ABSTRACT
Background
Functional Gait Disorders (FGD) are a common presentation of motor-Functional Neurological Disorders (motor-FND) that affect walking ability.
Aim
To provide a narrative review of the current literature on FGD.
Methods
A narrative overview of published literature was undertaken, based on a systematic search of relevant databases, authoritative texts and citation tracking.
Results
FGD is multidimensional and disabling, with numerous phenotypes described in the literature, including ‘knee buckling,’ ‘astasia-abasia’ and ‘excessive slowness.’ Motor symptoms such as weakness or tremor, and non-motor symptoms, such as pain and fatigue may contribute to the disability and distress in FGD. Phenotypic features and clinical signs are seen in FGD that demonstrate inconsistency and incongruity with structural disease. A limited number of treatment studies have specifically focussed on FGD, however, reporting of outcomes from motor-FND cohorts has demonstrated short and long-term improvements in walking ability through multidisciplinary rehabilitation.
Conclusions
The relative contribution of motor and non-motor symptoms in FGD remains unknown, but it is likely that non-motor symptoms increase the illness burden and should be considered during assessment and treatment. Recommended treatment for FGD involves multidisciplinary rehabilitation, but optimum treatment elements are yet to be determined.
Introduction
Functional neurological disorders (FND) are characterized by sensory, motor and cognitive symptoms that are unexplained by neuropathology (Citation1). People with motor-FND present with abnormalities of motor function, such as weakness or tremor, which when impacting gait can be specifically described as functional gait disorders (FGD) (Citation2). Functional gait disorders are common in outpatient settings, where they have been reported in up to 40% of people with motor-FND (Citation3,Citation4). Presentations of FGD have been long described in medical history, including Charcot’s works from the 19th century (Citation5), and the descriptions of shell shock from the first world war (Citation6). There has been a resurgence of interest in the field over the past two decades with particular advancements in both clinical and research domains across diagnosis, etiology and treatment (Citation7,Citation8). Descriptions of FGD in the literature have evolved over time with reported phenotypes including ‘knee buckling,’ ‘astasia-abasia’ and ‘excessive slowness’ amongst others. Functional gait disorders have been reported to occur in isolation, or as a combined presentation of impaired gait alongside other symptoms, such as functional tremor or dystonia (Citation9). They may also co-occur with other neurological conditions such as brain injury or multiple sclerosis (Citation10,Citation11). Presentations of FGD are understood to be part of the wider spectrum of FND symptoms, that also includes associated non-motor symptoms such as pain and fatigue.
This paper will provide an overview of the existing literature on FGD, including etiology, clinical presentation, phenotypes, diagnosis and treatment.
Methodology
A narrative review of published literature on FGD was undertaken. Papers included in this review were identified by searches of relevant databases (PubMed, CINAHL, Science Direct, Cochrane Library, Web of Science and Elsevier). In addition, a review of authoritative texts, reference checking and citation tracking took place. The following search terms were used: “functional gait disorder”; “functional neurological disorder”; “functional movement disorder”; “functional motor disorder”; “psychogenic motor disorder”; “psychogenic movement disorder”; “conversion disorder”; “psychogenic gait”; and “hysterical gait.” From the resultant articles, findings relating to FGD were synthesized and presented in this review.
Etiology and mechanism
The etiology of motor-FND is usually understood using a biopsychosocial model, where individuals have different predisposing and precipitating factors for developing symptoms, which are maintained by perpetuating factors (Citation12). Each of these etiological factors may be considered in terms of biological, psychological or social domains. Examples of predisposing factors could include biological vulnerabilities in the nervous system, emotional disturbance or adverse life events (Citation12). In this context, the presence of neurological disease or injury can be considered a predisposing risk factor for developing FND (Citation13). Precipitating events may include injury, illness, dissociation, trauma, or other physical or psychological events (Citation13–16). For example, functional symptoms can occur following a mild traumatic brain injury (Citation17). Perpetuating factors may include learnt habitual movements, illness beliefs and social factors (Citation12). This model allows for the integration of both physical and psychological factors when accounting for symptoms, without emphasis on psychological factors, which were previously considered a requirement for diagnosis (Citation18).
The mechanism for symptoms of FND has been suggested to follow a hierarchical Bayesian model of altered higher-level feed-forward control with impaired intentional movement and sensory processing. During normal movement, our nervous system predicts a certain response from the intended action, and the prediction error which arises can be explained as the difference between what you expect to sense and what you sense (Citation15). The Bayesian model proposes that the aim of normal movement is to minimize the prediction errors at each level of control (Citation19). Predictions of the sensory consequences of intended movement occur at a high level in the cortical hierarchy and are transmitted down the descending motor pathways, producing movements that follow these prediction errors (Citation15). Theoretically, in motor-FND, an abnormal prior expectation occurs in an intermediate motor area, which is given excessive attention and precision, that leads to a prediction error, that is corrected through symptom production (e.g., added movements such as tremor or dystonia) (Citation15).
The altered prior expectation can follow certain beliefs and events. For example, beliefs about illness can occur following a physical injury (Citation20), health scares in the media (Citation21), or concern over inheritable family illness (Citation22). Motor-FND may occur when this belief is combined with a precipitating event, such as a painful injury to a limb resulting in functional weakness (Citation23).
