ABSTRACT
Objective
This study aims to explore the function of circRIMS in cerebral ischemia/reperfusion (CIR) and its regulatory mechanism.
Method
The expression of the circRIMS was examined in GEO chip data and validated by qRT-PCR analysis. A middle cerebral artery occlusion/repression (MCAO/R) model was developed using C57BL/6J mice. Starbase and circinteractome were employed to identify the target miRNA and mRNA. The result was confirmed by dual-luciferase reporter assay, and biotinylated RNA-pulldown assay. The cell viability and apoptosis were confirmed through CCK-8 and flow cytometry assay.
Results
This study revealed that circRIMS expression was upregulated in MCAO mice model and OGD/RX-simulated cell model. Knockdown circRIMS demonstrated the functional of circRIMS in increasing cell viability, reducing apoptosis, LDH activity and inflammatory factors secretion in OGD/RX-simulated CIR injury in vitro. Additionally, miR-96-5p was identified as a target of circRIMS, while the STAT1 gene is a downstream gene of miR-96-5p, and JAK was also considered to be a downstream gene of the JAK-STAT pathway. Furthermore, inhibition of miR-96-5p or overexpression of STAT1 promoted the progression of CIR injury by elevating apoptosis, reducing cell viability, and increasing the secretion of inflammatory cytokines.
Conclusion
CircRIMS contributes to the progression of CIR injury via regulating miR-96-5p/JAK/STAT1 axis.
Introduction
Cerebral ischaemia/reperfusion (CIR) injury occurs when blood supply is restored after transient cerebral tissue ischaemia but leads to failure in physiological recovery leads to severe brain damage (Citation1). It is associated with increased rates of disability, morbidity and mortality (Citation2); The combination of environmental factors and genes promotes its development; however, its pathogenesis is still uncertain. Globally, stroke is the leading cause of severe disability and mortality (Citation3,Citation4). Approximately 80–85% of strokes are the result of cerebral ischaemia, mainly triggered by embolic occlusion and primary cerebral aortic thromboembolism (Citation3,Citation5). Several studies have revealed that CIR injury typically involves a vast number of damaging mechanisms, such as oxidative stress and inflammation, which ultimately lead to necrosis, apoptosis, and acute autophagy in the ischemic cerebrum (Citation6–8). Currently, drug or mechanical reperfusion therapy is usually used as first-line care for acute ischemic stroke. Although clinical trials have shown that reperfusion can be achieved in most cases, ischaemia-reperfusion injury remains an important factor (Citation9–13). Therefore, it is important to study the molecular mechanism of CIR injury.
With the development of new-generation sequencing (NGS) technology, circular RNAs (circRNAs) have been proven to be an important regulatory RNAs type, with a loop structure and lacking 5’-3’ poly-adenylated tails (Citation14,Citation15). It is an internal non-coding RNAs (ncRNAs), single-stranded, closed loop with high stability, and between 100 b and 4 kb in size (Citation16). CircRNAs are abundant in the eukaryotic transcriptome and has stable specific expression in the tissue and developmental stages (Citation17). They act as sponges for microRNA (miRNA), which play an significant regulatory role in gene expression during development, while also serving as biomarkers and endogenous RNAs (Citation18). Previous research have revealed the carcinogenic role of circRIMS in gastric cancer (Citation19). Furthermore, circDLGAP has been shown to sponge miR-143, promote HECTD1 expression, enhance neurological damage initiated by ischemic stroke, and thereby reduce cerebral infarction (Citation20).
MicroRNAs (miRNAs) consist of about 20 nucleotides and are highly conserved small non-coding RNAs (ncRNAs) capable of controlling physiological functions, including synaptic formation and brain neurogenesis (Citation21,Citation22). Duan et al. (2019) reported upregulation of miR-96-5p in MCAO rats, particularly in infarcted areas. Furthermore, Xu et al. reported that upregulation of miR-96-5p inhibits T lymphocyte proliferation and viability by inhibiting HBEGF expression. MiRNAs and circRNAs play significant roles in CIR injury modulation (Citation23–26). Meanwhile, CircRIMS (circ -014,781) is highly expressed in an in vitro cerebral ischemia model, and knocking out circRIMS could effectively reduce apoptosis and inflammation of astrocytes. It has been found to target miR-96-5p adsorption and regulate STAT1 (signal transducer and transcription activator) expression and further inhibit apoptosis in gastric adenocarcinoma cells (Citation27) and renal cells (Citation28).
In this study, we investigated the roles and mechanisms of circRIMS, miR-96-5p, and JAK/STAT expression in CIR injury, and explored the possible mechanisms of named molecules in CIR-mediated catheterocytosis and inflammation. These data provide potential therapeutic targets and strong data support for future clinical treatment of CI/R injury.
