ABSTRACT
Background
Mild traumatic brain injury (mTBI) can cause persistent symptoms suggestive of oculomotor deficits. This research synthesized evidence on restitutive interventions for reducing oculomotor deficits in adults with mTBI to understand if these interventions have clinical utility for improving recovery.
Methods
Medline, EMBASE, CINHAL, PsychInfo, and Scopus, databases were searched for experimental studies published in English. We rated risk of bias (RoB) using recommended tools, and the certainty of the evidence according to GRADE guidelines. We conducted meta-analyses for similar outcomes reported in at least two studies.
Results
Out of 5,328 citations, 12 studies (seven case series and five crossover design), with a combined sample size of 354 participants; (43% males) met the inclusion criteria and were analyzed. The analysis revealed a trend toward improvement of oculomotor deficits and visual tasks in response to restitutive intervention. None of the studies addressed sex or gender effects. All studies had high RoB, suggesting low certainty in the reported results.
Discussion
Restitutive interventions may be beneficial for adults with oculomotor deficits after mTBI, however overall certainty of the evidence remains low. Future efforts must include enhancing attention to study methodology and reporting, sex and gender analyses, and reaching a consensus on outcome measures.
PROSPERO registration number
CRD42022352276
Introduction
Mild traumatic brain injury (mTBI) is a significant public health concern, with an estimated annual incidence of over four million cases across Canada and the United States combined (Citation1,Citation2).
Prognosis for mTBI is variable and research suggests that only 27% of individuals recover within three years after injury, with more symptoms being predictive of a longer recovery (Citation3). Some of the most concerning symptoms reported by individuals after mTBI are those suggestive of oculomotor deficits, which often present as blurred vision, headaches when reading, and difficulty functioning in visually stimulating environments (Citation4,Citation5). Research has reported oculomotor deficits being present in up to 85% of individuals who have sustained a mTBI during both acute and chronic stages after injury (Citation4–7). This places a significant burden on individuals in terms of days lost from work, disruption in social roles, and return to purposeful life (Citation3).
Oculomotor deficits following mTBI may present as measurable impairments in vergence, accommodation, saccades, gaze stability and smooth pursuits (Citation6,Citation7), as well as difficulties in visual tasks such as reading (Citation7). This is not surprising given that eye movements are coordinated by more than 40% of the human brain (Citation8). Disruption to any of the brain areas in the form of diffuse axonal injury secondary to mTBI has the potential to impair oculomotor function and tasks related to vision (Citation8). Historically, rehabilitation of patients with mTBI has focused on addressing oculomotor deficits through rest or reduction of visually stimulating activities such as computer use, with less emphasis on oculomotor training, even though evidence suggests that oculomotor rehabilitation applied after injury may support recovery (Citation9–11).
Oculomotor rehabilitation
Rehabilitative approaches for oculomotor deficits can be categorized as restitutive, compensatory, substitutive, and pharmacological (Citation12,Citation13) Supplementary Material: Table S1).
These approaches are not mutually exclusive, and their strategies may fit into one or more categories (Citation13). Restitutive approaches, the focus of this review, involve strategies that support biochemical events to help restore function in the neural tissue and facilitate recovery, for example, repetitive training of a compromised eye movement (Citation12,Citation13).
Over the last several decades, significant effort has been placed into developing restitutive approaches that remediate oculomotor deficits and alleviate the associated symptoms in individuals with mTBI (Citation14–16). A common limitation among these studies, however, is the challenge of establishing controlled conditions (Citation14–16) and the relatively small sample sizes involving select groups of patients (i.e., sport-related concussions), impeding the generalizability of findings to all patients with mTBI (Citation16). Furthermore, the duration of follow-up in these studies varied, and the separation of the early and late application of the interventions remains unexplored (Citation14–16). Moreover, previous attempts to consolidate research on rehabilitation for oculomotor deficits in the form of evidence synthesis (Citation9–11) come with methodological limitations including (i) the amalgamation of patients with various TBI severities, ranging from mild to severe, and types of brain injuries, both traumatic and non-traumatic in origin (Citation9–11); (ii) combining restitutive, compensatory, substitutive and pharmacological approaches (Citation10), and (iii) not having an adequate number of studies to perform a meta-analysis (Citation10).