These models provide a theoretical basis for the etiology and mechanisms of FGD, but whether there are additional factors contributing to symptoms unique to FGD remains unknown.
Clinical presentation
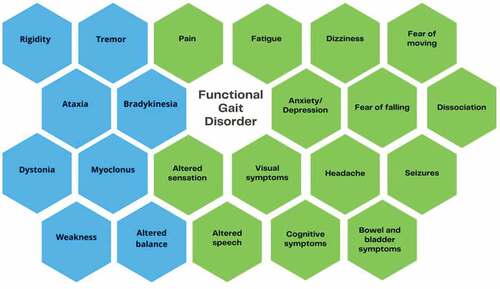
Functional gait disorders are diverse and may vary over time in their clinical presentation. People with FGD usually present with motor and non-motor symptoms and most have other co-morbid conditions, making it a complex and multidimensional disorder.
Motor symptoms
Common functional motor symptoms include weakness, tremor, dystonia and myoclonus (), that may be present in people with FGD (Citation24). It is clear that these motor symptoms will contribute to the disruption of normal gait kinetics and kinematics. Motor symptoms have been found to persist in the long term, as shown in a 14-year-long case-control study with 76 participants with functional weakness, where complete resolution occurred in 20% of their participants, improvement in 31% and worsened or remained stable in 49% (Citation25).
Non-motor symptoms
Non-motor symptoms are common in people with motor-FND, including those with FGD Citation26 (). Tinazzi et al. (Citation27) investigated the clinical correlates of motor-FND in a cohort of 410 patients. The most common non-motor symptoms were anxiety (52.1%), fatigue (45.1%), and pain (41.9%). Chronic pain was also shown to be highly prevalent in patients attending specialist FND clinics, affecting 56% and 79% in a Canadian and United Kingdom clinic-cohort respectively (Citation28). Other symptoms found by Tinazzi et al. Citation27 included somatosensory symptoms (25.3%), functional visual symptoms (11.4%) and cognitive symptoms (10.9%). Each of these symptoms may have a direct impact on walking ability.
Two recent studies have explored the impact of non-motor symptoms in people with motor-FND. One study with 61 participants reported that health-related quality of life scores negatively correlated with depression, anxiety and pain, with no correlation found between health-related quality of life and motor symptom severity (Citation26). Similarly, in 181 participants with motor-FND, Gelauff et al. (Citation24) reported that quality of life was negatively associated with fatigue and depression but not self-rated motor symptom severity. These findings indicate a multifaceted interplay of FND symptoms and suggest that the non-motor symptoms may have a greater impact on quality of life than motor symptoms.
Other non-motor features that may be associated with FGD include fear of falling, kinesiophobia and dizziness. Fear of falling is a common feature in the community-dwelling elderly, especially following falls (Citation29). The term “cautious gait” describes a response to perceived disequilibrium or a postural threat (Citation30) and is associated with an increased risk of falling (Citation31). Fear of falling may be an important consideration during assessment and treatment in FGD. In persistent pain cohorts, kinesiophobia is defined as fear of pain associated with movement, leading to avoidant disuse and central sensitization of pain (Citation32). Given the high incidence of persistent pain in people with FND, it is possible that kinesiophobia may occur in some cases of FGD. Another non-motor symptom that can impact gait is functional dizziness (Citation33), but is often not considered in classical descriptions of FGD. Functional dizziness (also known as persistent postural-perceptual dizziness) commonly follows vestibular disorders, such as benign paroxysmal positional vertigo or vestibular neuritis (Citation33,Citation34).
Further research into the prevalence, severity and impact of motor and non-motor symptoms in FGD may help to inform the etiology and mechanisms to support targeted assessment and individualized treatment.
Functional gait disorder phenotypes and classification
Over the years many subtypes of FGD have been described. The term “astasia-abasia,” first used in the nineteenth century, is an early description of a functional gait disorders, with astasia relating to the inability to stand upright and independently, and abasia denoting the inability to walk in a coordinated manner (Citation35). Charcot described clinical observations of “dragging gait” in patients with functional paralysis where the affected leg was dragged behind with the forefoot in contact with the ground (Citation36,Citation37). This phenotype is still reported today (Citation6), which suggests the stability of this phenotype over time.
A range of FGD phenotypes have been described over the years and there have been attempts to develop classification systems with various objectives, such as reporting characteristics, supporting diagnosis or to provide phenomenological classifications.
Tinazzi et al. (Citation38) investigated 109 participants with FGD and reported “slow gait” (n = 43, 39.4%), “astasia-abasia” (n = 26, 23.8%), and “knee buckling” (n = 24, 22%) as the most common phenotypes. Lempert et al. (Citation39) classified the gait disorder in 37 patients and found that 97% of their sample could be categorized into one of six groups (momentary fluctuation of gait and stance, excessive slowness, psychogenic rhomberg, uneconomic postures, walking on ice, and sudden buckling of knees without falls). Jordbru et al. (Citation40) further developed this work by testing the inter-rater reliability of these phenotypes in a sample of 30 patients with FGD. Good inter-rater reliability and agreement was found using the three most common phenotypes in their sample (limping/dragging of one leg, walking on ice/slow gait, and truncal ataxia/imbalance).