Materials and methods
Middle cerebral artery occlusion/reperfusion mouse model
For CIR injury, mice (C57BL/6J) were exposed to MCAO (middle cerebral artery occlusion), as previously described (Citation29,Citation30). Briefly, mice were anesthetized by intraperitoneal administration of 4% chloral hydrate (Sigma-Aldrich, USA), then placed on an operating heating table and subsequently incubated for life sustainability. The internal carotid artery (left) was exposed, and a 4-0 nylon suture was placed and moved through the carotid bifurcation till the middle cerebral artery (MCA) origin was blocked. After 1 h of occlusion, reperfusion of mice was done by removing sutures from the vessels. Sham-operated mice were placed under the same procedure without MCAO.
Evaluation of neurological deficits
Neurological deficits were evaluated by a scientist blinded to the experimental design 24 h post-reperfusion as previously described (Citation31). The scored deficits were on a scale of 0–5 (0, no deficits; 1, difficulty in full contra-lateral forelimb extension; 2, inability of contra-lateral forelimb extension; 3, mild circling to contra-lateral side; 4, severe circling; and 5, falling to contra-lateral side), with a higher score denoting more severe damage of movement.
Cell culture and treatment
Primary astrocytes were removed from the C57L/6J mice cerebellum and cultured using Dulbecco Modified Eagle’s Medium (DMEM; Gibco, USA), enriched with Fetal bovine serum (FBS, 10%; Gibco, USA), 5% CO2 at 37 °C. TNF- α, IL-1β, and −6 levels were estimated with ELISA kits (Sangon Biotech, China). In accordance with the instructions, cells were transfected with Lipofectamine 3000 (Invitrogen, USA) with shRNA-NC, circRIMS, and miR-96-5p inhibitors.
Oxygen glucose deprivation-reperfusion (OGD/RX) was performed as described (Citation32). Briefly, in the absence of glucose and FBS, cells culture is performed in DMEM (Gibco, USA) for 48 h with pre-mixed gas (95% N2 and 5% CO2) in an incubator at 37 °C. Subsequently, cells were exposed to normal DMEM (Gibco, USA), FBS (10%, Gibco, USA), and placed in an incubator containing air (95%) and CO2 (5%). Simulated group cells were only cultured in normal DMEM (Gibco, USA) and FBS (fetal bovine serum, 10%; Gibco, USA).
Real-time quantitative polymerase chain reaction (qRT-PCR)
Total RNA isolation from astrocytes using Trizol reagent (Life Technologies Corporation, USA). Nanodrop spectrophotometer (ND-100, ThermoFisher, USA) was utilized to determine the concentration and quality of RNA at 280 nm. Subsequently, RNase R (ThermoFisher Scientific, USA) was employed to check the presence of circRIMS (circ - 014781) and removed the effect of linear RNAs. Primers of circRIMS, STAT1, miR-96-5p were acquired from Shanghai ShineGene (China). One-Step SYBR Prime-Script RT-PCR Kit (Takara Biomedical Technology, China) was used for evaluation of circRIMS, STAT1 and miR-96-5p; GADPH used as the internal control.
Western blot analysis
Total protein were extracted from cells using RIPA buffer (Promega, USA) and quantified using the Bicinchoninic Acid (BCA) Protein Quantification Kit (Bio-Rad, USA). At the same concentration, the proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, USA) and blocked with 5% milk. Incubation with primary antibodies against STAT1 (Abcam, USA) overnight at 4 °C with GADPH as an endogenous control. Subsequently, secondary antibody (Abcam, USA) was utilized for the incubation of the membrane for 1 h at room temperature and visualization with a chemiluminescence detection kit (Beyotime, China).
Lactate dehydrogenase (LDH) activity
Lactate dehydrogenase (LDH) activity was performed in cell culture medium using the LDH colorimetric kits (Jiancheng Biotechnology, China) in accordance with the producer’s manual.
Cell transfection
CircRIMS-specific short hairpin RNA (sh-circ-RIMS), negative control (sh-NC), miR-96-5p overexpression vector and inhibitor were obtained from Genepharma (China) and transfected using Lipofectamine 3000 (Invitrogen, China). Approximately oligonucleotides (50 nM) were mixed with Opti-MEM (50 μL; Invitrogen, USA). Similarly, of Lipofectamine 3000 (5 μL; Invitrogen, USA) were mixed with Opti-MEM (50 μL). Then, the two portions were mixed after 5 min. After 20 min of waiting, the transfection mixture along with the supernatant were added together; 6–8 h after transfection, the culture medium was substituted with a fresh complete medium.
Flow cytometry assay
Cell harvesting was performed post-transfection with trypsinization method and Annexin V-fluorescein isothiocyanate (FITC) and 7-amino-actinomycin D (7-AAD) stain in accordance with the Apoptosis Detection Kit (BD Biosciences, USA) instructions. Cell harvested from astrocytes were evaluated post-transfection using flow cytometry (FAC-Scan, BD Biosciences, USA) with Cell Quest 3.0 Software (BD Biosciences, USA).
Cell viability assay
Cell viability assay was achieved with cell counting kit-8 (CCK-8, Dojindo Molecular Technologies, China). In brief, post-transfection, 2 × 105 cells were inserted into a 96-well plate and then cultivated in an incubator consisting 5% CO2 for 0, 24, 48, and 72 h with the addition of CCK-8 solution (10 μL). Cultivation of cells was done at 37 °C for an additional 4 h and absorbance was determined at 450 nm using a microplate reader (Bio-Rad, USA).
