation study. Future studies could use confirmatory methods, including in a longitudinal study, as this would clarify the mRPQ’s dimensions over time, including the timeframes across which the summary scores are indicated.
SVIs are easily critiqued if they lack sufficient reliability and validity and are not developed according to systematic scale development processes (Citation56), and structural analyses are key in this regard. The mRPQ was initially developed to improve the clinical interpretation of postconcussion symptoms when used in conjunction with other clinical data. As already noted, the reporting of atypical symptoms in and of themselves is not clinically meaningful as there are many reasons why such symptoms could be endorsed. In a treatment context, the identification of such symptoms can and should still inform and support patient-centered decisions about further assessment or care, even if interpreted as indicating an issue with validity (Citation57,Citation58). For example, Sherer and colleagues observed that if a person’s profile is positive for invalidity, there may be challenges for engaging with standard therapies. Guided by clinical experience, they suggest that all complaints can be acknowledged, and potentially further explored through a process of psychoeducation (Citation57). A modified “trauma-focussed” therapy for this group has also found empirical support (Citation58). In this way, a reliable and valid measure for assessing atypical PCS symptoms has the very strong potential to contribute important information about the way forward for people with persistent symptoms following mTBI.
Acknowledgments
This project was approved by the QUT Human Research Ethics Committee (HREC approval number: 1900001173). The risk assessment for this project was approved by the QUT (approval number: 1331). The QUT School of Psychology and Counselling funded the gift cards for this project. The authors did not receive external funding for this research. The authors have no conflicts of interest to declare.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes
1. Plass et al. (2019) used a non-English version of the RPQ in a sample of mild-to-severe TBI patients.
2. Transforming Research And Clinical Knowledge in Traumatic Brain Injury [(TRACK)-TBI] refers to a large, multi-site, longitudinal investigation of TBI outcomes in North America.
References
- Cassidy JD, Carroll L, Peloso P, Borg J, Von Holst H, Holm L, Kraus J, Coronado VG. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehab Med. 2004;36:28–60. doi:10.1080/16501960410023732.
- Prince C, Bruhns ME. Evaluation and treatment of mild traumatic brain injury: the role of neuropsychology. Brain Sci. 2017;7(8):105. doi:10.3390/brainsci7080105
- Dikmen S, Machamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. J Int Neuropsych Soc. 2010;16(3):401–11. doi:10.1017/S1355617710000196
- Theadom A, Starkey N, Barker-Collo S, Jones K, Ameratunga SFV. Population-based cohort study of the impacts of mild traumatic brain injury in adults four years post-injury. PLoS One. 2018;13(1):1–13. doi:10.1371/journal.pone.019
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed ed. Washington, DC: American Psychiatric Association; 1994.
- Iverson GL. Network analysis and precision rehabilitation for the post-concussion syndrome. Front Neurol. 2019;10(489). doi:10.3389/fneur.2019.00489
- Donnell AJ, Kim MS, Silva MA, Vanderploeg RD. Incidence of postconcussion symptoms in psychiatric diagnostic groups, mild traumatic brain injury, and comorbid conditions. Clin Neuropsychol. 2012;26(7):1092–101. doi:10.1080/13854046.2012.713984
- Quinn DK, Mayer AR, Master CL, Fann JR. Prolonged postconcussive symptoms. Am J Psychiatry. 2018;175(2):103–11. doi:10.1176/appi.ajp.2017.17020235
- Smith-Seemiller L, Fow NR, Kant R, Franzen MD. Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Inj. 2003;17(3):199–206. doi:10.1080/0269905021000030823
- Carroll L, Cassidy JD, Peloso P, Borg J, Von Holst H, Holm L, Paniak C, Pépin M. Prognosis for mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehab Med. 2004;36:84–105. doi:10.1080/16501960410023859.
- Iverson GL, Lange RT. Post-concussion syndrome. In: Schoenberg MR, Scott JG, editors. The little black book of neuropsychology: a syndrome-based approach. Boston, MA: Springer US; 2011. p. 745–63.