Nonnekes et al. (Citation41) developed a sign-based approach to support the diagnosis of FGD using clinical features that demonstrate inconsistencies and incongruencies with neurological disease. The authors suggest that seven broad categories capture the diverse clinical spectrum of FGD (ataxic gait, spastic gait, weak gait, antalgic gait, parkinsonian gait, hemiparetic gait, and dystonic gait). includes a summary of the reported phenotypes of FGD.
Table 1. A summary of the reported phenotypes of functional gait disorders.
Functional gait disorders are difficult to categorize because of their complexity, variability and heterogeneity, as highlighted by the broad range of presentations reported in . However, difficulty with classification is not unique to FGD. In dystonia, phenotypic categorization has proven to be challenging due to a large degree of variability among presentations, resulting in different methods of classification based on etiology, age at onset or body distribution (Citation44). Classification of FGD into distinct subtypes may have its limitations, as features can be heterogenous, however, gait analysis and validation of phenotypes in a large cohort may inform a system that supports treatment planning.
Terminology and diagnosis
Terminology used to describe FND has evolved over the editions of the International Classification of Diseases (ICD) and the Diagnostic and Statistical Manual of Mental Disorders (DSM). Having previously been known as ‘hysteria,’ it came to be referred as ‘Conversion disorder’ (DSM-4) or ‘Dissociative (conversion) disorder’ (ICD-10), which evolved to ‘Functional neurological symptom disorder (Conversion disorder)’ in DSM-5TR, and ‘Dissociative neurological symptom disorder’ in ICD-11 (Citation45,Citation46). The term ‘functional’ has become preferred among neurologists (Citation47,Citation48) and people diagnosed with the condition (Citation49).
The diagnosis of FND is usually made by neurologists, especially when motor symptoms are the dominant presentation (Citation43,Citation50). Psychiatrists may have a role in diagnosis, especially for psychological formulation (Citation51). Where possible, the diagnosis should be made based on the identification of positive clinical signs, such as Hoover’s sign for functional weakness, as well as inconsistency of the presentation or incongruency with structural disease (Citation38,Citation52). Examples of inconsistency include a disparity between gait patterns in different environments; variability of symptoms over short periods of time; sudden changes in the frequency or amplitude of a tremor; and a difference between clinical assessment and function, for instance, an inability to access movement during a formal assessment that returns to normal during spontaneous movement (Citation53–55). An example of incongruency is a delayed onset of motor symptoms following minor injury (Citation41). Information from the subjective history provides supporting evidence for the diagnosis, such as transitory episodes of spontaneous remission (Citation41).
The diagnosis can be difficult to distinguish from other conditions, such as movement disorders, because the phenotypes can be similar. Additionally, FGD can coexist with other neurological disease, such as brain injury (Citation11), multiple sclerosis (Citation10) or parkinsonism (Citation38). It can often be pertinent to make two diagnoses in these cases (Citation56). Eames (Citation11), for example, identified that 54 patients from a cohort of 167 (32.3%) with brain injury developed functional symptoms. Associations were found in those with diffuse forms of brain injury, such as hypoxia, and the author also identified a higher incidence of extrapyramidal disorders in those with functional symptoms. Stone and colleagues (Citation10) found that 11.9% of patients in their cohort, with a confirmed neurological diagnosis, also had symptoms that were ‘somewhat’ or ‘not at all’ explained by the neurological disease, effectively describing concurrent FND and structural disease. Owing to this concurrence, the differentiation of symptoms can be challenging, especially in settings where clinicians’ knowledge of and training in FND is limited. However, literature describing validated positive clinic signs for FND has been reported to support the diagnosis (Citation52,Citation57). Additionally, investigations including neuroimaging may be important to rule out other potential causes for symptoms, alongside a thorough neurological examination.
People with FND may initially voice disbelief in the diagnosis, which may be related to stigma or an expectation that an alternative explanation for their symptoms may appear over time. However, misdiagnosis rates are low when the diagnosis is made in a tertiary setting (Citation58). Helping patients understand their diagnosis is an important first step in treatment as acceptance is associated with improved prognosis (Citation48) and greater benefit from treatment (Citation59). Patients may be more accepting of the diagnosis if it is communicated clearly, including an explanation of how the diagnosis was made based on positive clinical signs (Citation60).
Treatment
Consensus from the experts in the field recommend multidisciplinary treatment for motor-FND, including FGD, which includes input from physicians, physiotherapists, occupational therapists and psychologists, based on a biopsychosocial framework (Citation61,Citation62). Studies have reported favorable short- and long-term outcomes following therapy for people with motor-FND, but few trials have focussed specifically on those with FGD.