Luciferase reporter gene assay
The luciferase reporter gene assays were achieved with the Dual-luciferase Reporter Assay System (Promega, USA). Transfection of cells was achieved using pmirGLO-circRIMS, and miR-96-5p inhibitors with riboFECT™ CP Transfection Kit (RiboBio, China) and luciferase activities assessment based on the Dual-luciferase Reporter Assay System (Promega, USA). Renilla luciferase activated was estimated as the control.
RNA immunoprecipitation (RIP) assay
The RIP assay was used to assess the binding of circRIMS to miR-96-5p. After lysis in RIP the cell extract was incubated with magnetic beads conjugated with anti‑Ago2 (1:2000; Abcam, USA) or normal anti-IgG (1:2000; Abcam, USA) at 4°C for 4 h. Finally, RNA was extracted from the beads followed by qRT-PCR to determine the relative enrichment of circRIMS or miR-96-5p.
Bioinformatics analysis
CircRIMS expression profiles of cells of three cases of MCAO and sham mice were attained from the Gene Expression Omnibus database (GEO) chip data with the accession number GSE115697. The Limma package R software (version 3.4.1) was utilized to visualize (heat map analysis) the differentially expressed circRNA between the cells.
Target gene prediction
Circ -014781 (circ-RIMS) prediction target miRNA (miR-96-5p) was done with circRNA interactome (https://circinteractome.nia.nih.gov/). StarBase v2.0 (http://starbase.sysu.edu.cn/) was employed for predicting the miRNAs target 3’UTR region of mRNA (STAT1).
Statistical analysis
Data were represented as mean ± SD, and GraphPad prism 7 was used for statistical analysis. To compare the two groups, an unpaired Student’s t-test was used, followed by one-way ANOVA for comparison among multiple groups. The values at p < 0.05 were taken to be statistically significant.
Results
CircRIMS is significantly upregulated in MACO mice model and OGD/RX-stimulated cerebral ischemia model in vitro
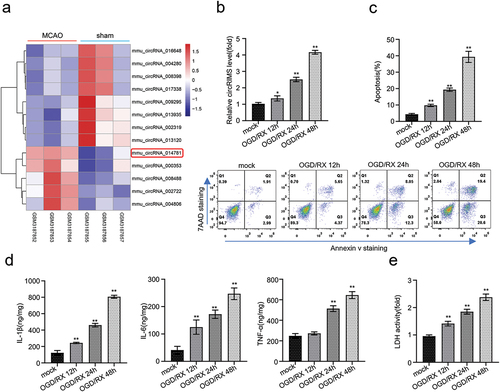
Heat map analysis was employed to reveal the differential expression of circRNAs in the GEO chip data (GSE115697) containing three cases of MCAO model and sham. The results showed that five circRNAs were upregulated and 8 were downregulated (p < 0.05, ). qRT-PCR showed a significant increase in the expression of circRIMS in OGD/RX-treated astrocytes in a time-dependent manner compared with the normal control group, with the highest expression observed after 48 h of OGD/RX treatment (p < 0.01, ). Furthermore, we conducted flow cytometry experiment to examine the apoptosis of astrocytes during different OGD/RX treatments. The outcome revealed a remarkable increase in apoptosis of the OGD/RX-treated astrocytes with respect to time, and the highest apoptosis was spotted after 48 h of treatment (p < 0.01, ). ELISA assay revealed that the expression of inflammatory factors in OGD/RX-treated astrocytes was remarkably elevated in a time-dependent manner (p < 0.01, ). Meanwhile, LDH analysis showed a significant increase in LDH release from OGD/RX-treated stellate cells in a time-dependent manner, indicating increased tissue damage in the presence of a severe underlying illness or disease (p < 0.01, ).
Figure 1. CircRIMS is significantly upregulated in MACO mice model and OGD/RX-stimulated cerebral ischemia model in vitro.
