ABSTRACT
Objectives
Examine quality of life (QoL) and psychological health after mild traumatic brain injury (mTBI) in older people (65+ years) at 3- and 6-month follow-up and explore which injury factors predicted QoL.
Methods
mTBI patients were compared to trauma comparison (TC) and community comparison (CC) groups. QoL and psychological health were measured at both timepoints. After accounting for 3-month psychological health, injury severity, neuroimaging, and 3-month neuropsychological performance were assessed as predictors of 6-month QoL.
Results
Overall 3-month QoL was lower for mTBI (Cohen’s d = 0.938) and TC (Cohen’s d = 0.485) groups compared to CCs, but by 6 months only mTBI patients continued to report poorer overall QoL (Cohen’s d = 0.577) and physical QoL (Cohen’s d = 0.656). Despite group differences, QoL for most (~92%) was within normative limits. 3-month psychological health predicted QoL 6-months postinjury (β = -.377, 95% CI −.614, −.140) but other proposed risk factors (GCS <15, neuroimaging, 3-month neuropsychological performance) did not uniquely predict QoL.
Conclusions
Older adults following mTBI reported lower QoL up to 6-months postinjury compared to non-injured peers, indicating that mTBI patients were particularly susceptible to ongoing differences in QoL 6-months postinjury.
Globally, communities are aging, and it is estimated that two billion people will be 60 years or older by 2050 (Citation1). Public health policies promote the positive impact of active lifestyles in maintaining health and life satisfaction in older age (Citation2,Citation3). However, more older people are presenting at Emergency Departments as a result of falling (Citation4,Citation5). In addition to a range of possible orthopedic injuries, a common consequence is traumatic brain injury (TBI) (Citation4). Outcome after TBI in older people has often been measured in terms of mortality after severe injury (Citation6). More recently there has been a greater focus on understanding changes in functioning after milder injury, as older people seek to return to their previous lifestyles. Quality of life (QoL) is a postinjury outcome that is important to inform patient-centered care and provide appropriate rehabilitation services, if needed. QoL refers to an individual’s perception of their position in life in the context of their personal and cultural expectations, standards, and goals (Citation7). Despite the increase in older people presenting with injury after falling, there is limited information about QoL after mild TBI (mTBI) and injury factors that may adversely impact outcome (Citation8).
Quality of life and risk factors
In a previous preliminary study from our research group, it was noted that in an older age group physical QoL at 3-month post-mTBI remained lower than non-injured peers, whereas psychological QoL was less impacted (Citation9). In terms of recovery, a further research group using a single cohort study reported that QoL significantly improved for older adult patients between discharge from hospital and 6-months postinjury (Citation10). More recent evidence, however, suggests that 41% of older adults still report poor physical QoL 6 months after mTBI and 22% also report poor psychological QoL, indicating that a subset of older people may continue to have poor QoL up to 6 months postinjury (Citation11). We hypothesized that further investigation, using appropriate comparison groups, will allow for a greater understanding of the specific impact of mTBI on QoL in older age.
Another challenge is to identify possible risk factors associated with suboptimal outcome for those reporting lower QoL after injury. After mixed severity TBI in older age, several premorbid risk factors for poorer QoL have been suggested, including lower education, and poorer psychological and general health prior to injury (Citation11). In adult cohorts, risk factors have been extended to include injury-related variables such as Glasgow Coma Scale (GCS) score and extracranial injury as possible predictors of QoL (Citation12,Citation13). For example, one study suggested that injury-related factors may be important for physical QoL whereas mental QoL was influenced by preinjury patient characteristics (e.g., preinjury mental health and education) (Citation12).
Additionally, recent evidence suggests that cognition may contribute to poorer QoL in older adults after TBI (Citation14–16). In one study, cognitive status of older women (54–76 years of age) was associated with poorer health-related QoL 10 years after a TBI of any severity (Citation15,Citation16). However, findings were based on cross-sectional analysis, suggesting that found differences after an intervening decade could also be attributed to non-injury factors. Nevertheless, a further study of older adults found that after TBI, cognition was associated specifically with psychological QoL but not physical QoL or social participation (Citation14). Given these studies were based on mixed-severity samples, it is important to establish whether these relationships persist in a mTBI sample.
Psychological health after mTBI
After mTBI more specifically, research in adult cohorts has focused predominantly on the presence of persistent injury symptoms and post-traumatic distress, and the impact these may have on QoL (Citation17,Citation18). In this respect, older adults consistently report lower psychological distress than younger adults after mTBI, regardless of time since injury (Citation8,Citation19). Additionally, fewer older adults are diagnosed with major depressive disorder after mTBI, despite a higher history of depression and family history of mood disorder (Citation20). Rates of depression at discharge from hospital after mTBI and 6 months later have also been reported as similar, suggesting that older people are unlikely to develop late onset depression as a result of mTBI (Citation21). Nevertheless, persistent concussive symptoms (e.g., fatigue and difficulty concentrating) and the presence of psychological distress are commonly associated with lower QoL up to 6 months after mTBI across the lifespan (Citation17,Citation18,Citation22). This suggests that although older people generally report stable psychological health after injury, the presence of high levels of psychological distress, if reported, is likely to have an ongoing impact on QoL after mTBI.
Current aims
The primary aim of this study was to examine QoL and psychological health for older people 3- and 6-months after mTBI, as compared to a trauma comparison group and a non-injured community group. We expected that the mTBI group would report lower QoL compared to the trauma comparison and non-injured groups at 3-months postinjury but that differences would reduce by 6-months postinjury in line with recovery from injury. We also expected that psychological health would be similar across the three groups and relatively stable across time-points. The second aim was to explore whether injury-related factors (i.e., GCS score < 15, presence of abnormal neuroimaging, and 3-month neuropsychological performance) were associated with overall 6-month QoL, after accounting for the expected association with 3-month psychological health.