Another limitation of previous research is the lack of attention to sex and gender effects. Traditionally females have been underrepresented in mTBI research, despite knowledge that there are differences in injury presentation and recovery with women reporting higher rates of multisensory symptoms and those of child rearing age being at a higher risk for prolonged recovery compared to males (Citation17–19). Furthermore, the interplay between biological sex and gender on various aspects of behaviors has shown to influence the probability of sustaining mTBI, symptom load, entry to rehabilitation, response to treatment, experiences of disability and other parameters of recovery and outcomes (Citation17–20). Failing to explore and address these differences could lead to overlooking notable variations in the optimal timing (how soon after injury), dose and duration of treatment that differs between each sex. This in turn creates a missed opportunity for yielding research that has generalizable insights considering biological and sociocultural differences between men, women, and gender-diverse people (Citation17–20).
When considered together, restitutive approaches in rehabilitation are thought to remediate oculomotor deficits in individuals with mTBI. However, a more up-to-date systematic review and meta-analysis is required to improve our understanding of interventions’ specifics and which component(s) can influence the magnitude of the intervention effects.
Why this review is needed
To our knowledge this is the first systematic review and meta-analysis that specifically focuses on the efficacy of restitutive rehabilitation interventions for oculomotor deficits in adults with mTBI, taking into consideration sex and gender effects. The limitations of previous attempts to consolidate research on rehabilitation for oculomotor deficits in mTBI are considered in our systematic review. Furthermore, the Consensus Statement on Visual Rehabilitation in mTBI called for a systematic review and meta-analysis to clarify the state of the evidence regarding rehabilitation for visual symptoms following mTBI due to the existing uncertainty (Citation21). This was echoed in a recent guideline which stated that there is insufficient evidence to suggest for or against the use of any modality for the treatment of visual symptoms attributed to mTBI (Citation22). Therefore, despite its use in clinical practice, there remains uncertainty regarding the efficacy of oculomotor rehabilitation in mTBI for improving oculomotor function and patient centered outcomes such as return to work or sport (Citation9–11,Citation20,Citation21). Thus, our systematic review aims to fill this knowledge gap by providing a comprehensive evidence synthesis on the topic.
Objectives
The primary objectives of this systematic review were to: 1) provide a summary and critical evaluation of the evidence of the efficacy of restitutive oculomotor interventions in improving oculomotor function among adults with mTBI; 2) report on specific intervention characteristics that may have impact on results of interventions; and 3) incorporate a sex and gender lens in the analysis and reporting of results. The review considered research articles reporting on restitutive oculomotor interventions in adults with mTBI, including randomized controlled trials, quasi-experimental designs, case series, case cross-over.
Methods
This review was developed in compliance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Checklist (Citation23). The review was registered with the International Prospective Register of Systematic Reviews on August 23, 2022 (PROSPERO registry CRD42022352276). The methodology for the review was developed a priori and documented both in the registry and in a published protocol (Citation24).
Eligibility criteria
Inclusion criteria
To be considered for inclusion in this review, research had to be (Citation1) an original peer-reviewed English-language study; 2) an intervention (including randomized controlled trials, quasi-experimental designs, case series, case cross-over); 3) focus on rehabilitation of oculomotor deficits in adults with mTBI; and 4) evaluate the effectiveness of intervention.
Types of participants
We included studies that involved adults with mTBI (mean age 16 and over) of both sexes, with the presence of oculomotor deficits as determined by assessment by an optometrist or other regulated healthcare professional.