Jordbru et al. (Citation63) completed the only randomized trial explicitly investigating rehabilitation for FGD. The study randomized sixty people to a three-week inpatient multidisciplinary rehabilitation program or to a waiting list control group. The treatment was described as adapted physical activity within a cognitive behavioral framework. Significant between-group improvements were reported immediately after treatment in the Functional Mobility Scale, Functional Independence Measure and the Physical Domain of the SF-12. Benefits from treatment were mostly maintained at 12-month follow up, with some loss of treatment effect in measures of mental health.
provides an outline of the results from multidisciplinary intervention programs that have focussed on outcomes in motor-FND, which includes participants with FGD. Positive outcomes were reported in all studies, with most adopting small cohort designs, some with long-term follow up. Consistent themes are evident that can be applied to treatment of FGD including 1) multidisciplinary interventions, 2) motor retraining, 3) goal setting with a graded approach, and 4) an individualized treatment tailored to the patients’ needs.
Table 2. Multidisciplinary treatment studies in motor-FND (including FGD).
Physiotherapy is an integral part of the rehabilitation of gait in people with FGD. Nielsen et al (Citation68) conducted a pilot randomized study of specialist physiotherapy for motor-FMD. Participants were randomized to the treatment group (n = 30, specialized physiotherapy) or control group (n = 30, treatment as usual). Results indicated high acceptability of the treatment and no adverse events, with 72% of the treatment group rating their symptoms as improved at 6 months, compared to 18% in the control group, and moderate to large treatment effect across a range of outcomes, including the physical domains of the Short Form-36 (Cohen’s d = 0.46–0.79). Consequently, a powered randomized controlled trial is underway (Citation69).
Owing to the multidimensional nature of FGD and the contribution of non-motor symptoms, the involvement of other multidisciplinary disciplines is an essential part of the treating team. Occupational therapists are key members of the treating team involved with people with FGD, often addressing both motor and non-motor symptoms and the impact on independence in daily function. Consensus expert recommendations for occupational therapy have been described, which are supported by evidence from multidisciplinary treatment trials (Citation70,Citation71). Similarly, psychologists are integral to the treating team and provide psychotherapy to address FND symptoms, as well as comorbid mental illness, such as depression and anxiety, which commonly occur in this population (Citation72). A recent systematic review of psychotherapy treatment for adults with FND indicated that both cognitive behavioral therapy and psychodynamic therapy were potentially effective treatments, although further controlled trials and long-term follow-up are needed (Citation73). Ideally, this interdisciplinary care is best provided within the context of a specialty FND service, either in hospital-based or community- based settings, with leadership and care coordination from a rehabilitation physician, including communication with the patient’s community-based primary care giver. However, these specialty services are rare and many challenges impact how this treatment is delivered, including limitations around resources and clinicians’ knowledge of FND (Citation28). The rehabilitation of people with FND may occur more commonly in typical neurorehabilitation settings. It is vital to recognize that FND may not respond to typical approaches of treatment, and that treatment modifications are needed to address mechanistic drivers of these symptoms, such as attention (i.e., the reversibility of symptoms with diverted attention) but also psychological factors such as anxiety. It is for these reasons that improved awareness of the assessment and management of FND amongst clinicians in these treatment settings is vital to improved patient outcomes.
This review found there is a growing evidence base for the treatment of FGD, but there is a lack of well-powered randomized controlled trials. More research is needed to determine the optimal treatment parameters for FGD, including the type of therapy, dosage, setting and intensity.
Conclusion
Functional gait disorders are multidimensional and disabling, with numerous phenotypes described in the literature. Both motor and non-motor symptoms contribute to FGD, but their relative contribution needs further investigation. Non-motor symptoms have been shown to be associated with increased illness burden and should be carefully considered during assessment and treatment. The current recommended treatment for FGD involves multidisciplinary rehabilitation, but optimum treatment elements are yet to be determined. Future research should focus on further characterization of the motor and non-motor symptoms in FGD and their impact on quality of life, gait and participation, to inform future treatment studies.
Key points
Diagnosis of FGD should be based on the clinical examination identifying positive clinical signs of FND (e.g., Hoover’s sign for functional weakness) and symptoms that are inconsistent and incongruent with structural disease.
Functional gait disorders may occur alongside other neurological diseases, such as brain injury. Up to 12% of patients with neurological disease may have functional neurological symptoms.
The aetiology of FND is best understood using a biopsychosocial model that considers predisposing, precipitating and perpetuating factors.
Non-motor symptoms are common in people with functional gait disorders, and these may account for a greater proportion of the experienced disability and distress than motor symptoms. These symptoms should be considered during assessment and treatment.
Multidisciplinary rehabilitation is recommended for people with FGD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hallett M, Aybek S, Dworetzky BA, McWhirter L, Staab JP, Stone J. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022 Jun 1;21(6):537–50. 10.1016/S1474-4422(21)00422-1.
- Edwards MJ, Bhatia KP, Edwards MJBK. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012 Mar 1;11(3):250–60. 10.1016/S1474-4422(11)70310-6.
