ABSTRACT
Background
The cardiac autonomic control system function is frequently impaired after brain injury. An association exists between the cardiac autonomic control system and endurance performance.
Aim
To evaluate the association between cardiac autonomic control system indices at the beginning of the inpatient rehabilitation and walking endurance two months later among children and adolescents following acquired brain injury.
Methods
A prospective study included 28 children and adolescents following acquired brain injury in the sub-acute phase. A Polar device (RS800CX) records heart rate as a proxy measure of autonomic function at sitting and lying supine on admission and two months later. The 6-minute walk test was assessed at the second assessment in 25 participants. Non-parametric tests were used with statistical significance defined as p < 0.05.
Results
There were statistically significant differences in heart rate variability between lying and sitting positions, which were positively correlated with the 6-minutes walk test results two months later, mainly in the parasympathetic components (rs = 0.51 p-value <0.01).
Conclusions
At the beginning of the rehabilitation of children and adolescents following acquired brain injury, a simple manipulation – position change from sitting to lying, activates cardiac autonomic control system responses. These responses are positively associated with walking endurance two months later.
Introduction
The primary role of the autonomic nervous system is to ensure homeostasis by regulating the internal organs through its three subdivisions; the sympathetic system (SNS), the parasympathetic system (PNS), and the enteric system (Citation1). The prefrontal and insular cortexes, cingulate gyrus, amygdala, and the hypothalamus are all involved in heart rate regulation (Citation2). The brainstem is an interface between afferent information from the heart, lungs, and other body systems and efferent neuronal activity (Citation1).
Heart rate variability (HRV) is a measure that quantifies the variability of the heart rate around its average and therefore expresses the balance between the PNS system, which slows the heart rate (HR) and increases HRV, and the SNS, which increases HR and reduces its variability (Citation3). Therefore, HRV is considered a neuro-cardiac function measure reflecting heart and brain interactions (Citation2) and is frequently used in studying children following brain injury (Citation4–7).
Acquired Brain Injury (ABI) is a sudden, non-progressive injury to the brain resulting from either a traumatic or a non-traumatic event and is one of the leading causes of disability among children and adolescents (Citation8). Children post-ABI demonstrate reduced HRV (Citation5) and low endurance capacity (Citation9) compared to typically developed children.
Performing aerobic or endurance tasks leads to elevated sympathetic activity, thus increasing heart rate and vasoconstriction to visceral organs to promote blood flow to the activated tissues. At the early stages of exercise recovery, the parasympathetic system dominates to permit cardiac deceleration, followed by a sympathetic withdrawal (Citation1). Impairment in the central autonomic control system is associated with impaired autonomic regulation during exercise, leading to reduced performance. In children after Traumatic Brain Injury (TBI) years post the trauma, an association was found between HRV and endurance abilities (Citation5). Following an acute hospitalization phase, children and young adults post moderate to severe ABI are admitted to an intensive inpatient rehabilitation program (sub-acute phase), consisting of at least three active therapies per day, to regain their functional abilities and to return to their homes and community (Citation10). In post-stroke patients admitted to inpatient rehabilitation, a significant moderate-strong association was found between HRV parameters at admission and both motor function and aerobic capacity at discharge (Pearson correlation coefficient = 0.53–0.64, p < 0.05) (Citation11,Citation12).
The aims of the current study are (a) to describe the function of the cardiac autonomic control system (CACS) by presenting HRV measures during the subacute rehabilitation phase, (b) to describe motor function using the Gross Motor Function Measure −88 during the subacute rehabilitation phase, (c) to evaluate the association between cardiac autonomic control system indices at the beginning of the subacute rehabilitation phase and walking endurance two months later in children and adolescents following acquired brain injury.
Methods
Sample size
The sample size calculation was based on the assumption that a moderate- strong association (Pearson correlation coefficient = 0.5) exists between autonomic impairment at the beginning of the rehabilitation and walking endurance two months later in people following brain damage, as noted before in people post-stroke (Pearson correlation coefficient = 0.53–0.64) (Shapira-Vadler et al. (2015), (Citation11). Under the accepted assumption for a type 1 error of 5% and test power of 80%, the required sample size is 30 children and adolescents.