Types of interventions
We included studies of any restitutive oculomotor-based intervention compared to usual care, other interventions (compensatory, substitutive, pharmacological), placebo, or no comparator. Interventions were classified as restitutive if they included strategies aimed at restoring oculomotor function using various eye movement exercise approaches.
Types of outcomes
We included studies reporting on the effect of oculomotor rehabilitation on both oculomotor deficits and visually related tasks, including visual search and attention and reading rate.
Exclusion criteria
We excluded studies that: 1) were conference abstracts, theses, reviews, non-peer-reviewed articles, gray literature, descriptive or editorials, as these sources are unlikely to present the information at the level of detail and rigor for the purposes of a systematic review; 2) included moderate and severe traumatic brain injury, or other categories of acquired brain injury without providing results for mTBI participants separately, 3) examined interventions other than oculomotor rehabilitation; or 4) were qualitative research studies.
Information sources
The lead author, in collaboration with an information specialist at the institution with which authors were affiliated, developed a comprehensive search strategy. Search terms were identified by reviewing the literature and existing reviews on similar topics. The reproducible strategies are available https://figshare.com/s/0026079cac43d2cc17fd.
Search strategy
Electronic searches
The following databases were searched: Medline Ovid, Embase Ovid, PsychINFO Ovid, Cumulative Index to Nursing and Allied Health Literature, and Scopus. The searches were completed on March 15, 2023, using each database’s inception date as a starting point to maximize search breadth (e.g., 1946 for Medline, 1967 for PsycINFO, etc.). Citations were saved in Endnote 20 software and duplicates were removed by the lead author. The search strategy used both text and index terms to capture the concepts of ‘oculomotor,’ ‘rehabilitation’ and ‘mild traumatic brain injury.’
The reference lists of retrieved publications that passed the first screening (detailed below), and references of review articles on the topic of vision therapy and/or oculomotor interventions following mTBI were manually searched for additional studies. Additionally, experts in the field and researchers engaged in related work were contacted to identify any additional relevant works (Citation25,Citation26).
Searching other resources
Additional studies were identified through the following resources: 1) the reference lists of existing related reviews (Citation3,Citation21,Citation22); 2) Google Scholar (search conducted on March 1, 2023); and 3) clinical trial registries (https://who.int/ictrp/en, ClinicalTrials.gov, ukctg.nihr.ac.uk, anzctr.org.au).
Selection process
The screening and selection of articles was completed in two stages. Two review authors (MBi, ZG) first independently screened the titles and abstracts to identify relevant studies. Citations potentially meeting the inclusion and exclusion criteria were included for a second full text screen. During the second stage, an assessment of the full text by two independent reviewers (MBi, ZG) determined if the studies were to be included in the review. At both screening stages, the two independent reviewers assessed the studies’ eligibility against the inclusion and exclusion criteria. If discrepancies were found at any stage, the two review authors discussed and reached consensus. There was no need to consult a senior review author.
Data collection
Data were collected from studies passing the second screen and tabulated by two review authors independently (MBi, ZG) using standardized data extraction tables, developed in collaboration with a senior review author (TM).
Data items
Data items were extracted using the PICO framework into standardized tables and include information concerning: 1) participants: age, sex, mechanism of injury, time since injury, inclusion and exclusion criteria, confirmation of mTBI; 2) intervention: details of the intervention, frequency, and length of intervention and professional delivering the intervention, 3) comparison group: details of the comparison, frequency, and length of intervention and professional delivering the intervention; and 4) outcomes: key findings, significance and adverse events. Study location, setting and funding sources were also extracted. Data extraction and tabulation were conducted independently by two reviewers (Mbi, ZG) for study characteristics and outcome data. The study authors then combined and refined the content of the table with feedback from senior author (TM) until consensus was met with respect to quality and quantity of content.