- Baik JS, Lang AE, Baik JSLA. Gait abnormalities in psychogenic movement disorders. Mov Disord Clin Pract [Internet]. 2007 Feb 15 [cited 2020 Dec 20];22(3):395–99. Available from http://wiley.com/10.1002/mds.21283
- Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, Coleman R, Cull R, Murray G, Pelosi A, et al. Who is referred to neurology clinics? - The diagnoses made in 3781 new patients. Clin Neurol Neurosurg [Internet]. 2010 Nov [cited 2020 Nov 12];112(9):747–51. Available from https://pubmed.ncbi.nlm.nih.gov/20646830/10.1016/j.clineuro.2010.05.011
- Clinical lectures on the diseases of the nervous system : delivered at the infirmary of La Saltptrire/by J.M. Charcot translated by Thomas Savill : Charcot, J. M. (Jean Martin), 1825-1893 . Free download, borrow, and streaming : Internet archive [Internet]. [cited 2022 May 24]. Available from. https://archive.org/details/b21270211_001
- Stone J, Hewett R, Carson A, Warlow C, Sharpe M. The ‘disappearance’ of hysteria: historical mystery or illusion? J R Soc Med [Internet]. 2008 Jan [cited 2021 Dec 8];101(1):12. Available from /pmc/articles/PMC2235919/
- Perez DL, Edwards MJ, Nielsen G, Kozlowska K, Hallett M, Curt Lafrance W. Decade of progress in motor functional neurological disorder: continuing the momentum. J Neurol Neurosurg Psychiatry [Internet]. 2021 Jun 1 [cited 2022 Nov 21];92(6):668–77. doi:10.1136/jnnp-2020-323953.
- LaFaver K, Maurer CW, Nicholson TR, Perez DL, editors. Functional movement disorder. 2022 [cited 2022 Nov 21]; Available from https://link.springer.com/10.1007/978-3-030-86495-8
- Demartini B. Functional gait disorder. Curr Clin Neurol [Internet]. 2022 [cited 2022 Nov 7];135–45. Available from https://link.springer.com/chapter/10.1007/978-3-030-86495-8_11.
- Stone J, Carson A, Duncan R, Roberts R, Coleman R, Warlow C, Murray G, Pelosi A, Cavanagh J, Matthews K, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease. J Neurol [Internet]. 2012 Jan 16 [cited 2020 Dec 3];259(1):33–38. http://www.statsdirect.com.
- Eames P. Hysteria following brain injury. J Neurol Neurosurg Psychiatry [Internet]. 1992 [[cited 2022 Nov 7]];55:1046–53. http://jnnp.bmj.com/
- Carson AJ, Stone J. Functional and dissociative (psychogenic) neurological symptoms. In: Daroff R, Fenichel G, Jankovic J, Mazziotta J, editors. Bradley’s neurology in clinical practice. Philadelphia: Elsevier; 2012. p. 2147–62.
- Stone J, Warlow C, Sharpe M. Functional weakness: clues to mechanism from the nature of onset. J Neurol Neurosurg Psychiatry [Internet]. 2012 Jan 1 [cited 2021 Feb 16];83(1):67–69. doi:10.1136/jnnp-2011-300125.
- Pareés I, Brown H, Nuruki A, Adams RA, Davare M, Bhatia KP, Friston K, Edwards MJ. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain [Internet]. 2014 Nov [cited 2020 Dec 3];137(11):2916–21. Available from https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/awu237
- Edwards MJ, Adams RA, Brown H, Pareés I, Friston KJ.A Bayesian account of “hysteria. Brain. 2012;135(11):3495–512.doi:10.1093/brain/aws129.
- Ejareh Dar M, Kanaan RAA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging [Internet]. Vol. 12, Neuropsychiatric disease and treatment. Dove Medical Press Ltd.; 2016 cited 2021 Mar 19. 143–53. Available from https://go.gale.com/ps/i.do?p=AONE&sw=w&=11766328&v=2.1&it=r&id=GALE%7CA506651200&sid=googleScholar&linkaccess=fulltext
- Clark CN, Edwards MJ, Ong BE, Goodliffe L, Ahmad H, Dilley MD, Betteridge S, Griffin C, Jenkins PO . Reframing postconcussional syndrome as an interface disorder of neurology, psychiatry and psychology Keywords: mild traumatic brain injury; persistent symptoms; interface disorder; risk factors; imaging Abbreviations: mTBI = mild traumatic brain injury; PCS = postconcussional syndrome. [cited 2022 Nov 21]; DOI:10.1093/brain/awac149
- Stone J, LaFrance WC, Brown R, Spiegel D, Levenson JL, Sharpe M. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res [Internet]. 2011 Dec;71(6):369–76. Available from doi:10.1016/j.jpsychores.2011.07.005.
- Friston KJ, Daunizeau J, Kilner J, Kiebel SJ. Action and behavior: a free-energy formulation. Biol Cybern [Internet]. 2010 Mar 11 [cited 2020 Oct 28];102(3):227–60. Available from https://link.springer.com/article/10.1007/s00422-010-0364-z
- Stone J, Carson A, Aditya H, Prescott R, Zaubi M, Warlow C, Sharpe M. The role of physical injury in motor and sensory conversion symptoms: a systematic and narrative review. J Psychosom Res. 2009 May 1;66(5):383–90. doi:10.1016/j.jpsychores.2008.07.010.
- Stewart DE. The changing faces of somatization. Psychosomatics. 1990 May 1;31(2):153–58. doi:10.1016/S0033-3182(90)72188-3.