Participants
Children and adolescents admitted to the rehabilitation department in a tertiary rehabilitation hospital Lowenstein Rehabilitation Center between March 2018 and March 2020 were included following the inclusion criteria: (1) post-ABI (due to traumatic brain injury, stroke, encephalitis, brain tumor or anoxic brain damage) (2) aged 4–18 years (3) hospitalized for rehabilitation up to 45 days post-acute injury (4) able to cooperate, communicate and follow simple instructions. Excluded were children and adolescents: (1) those with former brain injury, (2) heart disease (3) use medications that could affect the autonomic nervous system, such as hypertensive medications.
This prospective study was approved by the Ethics Committee of the rehabilitation hospital and Tel Aviv university. After explaining the study procedure, guardians signed informed written consent, and participants gave verbal approval to participate.
Instruments
Primary outcome measures: Walking endurance was assessed by the 6 Minute Walk Test (6MWT). The test measures the maximal distance a person can walk in 6 minutes. The test was performed indoors in the rehabilitation department on a 70 – meter oval tract (Citation13,Citation14), marked every 3 meters, using verbal instructions based on the American Thoracic Society protocol (Citation15). The 6MWT was found to be highly reliable in children post ABI (Citation14) with a moderate-strong association with the gold standard Vo2 max and Vo2 peak among typically developed children and children with Cerebral Palsy (CP) (Citation16,Citation17).
The independent variables included the HR and HRV parameters monitored by the Polar watch RS800CX, 1000 Hz rating sample. Analysis was performed with the Polar software with a signal correction rate of up to 2% of all RR intervals. The Analysis includes both time domain and frequency domain measures. The time domain outcomes included the normal to normal RR interval standard deviation (SDNN) and the root mean square of successive RR interval differences (RMSSD). The SDNN expresses the long-term variability associated more with sympathetic system activity. The RMSSD evaluates the short-term variability of HRV and, therefore, is linked to parasympathetic system activity. Frequency measurements included the high frequency (HF), which again is more associated with the parasympathetic system activity, and the low frequency (LF), as recommended in a short recording of 5 minutes (Citation18,Citation19).
Covariates: The Gross Motor Function Measure −88 (GMFM) assessed motor function. The test assessed the gross motor function of children with CP. The test has 88 items in five domains, each in a different position (lying, sitting, kneeling, standing, and walking). Each item is scored 0 (unable to perform) to 3 (full performance) (Citation20). The test is highly reliable and valid for children post-TBI (Citation21) and is a recommended test for pediatric TBI (Citation22). The test has also been used to evaluate children following ABI (Citation23,Citation24). The level of independence in daily activities was assessed by the Functional Independence Measurement (FIM) and FIM for children (WeeFIM) (Citation25). The nursing staff in the rehabilitation department performed the assessment, which was collected from the medical records for the study.
Study procedure
Once the child met the inclusive criteria, the parents signed the informed consent, and the child gave verbal approval for participating, the child was enrolled in the study.
Each child was assessed twice: after admission to the department and two months later.
The tests that were performed in both assessments include:
RR interval recording: A trained, experienced physiotherapist collects the RR values while sitting and lying in a quiet room with a temperature between 21–26℃. The assessment was performed at least four hours after daily physiotherapy treatment and two hours after a meal. The manipulation, moving from sitting to lying, was chosen as it is feasible for the study participants. It has been shown that HRV increases when healthy subjects change their position from sitting to lying (Citation26).
Motor performance: The same therapist assessed the GMFM-88.
Functional performance: The nursing staff of the rehabilitation department assessed the FIM/WeeFIM.
At the second assessment, children who could walk independently performed the 6MWT under the supervision and guidance of the same physiotherapist.
During the two-month study period, the child received five days a week tailored rehabilitation program. The program included physiotherapy, occupational therapy, speech therapy, and psychological treatment based on each child’s needs.