Methods
This study was part of a larger prospective study investigating outcome for older adults following traumatic injury, some of which has been previously reported (Citation23,Citation24). This project was approved by the Alfred Health Ethics Committee and La Trobe University Ethics Committee (project ID 382/15). Included data were obtained in compliance with the Helsinki Declaration and institutional/national research standards for human research and informed consent was obtained from all participants.
Participants
All participants were aged ≥65 years and were fluent in English. Participants were also functionally independent (prior to injury) and resided within three hours of The Alfred Hospital, Melbourne Australia. Reasons for exclusion included a diagnosed life-threatening illness requiring treatment (e.g., current treatment for cancer), serious psychiatric condition (i.e., chronic schizophrenia or current depressive episode), a diagnosed cognitive condition (i.e., Alzheimer’s disease), or current reported alcohol misuse at the time of presentation to the emergency department (or at the time of assessment for non-injured older people). Other comorbid health conditions that commonly present in older age (e.g., high cholesterol and/or blood pressure stabilized and managed with medications, osteoarthritis, etc.) or previous conditions that were detailed as currently stable (e.g., prostate or breast cancer in long-term remission, joint replacement, etc.) were not considered to be severe health conditions and therefore were not part of the exclusion criteria for the current study.
Patients with acute injury were recruited from The Alfred Trauma Centre in Melbourne, Australia, and were allocated into one of two sub-groups: (i) mTBI, or (ii) trauma comparison (TC) group. A non-injured age-matched comparison group of community-dwelling older adults (labeled community comparison; CC) was also recruited, using researcher networks and local advertising. All eligible participants were contacted via post and those interested completed a telephone interview. This included a cognitive screening questionnaire (TELE, cutoff >17) (Citation25).
Consecutive trauma patients who presented to The Alfred Emergency & Trauma Centre between January 2016 and March 2019 were screened for eligibility using accessible medical records. Patients with mild TBI were identified using current criteria, which defines mTBI as blunt head trauma resulting in one or more of the following symptoms: (i) disorientation, (ii) loss of consciousness (LOC) ≤30 minutes, (iii) post-traumatic amnesia (PTA) ≤24 hours, and/or (iv) other transient neurological abnormalities (e.g., intracranial lesion not requiring surgery). Additionally, a Glasgow Coma Scale (GCS) score between 13 and 15 was recorded by 30 minutes postinjury or upon presentation to health services for treatment (Citation26,Citation27). Symptoms were not related to alcohol consumption, medications, drug use and were not caused by other injuries, preexisting medical conditions or penetrating craniocerebral injury (Citation26) based on available medical records.
Trauma comparison patients were those who sustained a mild orthopedic injury but without head injury. This was identified as: (i) abbreviated injury scale (AIS) score ≤ 3 in any body region (except the head region where AIS score was 0), (ii) GCS of 15, and (iii) no reported confusion around the incident. AIS scores were converted to a total Injury Severity Score (ISS) (Citation28). A peripheral ISS was also calculated to document extracranial injury (excluding the head region) for both trauma groups. Assessment and questionnaires were completed at 3 and 6 months after injury for the trauma groups, and questionnaires were completed 3 months apart for the non-injured CC group.
Measures
World Health Organisation Quality of Life Scale – shortened version (WHOQOL-BREF)
The WHOQOL-BREF assessed participants’ self-reported QoL in the preceding four weeks and was collected at both timepoints. The WHOQOL-BREF has adequate internal consistency and comparable discriminant validity to the original WHOQOL-100, discriminating well between ill and healthy subjects within all domains (Citation29). Participants rated their level of agreement for each item using a 5-point Likert scale (1 = strongly disagree/not at all/very dissatisfied; 5 = strongly agree/an extreme amount/extremely satisfied). Items were combined to calculate four domains of life quality: physical, psychological, social, and environmental. Domain scores were transformed into a scaled score between 0 and 100 (see WHOQOL manual for details) (Citation30). Scores were then converted to standardized z-scores, where positive z-scores indicated better QoL for each domain (i.e., positive outcome).
To calculate an overall QoL score, raw ordinal scores for items 3–26 were summed and converted to an interval score (range 24–120) (Citation31). This summary score was then transformed into a z-score for each timepoint, where positive scores indicated better overall QoL. Normative z-scores for each QoL domain were also calculated using Australian-based norms (Citation32), and then dichotomized based on scores above and below a z-score of −1.5 for each participant.
Psychological health (Depression, anxiety and stress scale; DASS-21)
The Depression Anxiety Stress Scale (21-item version; DASS-21) was used to measure current levels of psychological health at both timepoints. The DASS-21 has good psychometric properties, showing high internal consistency (α = .93) and adequate construct validity (r = .74 to .77) (Citation33,Citation34). Participants rated their severity of depressive, anxiety, and stress symptoms in the previous seven days using a 5-point Likert scale (0 = never; 4 = almost always). Scores were collated into three subscales: Depression, Anxiety, and Stress. A total score was also calculated to create an overall Psychological Health summary scale. Scores were converted to standardized z-scores, where positive z-scores indicated higher levels of psychological distress (i.e., poorer psychological health). Scores on each subscale were also dichotomized based on clinical cutoffs, whereby participants who endorsed moderate or above levels of depression, anxiety and/or stress symptoms were characterized as experiencing ‘psychological distress’ compared to those with normal or mild symptom severity.