Assessment of risk of bias in included studies
This review utilized the National Institute of Health risk of bias (RoB) assessment tool for before-after studies with no control group (Citation27). According to the tool, studies were graded by answering 12 questions regarding the objective, population, intervention, comparison, and outcomes. These questions were rated as yes (Y), no (N), cannot determine (CD), not applicable (NA), or not reported (NR). Specific questions and ratings are available in Supplementary material Table S3. For the crossover studies, the Cochrane RoB 2 for crossover studies was used examining biases in: 1) the randomization process; 2) carryover effects; 3) deviation from the intended interventions; 4) missing outcome data; 5) measurement of outcomes data; and 6) selection of reported result (Citation28). Detailed information is provided in Supplementary material Table S4.
Meta-analysis and synthesis methods
A best synthesis approach (i.e., tabulation) was used to organize study results (Citation29). Data were tabulated according to components of the research question and outcomes of interest. Meta-analyses were conducted using fixed-effects models, which allow conditional inference based on the k studies included in the analysis (Citation30). Outcomes were expressed as standardized mean differences (SMD), standard error of the SMD (SESMD) and 95% confidence intervals (CI). Studies with fewer than five participants were not included in the meta-analysis.
Measures of effect of treatment
This review employed fixed effects analyses, considering both within and between study heterogeneity. The SMD was utilized to assess the overall impact of the intervention in cases where two or more studies reported on the same continuous variable outcome and provided sufficient data for meta-analysis, including means and standard deviations. For outcomes reported in a single study, narrative analyses were conducted.
Assessment of heterogeneity
Heterogeneity in outcome effects was assessed using Cochrane’s Q and I2 (Citation31). In cases where I2 was negative, it was converted to 0, in line with recommendations by Higgins and colleagues (Citation31). All calculations were performed using Excel version 16.71.
Certainty assessment
We graded the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework (Citation32). The GRADE framework uses the domains of risk of bias, precision, consistency, directness, and publication bias to inform a rating of the evidence as very low, low, moderate, and high.
Dealing with missing data
In the event of missing data, we reached out to the study authors (Citation14–16,Citation33–35) by e-mail. For two studies missing data could not be obtained (Citation33, Citation34).
Summary of findings table
A ‘Summary of Findings’ for all pre-defined outcomes is presented in .
Table 1. Summary of main findings: setting and demographics. P: adults with mTBI I: oculomotor rehabilitation C: any comparator O: any oculomotor outcomes.
Table 2. Summary of findings: interventions and outcomes. P: adults with mTBI I: oculomotor rehabilitation C: any comparator O: any oculomotor outcomes.
Results
Study selection
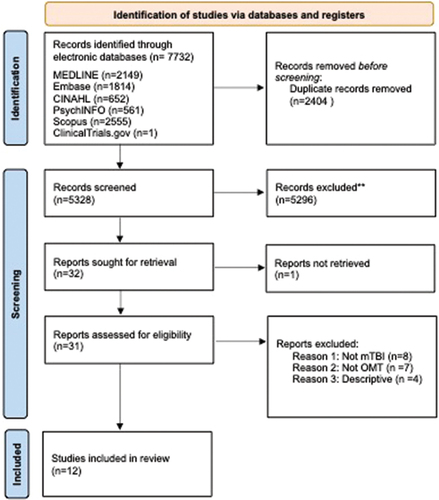
The electronic database search strategy and complementary searches yielded 5328 citations after removing duplicates. Two study authors (MBi, ZG) independently performed a title and abstract screen, identifying 31 citations for full text review. Of these, 12 publications met the inclusion criteria (Citation14–16, Citation32–34, Citation36–41). shows the flowchart of the literature search and selection process, along with the number of articles at each step.
Excluded studies
Nineteen studies did not meet the inclusion criteria and were excluded during the full-text review stage (Citation35,Citation42–59), as detailed in Supplementary Material Table S2. The primary reasons for study exclusion were: 1) inclusion of injury severities other than mTBI (Citation35,Citation42–46); 2) inclusion of interventions other than oculomotor (Citation47–52); and 3) studies that had a descriptive nature solely (Citation53–59).
