ABSTRACT
Aim
To explore the potential role of microRNA miR-221-5p on the angiopoietin-1 (Ang-1)/Ang-2/Tie-2 signaling axis after subarachnoid hemorrhage (SAH) in a rat model.
Methods
Aspects of the rat’s behavior were measured using the Kaoutzanis scoring system to test neurological responses. This included feeding behavior, body contraction, motor, and eye-opening responses. Brain sections were studied using transmission electron microscopy and Evans blue extravasation. Levels of Ang-1, Ang-2, and Tie-2 were determined by Western blot, while miR-221-5p was quantified using stem-loop real-time quantitative PCR (RT-qPCR).
Results
The SAH group responded worse to the neurological response test than the sham-operated group. The intercellular space was widened in the SAH group, but not in the sham-operated group. Evans blue dye leaked significantly more into brain tissue cells of the SAH group. Stem-loop qRT-PCR showed elevated miR-221-5p levels. Additionally, Ang-1 and Tie-2 were reduced but Ang-2 expression was increased after SAH. This led to a significant reduction of the Ang-1/Ang-2 ratio in the brain tissue, which was associated with the destruction of the blood-brain barrier.
Conclusion
The data indicate that miR-221-5p might regulate blood-brain barrier dysfunction through the Ang-1/Ang-2/Tie-2 signaling axis, suggesting that it should be further investigated as a potential novel biomarker.
Introduction
Subarachnoid hemorrhage (SAH) is characterized by the rupture of blood vessels in the space surrounding the brain, or on the surface of the spinal cord. SAH accounts for nearly 5% of all strokes, and the mortality rate and prognosis are poor (Citation1). Trauma and aneurysms are the most common causes of SAH (Citation2,Citation3). Moreover, more than 40% of all patients with SAH presented long-term neurological sequelae, whereas < 20% of those cases did not show any residual symptoms (Citation4). Therefore, understanding the pathophysiology of delayed neurological complications after SAH is crucial to identifying the patients at elevated risk for neurological complications, and early intervention should be performed to improve the prognosis of SAH (Citation5).
Patients with brain edema of the cytotoxic or vasogenic type are significantly associated with poor prognosis for SAH (Citation6). The occurrence of vasogenic edema is mainly attributed to blood – brain barrier (BBB) dysfunction (Citation7), which can be observed within hours after SAH. It is associated with several pathologic changes, including thrombosis, inflammation, and abnormal cerebral metabolism (Citation8–11). Moreover, BBB dysfunction is significantly associated with delayed cerebral ischemia in patients that experienced SAH, and epilepsy and neurodegeneration after a traumatic brain injury (Citation12–14).
Angiopoietin-1 (Ang-1) and Ang-2 are two of the most important proteins of the angiopoietin family, and their binding target is the Tie-2 receptor on the surface of vascular endothelial cells. Ang-1 binding to Tie-2 induces autophosphorylation of the receptor, which promotes endothelial cell maturation, stability, and tight intercellular junctions. On the other hand, Ang-2 is mainly responsible for loosening or breaking these connections between cells to allow migration of endothelial cells (Citation15–17). Therefore, high expression of Ang-1 and low expression of Ang-2 promote a high phosphorylation of Tie-2 in microvascular endothelial cells of the brain, maintaining the stability of the BBB. Previously, we found a significantly reduced ratio of Ang-1/Ang-2 in brain tissues of rats with SAH, which was accompanied by the destruction of the BBB and neurological impairment (Citation10). In contrast, it has been shown that exogenous Ang-1 supplementation can effectively promote Tie-2 phosphorylation of cerebral microvascular endothelial cells, reducing BBB damage after SAH in rats, and facilitating recovery of neural function (Citation18). Studies have found postangioplasty vessel injury exhibited various microRNAs (miR) expression profiles, and miR-221 plays an important role in regulating the vascular smooth muscle cell phenotypes (Citation19,Citation20). Moreover, miR-221 is involved in the regulation of angiogenesis mediated by endothelial cells, which plays an important role in the regulation of the BBB (Citation21). In a preliminary experiment, we found that the expression of miR-221 affected the Ang-1/Ang-2 ratio. Thus, the current study was undertaken to explore the potential role of miR-221-5p on BBB dysfunction after SAH in a rat model through the Ang-1/Ang-2/Tie-2 signaling axis.