- Hotopf M, Mayou R, Wadsworth M.Childhood risk factors for adults with medically unexplained symptoms: results from a national birth cohort study. Prim Care Companion J Clin Psychiatry. 2000;2(1):31.
- Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain [Internet]. 2004 Oct 1 [cited 2021 Sep 19];127(10):2360–72. doi:10.1093/brain/awh262.
- Gelauff JM, Kingma EM, Kalkman JS, Bezemer R, van Engelen BGM, Stone J, Tijssen MAJ, Rosmalen JGM. Fatigue, not self-rated motor symptom severity, affects quality of life in functional motor disorders. J Neurol. 2018 Aug 1;265(8):1803–09. doi:10.1007/s00415-018-8915-7.
- Gelauff JM, Carson A, Ludwig L, Tijssen MAJ, Stone J.The prognosis of functional limb weakness: a 14-year case-control study. Brain. 2019;142(7):2137–48.doi:10.1093/brain/awz138.
- Věchetová G, Slovák M, Kemlink D, Hanzlíková Z, Dušek P, Nikolai T, Růžička E, Edwards MJ, Serranová T. The impact of non-motor symptoms on the health-related quality of life in patients with functional movement disorders. J Psychosom Res. 2018 Dec 1;115:32–37. 10.1016/j.jpsychores.2018.10.001.
- Tinazzi M, Morgante F, Marcuzzo E, Erro R, Barone P, Ceravolo R, Mazzucchi S, Pilotto A, Padovani A, Romito LM, et al. Clinical correlates of functional motor disorders: an Italian multicenter study. Mov Disord Clin Pract. 2020 Nov 1;7(8):920–29. doi:10.1002/mdc3.13077.
- Aybek S, Lidstone SC, Nielsen G, Macgillivray L, Bassetti CL, Lang AE, Edwards MJ. What is the role of a specialist assessment clinic for FND? Lessons from three national referral centers. J Neuropsychiatry Clin Neurosci [Internet]. 2020 Jan 7 [cited 2021 Mar 16];32(1):79–84. https://psychiatryonline.org/doi/10.1176/appi.neuropsych.19040083
- Lavedán A, Viladrosa M, Jürschik P, Botigué T, Nuín C, Masot O, Lavedán R. Fear of falling in community-dwelling older adults: a cause of falls, a consequence, or both? PLoS One. 2018 Mar 1;13(3).
- Sudarsky L Psychogenic gait disorders [Internet]. Seminars in Neurology Semin Neurol; Jul, 2006 p. 351–56. Available from: https://pubmed.ncbi.nlm.nih.gov/16791781/
- Scheffer AC, Schuurmans MJ, Van Dijk N, VanVan der hooft T, De Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing [Internet]. 2008 Jan 1 [cited 2021 Nov 15];37(1):19–24. doi:10.1093/ageing/afm169.
- Popkirov S, Hoeritzauer I, Colvin L, Carson AJ, Stone J. Complex regional pain syndrome and functional neurological disorders - time for reconciliation. J Neurol Neurosurg Psychiatry. 2019 May 1;90(5):608–14. 10.1136/jnnp-2018-318298.
- Popkirov S, Staab JP, Stone J. Persistent postural-perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness [Internet]. Vol. 18, Practical Neurology. BMJ Publishing Group; 2018 cited 2021 Feb 16. 5–13. Available from http://pn.bmj.com/
- Seemungal BM, Passamonti L. Persistent postural-perceptual dizziness: a useful new syndrome. Pract Neurol [Internet]. 2018 Feb 1 [cited 2021 Mar 14];18(1):3–4. doi:10.1136/practneurol-2017-001817.
- Okun MS, Koehler PJ Paul Blocq and (psychogenic) astasia abasia [Internet]. Vol. 22, Movement Disorders. Mov Disord; 2007 [cited 2020 Aug 16]. p. 1373–78. Available from: https://pubmed-ncbi-nlm-nih-gov.epworth.idm.oclc.org/17516452/
- Clinical Lectures on Paralysis, Disease of the brain, and other affections … - Robert Bentley Todd - Google books [Internet]. [cited 2022 Jan 19]. Available from: https://books.google.com.au/books?hl=en&lr=&id=BjxpAAAAIAAJ&oi=fnd&pg=PR15&ots=rocBjH9Zhp&sig=A9q7eN0WUJdUT4gO-mNrmVq7HKo&redir_esc=y#v=onepage&q&f=false
- Nouvelle Iconographie de la Salpêtrière (Clinique des Maladies du Système Nerveux). Publiée sous la direction du Prof. Charcot (de l’Institut) par Paul Richer (chef du Laboratoire) Gilles de la Tourette (chef de Clinique) Albert Londe (Directeur du Service Photographique). Tome premier. Paris: lecroisnier et Babé, Place de l’Ecole de Médicine. 1888. J Ment Sci [Internet]. 1890 Jul [cited 2021 Dec 8]; 36(154):389–93. Available from https://www.cambridge.org/core/journals/journal-of-mental-science/article/abs/nouvelle-iconographie-de-la-salpetriere-clinique-des-maladies-du-systeme-nerveux-publiee-sous-la-direction-du-prof-charcot-de-linstitut-par-paul-richer-chef-du-laboratoire-gilles-de-la-tourette-chef-de-clinique-albert-londe-directeur-du-service-photographique-tome-premier-paris-lecroisnier-et-babe-place-de-lecole-de-medicine-1888/8A1C197B778E8B0B077B734E2CC132C2
- Tinazzi M, Pilotto A, Morgante F, Marcuzzo E, Cuoco S, Ceravolo R, Mazzucchi S, Padovani A, Romito LM, Eleopra R, Nicoletti A. Functional gait disorders: demographic and clinical correlations. Parkinsonism Relat Disord. 2021 Oct;91:32–36. doi:10.1016/j.parkreldis.2021.08.012.