Statistical analysis
Due to the small sample size, data were analyzed using non-parametric tests. Descriptive statistics included numbers and percentages for nominal variables and median and minimum-maximum values for ordinal and ratio variables. The Wilcoxon test was used to assess differences in outcome measures over time (two months) or between positions (lying-sitting) at each assessment. The correlations between HRV parameters at the first assessment and the 6MWT at the second, as well as between FIM and GMFM and HRV parameters, are shown by the Spearman correlation coefficient. Partial correlations were used to eliminate potential confounders in the association between HRV and 6MWT. Correlation coefficient strength was defined as moderate for 0.4–0.7 and strong for > 0.7 (Citation27). Statistical significance was defined as p < 0.05. Data were analyzed using the Statistical Package for Social Sciences (SPSS version 24).
Results
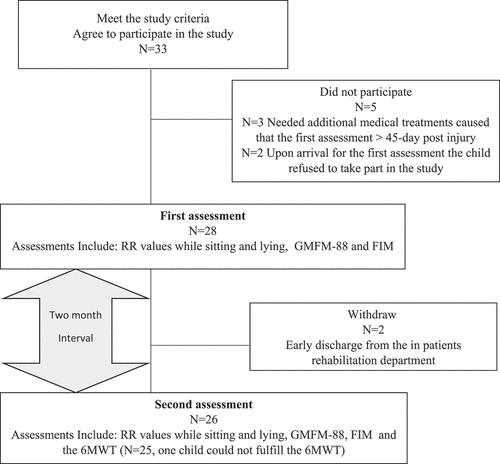
Thirty-three participants were enrolled and signed the informed consent. Five were excluded: Three needed additional medical treatments and were hospitalized in a general hospital after signing the consent so that when they returned to rehabilitation to join the study, the time that had passed since the injury was more than 45 days post-injury. Two other children refused to participate in the study on the first assessment day. Thus, at the first assessment, 28 participants were included. Two participants were discharged early from the department and did not complete the second assessment. Out of the remaining 26 children and adolescents, only one child, post-encephalitis, could not perform the 6MWT two months later ().
The participants’ demographic, anthropometric, and injury characteristics are presented in . The impairment profile was collected from the participant’s medical record based on the ICD 9 (international classification system revision 9) (Citation28). Participants were mostly males, with an extensive age range (5–18 years) and body mass index (14–29 Kg/m2). Seventy-five percent of the participants were post-closed TBI; three had a central nervous system tumor removed, three were post-stroke, and one had encephalitis. Half of the participants were admitted to the rehabilitation department between the third and fourth-week post-injury (inter-quartile range 13–28 days).
Table 1. Participant and injury characteristics at admission; in the total sample (N = 28) and in those who attend the second assessment and perform the 6MWT (N = 25).
Table 2. Participant impairment profile at admission.
presents the participant’s motor (GMFM-88) and functional (FIM) performances at admission (T1) and two months later (T2). A significant improvement occurred in the Activities of Daily Living (ADL) and functional tasks: the walking assessment of the GMFM-88 scale improved by 39.8%, and the median FIM improved by 36.4%. The median 6MWT distance (at the second assessment (T2) was 535 meters [in range 244–794 meters]. The 6MWT result was significantly associated with the participant’s age (rs = 0.45, p-value<0.05).
Table 3. Motor and functional performances at admission (T1) and two months later (T2).
The CACS function during the subacute phase following ABI
presents HRV parameters at sitting and lying positions at admission and two months later.
Table 4. HRV parameters sitting and lying in admission (T1) and two months later (T2).
At T1, the lowest HRV parameters were noted in two of the three children who had brain tumor surgery. Significant differences were found between positions, with higher values in lying in all parameters except LF. Two months later, significant differences were noted between positions only in parameters related to the parasympathetic system (RMSSD and HF). In both assessments, values were higher in the lying position. No significant difference was noted over time (T1-T2).
The association between CACS at the beginning of the rehabilitation phase and walking endurance and motor function two months later
This study aimed to assess the association between the CACS function and walking endurance and motor function in children and adolescents following ABI during the sub-acute phase. presents the significant correlations between HRV parameters and walking endurance assessed by the 6MWT and GMFM walking sub-scale results.
Table 5. Correlation coefficients between HRV parameters, FIM, GMFM-88 at admission (T1) and 6MWT and GMFM-88 two months later (T2).