3-month neuropsychological performance
We administered neuropsychological tests to measure cognitive changes after mTBI, using tasks involving processing speed, executive function, and memory (Citation35). These included the Hopkins Verbal Learning Test (Citation36), Trail Making Test (Citation37), Colour-Word Interference Task (Citation38), and WAIS-IV Coding subtest (Citation39) and are described in detail elsewhere (Citation23,Citation40). Z-scores from each test were averaged to calculate a standardized composite Z-score to reflect overall neuropsychological performance (Citation41). Positive z-scores indicated better neuropsychological performance.
Statistical analyses
First, Little MCAR’s analysis demonstrated that the data were missing at random, χ2 = 9770.867, df = 49695, p = 1.000, and we conducted multiple imputation analysis that used proxies (i.e., similar variables across timepoints) to predict and replace the missing data on both the independent and dependent variables (Citation42) using five imputed datasets. The average score for each datapoint from across the five datasets was calculated and included in the final dataset, resulting in 8% of the total dataset being imputed.
Variables were checked for normality, and non-normally distributed variables were transformed using square-root transformation. This included all three DASS-21 subscales and the psychological health summary scores for 3- and 6-month outcome, the physical and environmental subscales of the WHOQOL-BREF at both time-points, and the psychological QoL subscales for 3-month follow-up only. The calculated overall QoL summary score was also skewed and was normalized using Log10 transformation for 3-month data and square-root transformation for 6-month data. All variables were converted to standardized z-scores for ease of interpretation (see Measures section for details).
We used linear mixed modeling (LMM) to examine differences between groups across the two time-points, while accounting for within-subject correlations. LMM was chosen as the preferred statistical analysis because LMM can account for correlations between repeated measurements on the same subjects and has greater flexibility to measure longitudinal time effects (Citation43). We constructed LMMs for overall QoL, calculated using the WHOQOL-BREF scores, as well as for each of the WHOQOL-BREF subscales (i.e., physical, psychological, social and environmental QoL) and the DASS-21 psychological health summary score. The model for each outcome used group, time and a group (mTBI, TC, CC) x time (3-month and 6-month) interaction for fixed effects, with subject random effects adjustment. Significant interaction or main effects were followed by six planned contrasts with Holm’s adjustment for multiple testing (mTBI to TC, mTBI to CC, and TC to CC at both 3- and 6-month timepoints).
Chi-square statistics were used to examine group differences in the number of participants who endorsed significantly lower QoL across the four domains of the WHOQOL-BREF and greater severity of psychological symptoms (i.e., depression, anxiety, stress, and overall distress) compared to normative data.
In line with our second aim, we first examined correlation coefficients for relevant variables to determine the association between QoL and potential independent variables for the mTBI group only. Next, we conducted hierarchical regressions to determine whether injury severity characteristics predicted QoL after mTBI. The first step was to enter age, education, and gender using stepwise regression to control for these potentially confounding factors. Only variables that significantly contributed to the variance were kept in the model. At step 2, 3-month psychological health, GCS (dichotomized as GCS = 15 or GCS < 15), presence of abnormal neuroimaging, and 3-month neuropsychological performance were added to the final model.
LMM analyses were conducted using JASP v15.0 software. All other analyses were conducted using SPSS Version 27 software packaging (Citation44). A p-value of < .05 was defined to be statistically significant.
Results
Demographics
One-hundred and 42 trauma patients agreed to participate in the study. Twenty-one participants were excluded due to incomplete demographic information (n = 7), neurosurgery after injury (n = 2), injury severity considered greater than ‘mild’ (n = 10), or current alcohol misuse based on medical records (n = 2). Fifteen participants later withdrew, leaving a total of 106 trauma patients for analysis. Patients were assigned to either the mTBI group (n = 40) or trauma comparison group (TC; n = 66) based on injury characteristics. A community comparison group consisted of non-injured participants (CC; n = 47).
The three groups (i.e., mTBI, TC and CC) did not significantly differ on gender, age, years of education, or number of comorbidities (). As expected, trauma groups did differ on total ISS score (p < 0.001) but not on peripheral ISS score, indicating that mTBI patients had similar severity of non-head injury compared to the TC group. There were also no group differences for mechanism of injury, with approximately 75% of all trauma patients sustaining injuries due to falling. However, both trauma groups had lower cognitive performance 3-months after injury, as compared to the CC group ().
Table 1. Demographic and injury information for mTBI, trauma comparison (TC) and non-injured (CC) groups.
Quality of life at 3- and 6-month follow up
Estimated marginal means for the three groups on overall QoL and the WHOQOL-BREF domains are presented in .
Table 2. Z-score estimated marginal means (standard deviations) for mTBI, TC and CC groups on QoL and psychological health.
LMM analysis examined overall 3- and 6-month QoL and did not identify a group x time interaction effect, F (Citation2)150) = 2.483, p = 0.087, indicating that groups did not differ in their change across timepoints on overall QoL (See ).
Figure 1. Z-scores for physical and psychological quality of life at 3- and 6-month follow up for the mTBI, TC and CC groups.
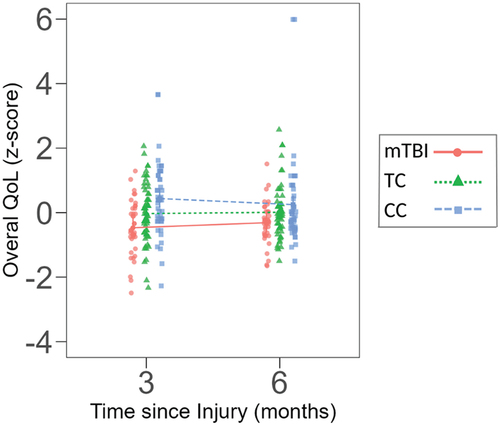
Results, however, demonstrated a significant main effect for group, F (Citation2)150) = 7.311, p = <.001, but not time, F (Citation1)150) = 0.002, p = 0.963. Planned contrasts demonstrated that at 3-months postinjury the mTBI (p = < .001) and TC (p = 0.046) groups reported lower overall QoL than the CC group, however, differences between the two trauma groups were non-significant (p = 0.069). By 6 months postinjury the mTBI group continued to report lower QoL than the CC group (p = 0.035), but the TC group did not differ from either the mTBI group (p = 0.190) or CC group (p = 0.197).