Knockdown of circRIMS reduces stellate cell apoptosis and OGD/RX-induced inflammation
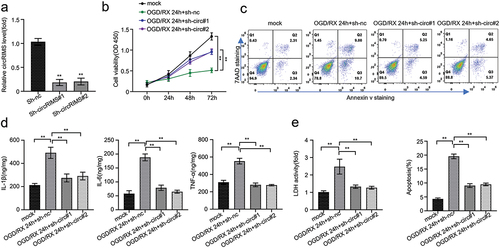
To ascertain the functional role and mechanism by which circRIMS can reduce apoptosis and inflammatory processes in astrocytes, we designed two shRNAs that can knockdown circRIMS, transfected them into OGD/RX-treated astrocytes, and measured the efficiency of knockdown. qRT-PCR results confirmed that sh-circRIMS significantly knocked down more than 50% of the circRIMS expression compared to negative control (Sh-NC) experiment (p < 0.01, ). The CCK8 assay was employed to uncover the cell viability of astrocytes after treatment. The outcome revealed that in a time-dependent manner (0, 24, 48, 72 h), the cell viability of astrocytes treated with OGD/RX at 24 h was markedly reduced compared to the mock (control), while cells transfected with sh-circ#1 and sh-circ#2 had significantly higher cell viability of at 24 h compared with the OGD/RX+sh-nc group, indicating that silencing circRIMS could increase the proliferation of astrocytes (p < 0.01, ). Additionally, flow cytometry assay indicated that OGD/RX-treated astrocytes recorded significantly higher programmed cell death at 24 h than that in the mock group, while 24 h transfected cells with sh-circ#1 and sh-circ#1 had significantly fewer programmed deaths compared to OGD/RX+ sh-nc-treated astrocytes (p < 0.01, ). Although inflammation is gender-dependent, our data from the ELISA assay indicated a significant increase in inflammatory factors secretion in the OGD/RX-treated group when compared with all other groups, and a significant decrease in cytoinflammatory factors secretion in the circRIMS-knockdown and OGD/RX-treated cells compared to all other groups, indicating that knockdown of circRIMS could effectively reduce inflammatory factors expression (p < 0.01, ). The LDH assay also revealed that LDH activity in the OGD/RX-treated cells at 24 h was significantly elevated than the mock group and other group cells whereas it was markedly reduced in circRIMS-knockdown and OGD/RX-treated cells when compared with the OGD/RX+sh-nc-treated cells indicating that knockdown of circRIMS could effectively ameliorate the rate of apoptosis in CIR injury (p < 0.01, ).
Figure 2. Knockdown of circRIMS can reduce stellate cell apoptosis and inflammation caused by OGD/RX.
CircRIMS directly targets and adsorb mir-96-5p
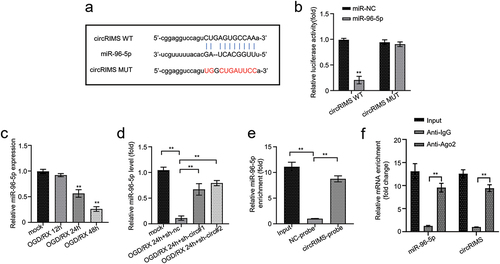
To evaluate molecular targets for circRIMS, we explored the starbase online bioinformatics analysis platform. The outcome indicated that circRIMS is an apparent target for miR-196-5p (). Fluorescein from the luciferase reporter assay revealed that overexpression of miR-96-5p effectively restricted the expression of luciferin in HEK cells transfected with circRIMS wild-type (WT) reporter gene compared to miR-NC (negative control), while there was no significant difference in luciferin activities of HEK cells transfected circRIMS mutant-type (MUT) sequence and overexpressed with miR-96-5p (p < 0.01, ). qRT-PCR revealed that miR-96-5p expression in the OGD/RX-treated astrocytes decreased significantly in an time-dependent manner compared with the normal control group, with the lowest expression observed after 48 h of OGD/RX treatment (p < 0.01, ). result shows that circRIMS and miR-96-5p are expressed inversely in OGD/RX-treated cells. In addition, qRT-PCR detected the expression of miR-96-5p in different groups of astrocytes. The result depicted that miR-96-5p expression in the OGD/RX+sh-nc-treated astrocytes was markedly diminished at 24 h compared with the mock group, and the expression was remarkably elevated in the circRIMS-knockdown astrocytes treated with OGD/RX compared with OGD/RX+sh-nc-treated astrocytes (p < 0.01, ). RNA pull-down analysis was carried out, and qRT-PCR revealed that circRIMS probes were efficient in enriching more miR-96-5p than empty or negative control probe (p < 0.01, ). Moreover, the RIP assay displayed that both miR-188-3p and circRIMS were effectively pulled down by Ago2, indicative of the direct interaction between them ().
Figure 3. CircRIMS directly targets and adsorb mir-96-5p.
Mir-96-5p targets and regulates the expression of STAT1
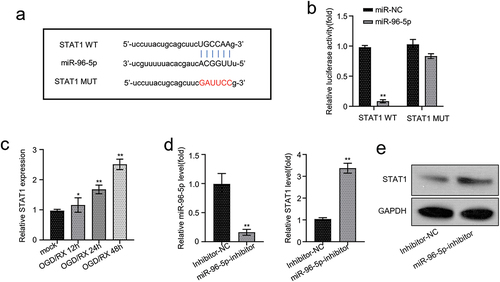
Starbase bioinformatics analysis predicted that STAT1 has miR-101-3p binding site in its 3’UTR noncoding region (). After transfecting the constructed STAT1 wild-type (WT) reporter plasmid or its mutated (MUT) form into the HEK-293T cell lines and co-transfecting with the miR-96-5p overexpression vector, we found that overexpressed miR-96-5p restricted the expression of luciferin in cells transfected with the STAT1 WT sequence compared to miR-NC. However, there was no significant difference in luciferase activity in HEK-293T cells transfected with the STAT1 MUT plasmid after co-transfection with miR-96-5p overexpression vector (p < 0.01, ). qRT-PCR revealed that STAT1 expression in the OGD/RX-treated astrocytes increased significantly in a time-dependent manner compared with the normal control group, with the highest expression observed after 48 h of OGD/RX treatment (p < 0.01, ). result shows that circRIMS and STAT1 are expressed similarly in OGD/RX-treated cells. qRT-PCR was further used to investigate the effect of miR-96-5p inhibitors in astrocytes. The outcome pointed out that miR-96-5p inhibitors undoubtedly restricted the miR-96-5p expression and upregulated the expression of STAT1 in astrocytes (p < 0.01, ). Western blot analysis was employed to deduce the expression of STAT1 protein in astrocytes incorporated with miR-96-5p inhibitors. We detected a significant increase in STAT1 expression in astrocytes, suggesting that miR-96-5p interacts with the STAT1 gene to regulate the STAT1 protein expression (p < 0.01, ).