Positive moderate and strong correlations were found between the change in RR, PNN50, and HF values due to position change (lying-sitting) at admission and the 6MWT results two months later. Positive moderate-strong correlations were found between the RR, RMSSD, PNN50, and HF values and their response to position change (lying-sitting) at admission and the GMFM-88 walking sub-item score two months later. Positive and strong correlations were found between FIM/WeeFIM and GMFM-88 results at admission and the 6MWT results two months later; GMFM sitting and 6MWT r = 0.56, GMFM standing and 6MWT r = 0.65, GMFM walking and 6MWT r = 0.64, (all p-value<0.05). (Data not presented in the table).
The added contribution of the CACS in predicting walking endurance above motor-functional measures was assessed by performing a partial correlation analysis, controlling for the impact of the GMFM – standing and walking at admission. The partial correlation reveals that the association between HRV parameters (lying-siting) at admission and 6MWT two months later decreased and was found insignificant (rs<0.25, p < 0.05). On the other hand, when performing partial correlation controlling the impact of the ΔHF (lying-siting) at admission, the association between GMFM standing and walking parts and the 6MWT decreased but remained moderate-strong and significant (rs<0.59, p < 0.01).
Discussion
This study aimed to evaluate the CACS function and its response to a mild position change, as assessed by HRV parameters, in children and adolescents post-ABI, during the subacute phase and to examine its association with walking endurance and motor abilities two months later.
The CACS responded to position change (sitting to lying) in both assessments: at admission and two months later. The response was more prominent in the parasympathetic HRV measures. HRV measures did not change significantly over time, while a significant improvement was noted in motor and function abilities during the two-month follow-up period. In addition, significant moderate-strong correlations were found between parasympathetic HRV parameters response to position change at admission and walking endurance and motor function two months later. However, the association between HRV and walking endurance did not remain significant after controlling for motor function at baseline.
Cardiac autonomic control system response to position change
Significantly lower HRV measures were found in sitting vs. lying positions at first and in the follow-up assessments among children following moderate-severe ABI during rehabilitation. The significant differences were mainly present in the parasympathetic HRV measures.
In previous studies in patients after brain injury (Citation6,Citation29), which assessed CACS reactivity during position change from lying to standing, the response was characterized by an increase in sympathetic function and a decrease in parasympathetic function. Hilz et al. (2017) assessed adults post mild to severe TBI, 6–94 months post the trauma during position change from lying to standing, and found a significant decrease of RR and RMSSD and a significant increase of LF/HF when standing in all TBI severity levels (Citation29). Likewise, Balestrini et al. (2019) demonstrated the CACS response to position change lying-sitting-standing in young athletes after head concussion in the sub-acute phase, as reflected in a reduction of the RMSSD parameter while standing (Citation6).
The current study adds to the above findings by showing that the CACS response to position change is also found in the early stages post-ABI. Moreover, the response is expressed even with attenuated manipulation – moving from sitting to lying.
The HRV parameters did not change during the two-month rehabilitation period
Previous studies demonstrated that people post-BI have impaired CACS activity (Citation30) and displayed lower HRV compared to healthy controls (Citation31). In the current study, the CACS responded to a minor autonomic manipulation- moving from a sitting to a lying position, which may imply that the CACS system has the potential to be trained. However, the HRV measures did not change over time. The motor and functional performance improved significantly during that period.
We postulated two hypotheses to explain this observation: The first is connected to the rehabilitation program, where less attention is given to the assessment and rehabilitation of the CACS, and more attention is given to achieving motor improvement and function. While the literature on motor and functional improvement is comprehensive, much less is known about autonomic rehabilitation in post-ABI individuals. The second hypothesis is time-related in that a more extended follow-up period may be needed to reveal CACS improvement.
One of the main strategies to improve CACS is by performing aerobic exercise (Citation32). Strong evidence indicates the positive effects of aerobic exercise training on cardiac autonomic function in healthy sedentary adults, patients with post-cardiac conditions, or people with diabetes (Citation33–35). Most studies that observed the effects of exercise on HRV parameters were performed at a moderate or high intensity for 20 to 60 minutes, 3 to 5 times a week for several weeks. These programs are not always suitable for ABI patients 2–3 months post-injury in duration and intensity. Some of them only regain independent walking during that time (Citation36). Therefore, further research is needed to alter the program’s intensity or address the cardiac autonomic function after achieving the necessary motor improvement.