LMM analysis for the specific QoL domains was also conducted. For physical QoL there was no group x time interaction, F (Citation2)150) = 0.640, p = 0.529. However, the mean score at 3- and 6-month timepoints differed between groups, F (Citation2)150) = 6.274, p = 0.002. Planned contrasts between each group indicated that the mTBI group reported lower physical QoL compared to the CC group at both 3 and 6 months (p = 0.002 and p = 0.012) but did not differ from the TC group (p = 0.176 and p = 0.176).
For psychological QoL, there was no group x time interaction effect, F (Citation2)150) = 1.590, p = 0.207. Again, we found a main effect for group, F (Citation2)150) = 3.086, p = 0.049, but not time, F (Citation1)150) = 0.010, p = 0.919. Planned contrasts indicated that the mTBI group reported lower psychological QoL compared to the CC group at 3-months (p = 0.025) but not 6-months postinjury (p = 0.429). Additionally, the TC group did not differ at either timepoint from the mTBI group (p = 0.606 and p = 0.710) or CC group (p = 0.160 and p = 0.462).
The results demonstrated non-significant group x time interaction effects for social QoL, F (Citation2)150) = 2.301, p = 0.104, and environmental QoL, F (Citation2)150) = 0.023, p = 0.978. Additionally, there were no significant main effects for social QoL between groups, F (Citation2)150) = 2.137, p = 0.122, or timepoints, F (Citation1)150) = 0.039, p = 0.844. There were also no significant main effects for environmental QoL between groups, F (Citation2)150) = 1.843, p = 0.162 or timepoints, F (Citation1)150) = 0.001, p = 0.996. This indicates that reported social and environmental QoL were similar between groups and did not change over time.
When scores on the four WHOQOL-BREF domains were compared to age- and gender-matched norms, the number of older adults who reported QoL significantly below normative levels (i.e., −1.5SDs below the mean) did not differ between groups for any domain and were less than 8% in any domain at both 3- and 6-month timepoints ().
Table 3. Frequency (%) of mTBI, trauma comparison and non-injured participants reporting poor quality of life and psychological health.
Psychological health at 3- and 6-month follow-up
For overall psychological health, LMM demonstrated no significant interaction effect for group x time, F (Citation2)150) = 0.619, p = 0.540. Additionally, there were no significant main effects for group, F (Citation2)150) = 1.829, p = 0.164, or time, F (Citation1)150) = 0.015, p = 0.901, indicating that older adults reported similar psychological health across time, regardless of injury status ().
Additionally, the number of older adults who endorsed moderate or higher levels of depression, anxiety and/or stress did not differ between groups and were generally low (less than 8%) at both 3- and 6-month follow-up ().
Predictors of quality of life 6 months after mTBI
First, Pearson’s correlations were analyzed using only mTBI patients and demonstrated significant associations between QoL, 3-month psychological health and 3-month neuropsychological performance (see supplementary Table S1). Next, a hierarchical regression was conducted to examine the association between injury-related variables and overall QoL at 6-months postinjury, after controlling for potential demographic factors and 3-month psychological health. Hierarchical regression analysis for 3-month QoL is presented in supplementary table S2.
For overall 6-month QoL, at the first step, age (β = −.390, 95% CI [−.419, −.361]) was the only significant demographic factor that predicted QoL (). When 3-month psychological health and other injury factors were added to the model in step 2, 3-month psychological health was the only significant predictor of 6-month QoL (β = −.377, 95% CI [−.614, −.140]), whereas age became non-significant and injury variables and 3-month neuropsychological performance did not uniquely contribute to the model, R2 =.278, F (Citation5,Citation34) = 2.613, p = 0.042.
Table 4. Regression coefficients for associations of 6-month QoL for patients with mTBI.
Given the significantly higher total ISS for the mTBI group, post-hoc regression analysis was conducted also including this variable. Results indicated that total ISS did not uniquely contribute to the final model (β = −.241, 95% CI [−.263, −.219]) and thus did not change the overall findings of the regression analysis.
Discussion
This study of older people after mTBI examined changes in QoL and psychological health across two timepoints (3 and 6 months postinjury) compared to a trauma comparison (TC) and a non-injured (CC) comparison group.
The findings indicated that older adults with either mTBI or traumatic injury experienced lower overall QoL at 3-months postinjury compared to non-injured peers, and that mTBI patients were particularly susceptible to ongoing differences in QoL 6-months postinjury. Our findings are similar to previous research examining global mTBI recovery in adults compared to an orthopedic injury group (Citation45). This research showed that rates of day-to-day functional limitations were initially similar between the two trauma groups, however, by 12-months postinjury the mTBI group reported significantly greater limitations on day-to-day functioning, possibly suggesting that mTBI may slow recovery from traumatic injury. In support of these findings, our current study in older adults, which included an additional non-injured peer group, demonstrated that both mTBI and TC patients require longer than the three-month timeframe for recovery that is often cited for mTBI adult cohorts more generally (Citation35,Citation46), and that mTBI patients (but not TC patients) may continue to report lower overall QoL 6-months postinjury. Whether differences in QoL between mTBI, TC, and non-injured peers become more pronounced with further follow-up (i.e., 12-months postinjury) or whether mTBI patients can eventually achieve similar levels of QoL compared to non-injured peers remains unclear and requires further investigation in future follow-up studies.