Figure 4. Mir-96-5p targets and regulates the expression of STAT1.
CircRIMS promotes CIR injury by targeting miR-96-5p/JAK/STAT1 axis to increase cell apoptosis
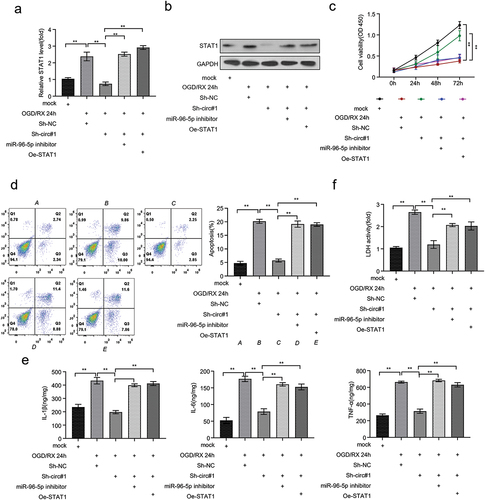
To increase our knowledge of the mechanisms by which circRIMS promotes CIR injury, we examined pathways associated with this disease progression. qRT-PCR and western blot analysis revealed that in the OGD/RX-treated astrocytes, mRNA and protein expression of STAT1 was significantly reduced after knocking down circRIMS. However, this condition was restored after inhibition of miR-96-5p and overexpression of STAT1 (p < 0.01, ). Furthermore, CCK-8 deduced the highest cell viability in the mock (control) group and OGD/RX+ Sh-circ#1-treated astrocytes, and significantly higher in cells transfected with miR-96-5p inhibitors or oe-STAT1(p < 0.01, ). Apoptosis detected by flow cytometry indicated that programmed cell death was lower in the circRIMS-knockdown group after OGD/RX treatment, but the apoptosis rate was significantly increased in the OGD/RX 24 h+Sh-circ#1+miR-96-5p inhibitor and OGD/RX 24 h+Sh-circ#1+Oe-STAT1 treatment group, indicating that inhibition of miR-96-5p and overexpression of STAT1 could restore the effect of OGD/RX induction on the cell (p < 0.01, ). Besides, ELISA assay indicated that inhibition of miR-96-5p and overexpression of STAT1 could restore the decreased inflammatory factors observed after knocking down circRIMS in OGD/RX-treated astrocytes (p < 0.01, ). Finally, the LDH assay deduced that LDH release was significantly lower in OGD/RX-treated astrocytes after circRIMS knockdown compared to the negative control group, and increased after miR-96-5p inhibition and STAT1 overexpression (p < 0.01, ).
Figure 5. CircRIMS promotes CIR injury by targeting miR-96-5p/JAK/STAT1 axis to increase cell apoptosis.
Discussion
Ischemic stroke is recognized as the leading cause disability and the second leading cause of mortality globally (Citation33). About 87% of all stroke cases are identified as ischemic stroke, with several factors suggesting contributing to its progression (Citation34,Citation35). In the current investigation, we found that circRIMS was remarkably elevated in tMACO mice models and OGD/RX-treated astrocytes and had a binding site that sponge miR-96-5p promoted the progression of CIR injury by accelerating programmed cell death. The mechanisms associated with early onset ischaemia-reperfusion have yet to be demystified, especially those in which noncoding regulatory RNAs play a role. Therefore, in-depth investigation into the role of these noncoding RNAs in the mechanisms of automatic stimulation in response to ischemic-reperfusion injury necessitates unraveling new therapies for stroke treatment and management (Citation36). Most circular RNAs have been categorized as novel collections of non-coding RNAs (ncRNAs) and formed by reverse splicing reactions (Citation37), accumulating evidence that circRNAs are crucial for regulating the pathophysiology of many disorders, including neurological disorders such as schizophrenia, depression, and ischemic stroke. For example, circRIMS was associated with gastric cancer promotion by isolating miR-148a-5p (Citation19). Similarly, overexpression of circRIMS was found to accelerate cell proliferation in esophageal squamous cell carcinoma by downregulating miR-613 (Citation38), while overexpression of circUCK2 effectively reduced apoptosis in CIR injury by binding miR-125b-5p (Citation39). From our investigation, we discovered that circRIMS expression is upregulated in CIR injury, ultimately leading to astrocytes apoptosis, secretion of inflammatory factors, and increased release of LDH. Interestingly, further experiments investigating the functional role of circRIMS revealed that knockdown of circRIMS could repress astrocytes apoptosis, secreted inflammatory factors, and LDH release, suggesting that circRIMS plays a major role in the progress of CIR injury.