- Lempert T, Brandt T, Dieterich M, Huppert D. How to identify psychogenic disorders of stance and gait - A video study in 37 patients. J Neurol [Internet]. 1991 Jun [[cited 2020 Aug 16]];238(3):140–46. Available from: https://link.springer.com/article/10.1007/BF00319680.
- Jordbru AA, Smedstad LM, Moen VP, Martinsen EW. Identifying patt erns of psychogenic gait by video-recording. J Rehabil Med. 2012 Jan;44(1):31–35. doi:10.2340/16501977-0888.
- Nonnekes J, Růžička E, Serranová T, Reich SG, Bloem BR, Hallett M. Functional gait disorders: a sign-based approach. Neurology. 2020 Jun 16;94(24):1093–99. 10.1212/WNL.0000000000009649.
- Baizabal-Carvallo JF, Alonso-Juarez M, Jankovic J, Baizabal-Carvallo JF, Alonso-Juarez MJJ. Functional gait disorders. Clinical phenomenology, and classification. Neurol Sci [Internet]. 2019 Apr 1 [cited 2020 Oct 6];14(4):911–15. Available from https://link.springer.com/article/10.1007/s10072-019-04185-8
- Fung VSC. Functional gait disorder. In: Handbook of clinical neurology, Elsevier B.V; 2016. 263–70.
- Albanese A, Bhatia K, Bressman SB, DeLong MR, Fahn S, Fung VSC, Hallett M, Jankovic J, Jinnah HA, Klein C, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord [Internet]. 2013 Jun 15 [cited 2021 Oct 25];28(7):863–73. https://onlinelibrary.wiley.com/doi/full/10.1002/mds.25475
- ICD-11 [Internet]. [cited 2021 Aug 2]. Available from: https://icd.who.int/en
- Psychiatry.org - DSM-5-TR Fact Sheets [Internet]. [cited 2022 Nov 21]. Available from: https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/dsm-5-tr-fact-sheets
- Friedman JH, LaFrance WC. Psychogenic disorders: the need to speak plainly [Internet]. Vol. 67, Archives of neurology. American Medical Association; 2010 cited 2021 Feb 14. 753–55. Available from https://jamanetwork.com/journals/jamaneurology/fullarticle/800272
- Espay AJ, Goldenhar LM, Voon V, Schrag A, Burton N, Lang AE. Opinions and clinical practices related to diagnosing and managing patients with psychogenic movement disorders: an international survey of movement disorder society members. Mov Disord [Internet]. 2009 Jul 15 [cited 2021 Feb 14];24(9):1366–74. Available from http://wiley.com/10.1002/mds.22618
- Ding JM, Kanaan RAA. Conversion disorder: a systematic review of current terminology. Gen Hosp Psychiatry. 2017 Mar 1;45;51–55. 10.1016/j.genhosppsych.2016.12.009
- Espay AJ, Lang AE. Phenotype-specific diagnosis of functional (Psychogenic) movement disorders. Curr Neurol Neurosci Rep. 2015 Jun 1;15(6). 10.1007/s11910-015-0556-y.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Daum C, Hubschmid M, Aybek S. The value of “positive” clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review [Internet]. Vol. 85, Journal of neurology, neurosurgery and psychiatry. BMJ Publishing Group; 2014 cited 2021 Jun 2. 180–90. Available from 10.1136/jnnp-2012-304607.
- Araújo R, Van De Warrenburg B, Lang A, Lees A, Bloem B. The waiting room: neurological observations made outside the movement disorder specialist’s consulting office. Pract Neurol [Internet]. 2019 Aug 1 [cited 2022 Jan 26];19(4):295–301. doi:10.1136/practneurol-2018-002110.
- Espay AJ, Aybek S, Carson A, Edwards MJ, Goldstein LH, Hallett M, LaFaver K, LaFrance CW, Lang AE, Nicholson T, Nielsen G, Reuber M, Voon V, Stone J, Morgante F. Current concepts in diagnosis and treatment of functional neurological disorders [Internet]. Vol. 75, JAMA neurology. American Medical Association; 2018 cited 2021 May 29. 1132–41. Available from https://jamanetwork.com/
- Hayes MW, Graham S, Heldorf P, De Moore G, Morris JGL. A video review of the diagnosis of psychogenic gait: appendix and commentary [Internet]. Movement Disorders. 1999;14:914–21. doi:10.1002/1531-8257(199911)14:6<914::AID-MDS1002>3.0.CO;2-B.
- Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol [Internet]. 2013 Apr 1 [cited 2022 Nov 21];13(2):104–13. doi:10.1136/practneurol-2012-000422.
- Aybek S, Perez DL. State of the art reVIeW diagnosis and management of functional neurological disorder. BMJ. 2022; 376:064. doi:10.1136/bmj.o64.
- Walzl D, Solomon AJ, Stone J. Functional neurological disorder and multiple sclerosis: a systematic review of misdiagnosis and clinical overlap [Internet]. Vol. 1, Journal of Neurology. Springer Science and Business Media Deutschland GmbH; 2021 cited 2021 Apr 8. 3. 10.1007/s00415-021-10436-6.
- Nielsen G, Stone J, Matthews A, Brown M, Sparkes C, Farmer R, Masterton L, Duncan L, Winters A, Daniell L, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry. 2015 Oct 1;86(10):1113–19. 10.1136/jnnp-2014-309255.
- Stone J. Functional neurological disorders: the neurological assessment as treatment. In: Vol. 16, Practical neurology, BMJ Publishing Group; 2016. 7–17.
- Gilmour GS, Nielsen G, Teodoro T, Yogarajah M, Coebergh JA, Dilley MD, Martino D, Edwards MJ. Management of functional neurological disorder. J Neurol [Internet]. 2020 Jul 1;267(7):2164–72. Available from 10.1007/s00415-020-09772-w.
- Greiner C, Schnider A, Leemann B. Functional neurological disorders: a treatment-focused review [Internet]. Vol. 167, Swiss archives of neurology, psychiatry and psychotherapy. EMH Media; 2016 cited 2020 Dec 3. 234–40. Available from https://sanp.ch/article/doi/sanp.2016.00441
- Jordbru AA, Smedstad LM, Klungsøyr O, Martinsen EW.Psychogen ic gait diso rde r: a random ized cont rolled trial of physical rehabilitation with one -year fo llow -up. J Rehabil Med. 2014;46(2):181–87.doi:10.2340/16501977-1246.
- Petrochilos P, Elmalem MS, Patel D, Louissaint H, Hayward K, Ranu J, Selai C. Outcomes of a 5-week individualised MDT outpatient (day-patient) treatment programme for functional neurological symptom disorder (FNSD). J Neurol [Internet]. 2020;267:2655–66. Available from doi:10.1007/s00415-020-09874-5.
- Demartini B, Batla A, Petrochilos P, Fisher L, Edwards MJ, Joyce E. Multidisciplinary treatment for functional neurological symptoms: a prospective study. J Neurol. 2014 Nov 25;261(12):2370–77. 10.1007/s00415-014-7495-4.
- Mccormack R, Moriarty J, Mellers JD, Shotbolt P, Pastena R, Landes N, Goldstein L, Fleminger S, David AS. Specialist inpatient treatment for severe motor conversion disorder: a retrospective comparative study. [cited 2020 Dec 3]; Available from: http://jnnp.bmj.com/
- Czarnecki K, Thompson JM, Seime R, Geda YE, Duffy JR, Ahlskog JE. Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Park Relat Disord [Internet]. 2012 Mar;18(3):247–51. Available from doi:10.1016/j.parkreldis.2011.10.011.
- Nielsen G, Buszewicz M, Stevenson F, Hunter R, Holt K, Dudziec M, Ricciardi L, Marsden J, Joyce E, Edwards MJ, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry. 2017 Jun 1;88(6):484–90. 10.1136/jnnp-2016-314408.
- Nielsen G, Stone J, Buszewicz M, Carson A, Goldstein LH, Holt K, Hunter R, Marsden J, Marston L, Noble H, et al. Physio4FMD: protocol for a multicentre randomised controlled trial of specialist physiotherapy for functional motor disorder. BMC Neurol. 2019 Oct 21;19(1). 10.1186/s12883-019-1461-9.
- Nicholson C, Edwards MJ, Carson AJ, Gardiner P, Golder D, Hayward K, Humblestone S, Jinadu H, Lumsden C, MacLean J, et al. Occupational therapy consensus recommendations for functional neurological disorder. J Neurol Neurosurg Psychiatry [Internet]. 2020 Oct 1 [cited 2022 Jan 19];91(10):1037–45. https://pubmed.ncbi.nlm.nih.gov/32732388/
- Gardiner P, Macgregor L, Carson A, Stone J. Occupational therapy for functional neurological disorders: a scoping review and agenda for research. CNS Spectr [Internet]. 2018 Jun 1 [cited 2021 Nov 19];23(3):205–12. doi:10.1017/S1092852917000797.
- Carson A, Stone J, Hibberd C, Murray G, Duncan R, Coleman R, Warlow C, Roberts R, Pelosi A, Cavanagh J, et al. Disability, distress and unemployment in neurology outpatients with symptoms “unexplained by organic disease.” [cited 2021 Jun 23]; Available from: http://jnnp.bmj.com/
- Gutkin M, McLean L, Brown R, Kanaan RA. Systematic review of psychotherapy for adults with functional neurological disorder. J Neurol Neurosurg Psychiatry [Internet]. 2021 Jan 1 [cited 2021 Nov 19];92(1):36–44. doi:10.1136/jnnp-2019-321926.