Interestingly, most older adults (regardless of injury) reported a normative level of QoL at 3 and 6 months postinjury – less than 8% reported ‘poor’ QoL (identified as normative scores −1.5 SDs below the mean) at follow-up. At face value, the normative findings may suggest a generally positive outlook for older people after injury. However, when examining group differences in specific domains of QoL, patients with mTBI reported lower physical QoL (at 3 and 6 months) and lower psychological QoL (at 3 months only) compared to non-injured peers. Therefore, the results suggest there are subtle but ongoing issues related to head injury up to 6-months postinjury. These findings are in line with previous research that demonstrates that a proportion of older adults report poor physical and psychological QoL up to six-months after mixed-severity TBI (Citation11). Reasons for the ongoing effect of mTBI, specifically in physical QoL, may relate to the type of mTBI symptoms reported by older people after injury. In the current study, physical QoL incorporated several aspects of physical functioning, including sleep quality, energy, mobility, and pain management (Citation30), all of which may be particularly susceptible to change after head injury (Citation22). For example, older people have been reported to be more likely than younger adults to endorse physical symptoms after mTBI, including fatigue, noise sensitivity and balance issues postinjury (Citation47).
From a psychological perspective, psychological QoL was lower for patients with mTBI compared to non-injured peers at 3-months postinjury but had improved by 6 months, indicating a possible trajectory of recovery. Likewise, older patients with mTBI reported similar psychological health (DASS 21) compared to non-injured peers and there was no delayed onset of psychological symptoms up to 6-months postinjury. Only a small number of older people (with and without injury) endorsed depressive, anxiety, or distress symptoms in the moderate or above clinical ranges (~3–7%). This is consistent with previously reported prevalence rates of depression and anxiety in older community-dwelling people (Citation48,Citation49). Some explanations for these findings may be that older people experience and report symptoms of psychological distress differently from younger cohorts; for example, and as noted above, a tendency post-trauma to focus on somatic symptoms rather than affective symptoms (Citation50,Citation51).
Notwithstanding the comments above about psychological health and recovery post-trauma, psychological health reported at 3-months postinjury did emerge as a predictor of overall QoL 6-months postinjury. This relationship is consistent with other studies investigating QoL in both young-middle aged mTBI cohorts and non-injured community-dwelling older age cohorts (Citation18,Citation52). In contrast, once 3-month psychological health was accounted for, demographic characteristics, injury factors (i.e., GCS < 15, presence of neuroimaging), and 3-month neuropsychological performance had minimal additional effect on overall QoL after mTBI. Injury factors like GCS score and presence of abnormal neuroimaging (i.e., complicated mTBI) have previously been identified as important predictors of TBI recovery in adult cohorts (Citation12,Citation13), however, there is also evidence that these initial indicators of severity are only weakly associated with longer-term QoL, and that premorbid health factors may emerge as more important (Citation53,Citation54). In this respect, a recent study examined QoL after mTBI in adults with persisting symptoms of concussion and identified that current levels of depression, anxiety and fatigue strongly predicted QoL, whereas demographic and injury-related factors did not significantly predict QoL (Citation55). Possible explanations for the negligible influence of GCS and abnormal neuroimaging findings in the current cohort of older adults may relate to the fact that we examined only older people who sustained a mild TBI (rather than mixed severity TBI or patients requiring neurosurgery) and participants in the current study were also considered to be generally healthy (i.e., without severe health problems and living independently) prior to injury. Therefore, it is possible that indices of severity may influence outcome if large enough patient samples/databases are available to undertake moderation/mediation analyses to provide a more complex analysis for how injury variables interact in determining outcome in older age. It will also be important in future controlled studies of more representative samples of older people to capture a range of pre- and postinjury variables to determine which factors (or interaction of factors) have the greatest predictive power for QoL after mTBI in older age cohorts.
Of specific note, although cognition has previously been associated with health-related QoL and psychological QoL after TBI (Citation14,Citation16), our findings suggest that after accounting for psychological distress, 3-month neuropsychological performance also has little effect on current or future QoL. Nevertheless, preliminary correlational analyses in the current study indicated a negative association between 3-month psychological health and 3-month neuropsychological performance for older age patients with mTBI. Therefore, further investigation is required to investigate the possibility that the association between 3-month neuropsychological performance on 6-month QoL may be mediated by 3-month psychological distress after injury. Further support for this position is provided by a recent study which identified that psychological support (i.e., cognitive-behavior therapy) may be 5–6 times more effective at reducing persisting symptoms after mTBI in adults compared to cognitive rehabilitation alone (Citation56). More generally, the findings from the current study highlight the continued importance of promoting good psychological health after mTBI to potentially improve QoL after injury.
Methodological issues
The design of our study needs to be considered when interpreting the findings. Firstly, differences in total ISS were expected as, by definition, the TC group did not have any injury to the head region thus making the total possible ISS lower for this group. Although we cannot rule out the possibility that total ISS may account for some differences identified for the mTBI group, post-hoc regression analysis indicated that total ISS did not significantly contribute to overall QoL after mTBI. Minimising differences in total ISS when using a trauma comparison group is an ongoing challenge in mTBI research more broadly and is an important consideration for future recruitment of mTBI cohorts.