To further elucidate the mechanism of this disease progression, we examined the putative target of circRIMS in OGD/RX-stimulated astrocytes in vitro. Online bioinformatics analysis revealed that circRIMS can target miR-96-5p. MiRNAs, which are small ncRNAs that regulate the post-transcriptional process by sponging the 3’UTR region of several mRNAs, and known to be associated with risk factors for stroke such as hypertension and atherosclerosis (Citation40). There is substantial evidence that miR-96-5p accelerates cell proliferation, migration and invasion in human breast cancer by activating the MEK/ERK signaling pathway and prevents autophagy and apoptosis in breast cancer (Citation41,Citation42). Furthermore, miR-96-5p was found to exert neuroprotective effects by increasing glutathione levels in dopaminergic cells (Citation43). Here, our results point out to a significant decrease in miR-96-5p expression in OGD/RX-treated astrocytes at 24 h compared to the mock control group, while its expression was remarkably elevated after circRIMS knockdown. Intriguingly, circRIMS probes were found to effectively enrich miR-96-5p compared to empty probe, indicating that circRIMS could prevent miR-96-5p expression to accelerate apoptosis inCIR injury.
Signal transducers and transcription activators (STAT) proteins are a family of transcription factors known for their special functions in cell growth and differentiation. STAT1, which plays a part in cytokine-signaling, is activated in neurons following ischemia, leading to ischemic brain injury (Citation44). Additionally, JAK-STAT has been identified as a major downstream signaling pathway for several inflammatory factors such as interleukin-6 (IL-6) (Citation45). In this investigation, the result points out that overexpressed miR-96-5p restricted the expression of luciferin in cells transfected with the STAT1 WT sequence compared to the MUT type. Subsequently, the incorporation of miR-96-5p inhibitors into the OGD/RX ischemic model resulted in downregulation of miR-96-5p expression, thereby increasing the expression of the STAT1 protein, suggesting an interaction between miR-96-5p and STAT1 protein. Similarly, overexpression of STAT1 and inhibition of miR-96-5p in OGD/RX ischemic models could reverse the effect of circRIMS knockdown, cumulatively increasing inflammatory factors, apoptosis, and stellate cell release, and indicate that the miR-96-5p/JAK/STAT1 axis are involved in promoting CIR injury.
It should be noted that our investigation is solely at the cellular level and has not been proved in vivo. We speculate that circRIMS/miR-96-5p/JAK/STAT1 has a similar effect in the clinic. It is necessary to explore the serum levels of circRIMS in different periods of CIR in follow-up studies, which may serve as a new biomarker of CIR. Given that stroke is a complex disease involving multiple pathways, more experiments are needed to validate the proposed pathway. In summary, in this study, we have been able to establish the interrelationship between circRIMS, miR-96-5p, and STAT1 protein. Cumulatively, our data indicate that circRIMS promotes the progression of CIR injury by elevating apoptosis through regulation of miR-96-5p/JAK/STAT1 axis and could be appropriated for diagnosis and targeted as a marker for the treatment of patients with CIR injury.
Conclusion
CircRIMS expression was implicated in the progress of CIR injury by elevating apoptosis via the regulation of the miR-96-5p/JAK/STAT1 axis suggesting that circRIMS could be appropriated for diagnosis and targeted as a marker for the treatment of patients with CIR injury.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yu L, Yang B, Wang J, Zhao L, Luo W, Jiang Q, Yang J. Time course change of COX2-PGI2/TXA2 following global cerebral ischemia reperfusion injury in rat hippocampus. Behav Brain Funct. 2014;10(1):42. doi:https://doi.org/10.1186/1744-9081-10-42.
- Lu C, Ha T, Wang X, L Liu, Zhang X, Kimbrough EO, Sha Z, Guan M, Schweitzer J, Kalbfleisch J, et al. The TLR9 ligand, CpG-ODN, induces protection against cerebral ischemia/reperfusion injury via activation of PI3K/Akt signaling. J Am Heart Assoc. 2014;3(2):e000629. doi:10.1161/JAHA.113.000629.
- Miller JB, Merck LH, Wira CR, Meurer WJ, Schrock JW, Nomura JT, Siket MS, Madsen TE, Wright DW, Panagos PD, et al. The advanced reperfusion era: implications for emergency systems of ischemic stroke care. Ann Emerg Med. 2017;69(2):192–201. doi:10.1016/j.annemergmed.2016.06.042.
- Chen B, Yang L, Chen J, Chen Y, Zhang L, Wang L, Li X, Li Y, Yu H. Inhibition of Connexin43 hemichannels with gap19 protects cerebral ischemia/reperfusion injury via the JAK2/STAT3 pathway in mice. Brain Res Bull. 2019;146:124–35.