Study characteristics
Included studies
Of 12 studies included, seven were pre-post intervention case series (Citation14–16,Citation33–36) and five crossover design studies (Citation33,Citation37–40). Characteristics of included studies are detailed in .
Study setting
Eleven studies took place in university-affiliated vision science clinics (Citation14,Citation15, Citation32–34, Citation36–41), and one study in a private optometry clinic (Citation16). Of the 12 studies, ten were conducted in the USA (Citation14,Citation16, Citation33, Citation34, Citation36–41), one in Sweden (Citation36) and one in Denmark (Citation15). Ten studies reported sources of funding, which included the Department of Defence (Citation33, Citation36–40), educational institutional funding (Citation15), government granting agencies (Citation14), and foundation grants (Citation36). Two studies did not report whether they were funded or not (Citation16, Citation34).
Participants
The total number of participants with mTBI and oculomotor deficits across 12 studies was 354. Across all studies, participants had a mean age of 25 years, with an age range from 6 to 72 years (Citation34). All studies included both males and female participants, with male participants making up on average 47% of participants (ranging from 13% (Citation33) to 50% (Citation15)).
Time since injury
Time since injury at baseline assessment was reported in all studies. The minimum time since injury was one week (Citation16), and the mean number of weeks since injury across studies was 9.2 weeks (standard deviation (SD) ±4.3 weeks). Eleven of the 12 studies indicated that participants sustained a medically documented mTBI prior to enrolling in the study, one study (Citation15) did not report how mTBI was ascertained. Nine of the 12 studies reported exclusion criteria, such as neurological stability, other ocular-health related exclusions, and age limits, while three (Citation16,Citation36,Citation39) did not provide information on exclusion criteria.
Intervention
There was variability in the delivery of the oculomotor-based interventions with respect to several parameters: 1) the frequency included weekly (Citation14), bi-weekly (Citation37) and irregular frequency (Citation36); 2) the duration: sessions ranged from 40 minutes (Citation16) to 60 minutes (Citation36) (mean 44.5 ± 5.0); 3) the total number of weeks of intervention: ranging from 4.5 weeks (Citation16) to 13.6 weeks (Citation14); and 4) the number of sessions: mean number was 9.3 (standard deviation (SD): ±4.3; range: 3–19) (Citation34, Citation36). Among the 12 studies, five incorporated a homework component, with durations ranging from five to 15 minutes per day, completed between treatment sessions (Citation28–30, Citation33, Citation34). In one study, compliance was monitored using a diary (Citation30). The remaining studies did not provide information regarding the way compliance was ascertained. Eleven studies provided one-on-one sessions (Citation14,Citation16, Citation32–34, Citation36–41), and one study (Citation15) delivered the intervention in groups of four to six individuals. One study included both in-person (face-to-face) and virtual sessions (Citation36).
Delivery of intervention
The intervention was administered by a variety of healthcare professions, with some studies involving multiple healthcare professionals:
Optometrists (Citation14–16, Citation32–34,Citation36–41)
Orthoptist (Citation34)
Para-optometric technician (Citation16)
Speech language pathologist (Citation15)
Vision therapist (Citation36)
Content of the intervention
Oculomotor-based rehabilitation encompasses a variety of activities, with adaptation of exercises (i.e., target size (Citation39), and endurance (Citation40)) to the specific needs of individual participants. All studies progressed participants to more challenging activities (i.e., reducing the target size (Citation39)), as participants were able to tolerate the activities. Eleven studies included a component of the intervention delivered via computer program (Citation14, Citation32–34, Citation36–41). Oculomotor-based exercises included a variety of skills training, including but not limited to fixation (Citation37), vergence (Citation41), version (Citation40), accommodation (Citation34) activities, and saccades (Citation15). Of the 12 studies, one did not report the use of prisms as part of the intervention (Citation15); the rest reported using prisms (Citation14,Citation16, Citation32–34, Citation36–41).