As mentioned above, our sample consisted of older people who were independent and considered to be generally healthy prior to injury, to control for preinjury health factors that could confound the outcome. We excluded people based on several health-related conditions such as cognitive impairment (i.e., Alzheimer’s disease), serious psychiatric conditions, and functional dependence prior to injury. However, many older adults who present at emergency departments have these premorbid health conditions (Citation57), and recent evidence suggests that severe systemic disease and frailty may negatively impact functional outcome after TBI in older people (Citation11,Citation58). Nevertheless, in this relatively small sample size study, we elected to investigate mTBI in older people by excluding, as far as possible, the range of confounding variables related to preinjury health status. We acknowledge that this places some limitations on the generalizability of our findings to routine clinical populations, but it also directs attention to the possibility of subtle ongoing differences in QoL after mTBI, even for those without significant additional preinjury risk-factors. The results provide baseline information about expected QoL for independent, generally healthy older people after mTBI. Future research could expand this to include those with significant comorbidities in order to increase our understanding of outcome in more vulnerable cohorts. For example, it is likely that various preinjury health factors (i.e., frailty prior to injury, sleep quality, and presence of pain) will interact with injury to negatively impact QoL after injury. Further investigation is important and if these factors, in addition to postinjury psychological health, prove to significantly impact QoL they can provide targets for treatment after mTBI.
Conclusion
In this cohort of older adults aged 65+ years, most older people reported QoL and psychological health within normative ranges, regardless of injury status (i.e., mTBI, TC or no injury). Although this may suggest a positive outlook after injury, there were significant differences between injured and non-injured groups, indicating that changes in QoL after injury requires consideration in managing post-trauma outcome. The findings demonstrate that mTBI and TC patients require longer than the usual three-month timeframe for recovery and that mTBI patients can continue to report poorer QoL up to 6-months postinjury compared to non-injured peers. 3-month psychological health was a significant predictor of overall QoL 6-months after mTBI, however, other injury severity characteristics and 3-month neuropsychological performance had little additional unique contributions to QoL after mTBI. This indicates that injury severity may have less influence on QoL than initially proposed but also suggests that promoting strategies for maintaining good psychological health in older adults after mTBI will positively impact overall QoL up to 6-months postinjury.
Acknowledgment
Thank you to the participants for their contribution to the study and also Dr Lei Gryffydd for assistance with recruitment and data collection. The authors report there are no competing interests to declare. Researcher C.H.H. was supported by an Australian Postgraduate Award (APA) Scholarship.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- United Nations D of E and SAPD. World population ageing 2017 - highlights. New York, NY: United Nations; 2017.
- Batsis JA, Daniel K, Eckstrom E, Goldlist K, Kusz H, Lane D, Loewenthal J, Coll PP, Friedman SM. Promoting healthy aging during COVID-19. J Am Geriatr Soc. 2021 Mar 1;69(3):572–80. doi:10.1111/jgs.17035.
- Rainero I, Summers MJ, Monter M, Bazzani M, Giannouli E, Aumayr G. The My Active and Healthy Aging ICT platform prevents quality of life decline in older adults: A randomised controlled study. Age Ageing. 2021 Jul 1;50(4):1261–67. doi:10.1093/ageing/afaa290.
- Heydari F, Golban M, Majidinejad S. Traumatic brain injury in older adults presenting to the emergency department: Epidemiology, outcomes and risk factors predicting the prognosis. Adv J Emerg Med. 2020;4(2):19. doi:10.22114/ajem.v0i0.170.
- Liew TYS, Ng JX, Jayne CHZ, Ragupathi T, Teo CKA, Yeo TT. Changing demographic profiles of patients with traumatic brain injury: An aging concern. Front Surg. 2019 Jul 3; 6;6. doi:10.3389/fsurg.2019.00037.
- McIntyre A, Mehta S, Aubut JA, Dijkers M, Teasell RW. Mortality among older adults after a traumatic brain injury: A meta-analysis. Brain Inj. 2013;27(1):31–40. doi:10.3109/02699052.2012.700086.
- Karimi M, Brazier BJ. Health, health-related quality of life, and quality of life: What is the difference? Pharmacoeconomics. 2016 Jul 1;34(7):645–49. doi:10.1007/s40273-016-0389-9.
- Hume CH, Wright BJ, Kinsella GJ. Systematic review and meta-analysis of outcome after mild traumatic brain injury in older people. J Int Neuropsychol Soc. 2021;28(7):736–55. doi:10.1017/S1355617721000795.
- Kinsella GJ, Olver J, Ong B, Gruen R, Hammersley E. Mild traumatic brain injury in older adults: Early cognitive outcome. J Int Neuro-psychol Soc. 2014;20(6):663–71. doi:10.1017/S1355617714000447.
- Kristman VL, Brison RJ, Bedard M, Reguly P, Chisholm S. Prognostic markers for poor recovery after mild traumatic brain injury in older adults: A pilot cohort study. J Head Trauma Rehabil. 2016 Nov 13;31(6):E33–43. doi:10.1097/HTR.0000000000000226.
- van der Vlegel M, Mikolić A, Hee QL, Kaplan ZLR, Retel Helmrich IRA, van Veen E, IRAR H, van Veen E, Andelic N, Steinbuechel NV, et al. Health care utilization and outcomes in older adults after Traumatic Brain Injury: a CENTER-TBI study. Injury [Internet]. 2022, May;53(8):2774–82. doi: 10.1016/j.injury.2022.05.009.
- Helmrich IRAR, van Klaveren D, Dijkland SA, Lingsma HF, Polinder S, Wilson L, von Steinbuechel N, van der Naalt J, Maas AIR, Steyerberg EW, et al. Development of prognostic models for health-related quality of life following traumatic brain injury. Qual Life Res. 2022 Feb 1;31(2):451–71. doi:10.1007/s11136-021-02932-z.
- Yousefzadeh-Chabok S, Kapourchali FR, Ramezani S. Determinants of long-term health-related quality of life in adult patients with mild traumatic brain injury. Eur J Trauma Emerg Surg. 2021 Jun 1;47(3):839–46. doi:10.1007/s00068-019-01252-9.