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18. doi:https://doi.org/10.1056/NEJMoa1414792.
- Stegner D, Klaus V, Nieswandt B. Platelets as modulators of cerebral ischemia/reperfusion injury. Front Immunol. 2019;10:2505. doi:10.3389/fimmu.2019.02505.
- ZHAO Y, HUANG G, CHEN S, GOU Y, DONG Z, Zhang X. Homocysteine aggravates cortical neural cell injury through neuronal autophagy overactivation following rat cerebral ischemia-reperfusion. IJMS. 2016;17(8):1196. doi:https://doi.org/10.3390/ijms17081196.
- LIU AF, ZHAO FB, WANG J, LU YF, TIAN J, Zhao Y, Gao Y, Hu X-J, Liu X-Y, Tan J, et al. Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J Transl Med. 2016;14(1):101. doi:https://doi.org/10.1186/s12967-016-0858-0.
- WANG Y, JASPER H, TOAN S, MUID D, CHANG X, Zhou H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021;45:102049. doi:10.1016/j.redox.2021.102049.
- CHANG X, LOCHNER A, WANG HH, WANG S, ZHU H, Ren J, Zhou H. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11(14):6766–85. doi:https://doi.org/10.7150/thno.60143.
- ZHOU H, REN J, TOAN S, MUI D. Role of mitochondrial quality surveillance in myocardial infarction: from bench to bedside. Ageing Res Rev. 2021;66:101250. doi:10.1016/j.arr.2020.101250.
- TAN Y, MUI D, TOAN S, ZHU P, LI R, Zhou H. SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol Ther Nucleic Acids. 2020;22:696–707. doi:10.1016/j.omtn.2020.09.013.
- WANG J, ZHOU H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia–reperfusion injury. Acta Pharm Sin B. 2020;10(10):1866–79. doi:https://doi.org/10.1016/j.apsb.2020.03.004.
- SALZMAN J. Circular RNA expression: its potential regulation and function. Trends Genet. 2016;32(5):309–16. doi:10.1016/j.tig.2016.03.002.
- MENG X, LI X, ZHANG P, WANG J, ZHOU Y, Chen M. Circular RNA: an emerging key player in RNA world. Brief Bioinform. 2017;18:547–57. doi:10.1093/bib/bbw045.
- MEMCZAK S, JENS M, ELEFSINIOTI A, TORTI F, KRUEGER J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–38. doi:10.1038/nature11928.
- HUANG S, YANG B, CHEN BJ, BLIIM N, UEBERHAM U, Arendt T, Janitz M. The emerging role of circular RNAs in transcriptome regulation. Genomics. 2017;109(5–6):401–07. doi:https://doi.org/10.1016/j.ygeno.2017.06.005.
- HAN B, CHAO J, YAO H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31–44. doi:10.1016/j.pharmthera.2018.01.010.
- LIN J, ZHANG Y, ZENG X, XUE C, LIN X. CircRNA CircRIMS acts as a MicroRNA sponge to promote gastric cancer metastasis. ACS Omega. 2020;5(36):23237–46. doi:https://doi.org/10.1021/acsomega.0c02991.
- BAI Y, ZHANG Y, HAN B, YANG L, CHEN X, Huang R, Wu F, Chao J, Liu P, Hu G, et al. Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood–brain barrier integrity. J Neurosci. 2018;38(1):32–50. doi:https://doi.org/10.1523/JNEUROSCI.1348-17.2017.
- CHEN JA, HUANG YP, MAZZONI EO, TAN GC, ZAVADIL J, Wichterle H. Mir-17-3p controls spinal neural progenitor patterning by regulating Olig2/Irx3 cross-repressive loop. Neuron. 2011;69(4):721–35. doi:https://doi.org/10.1016/j.neuron.2011.01.014.
- DELALOY C, LIU L, LEE JA, SU H, SHEN F, Yang G-Y, Young WL, Ivey KN, Gao F-B. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6(4):323–35. doi:https://doi.org/10.1016/j.stem.2010.02.015.
- YANG H, ZHANG L, AN J, ZHANG Q, LIU C, He B, Hao D-J. MicroRNA-Mediated reprogramming of somatic cells into neural stem cells or neurons. Mol Neurobiol. 2017;54(2):1587–600. doi:https://doi.org/10.1007/s12035-016-0115-9.
- OLSCHEWSKI DN, RUEGER MA. The silencing of circular RNA in neural stem cells - a gateway to new therapeutic strategies in cerebral ischemia? EBioMedicine. 2020;53:102705. doi:10.1016/j.ebiom.2020.102705.
- HU Y, DENG H, XU S, ZHANG J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci. 2015;16(10):24895–917. doi:10.3390/ijms161024895.
- YANG J, CHEN M, CAO RY, LI Q, ZHU F. The role of circular RNAs in cerebral ischemic diseases: ischemic stroke and cerebral ischemia/reperfusion injury. Adv Exp Med Biol. 2018;1087:309–25. doi:10.1007/978-981-13-1426-1_25.