Feedback such as encouragement and verbal motivation were reported in three studies (Citation16, Citation34, Citation36). Three studies (Citation14,Citation16, Citation34) drew on the Convergency Symptom Insufficiency Treatment Trial (CITT) (Citation60,Citation61,Citation62) as a basis for intervention component selection (Citation14,Citation16, Citation34).
Results of studies
Oculomotor outcomes
There was heterogeneity between studies in the choice of oculomotor outcomes. Studies examined several outcomes not limited to near point of convergence (NPC) break and recovery, symptoms suggestive of convergency insufficiency (CISS), near point of accommodation (NPA), amplitude of accommodation (AA), positive and negative fusional vergence (PFV, NFV), vergence facility (VF), reading rate and visual search and attention test (VSAT) (see : Summary of main findings).
Meta-analysis
The meta-analytic component of this review aimed to identify possible differences from pre- to post-intervention in measures of oculomotor function and visual tasks (see ).
Five studies reported on NPC break (Citation14,Citation32–34,Citation38) and CISS (Citation14,Citation32–34,Citation37), three reported on PFV (Citation14,Citation32,Citation34), and two reported on VF (Citation14,Citation32), reading rate (Citation15,Citation38) and VSAT (Citation40,Citation41) each. Results revealed significant differences from pre to post-intervention in each of these measures: NPC break SMD: −5.24 (n = 17, CI: −6.71, −3.91; SESMD: 0.71, I2 = 62.2) (Citation14,Citation38); CISS SMD: −4.87 (n = 17, CI: −6.16 to −3.59; SESMD: 0.65, I2 = 0) (Citation14,Citation37); PFV SMD: 5.5 (n = 17, CI: 4.09, 6.91; SESMD: 0.72, I2=0) (Citation14,Citation37); VF SMD: 5.65 (n = 17, CI: 4.19, 7.11; SESMD: 0.70, I2 = 53.7) (Citation14,Citation37); reading rate SMD: 1.26 (n = 36, CI: 0.62, 1.61; SESMD: 3.72, I2 = 38.67) (Citation15,Citation38); and VSAT SMD: 2.37 (n = 19, CI: 1.52, 3.23; SESMD: 0.44, I2 = 87.15) (Citation35,Citation40). Please refer to Figures S1 to S6 and the supplementary material for additional information.
Requirements for meta-analysis were not met for NFV (Citation33,Citation39), AF (Citation32–34), and AA (Citation33,Citation41) and NPA (Citation33,Citation37).
Patient-centered outcomes
Assessment of the influence of the intervention on occupation-based outcomes was reported in one study (Citation15). This study (Citation15) included the Canadian Occupational Performance Measure (Citation63), which incorporates the individual’s own perception of meaning, performance, and satisfaction on different chosen activities. The results showed improvement in items meaningful to the participant. One study reported on time to resolution of deficits and return to sport (Citation16). Participants were required to report improvement in symptoms and demonstrate resolution of deficits using traditional oculomotor tests and sport-specific challenges (Citation16).
Sex and gender considerations
Of the 12 studies, nine (Citation32–34,Citation36–41) provided a breakdown of the number of males and females in the study. Three study authors provided additional sex-based data that was not reported in the publication (Citation14–16). There was no reporting on sex differences in the results, and no discussion on gender-related characteristics that might have impacted the outcomes.
Adverse events and participant experience
No adverse effects related to study intervention were explicitly reported for any of the included studies. Two studies reported that participants tolerated the interventions well (Citation14,Citation15). One study involving a case series of three participants described that the participants reported a ‘positive experience’ and an increased understanding of their visual function in relation to their recovery (Citation36).