Correlation between CACS response to a position change and walking endurance
Associations between HRV and functional outcomes have been described before; in patients following traumatic brain injury at the acute, sub-acute, and chronic phases (Citation37) and in patients post-stroke (Citation11). Similarly, the current study found significant, moderate-strong associations between the HRV measures at baseline and the GMFM sub-categories two months later.
In this study, the response of the parasympathetic measures to position change early in rehabilitation was associated with walking endurance two months later. Previous studies highlighted that brain injury is associated with incremental changes in sympathetic activity and with changes in the fine balance between the sympathetic and parasympathetic systems of the autonomic nervous system (Citation30). As presented in the current study, the early response of the parasympathetic system may signify better recovery and improved walking endurance. It is important to note that no associations were found between HRV at rest and walking endurance. But even a very mild manipulation – moving from a sitting to a lying position- was associated with a change in parasympathetic measures that were associated with walking endurance two months later
The current study shows that the walking endurance test is associated with motor and cardiorespiratory abilities. When controlling the impact of motor components, the associations between the parasympathetic HRV parameters and the 6MWT were reduced and became insignificant. It is clear and not surprising; first of all, there is a need for the motor ability to perform the activity, but at the same time, there is a need for autonomous power to support it.
This topic needs more extensive follow-up studies, with HRV monitoring and CACS triggering during the rehabilitation program.
Limitations
This work has several limitations. First, there might be a selection bias as the participants were all from a single rehabilitation center. However, since the rehabilitation center is a tertiary center admitting patients from all areas and all healthcare organizations, participants represent the country’s diverse populations. Since the demographic characteristics of participants (for example, dominantly males) concur with other studies (Citation38), we believe they may have external validation.
In addition, although the response rate was high (only two parents and their children did not agree to participate), some data were missing at the first or second assessment. Lastly, the sample size is small and heterogeneous. Only 25 of the participants performed the HRV measures and the 6MWT (sample size needed was 30 participants); moreover, 75% of the participants were post-traumatic brain injury, the other causes of injury were heterogeneous, the age range was wide, and the participants were assessed at different times since the injury.
Conclusions and implications for clinicians and researchers
The main finding of the current study is that in children and adolescents following ABI, the CACS responded to a mild position change, even in the early stages of rehabilitation. These changes are associated with walking endurance two months later. In addition, while daily and motor functions show improvement over time, no improvement was found in HRV measures during the two-month follow-up.
These findings raise questions regarding the assessment and the treatment related to CACS during the rehabilitation process and the need to refer to it during the sub-acute rehabilitation period. A system like the CACS that does not undergo a proper evaluation cannot receive the proper treatment.
Further studies are needed to evaluate the function and changes in the CACS as early as possible and identify the best means of treatment for its rehabilitation.
Disclosure statement
The authors report no conflict of interest
Additional information
Funding
References
- Gibbons CH, Basics of autonomic nervous system function. Handb Clin Neurol. 2019;160:407–18.
- Silvani A, Calandra-Buonaura G, Dampney RA, Cortelli P, Brain-heart interactions: physiology and clinical implications. Philos Trans A Math Phys Eng Sci. 2016;374(2067):20150181. doi:10.1098/rsta.2015.0181.
- Lahiri MK, Kannankeril PJ, Goldberger JJ, Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51(18):1725–33. doi:10.1016/j.jacc.2008.01.038.
- Sorek G, Shaklai S, Meyer S, Katz-Leurer M, Autonomic cardiac control response to walking and executive cognitive task in adolescents with acquired brain injury and typically developed controls. Brain Inj. 2018;32(6):770–75. doi:10.1080/02699052.2018.1450993.
- Katz-Leurer M, Rotem H, Shofer M, Meyer S, Pediatric cardio-autonomic response to variable effort after severe traumatic brain injury. Brain Inj. 2016;30(10):1239–42. doi:10.1080/02699052.2016.1179343.