Materials and methods
Reagents and instruments
Chloral hydrate (Real-time preparation); Evans blue dye (CAS:314-13-6; Sigma-Aldrich, St. Louis, MO, USA); micro hemostatic clamp (batch #: 200921; Ningbo Medical Needle Co. Ltd, Ningbo, China); a Canon 550D digital camera; line switch (model #: 2636-A4; Beijing Xinong Technology Co. Ltd, Beijing, China); refrigerator (BCD-216; Qingdao Haier Co. Ltd, Qingdao, China); Philips NV transmission electron microscope (TECNAI 10); absolute ethyl alcohol (batch #: 20130130; Chengdu Kelon Chemical Reagent Factory, Chengdu, China); Osmic acid, uranyl acetate, and acetone were all provided by the laboratory of the Medical School at Zhejiang University (Zhejiang, China).
Laboratory conditions for housing rats
A total of 10 male SPF Sprague Dawley rats weighing between 180–220 g were purchased from Shanghai Slacker Co. Ltd (License #: SCXK (Shanghai, China) 2017–0005, certificate no.: 20170005058904). They were housed in the laboratory animal room with license # SYXK (Zhejiang, China) 2020–0013. Sterilized ultrapure drinking water (grade 2) was provided ad libitum. This water quality is in accordance with the national ‘Drinking Water Hygiene Standard’ (GB5749–2006) of the People’s Republic of China. The temperature in the feeding environment ranged between 20 and 25 °C, and the relative humidity was between 40–70%. The rats were acclimatized to the animal house for one week prior to the experiment. Experimental animals were selected from 10 adult male Sprague Dawley rats (300–350 g), which were randomly divided into the SAH group (n = 5) and sham-operated group (n = 5) after adaptive feeding for one week.
Endovascular puncture model in the rat
A rat model of SAH was constructed using the endovascular perforation technique (Citation22): (1) the rats were anesthetized with intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg); (2) the limbs of anesthetized rats were fixed on the operating table, the incisors were hooked with rubber bands to make the head level with the table, and the neck hair was removed and the skin was disinfected with alcohol; (3) the neck was opened to find the carotid artery and its associated nerves on the left side. The nerve was carefully separated from the artery from the proximal heart to the bifurcation of the carotid artery; (4) two surgical lines were passed through the proximal carotid artery for standby use, and one surgical line was passed through the bifurcation of the internal and external carotid arteries to ligate the external carotid artery and close the internal carotid artery with a miniature vascular clip; (5) the proximal carotid artery was ligated with the bottom line, and the top line was tied without ligating; (6) a small incision between the two lines was made and a 3–0 monofilament nylon line (the front end is beveled and marked with black marker at 2 cm) was inserted. The nylon line was gently tied on the top line. The vascular clip was opened, and the lature line tightened at the bottom to make it at a 45 degree angle with the trachea, pushing the nylon line slowly and gently. When the mark was close to the intersection of the artery, a little resistance was felt. At this point, the rats will continue to push 2 mm, breathing will change; (7) the nylon line was withdrawn after 10 seconds, and the upper line was ligated and sutured; and (8) the rat was placed on a heating pad until it woke up. The eye on the operative side of the rat appeared dim during the process.
Neurological assessment
The neurological assessment of the rats was performed using the scoring system established by Kaoutzanis (Citation23). It included the rat’s feeding behavior (free eating was scored as 2, refusal of food was scored as 1); motor response (walking freely was scored as 5, walking with difficulty scored as 4, and inability to walk was scored as 3); body contraction (limb contraction was scored as 2, and no response during pricking was scored as 1); and eye opening response (self-opening was scored as 4, auditory stimulation was scored as 3, pricking pain was scored as 2, and inability to open eyes was scored as 1).