- Caron L, Ouellet MC, Hudon C, Predovan D, Sirois MJ, de Guise É, Lamontagne M-È, Émond M, Le Sage N, Beaulieu-Bonneau S, et al. Cognitive functioning following traumatic brain injury in older adults: associations with social participation and health-related quality of life. Brain Inj. 2022;36(9):1099–108. doi:10.1080/02699052.2022.2110284.
- Rauen K, Späni CB, Tartaglia MC, Ferretti MT, Reichelt L, Probst P, Schäpers B, Müller F, Jahn K, Plesnila N, et al. Quality of life after traumatic brain injury: a cross-sectional analysis uncovers age- and sex-related differences over the adult life span. Geroscience. 2021 Feb 1;43(1):263–78. doi:10.1007/s11357-020-00273-2.
- Rauen K, Reichelt L, Probst P, Schäpers B, Müller F, Jahn K, Plesnila N. Quality of life up to 10 years after traumatic brain injury: A cross-sectional analysis. Health Qual Life Outcomes. 2020;Jun 4 18(1). 10.1186/s12955-020-01391-3.
- Voormolen DC, Polinder S, von Steinbuechel N, Vos PE, Cnossen MC, Haagsma JA. The association between post-concussion symptoms and health-related quality of life in patients with mild traumatic brain injury. Injury. 2019 May 1;50(5):1068–74. doi:10.1016/j.injury.2018.12.002.
- Haagsma JA, Scholten AC, TMJC A, Vos PE, van Beeck EF, Polinder S, Van Beeck EF. Impact of Depression and Post-Traumatic Stress Disorder on Functional Outcome and Health-Related Quality of Life of Patients with Mild Traumatic Brain Injury. J Neurotrauma. 2015 Jun 1;32(11):853–62. doi:10.1089/neu.2013.3283.
- Richey LN, Rao V, Roy D, Narapareddy BR, Wigh S, Bechtold KT, Sair HI, Van Meter TE, Falk H, Leoutsakos J-M, et al. Age differences in outcome after mild traumatic brain injury: results from the HeadSMART study. Int Rev Psychiatry [Internet]. 2020;32(1):22–30. Available from. doi:10.1080/09540261.2019.1657076.
- Rapoport MJ, Kiss A, Feinstein A. The impact of major depression on outcome following mild-to-moderate traumatic brain injury in older adults. J Affect Disord. 2006;92(2–3):273–76. doi:10.1016/j.jad.2005.05.022.
- Asselstine J, Kristman VL, Armstrong JJ, Dewan N. 2020. The rivermead post-concussion questionnaire score is associated with disability and self-reported recovery six months after mild traumatic brain injury in older adults. Brain Inj [Internet]. 34(2):195–202. Available from. doi: 10.1080/02699052.2019.1682670
- Chung JW, Liu D, Wei L, Wen YT, Lin HY, Chen HC, Chiu H-Y. Postconcussion symptoms after an uncomplicated mild traumatic brain injury in older adults: Frequency, risk factors, and impact on quality of life. J Head Trauma Rehabil [Internet] 2021;37(5):278–84. doi:10.1097/HTR.0000000000000733.
- Hume CH, Mitra B, Wright BJ, Kinsella GJ. Cognitive performance in older people after mild traumatic brain injury: Trauma effects and other risk factors. J Int Neuropsychol Soc. 2022;29(7):651–61. doi:10.1017/S1355617722000674.
- Gryffydd L, Mitra B, Wright BJ, Kinsella GJ. 2021. Cognitive performance in older adults at three months following mild traumatic brain injury. J Clin Exp Neuropsychol [Internet]. 43(5):481–96. Available from. doi: 10.1080/13803395.2021.1933915
- Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. Int Psychogeriatr. 1995;7(3):429–38. doi:10.1017/S1041610295002171.
- Lefevre-Dognin C, Cogné M, Perdrieau V, Granger A, Heslot C, Azouvi P. Definition and epidemiology of mild traumatic brain injury. Neurochir [Internet]. 2021;67(3):218–21. Available from. doi:10.1016/j.neuchi.2020.02.002.
- Kristman VL, Borg J, Godbolt AK, Salmi LR, Cancelliere C, Carroll LJ, Holm LW, Nygren-de Boussard C, Hartvigsen J, Abara U, et al. Methodological issues and research recommendations for prognosis after mild traumatic brain injury: Results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. 2014;95(3):S265–S77. doi:10.1016/j.apmr.2013.04.026.
- Copes WS, Champion HR, Sacco WJ, Lawnick MM, Keast SL, Bain LW. The injury severity score revisited. J Trauma. 1988;28(1):69–77. doi:10.1097/00005373-198801000-00010.
- Harper A, Power M, Group WHOQOL. 2013. Development of the World Health Organization WHOQOL-BREF quality of development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 28(3):551–58. September 2000. doi: 10.1017/S0033291798006667
- World Health Organisation Group. World Health Organization Quality of Life (WHOQOL) User Manual. Geneva, Switzerland: World Health Organisation; 2012.
- Balalla SK, Medvedev ON, Siegert RJ, Krägeloh CU. Validation of the WHOQOL-BREF and shorter versions using rasch analysis in traumatic brain injury and orthopedic populations. Arch Phys Med Rehabil. 2019 Oct 1;100(10):1853–62. doi:10.1016/j.apmr.2019.05.029.
- Hawthorne G, Herrman H, Murphy B. Interpreting the WHOQOL-Brèf: Preliminary population norms and effect sizes. Soc Indic Res. 2006 May;77(1):37–59. doi:10.1007/s11205-005-5552-1.
- Henry JD, Crawford JR. The short-form version of the depression anxiety stress scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Brit J Clin Psychol. 2005;44(2):227–39. doi:10.1348/014466505X29657.