Risk of bias assessment
Risk of bias assessment found an overall high risk of bias for pre- and post-intervention studies with no comparison group (Citation14–16,Citation32–35). The studies ranged from a score of three to seven out of 12 on questions assessing internal and external validity. Similarly, the RoB assessment for the five crossover studies revealed a high risk of bias on the basis that there were ‘some concerns’ and ‘high’ RoB for multiple domains (Citation36–40). The final categorization of studies as low, medium, or high RoB resulted from continuous discussion between the authors. Please refer to the supplementary material: Table S3 and Table S4 for individual study scores.
Certainty of the evidence
Using the GRADE approach, we determined the certainty of the evidence to be low with respect to oculomotor rehabilitation conferring improvement in NPC, CISS, PFV, VF, VSAT, and reading rate in adults with mTBI due to: 1) high risk of bias; 2) imprecise effect estimates; 3) indirectness for studies with no comparison group; 4) inconsistency as indicated by a moderate I2 for two outcomes and a high I2 for two outcomes out of the six outcomes included in the meta-analysis; and 5) potential for publication bias due to an absence of study protocols published a priori across all studies (Citation64–66). Please refer to the supplementary material Table S5.
Discussion
Results in context
This systematic review and meta-analysis synthesized evidence for the efficacy of restitutive oculomotor interventions in adults with mTBI. The review included 12 studies with a total of 354 participants, reporting on the effects of restitutive oculomotor rehabilitation in adults with mTBI, with variations in content, timing, and duration. Sex and gender differences in response to intervention were not investigated in the studies included in this review. The limited published research on the topic is alarming, considering the frequency with which symptoms suggestive of oculomotor deficits present in individuals with mTBI, and that research into these interventions started over ten years ago (Citation7,Citation37).
While the available evidence suggests a direction of improvement in all outcomes that were included for meta-analysis (NPC break, CISS, PFV, VF, reading rate and VSAT) in the presence of oculomotor-based rehabilitation, it is important to note that there was a high RoB in all studies. The meta-analyses indicated a small size improvement in reading rate and VSAT and a moderate size improvement in NPC, CISS, PFV and VF. However, caution is warranted when interpreting the estimated effects in a meta-analysis of small sample size studies, as in our case. It is advisable to focus on the confidence intervals, where a narrow confidence interval provides greater confidence that the estimated effect is stable (Citation67). In the case of our analysis, confidence intervals of the effect (SMD) for reading rate and VSAT were narrow (less than one standard deviation); and for NPC, CISS, PFV and VF were moderate (between one and two standard deviations) (Citation65). These effects were accompanied with an improvement in patient-centered outcomes, such as the knowledge and understanding of the visual component of symptoms on the Canadian Occupational Performance Measure (Citation15) and return to sport (Citation63). However, due to a lack of high-quality studies, variations in the efficacy, timing, and characteristics of the interventions, inadequate reporting of data, and a lack of attention to sex and gender differences in outcomes, the clinical certainty in the reported results and generalizability to all people with mTBI in clinical settings is low.
Agreements and disagreements with other studies or reviews
Findings from our systematic review are consistent with findings from previously published systematic reviews and results of the recent Consensus Statement on Visual Rehabilitation in mTBI (Citation10,Citation11,Citation21). In a recent systematic review encompassing studies of individuals with acquired brain injuries of diverse etiologies including stroke and traumatic brain injury, Watabe and colleagues found nine studies reporting on interventions ranging from three to ten weeks with two to five intervention sessions per week (Citation11). However, in our systematic review in the context of mTBI, the reported frequency was between one and two sessions per week. This emphasizes the importance of considering individuals with mTBI separately from other types of TBI, as different frequencies and durations of rehabilitation may be needed.