Transmission electron microscopy of hippocampal tissue
The samples were obtained from both the model and the sham-operated groups. Humane euthanasia was performed after neurological assessment. The model rats were put into a closed foam box and an appropriate amount of isoflurane was added. The dead rats were removed 5 min later, and the chest was opened and slowly irrigated with 40 mL normal saline. After that step, the brain was opened, the cranial roof was cut open, the brain was removed. The fresh brain tissue can be processed as needed. One-day-old bacterial suspension was added to 2.5% glutaraldehyde phosphate buffer and fixed for 3 h, then rinsed twice with 0.1 M phosphate-buffered saline for 15 min. After adding 1% osmium tetroxide, the samples were fixed for 1 h, and then rinsed twice with 0.1 M phosphate-buffered saline for 15 min. Then, using 50%, 70%, and 90% alcohol, the samples were dehydrated successively once for 15 min. A single dehydration step with 100% alcohol was carried out for 20 min. Next, the samples were washed twice with 100% acetone for 20 min. Samples were then placed in a 1:1 mix of anhydrous acetone and embedding agent and shaken for 2 h to allow the mix to penetrate the tissue. The pure embedding agent penetrates the tissue and oscillates for 2 h. After 2 h, the samples were placed in the oven to polymerize. This was done in three steps: at 37 °C for 24 h, at 45 °C for 24 h, and at 60 °C for 48 h. Block repair and cut the samples into ultrathin sections of approximately 120 nm. Next, the sections were stained with 4% uranium acetate for 20 min and with lead citrate for 5 min. Finally, the stained, ultrathin sections were placed on a single-hole copper net and observed and photographed under a Philips TECNAI 10 transmission electron microscope.
Evans blue extravasation
After the rat model of SAH had been constructed, the vascular permeabilization of the BBB was assessed using a modified version of the Evans blue extravasation method. First, 1 mL 2% Evans blue was injected intravenously into the tail vein of the model rats, and the rats were killed 3 h later. Then, 4 mL/kg Evans blue dye at a concentration of 2% in 0.9% normal saline solution was injected into the right femoral vein over 5 min. Subsequently, 300 mL of saline was perfused to wash out the remaining dye in the blood vessels after 2 h. After this, the brains were removed and cut into 2 mm-thick sections, which were then weighted and immersed in formamide. The absorbance of Evans blue in the supernatant was assessed spectrophotometrically (OptiMax, Molecular Devices, Sunnyvale, CA, USA) at 610 nm, which was applied to assess the BBB permeability in the brain motor cortex tissue.
MiRNA quantification using real-time quantitative PCR
Stem-loop real-time quantitative PCR (RT-qPCR) was used to quantify miR-221-5p in the tissue samples. Specifically, RNA was extracted using a miRNeasy Micro Kit (Qiagen, Limburg, Netherlands), then the stem-loop reverse transcription primers were hybridized to the miRNAs in the sample and reverse transcribed. The samples were incubated at room temperature for 5 min and then centrifuged at 10,000 × g, 4 °C for 15 min. Then the supernatant was transferred and incubated at room temperature for 10 min, and centrifuged at 10,000 × g, 4 °C for 10 min. The RNA reverse transcription reaction was performed with the ReverTra Ace qPCR RT Master mix with gDNA Remover (Toyobo Life Science). The QuansStudio 5 instrument (Thermo Fisher Scientific, Inc.) was used during RT-qPCR to measure the RNA expression level. The reverse transcription product was amplified via RT-qPCR using a miR-221-5p-specific forward primer and a universal reverse primer. The forward primer of miR-221-5p was 5’-GCCGAGACCTGGCATACAAT-3,“ while the reverse primer was 5”-CTCAACTGGTGTCGTGGA-3.“ Moreover, the forward and reverse primers of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 5”-CTCATGACCACAGTCCATGC-3,“ and 5”-TTCAGCTCTGGGATGACCTT-3,’ respectively.
Western blot analysis
The frozen tissue samples were solubilized on ice in radioimmunoprecipitation assay buffer using a tissue homogenizer. Once thawed, 60 µg/lane of protein samples were separated with a sodium dodecyl sulfate – polyacrylamide gel (10% or 12% acrylamide) and then transferred to polyvinylidene fluoride membranes by electrolysis. Next, the samples were incubated in a blocking solution (5% nonfat milk in 20 mM Tris(hydroxymethyl)aminomethane hydrochloride, 150 mM natrium chloride, 0.1% Tris-buffered saline with Tween 20 detergent [TBST; Sigma-Aldrich, St. Louis, MO, USA]) for 2 h. After blocking, the primary antibodies against Ang-1, Ang-2, Tie-2, and GAPDH were incubated overnight at 4 °C. Additionally, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies after rinsing with TBST buffer. The antigen-specific bands were assessed using a chemiluminescence kit (Heliosense Biotechnologies Inc., Xiamen, China) and X-ray film (Heliosense Biotechnologies Inc.). Quantity one software (version: 4.62; Bio-Rad, Hercules, CA, USA) was used to analyze the density of the bands.