- Crawford JR, Henry JD. The depression anxiey stress scales (DASS): Normative data and latent structure in a large non-clinical sample. Brit J Clin Psychol. 2003;42(2):111–31. doi:10.1348/014466503321903544.
- Carroll LJ, Cassidy JD, Cancelliere C, Côté P, Hincapié CA, Kristman VL, Holm LW, Borg J, Nygren-de Boussard C, Hartvigsen J, et al. Systematic review of the prognosis after mild traumatic brain injury in adults: Cognitive, psychiatric, and mortality outcomes: Results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. 2014;95(3):S152–S73. doi:10.1016/j.apmr.2013.08.300.
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test - Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. doi:10.1076/clin.12.1.43.1726.
- Reitan RM, Wolfson D. Category test and trail making test as measures of frontal lobe functioning. Clin Neuropsychol. 1995;9(1):50–56. doi:10.1080/13854049508402057.
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) [Database record]. Washington, DC: PsycTESTS; 2001.
- Weschler D. Weschler Adult Intelligence Scale - Fourth Edition (WAIS-IV) [Database record]. Sydney, Australia: Pearson Clinical Assessment; 2008.
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 5th ed. New York, NY: Oxford University Press; 2012.
- Silverberg ND, Crane PK, Dams-O’Connor K, Holdnack J, Ivins BJ, Lange RT, Manley GT, McCrea M, Iverson GL. Developing a cognition endpoint for traumatic brain injury clinical trials. J Neurotrauma. 2017;34(2):363–71. doi:10.1089/neu.2016.4443.
- Crossley TF, Levell P, Poupakis S. Regression with an imputed dependent variable. J of Appl Econ. 2022 Nov 1;37(7):1277–94. doi:10.1002/jae.2921.
- Muth C, Bales KL, Hinde K, Maninger N, Mendoza SP, Ferrer E. Alternative models for small samples in psychological research: applying linear mixed effects models and generalized estimating equations to repeated measures data. Educ Psychol Meas. 2016 Feb 1;76(1):64–87. doi:10.1177/0013164415580432.
- IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp; 2020.
- Nelson LD, Temkin NR, Dikmen S, Barber J, Giacino JT, Yuh E, Levin HS, McCrea MA, Stein MB, Mukherjee P, et al. Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study. JAMA Neurol. 2019;76(9):1049–59. doi:10.1001/jamaneurol.2019.1313.
- Karr JE, Areshenkoff CN, Garcia-Barrera MA. The neuropsychological outcomes of concussion: A systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology. 2014;28(3):321–36. doi:10.1037/neu0000037.
- Thompson HJ, Rivara FP, Wang J. Effect of age on longitudinal changes in symptoms, function, and outcome in the first year after mild-moderate traumatic brain injury. J Neurosci Nurs. 2020 Apr 1;52(2):46–52. doi:10.1097/JNN.0000000000000498.
- Crawford JR, Henry JD, Crombie C, Taylor EP Brief report normative data for the HADS from a large non-clinical sample.
- Pirkis J, Pfaff J, Williamson M, Tyson O, Stocks N, Goldney R, Draper B, Snowdon J, Lautenschlager N, Almeida OP, et al. The community prevalence of depression in older Australians. J Affect Disord. 2009 May;115(1–2):54–61. doi:10.1016/j.jad.2008.08.014.
- Wuthrich VM, Johnco CJ, Wetherell JL. Differences in anxiety and depression symptoms: Comparison between older and younger clinical samples. Int Psychogeriatr. 2015;27(9):1523–32. doi:10.1017/S1041610215000526.
- Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5(1):363–89. doi:10.1146/annurev.clinpsy.032408.153621.
- Chang YC, Yao G, Hu SC, der WJ. Depression affects the scores of all facets of the WHOQOL-BREF and may mediate the effects of physical disability among community-dwelling older adults. PLoS One. 2015 May 26;10(5):e0128356. doi:10.1371/journal.pone.0128356.
- Settervall CHC, Cardoso De Sousa RM, Helena C, Settervall C, Enferm AP. Escala de coma de Glasgow e qualidade de vida pós-trauma cranioencefálico. Acta Paulista de Enfermagem. 2012;25(3):364–70. doi:10.1590/S0103-21002012000300008.
- von Steinbüchel N, Wilson L, Gibbons H, Hawthorne G, Höfer S, Schmidt S, von Steinbüchel N, Bullinger M, Maas A, Neugebauer E, et al. Quality of life after brain injury (QOLIBRI): Scale validity and correlates of quality of life. J Neurotrauma. 2010 Jul 1;27(7):1157–65. doi:10.1089/neu.2009.1077.
- Popov N, Mercier LJ, King R, Fung T, Debert CT. Factors associated with quality of life in adults with persistent post-concussion symptoms. Can J Neurol Sci. 2022 Jan 26;49(1):109–17. doi:10.1017/cjn.2021.53.
- Vanderploeg RD, Belanger HG, Curtiss G, Bowles AO, Cooper DB. Reconceptualizing rehabilitation of individuals with chronic symptoms following mild traumatic brain injury. Rehabil Psychol. 2019 Feb 1;64(1):1–12. doi:10.1037/rep0000255.
- Karr JE, Iverson GL, Isokuortti H, Kataja A, Brander A, Öhman J. Preexisting conditions in older adults with mild traumatic brain injuries. Brain Inj. 2021;35(12–13):1607–15. doi:10.1080/02699052.2021.1976419.
- Abdulle AE, de Koning ME, van der Horn HJ, Scheenen ME, Roks G, Hageman G, Spikman JM, van der Naalt J. Early predictors for long-term functional outcome after mild traumatic brain injury in frail elderly patients. J Head Trauma Rehabil. 2018;33(6):E59–E67. doi:10.1097/HTR.0000000000000368.