- Iverson GL, Zasler ND, Lange RT. Post-concussive disorder. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain injury medicine: principles and practice. New York, United States: Demos Medical Publishing; 2007. p. 373–406.
- Silver JM. Effort, exaggeration and malingering after concussion. J Neurol Neurosur Ps. 2012;83(8):836–41. doi:10.1136/jnnp-2011-302078
- Ettenhofer ML, Barry DM. A comparison of long-term postconcussive symptoms between university students with and without a history of mild traumatic brain injury or orthopedic injury. J Int Neuropsych Soc. 2012;18(3):451–60. doi:10.1017/S1355617711001895
- Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, Chapman J, Gurka J, Dawson K, Capon L, Marosszeky JE. Mild traumatic brain injury does not predict acute postconcussion syndrome. J Neurol Neurosur Ps. 2008;79(3):300. doi:10.1136/jnnp.2007.126565.
- Iverson GL, McCracken LM. ‘Postconcussive’symptoms in persons with chronic pain. Brain Inj. 1997;11(11):783–90.doi:10.1080/026990597122990.
- Gouvier WD, Uddo-Crane M, Brown LM. Base rates of post-concussional symptoms. Arch Clin Neuropsychol. 1988;3(3):273–78.doi:10.1093/arclin/3.3.273.
- Iverson GL, Lange RT. Examination of” postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003;10(3):137–44.doi:10.1207/S15324826AN1003_02.
- Edmed SL, Sullivan KA. Method of symptom assessment influences cognitive, affective and somatic post-concussion-like symptom base rates. Brain Inj. 2014;28(10):1277–82. doi:10.3109/02699052.2014.915988
- Merritt VC, Arnett PA. Premorbid predictors of postconcussion symptoms in collegiate athletes. J Clin Exp Neuropsyc. 2014;36(10):1098–111. doi:10.1080/13803395.2014.983463
- Dunn JT, Lees‐Haley PR, Brown RS, Williams CW, English LT. Neurotoxic complaint base rates of personal injury claimants: implications for neuropsychological assessment. J Clin Psychol. 1995;51(4):577–84.doi:10.1002/1097-4679(199507)51:4<577::AID-JCLP2270510418>3.0.CO;2-E.
- Iverson GL. A biopsychosocial conceptualization of poor outcome from mild traumatic brain injury. In: Vasterling JJ, Bryant RA, Keane TM, editors. PTSD and mild traumatic brain injury. New York: The Guilford Press; 2012. p. 36–60.
- Kenzie ES, Parks EL, Bigler ED, Lim MM, Chesnutt JC, Wakeland W. Concussion as a multi-scale complex system: an interdisciplinary synthesis of current knowledge. Front Neurol. 2017;8(513). doi:10.3389/fneur.2017.00513
- Potter S, Leigh E, Wade D, Fleminger S. The rivermead post concussion symptoms questionnaire. J Neurol. 2006;253(12):1603–14. doi:10.1007/s00415-006-0275-z
- King NS, Crawford S, Wenden FJ, Moss NEG, Wade DT. The Rivermead Post-concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–92. doi:10.1007/bf00868811
- Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehab. 1995;10(3):1–17. doi:10.1097/00001199-199506000-00002
- Eyres S, Carey A, Gilworth G, Neumann V, Tennant A. Construct validity and reliability of the Rivermead Post-concussion Symptoms Questionnaire. Clin Rehabil. 2005;19(8):878–87. doi:10.1191/0269215505cr905oa
- Herrmann N, Rapoport M, Rajaram R, Chan F, Kiss A, Ma A, Feinstein A, Mc Cullagh S, Lanctôt KL. Factor analysis of the rivermead post-concussion symptoms questionnaire in mild-to-moderate traumatic brain injury patients. J Neuropsych Clin N. 2009;21(2):181–88. doi:10.1176/appi.neuropsych.21.2.181.