Dual-luciferase reporter gene assays
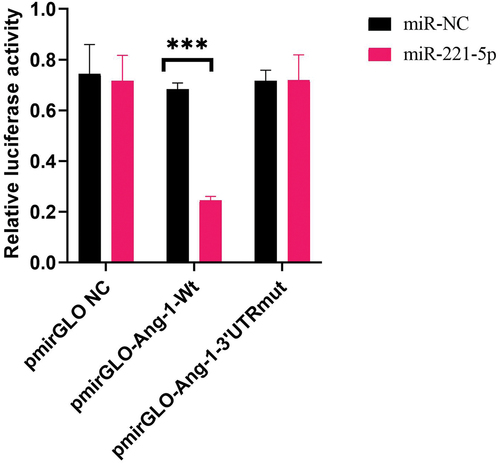
Lipofectamine 3000 was used to transfect the cells, and miR-221-5p and miR-NC were transfected simultaneously. A dual luciferase reporter assay was used to detect the targeted regulatory association of miR-221-5p against Ang-1. Wild type (pmirGLO-Ang-1-Wt) and mutant (pmirGLO-Ang-1–3’UTRmut) reporter plasmids containing the binding sites of wild and mutant miR-221-5p were synthesized.
Statistical analysis
All analyses in our study were performed using IBM SPSS Statistics software (version: 19.0; IBM Corp., Armonk, NY, USA). The data were described using mean and standard deviation, and independent-sample t-tests were run to compare the differences between the model and the sham-operated group. All reported P-values were 2-tailed, and the significance level was set to 0.05.
Results
Neurological assessment
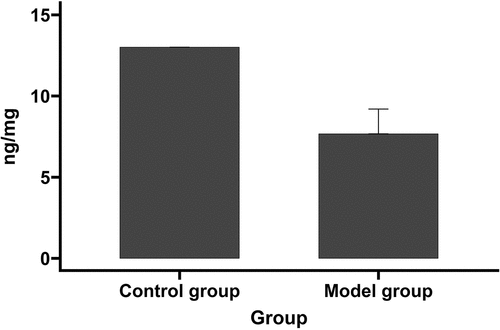
The rats in the SAH model group and the sham-operated control group were scored using Kaoutzanis’ scoring system (). Compared with the sham-operated group, there was an elevated rate at which rats in the model group refused to eat, had difficulty or were unable to walk, contracted their limbs when pricked, and showed signs of pricking pain when opening their eyes. The autopsy found blood on the underside of the brain. The overall scores of the model group were significantly lower than that of the sham-operated group (7.7 ± 1.5 vs 13.0 ± 0.0; P < 0.05; ).
Table 1. The Kaoutzanis scoring system in model and sham-operated groups.
Transmission electron microscopy of hippocampal tissue
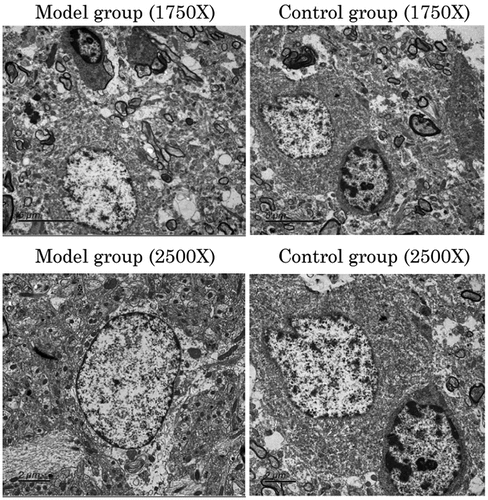
The details of the hippocampal tissue in the SAH model and sham-operated groups were assessed by transmission electron microscopy (). The intercellular space of the cerebral tissue samples in the model group was wider than that in the sham-operated group.