- ZHOU HY, WU CQ, BI EX. MiR-96-5p inhibition induces cell apoptosis in gastric adenocarcinoma. World J Gastroenterol. 2019;25(47):6823–34. doi:10.3748/wjg.v25.i47.6823.
- LI S, YANG Y, SHI MH, WANG JF, Ran XQ. RAN XQ. miR-96-5p attenuates malathion-induced apoptosis of human kidney cells by targeting the ER stress marker DDIT3. J Environ Sci Health B. 2020;55(12):1080–86. doi:https://doi.org/10.1080/03601234.2020.1816092.
- ZHUANG P, WAN Y, GENG S, HE Y, FENG B, Ye Z, Zhou D, Li D, Wei H, Li H, et al. Salvianolic acids for injection (SAFI) suppresses inflammatory responses in activated microglia to attenuate brain damage in focal cerebral ischemia. J Ethnopharmacol. 2017;198:194–204.
- LI Q, HE Q, BARAL S, MAO L, LI Y, Jin H, Chen S, An T, Xia Y, Hu B. MicroRNA-493 regulates angiogenesis in a rat model of ischemic stroke by targeting MIF. FEBS J. 2016;283(9):1720–33. doi:https://doi.org/10.1111/febs.13697.
- LI Y, CHOPP M, CHEN J, WANG L, GAUTAM SC, Xu Y-X, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20(9):1311–19. doi:https://doi.org/10.1097/00004647-200009000-00006.
- HAN B, ZHANG Y, ZHANG Y, BAI Y, CHEN X, Huang R, Wu F, Leng S, Chao J, Zhang JH, et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy. 2018;14(7):1164–84. doi:https://doi.org/10.1080/15548627.2018.1458173.
- FAN Y, DING S, SUN Y, ZHAO B, PAN Y, Wan J. MiR-377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J Cell Biochem. 2018;119(1):327–37. doi:https://doi.org/10.1002/jcb.26181.
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi:https://doi.org/10.1161/CIR.0000000000000558.
- TOURNIER-LASSERVE E. New players in the genetics of stroke. N Engl J Med. 2002;347(21):1711–12. doi:10.1056/NEJMcibr022035.
- LIAO H, ZHANG S, QIAO J. Silencing of long non-coding RNA MEG3 alleviates lipopolysaccharide-induced acute lung injury by acting as a molecular sponge of microRNA-7b to modulate NLRP3. Aging (Albany NY). 2020;12(20):20198–211. doi:10.18632/aging.103752.
- MENG F, ZHOU Y, DONG B, DONG A, ZHANG J. Long non-coding RNA LINC01194 promotes the proliferation, migration and invasion of lung adenocarcinoma cells by targeting miR-641/SETD7 axis. Cancer Cell Int. 2020;20(1):588. doi:10.1186/s12935-020-01680-3.
- WAN H, YUAN B, JIANG K, WEI J, FENG X, Sun B, Wang F. CircRNA CircRIMS is overexpressed in esophageal squamous cell carcinoma and downregulate miR-613 through methylation to increase cell proliferation. CMAR. 2021;13:4587–95. doi:10.2147/CMAR.S282983.
- CHEN W, WANG H, FENG J, CHEN L. Overexpression of circRNA circUCK2 attenuates cell apoptosis in cerebral ischemia-reperfusion injury via miR-125b-5p/GDF11 signaling. Mol Ther Nucleic Acids. 2020;22:673–83. doi:10.1016/j.omtn.2020.09.032.
- KOUTSIS G, SIASOS G, SPENGOS K. The emerging role of microRNA in stroke. Curr Top Med Chem. 2013;13(13):1573–88. doi:10.2174/15680266113139990106.
- QIN WY, FENG SC, SUN YQ, JIANG GQ. MiR-96-5p promotes breast cancer migration by activating MEK/ERK signaling. J Gene Med. 2020;22(8):e3188. doi:https://doi.org/10.1002/jgm.3188.
- SHI Y, ZHAO Y, SHAO N, YE R, LIN Y, Zhang N, Li W, Zhang Y, Wang S. Overexpression of microRNA-96-5p inhibits autophagy and apoptosis and enhances the proliferation, migration and invasiveness of human breast cancer cells. Oncol Lett. 2017;13(6):4402–12. doi:https://doi.org/10.3892/ol.2017.6025.
- KINOSHITA C, AOYAMA K, MATSUMURA N, KIKUCHI-UTSUMI K, WATABE M, Nakaki T. Rhythmic oscillations of the microRNA miR-96-5p play a neuroprotective role by indirectly regulating glutathione levels. Nat Commun. 2014;5(1):3823. doi:https://doi.org/10.1038/ncomms4823.
- TAKAGI Y, HARADA J, CHIARUGI A, MOSKOWITZ MA. STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. J Cereb Blood Flow Metab. 2002;22(11):1311–18. doi:10.1097/01.WCB.0000034148.72481.F4.
- SUZUKI S, TANAKA K, NOGAWA S, DEMBO T, KOSAKAI A, Fukuuchi Y. Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol. 2001;170(1):63–71. doi:https://doi.org/10.1006/exnr.2001.7701.