- Lannsjö M, Af Geijerstam JL, Johansson U, Bring J, Borg J. Prevalence and structure of symptoms at 3 months after mild traumatic brain injury in a national cohort. Brain Inj. 2009;23(3):213–19. doi:10.1080/02699050902748356
- Styrke J, Sojka P, Björnstig U, Bylund P, Stålnacke B. Sex-differences in symptoms, disability, and life satisfaction three years after mild traumatic brain injury: a population-based cohort study. J Rehab Med. 2013;45(8):786–94.doi:10.2340/16501977-1215.
- Barker-Collo S, Theadom A, Starkey N, Kahan M, Jones K, Feigin V. Factor structure of the Rivermead Post-concussion Symptoms Questionnaire over the first year following mild traumatic brain injury. Brain Inj. 2018;32(4):453–58. doi:10.1080/02699052.2018.1429659
- Thomas M, Skilbeck C, Cannan P, Slatyer M. The structure of the Rivermead Post-concussion Symptoms Questionnaire in Australian adults with traumatic brain injury. Brain Impair. 2018;19(2):166–82. doi:10.1017/BrImp.2017.26
- Plass AM, Van Praag D, Covic A, Gorbunova A, Real R, von Steinbuechel N, Sirén A-L, Dutch and Flemish CENTER TBI investigators. The psychometric validation of the Dutch version of the Rivermead Post-concussion Symptoms Questionnaire (RPQ) after traumatic brain injury (TBI). PLoS One. 2019;14(10):e0210138–e. doi:10.1371/journal.pone.0210138.
- Barker-Collo S, Theadom A, Starkey NJ, Kahan M, Jones K, Feigin V. Long-term factor structure of the Rivermead Post-concussion Symptom Questionnaire in mild traumatic brain injury and normative sample. Brain Inj. 2019;33(5):618–22. doi:10.1080/02699052.2019.1570339
- Agtarap S, Kramer MD, Campbell-Sills L, Yuh E, Mukherjee P, Manley GT, McCrea MA, Dikmen S, Giacino JT, Stein MB, et al. Invariance of the bifactor structure of mild traumatic brain injury (MTBI) symptoms on the Rivermead Postconcussion Symptoms Questionnaire across time, demographic characteristics, and clinical groups: a TRACK-TBI study. Assessment. 2020;28(6):1656–70. doi:10.1177/1073191120913941.
- Balalla S, Krägeloh C, Medvedev O, Siegert R. Is the Rivermead Post-concussion Symptoms Questionnaire a reliable and valid measure to assess long-term symptoms in traumatic brain injury and orthopedic injury patients? A novel investigation using rasch analysis. Neurotraum Rep. 2020;1(1):63–72. doi:10.1089/neur.2020.0017
- Brooks KJL, Sullivan KA. Validating the modified Rivermead Post-concussion Symptoms Questionnaire (mRPQ). Clin Neuropsychol. 2023;37(1): 207–226. doi:10.1080/13854046.2021.1942555.
- Cooper DB, Nelson L, Armistead-Jehle P, Bowles AO. Utility of the mild brain injury atypical symptoms scale as a screening measure for symptom over-reporting in operation enduring freedom/operation Iraqi freedom service members with post-concussive complaints. Arch Clin Neuropsychol. 2011;26(8):718–27. doi:10.1093/arclin/acr070
- Vanderploeg RD, Cooper DB, Belanger HG, Donnell AJ, Kennedy JE, Hopewell CA, Scott SG. Screening for postdeployment conditions: development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. J Head Trauma Rehab. 2014;29(1):1–10. doi:10.1097/HTR.0b013e318281966e.
- Windle K, Sullivan KA. Towards an embedded symptom validity indicator for the Rivermead Post-concussion Symptom Questionnaire. Appl Neuropsychol. 2019;1–13. doi:10.1080/23279095.2019.1660880.