Evans blue extravasation
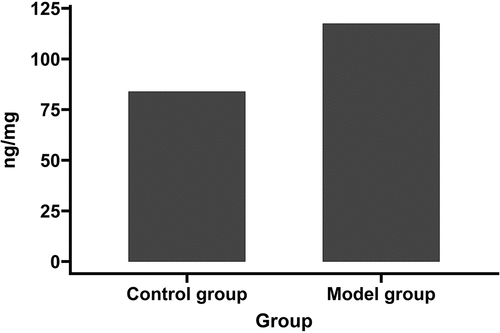
shows that the Evans blue dye leaked in both the SAH and sham-operated groups; however, it leaked to a greater extent in the SAH group than in the sham-operated group.
MiR-221-5p expression
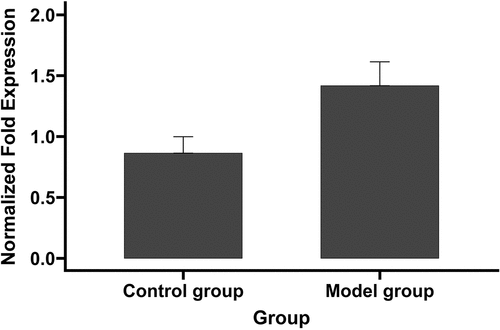
RT-qPCR was used to quantify miR-221-5p expression (). The results showed that miR-221-5p expression was significantly elevated in the model group (1.41730 ± 0.19663 vs 0.86344 ± 0.13456; P < 0.05).
Ang-1/Ang-2/Tie-2 expression
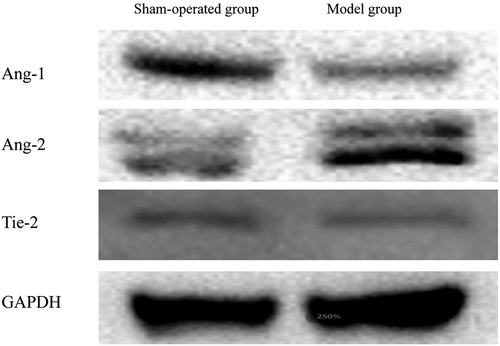
The expression of Ang-1, Ang-2, and Tie-2 were assessed using Western blot analysis, and the results of the SAH model group are shown in . The expression of Ang-1 and Tie-2 were reduced in model group, while that of Ang-2 was significantly increased in the SAH model group.
MiR-221-5p overexpression inhibits Ang-1 expression
A dual luciferase reporter assay was employed to detect the possible activation of miR-221-5p on target reporter genes (Ang-1). The results showed that the relative fluorescence activity was significantly decreased in cells transfected with pmirGLO-Ang-1-Wt and miR-221-5p, which suggested a targeted regulation of Ang-1 by miR-221-5p ().
Discussion
The BBB is a structure that is mainly composed of cerebral microvascular endothelial cells, astrocyte terminal feet, extracellular matrix, and pericytes. Under normal conditions, cerebral homeostasis is maintained by preventing neurotoxic plasma components, blood cells, and pathogens from entering the peripheral blood (Citation24,Citation25). The pathophysiology of secondary nerve injury after SAH can roughly be divided into early brain injury and delayed cerebral ischemia depending on the time of onset, both of which can lead to BBB dysfunction. Once the BBB integrity has been damaged, the brain tissue is directly exposed to neurotoxic blood contents and immune cells, leading to secondary brain injury, including inflammation and oxidative stress (Citation26). Therefore, the underlying mechanisms leading to BBB dysfunction after SAH should be identified.
In this study, we found that motor and sensory functions (evaluated using the Kaoutzanis scoring system) were significantly worse in the model group compared to the sham-operated group. Moreover, Evans blue dye leaked significantly more into tissue samples of rats from the model group compared to those of the sham-operated group. Altogether, this suggests that the rat model was constructed correctly and that BBB dysfunction occurred after SAH.
Recently, the detection of BBB permeability after SAH has become an indicator to predict the secondary risk and recovery of neurological function. Parameters of computed tomography perfusion analysis and magnetic resonance imaging, including Kep and Ktrans (d), can show the stability and the degree of damage to the BBB. These techniques have proven to predict delayed cerebral ischemia or infarction and the prognosis of SAH (Citation27,Citation28). Moreover, studies have demonstrated that reducing the permeability of human cerebrovascular endothelial cells and the BBB can improve long-term neurological deficits (Citation29). Although the correlation between the permeability of the BBB and secondary neurological impairment and neurological recovery has been shown, it is still an important intervention method. Future research should focus on improving the prognosis of patients after SAH by improving the permeability of the BBB and reducing any existing damage to the BBB.