- Balestrini CS, Moir ME, Abbott KC, Klassen SA, Fischer LK, Fraser DD, Shoemaker JK. Autonomic dysregulation in adolescent concussion is sex- and posture-dependent. Clin J Sport Med. 2021;31(3):257–65. doi:10.1097/JSM.0000000000000734.
- Katz-Leurer M, Rotem H, Keren O, Meyer S, Heart rate and heart rate variability at rest and during exercise in boys who suffered a severe traumatic brain injury and typically-developed controls. Brain Inj. 2010;24(2):110–14. doi:10.3109/02699050903508234.
- Teasell R, Bayona N, Marshall S, Cullen N, Bayley M, Chundamala J, Villamere J, Mackie D, Rees L, Hartridge C, et al. A systematic review of the rehabilitation of moderate to severe acquired brain injuries. Brain Inj. 2007;21(2):107–12. doi:10.1080/02699050701201524.
- Mossberg KA, Amonette WE, Masel BE, Endurance training and cardiorespiratory conditioning after traumatic brain injury. J Head Trauma Rehabil. 2010;25(3):173–83. doi:10.1097/HTR.0b013e3181dc98ff.
- Gmelig Meyling C, Verschuren O, Rentinck IR, Engelbert RH, Gorter JW, Physical rehabilitation interventions in children with acquired brain injury: a scoping review. Dev Med Child Neurol. 2022;64(1):40–48. doi:10.1111/dmcn.14997.
- Lees T, Shad-Kaneez F, Simpson AM, Nassif NT, Lin Y, Lal S, Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark Insights. 2018;13:1177271918786931. doi:10.1177/1177271918786931.
- Shapira-Vadler O, Treger I, Katz-Leurer M, Muscle strength, function and heart autonomic regulation system recovery at the sub-acute stage post stroke. Eur Neurol. 2015;74(3–4):154–57. doi:10.1159/000440953.
- Mossberg KA, Fortini E, Responsiveness and validity of the six-minute walk test in individuals with traumatic brain injury. Phys Ther. 2012;92(5):726–33. doi:10.2522/ptj.20110157.
- Fadida Y, Shapira-Vadler O, Spasser R, Frenkel-Toledo S, Reproducibility and smallest real differences of walking and Energy Expenditure Index in children and adolescents with an acquired brain injury. NeuroRehabilitation. 2019;45(1):19–24. doi:10.3233/NRE-192716.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test [published correction appears in Am J Respir Crit Care Med. 2016 15 May;193(10):1185]. Am J Respir Crit Care Med. 2002;166(1):111–17.doi:10.1164/ajrccm.166.1.at1102.
- Li AM, Yin J, Yu CC, Tsang T, So HK, Wong E, Chan D, Hon EK, Sung R. The six-minute walk test in healthy children: reliability and validity. Eur Respir J. 2005;25(6):1057–60. doi:10.1183/09031936.05.00134904.
- Maher CA, Williams MT, Olds TS, The six-minute walk test for children with cerebral palsy. Int J Rehabil Res. 2008;31(2):185–88. doi:10.1097/MRR.0b013e32830150f9.
- Kleiger RE, Stein PK, Bigger JT, Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. doi:10.1111/j.1542-474X.2005.10101.x.
- Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–81. doi:10.1093/oxfordjournals.eurheartj.a014868.
- Russell DJ, Rosenbaum P, Wright M, Avery LM. Gross motor function measure (GMFM-66 & GMFM-88) users manual. London: Mac Keith Press; 2002.
- Linder-Lucht M, Othmer V, Walther M, Vry J, Michaelis U, Stein S, Weissenmayer H, Korinthenberg R, Mall V. Validation of the gross motor function measure for use in children and adolescents with traumatic brain injuries. Pediatrics. 2007;120(4):e880–e886. doi:10.1542/peds.2006-2258.
- McCauley SR, Wilde EA, Anderson VA, Bedell G, Beers SR, Campbell TF, Chapman SB, Ewing-Cobbs L, Gerring JP, Gioia GA, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012;29(4):678–705. doi:10.1089/neu.2011.1838.