- Lishman WA. Physiogenesis and psychogenesis in the ‘post-concussional syndrome’ Brit J Psychiat. 1988;153(4):460–69. doi:10.1192/bjp.153.4.460
- Rogers R, Bender SD. Clinical assessment of malingering and deception. 4th ed ed. London, England: The Guilford Press; 2019.
- Flora D, LaBrish C, Chalmers R. Old and new ideas for data screening and assumption testing for exploratory and confirmatory factor analysis. Front Psych. 2012;3(55). doi:10.3389/fpsyg.2012.00055
- Muthén B, Kaplan D. A comparison of some methodologies for the factor analysis of non-normal likert variables. Brit J Math Stat Psy.1985;38(2):171–89. doi:10.1111/j.2044-8317.1985.tb00832.x
- Muthén B, Kaplan D. A comparison of some methodologies for the factor analysis of non-normal Likert variables: a note on the size of the model. Brit J Math Stat Psy.1992;45(1):19–30. doi:10.1111/j.2044-8317.1992.tb00975.x
- Lorenzo-Seva U, Ferrando PJ. Factor version 10.10.02 64bits. 2020 February; [accessed 2020 Nov 20]. psico.fcep.urv.cat/utilitats/factor
- Lloret A, Ferreres A, Hernández A, Tomás I. The exploratory factor analysis of items: guided analysis based on empirical data and software. An Psicol.2017;33(2):417–32. doi:10.6018/analesps.33.2.270211
- Lorenzo-Seva U, Ferrando PJ. Not positive definite correlation matrices in exploratory item factor analysis: causes, consequences and a proposed solution. Struct Equ Modeling. 2020;1–10. doi:10.1080/10705511.2020.1735393.
- Velicer WF. Determining the number of components from the matrix of partial correlations. Psychometrika. 1976;41(3):321–27. doi:10.1007/BF02293557
- Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assess. 1995;7(3):286–99. doi:10.1037/1040-3590.7.3.286
- Stubbs JL, Green KE, Silverberg ND, Howard A, Dhariwal AK, Brubacher JR, Garraway N, Heran MKS, Sekhon MS, Aquino A, et al. Atypical somatic symptoms in adults with prolonged recovery from mild traumatic brain injury. Front Neurol. 2020;11:43.
- Sullivan K, Keyter A, Jones K, Ameratunga S, Starkey N, Barker-Collo S, Webb J, Theadom A. A typical symptom reporting after mild traumatic brain injury. Brain Impair. 2021:1–10. doi:10.1017/BrImp.2021.30.
- Ayr LK, Yeates KO, Taylor HG, Browne M. Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. J Int Neuropsych Soc. 2009;15(1):19–30. doi:10.1017/S1355617708090188
- Bryant FB, Yarnold PR. Principal-components analysis and exploratory and confirmatory factor analysis. In: Grimm L, Yarnold PR, editors. Reading and understanding multivariate Statistics. Washington, DC, US: American Psychological Association; 1995. p. 99–136.
- McLeod TCV, Leach C. Psychometric properties of self-report concussion scales and checklists. J Athl Training. 2012;47(2):221–23. doi:10.4085/1062-6050-47.2.221
- Sherer M, Sander AM, Ponsford J, Vos L, Poritz JMP, Ngan E, Leon Novelo L. Patterns of cognitive test scores and symptom complaints in persons with TBI who failed performance validity testing. J Int Neuropsych Soc. 2020;26(9):932–38. doi:10.1017/S1355617720000351.
- Jurick SM, Crocker LD, Merritt VC, Hoffman SN, Keller AV, Eglit GML, Thomas KR, Norman SB, Schiehser DM, Rodgers CS, et al. Psychological symptoms and rates of performance validity improve following trauma-focused treatment in veterans with PTSD and history of mild-to-moderate TBI. Journal of the International Neuropsychological Society: JINS. 2020;26(1):108–18. doi:10.1017/S1355617719000997.