Ang-1 and Ang-2, as primary physiological ligands of the endothelial-specific receptor Tie-2, are abundant in endothelial cells and periendothelial cells (Citation30–33). The Ang-1/Ang-2/Tie-2 signaling axis is significantly involved in the process of vasculogenesis and normal vascular maturation, and is needed for maintenance and remodeling (Citation34,Citation35). Because Ang-1 is essential for vascular morphogenesis, the Ang-1/Tie-2 signaling axis is a promising therapeutic target for angiogenesis and vascular remodeling. Furthermore, the expression of Ang-1 and Ang-2 might be able to regulate vascular permeability and lymphatic integrity. In addition, Ang-2/Tie-2 signaling induces vascular destabilization through the loss of cell-to-cell contacts, and Ang-2 could be considered a Tie-2 antagonist (Citation35). Our study found that Ang-1 expression was reduced, while that of Ang-2 was elevated after SAH. These results suggest that Ang-1 plays a role in promoting angiogenesis at the early stage of SAH, which may be involved in inducing endothelial progenitor cells to return to the lesion, thereby promoting BBB repair. Moreover, Ang-2 is less responsive to EBI than Ang-1. The expression level of the receptor Tie-2 increased at first and then decreased slightly, which may be related to a saturation of the receptor. Further aggravation of vascular endothelial cell injury may lead to receptor depletion. Finally, we hypothesized that miR-221-5p might target the Ang-1/Ang-2/Tie-2 signaling axis because the expression of miR-221-5p was significantly increased in the SAH model group compared to the sham-operated group. This illustrates why miR-221-5p might be a potential new biomarker and therapeutic target, especially considering that miRNAs are released into stable body fluids.
Our study found that the neurological responses of rats were negatively affected by SAH, and that BBB dysfunction occurred. This was highlighted by the widened intercellular space in cerebral samples of rats in the SAH model group. Damage to the wall lining layer after aneurysm rupture, middle, and smooth muscle cell apoptosis are common consequences of SAH and are further compromised by poor repair of the damaged structure, followed by new damage. A process that might repeat itself many times. [Ang-1/Ang-2 expression in vessel wall injury]. In our study, inflammatory infiltration occurred more frequently, which could mean that the tumor wall of a local vessel was thinner. Considering that the direction of blood flow during shock is generally directed upwards, the apical rupture of a tumor accounts for the highest proportion of bleeding (Citation36). The elevated level of Ang-1 expression measured in serum samples of rats at an early stage of SAH is beneficial for the prognosis of people with SAH. The imbalance of the Ang-1/Ang-2 ratio (reduced or increased inverted) reflects the degree of structural disorder in the tumor wall. This may be an important reason for the formation and rupture of ICA, because the decrease of the Ang-1/Ang-2 ratio, or the increase of Ang-2/Ang-1, may determine the probability of rupture hemorrhage and the degree of brain injury. It would also directly affect the severity of brain injury after SAH, thus determining the patient’s state of consciousness. This would significantly affect the prognosis of a patient after SAH.
The limitations of this study should be highlighted. Although the luciferase experiment demonstrated that miR-221-5p can interact with Ang-1, the impact of this interaction on SAH outcomes (behavioral or pathophysiologic) was not described. Further investigations should examine the potential link between the role of miR-221-5p on the outcomes of SAH.
Conclusions
This study found poor neurological responses and BBB dysfunction in a rat model after SAH, which appeared to be regulated via the Ang-1/Ang-2/Tie-2 signaling axis. MiR-221-5p should be investigated as a biomarker that might be able to regulate Ang-1/Ang-2/Tie-2 signaling with the aim to improve the prognosis of SAH.
Ethics approval
All experiments were approved by the animal care committee of Huzhou Normal University (HZYY-LL-KY-006-01) and performed in compliance with the national institutes of health and institutional guidelines for the humane care of animals.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2012;43(6):1711–37. doi:10.1161/STR.0b013e3182587839.