- Kelly G, Mobbs S, Pritkin JN, Mayston M, Mather M, Rosenbaum P, Forsyth R, Forsyth R, Gross motor function measure‐66 trajectories in children recovering after severe acquired brain injury. Dev Med Child Neurol. 2015;57(3):241–47. doi:10.1111/dmcn.12592.
- Ryan JL, Zhou C, Levac DE, Fehlings DL, Beal DS, Hung R, Wright FV, Gross motor change after inpatient rehabilitation for children with acquired brain injury: a 10‐year retrospective review. Dev Med Child Neurol. 2022. doi:10.1111/dmcn.15471.
- Deutsch A, Braun S, Granger C, The functional Independence measure (FIMSM Instrument) and the functional Independence measure for children (WeeFIM® Instrument): ten years of development. Crit Rev Phys Rehabil Med. 1996;8(4):267–81.
- Acharya UR, Kannathal N, Hua LM, Yi LM, Study of heart rate variability signals at sitting and lying postures. J Bodyw Mov Ther. 2005;9(2):134–41. doi:10.1016/j.jbmt.2004.04.001.
- Dancey CP, Reidy J. Statistics without maths for psychology. Harlow, United Kingdom: Pearson Education; 2007.
- Zima BT, Gay JC, Rodean J, Doupnik SK, Rockhill C, Davidson A, Hall M, Classification system for international classification of diseases, ninth revision, clinical modification and tenth revision pediatric mental health disorders. JAMA Pediatr. 2020;174(6):620–22. doi:10.1001/jamapediatrics.2020.0037.
- Hilz MJ, Wang R, Markus J, Ammon F, Hösl KM, Flanagan SR, Winder K, Koehn J. Severity of traumatic brain injury correlates with long-term cardiovascular autonomic dysfunction. J Neurol. 2017;264(9):1956–67. doi:10.1007/s00415-017-8581-1.
- Khalid F, Yang GL, McGuire JL, Robson MJ, Foreman B, Ngwenya LB, Lorenz JN. Autonomic dysfunction following traumatic brain injury: translational insights. Neurosurg Focus. 2019;47(5):E8. doi:10.3171/2019.8.FOCUS19517.
- Keren O, Yupatov S, Radai MM, Elad-Yarum R, Faraggi D, Abboud S, Ring H, Groswasser Z. Heart rate variability (HRV) of patients with traumatic brain injury (TBI) during the post-insult sub-acute period. Brain Inj. 2005;19(8):605–11. doi:10.1080/02699050400024946.
- Bellenger CR, Fuller JT, Thomson RL, Davison K, Robertson EY, Buckley JD, Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta-analysis. Sports Med. 2016;46(10):1461–86. doi:10.1007/s40279-016-0484-2.
- Albinet CT, Boucard G, Bouquet CA, Audiffren M, Increased heart rate variability and executive performance after aerobic training in the elderly. Eur J Appl Physiol. 2010;109(4):617–24. doi:10.1007/s00421-010-1393-y.
- Pearson MJ, Smart NA, Exercise therapy and autonomic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2018;23(1):91–108. doi:10.1007/s10741-017-9662-z.
- Picard M, Tauveron I, Magdasy S, Benichou T, Bagheri R, Ugbolue UC, Navel V, Dutheil F. effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0251863. doi:10.1371/journal.pone.0251863.
- Beretta E, Cimolin V, Piccinini L, Carla Turconi A, Galbiati S, Crivellini M, Strazzer S, Strazzer S, Assessment of gait recovery in children after traumatic brain injury. Brain Injury. 2009;23(9):751–59. doi:10.1080/02699050903133988.
- Hasen M, Almojuela A, Zeiler FA, Autonomic dysfunction and associations with functional and neurophysiological outcome in moderate/severe traumatic brain injury: a scoping review. J Neurotrauma. 2019;36(10):1491–504. doi:10.1089/neu.2018.6073.
- Dahl HM, Andelic N, Løvstad M, Holthe IL, Hestnes M, Diseth TH, Myhre MC, Epidemiology of traumatic brain injury in children 15 years and younger in South-Eastern Norway in 2015–16. Implications for prevention and follow-up needs. Eur J Pediatr Neurol. 2021;31:70–77.doi:10.1016/j.ejpn.2021.02.002.