- Muehlschlegel S. Subarachnoid Hemorrhage. Continuum. 2018;24(6):1623–57. doi:10.1212/CON.0000000000000679.
- Haeren RHL, Jahromi BR, Niemela M. Posttraumatic subarachnoid hemorrhage related to concomitant carotid artery dissection and ruptured basilar trunk aneurysm: a case report and literature review. Surg Neurol Int. 2021;12:344. doi:10.25259/SNI_193_2021.
- Hong CM, Tosun C, Kurland DB, Gerzanich V, Schreibman D, Simard JM. Biomarkers as outcome predictors in subarachnoid hemorrhage–a systematic review. Biomarkers. 2014;19(2):95–108. doi:10.3109/1354750X.2014.881418.
- Dreier JP, Fabricius M, Ayata C, Sakowitz OW, William Shuttleworth C, Dohmen C, Graf R, Vajkoczy P, Helbok R, Suzuki M, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. 2017;37(5):1595–1625. doi:10.1177/0271678X16654496.
- Dreier JP, Lemale CL, Kola V, Friedman A, Schoknecht K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology. 2018;134:189–207. doi:10.1016/j.neuropharm.2017.09.027.
- Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33(5):1225–32. doi:10.1161/01.STR.0000015624.29071.1F.
- Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28(4):399–414. doi:10.1179/016164106X115008.
- Song JN, Chen H, Zhang M, Zhao Y-L, Ma X-D. Dynamic change in cerebral microcirculation and focal cerebral metabolism in experimental subarachnoid hemorrhage in rabbits. Metab Brain Dis. 2013;28(1):33–43. doi:10.1007/s11011-012-9369-8.
- Gu H, Fei ZH, Wang YQ, Yang J-G, Zhao C-H, Cai Y, Zhong X-M. Angiopoietin-1 and angiopoietin-2 expression imbalance influence in early period after subarachnoid hemorrhage. Int Neurourol J. 2016;20(4):288–95. doi:10.5213/inj.1632692.346.
- Ivanidze J, Ferraro RA, Giambrone AE, Segal AZ, Gupta A, Sanelli PC. Blood-brain barrier permeability in Aneurysmal Subarachnoid hemorrhage: correlation with clinical outcomes. AJR Am J Roentgenol. 2018;211(4):891–95. doi:10.2214/AJR.17.18237.
- Russin JJ, Montagne A, D’Amore F, He S, Shiroishi MS, Rennert RC, Depetris J, Zlokovic BV, Mack WJ. Permeability imaging as a predictor of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2018;38(6):973–79. doi:10.1177/0271678X18768670.
- Friedman A, Kaufer D, Heinemann U. Blood–brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85(2–3):142–49. doi:10.1016/j.eplepsyres.2009.03.005.
- Bar-Klein G, Lublinsky S, Kamintsky L, Noyman I, Veksler R, Dalipaj H, Senatorov VV, Swissa E, Rosenbach D, Elazary N, et al. Imaging blood–brain barrier dysfunction as a biomarker for epileptogenesis. Brain. 2017;140(6):1692–705. doi:10.1093/brain/awx073.
- Sabirzhanov B, Faden AI, Aubrecht T, Henry R, Glaser E, Stoica BA. MicroRNA-711–Induced downregulation of angiopoietin-1 mediates neuronal cell death. J Neurotrauma. 2018;35(20):2462–81. doi:10.1089/neu.2017.5572.
- Wang L, Zhang X, Liu X, Feng G, Fu Y, Milner R, Li L. Overexpression of α5β1 integrin and angiopoietin-1 co-operatively promote blood-brain barrier integrity and angiogenesis following ischemic stroke. Exp Neurol. 2019;321:113042. doi:10.1016/j.expneurol.2019.113042.
- Li Z, Korhonen EA, Merlini A, Strauss J, Wihuri E, Nurmi H, Antila S, Paech J, Deutsch U, Engelhardt B, et al. Angiopoietin-2 blockade ameliorates autoimmune neuroinflammation by inhibiting leukocyte recruitment into the CNS. J Clin Invest. 2020;130(4):1977–1990. doi:10.1172/JCI130308.
- Gu H, Wang YQ, Zhao CH, Zhong X-M, Yang J-G. The decrease of tie-2 receptor phosphorylation in microvascular endothelial cells is involved in early brain injury after subarachnoid hemorrhage. Artery Res. 2018;23(C):45–51. doi:10.1016/j.artres.2018.07.004.
- Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121(8):1022–32. doi:10.1161/CIRCULATIONAHA.109.889048.
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284(6):3728–38. doi:10.1074/jbc.M808788200.
- Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104(4):442–54. doi:10.1161/CIRCRESAHA.108.191270.
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26(6):1086–91. doi:10.1161/01.STR.26.6.1086.
- Nishizawa S. The roles of early brain injury in cerebral vasospasm following subarachnoid hemorrhage: from clinical and scientific aspects. Acta Neurochir Suppl. 2013;115:207–11.
- Wei C, Guo S, Liu W, Jin F, Wei B, Fan H, Su H, Liu J, Zhang N, Fang D, et al. Resolvin D1 ameliorates inflammation-mediated blood-brain barrier disruption after subarachnoid hemorrhage in rats by modulating A20 and NLRP3 inflammasome. Front Pharmacol. 2021;11:610734.
- Xu H, Cai Y, Yu M, Sun J, Cai J, Li J, Qin B, Ying G, Chen T, Shen Y, et al. Celastrol protects against early brain injury after subarachnoid hemorrhage in rats through alleviating blood-brain barrier disruption and blocking necroptosis. Aging (Albany NY). 2021;13(12):16816–16833. doi:10.18632/aging.203221.
- Li Y, Wu P, Bihl JC, Shi H. Underlying mechanisms and potential therapeutic molecular targets in blood-brain barrier disruption after subarachnoid hemorrhage. Curr Neuropharmacol. 2020;18(12):1168–79. doi:10.2174/1570159X18666200106154203.
- Amoo M, Henry J, Pender N, Brennan P, Campbell M, Javadpour M. Blood-brain barrier permeability imaging as a predictor for delayed cerebral ischaemia following subarachnoid haemorrhage. A narrative review. Acta Neurochir (Wien). 2021;163(5):1457–67. doi:10.1007/s00701-020-04670-6.
- Chen S, Xu P, Fang Y, Lenahan C. The updated role of the blood brain barrier in subarachnoid hemorrhage: from basic and clinical studies. Curr Neuropharmacol. 2020;18(12):1266–78. doi:10.2174/1570159X18666200914161231.
- Sun JY, Zhao SJ, Wang HB, Hou Y-J, Mi Q-J, Yang M-F, Yuan H, Ni Q-B, Sun B-L, Zhang Z-Y, et al. Ifenprodil Improves Long-Term Neurologic Deficits Through Antagonizing glutamate-induced excitotoxicity after experimental subarachnoid hemorrhage. Transl Stroke Res. 2021;12(6):1067–1080. doi:10.1007/s12975-021-00906-4.
- Venkat P, Yan T, Chopp M, Zacharek A, Ning R, Van Slyke P, Dumont D, Landschoot-Ward J, Liang L, Chen J, et al. Angiopoietin-1 mimetic peptide promotes neuroprotection after stroke in type 1 diabetic rats. Cell Transplant. 2018;27(12):1744–1752. doi:10.1177/0963689718791568.
- Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165(5):1557–70. doi:10.1016/S0002-9440(10)63413-X.
- Pang D, Wang L, Dong J, Lai X, Huang Q, Milner R, Li L. Integrin α5β1-Ang1/Tie2 receptor cross-talk regulates brain endothelial cell responses following cerebral ischemia. Experimental & Molecular Medicine. 2018;50(9):1–12. doi:10.1038/s12276-018-0145-7.
- Chen J, Cui X, Zacharek A, Chopp M. Increasing Ang1/Tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. J Cell Mol Med. 2009;13(7):1348–57. doi:10.1111/j.1582-4934.2008.00380.x.
- Li W, Kandhare AD, Mukherjee AA, Bodhankar SL. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: role of TGF-ß/Smads and ang-1/Tie-2 signaling pathways. Excli J. 2018;17:399–419. doi:10.17179/excli2018-1036.
- Biel NM, Siemann DW. Targeting the angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 2016;380(2):525–533. doi:10.1016/j.canlet.2014.09.035.
- Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, Uno M, Nagahiro S. Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens. 2009;27(6):1284–92. doi:10.1097/HJH.0b013e328329d1a7.