An earlier systematic review by Rowe and colleagues evaluated the effectiveness of both pharmacological and non-pharmacological interventions in persons with acquired brain injury with mixed etiologies (Citation10). The review included only randomized controlled trials published before 2017 and included only one study of oculomotor rehabilitation (Citation10). In line with our findings, the authors reported that the overall quality of the evidence was low, due to small sample sizes (imprecision), high RoB risk due to selective reporting that did now allow for the calculation of effect sizes and an absence of published protocols (Citation10). The authors concluded, in that while insufficient evidence exists to truly guide treatment decisions, oculomotor-based rehabilitation demonstrates promising efficacy reducing oculomotor deficits in individuals with mTBI (Citation10,Citation11). Our results align with this conclusion.
Finally, the Consensus Statement on Visual Rehabilitation in mTBI provided a narrative literature review and critique of the current landscape of evidence (Citation21). Specific to oculomotor rehabilitation, the consensus cited that while it is a popular approach which has had success in other populations, such as children and young adults with convergence insufficiency, evidence for its use in mTBI is lacking (Citation21,Citation60). The consensus, citing Cochrane, assigned a low certainty rating to the existing evidence, attributed to factors such as small sample size and suboptimal statistical analyses, including the use of uncorrected t-tests for multiple comparisons (Citation21). Our results support the consensus’ conclusions and emphasize the need for methodologically stronger rehabilitation research targeting oculomotor deficits in individuals with mTBI. In alignment with our findings, previous reviews also noted the absence of adverse events and observed a positive trend toward the efficacy of oculomotor rehabilitation (Citation16). This is especially pertinent in light of the increasing frequency in which oculomotor rehabilitation is used by clinicians in individuals with mTBI, and its potential for improving oculomotor deficits that may contribute to headaches, fatigue, feeling overstimulated in visually stimulating environments that may interfere with activities of daily living and quality of life (Citation9).
Sex- and gender-based analyses
The design and analysis of the included studies did not account for sex and/or gender effects. Despite the inclusion of both males and females in all studies, none of the results were stratified by sex, and the authors did not provide any justification for the absence of sex-stratified analysis. Gender-related characteristics (i.e., roles, responsibilities, identities, etc.) of research participants were also not reported across studies. The Sex and Gender Equity in Research Guidelines (Citation68,Citation69) were published in 2016, yet none of the studies included in the review published after the guideline’s release, adhered to the recommended guidelines. The absence of research that considers the unique experiences of people of different sexes and genders has led to an incomplete understanding of mTBI, oculomotor deficits, and intervention effectiveness. This underscores the continued need for intentional implementation of sex and gender considerations in research design and reporting in oculomotor research in individuals with mTBI (Citation69). This is especially relevant given the known physiological differences, such as longer recovery times, and social differences, including gender roles such as occupation, in mTBI injury experiences, which may play a role in research participation, adherence to recommended exercises, and response to oculomotor rehabilitation efforts (Citation70,Citation71).
Overall completeness and applicability of the evidence
There was variation in the study samples’ characteristics in terms of patient population (age range, mTBI definition, percent male), intervention (timing of application, frequency) and studied outcomes (convergence, accommodation, patient-specific outcomes) which affected the efficacy of the results.
Patient population
There are numerous patient characteristics that might potentially influence the efficacy of restitutive interventions. While there were some younger and older participants included (Citation16,Citation34), the mean age across studies was young adults (25 years) having sustained a mTBI, and all studies included both sexes. Despite age related changes in ocular function, we did observe in one case series with a participant over the age of 50 years old, and, in a retrospective chart review, which included individuals up to 72 years of age, improvements in measures of convergence and accommodation (Citation34,Citation36). The range of relevant patient characteristics were likely be related to the complexity and intensity of interventions; however, researchers did not discuss how multiple relevant patient characteristics (sex, age, level of baseline deficit, applied effort) interacted with the multiple potential rehabilitation components (number of sessions, choice of intervention), making the detection of intervention effect at the level of individual complex.
Intervention
Oculomotor deficits in people with mTBI may result from damage that occurs through a variety of mechanisms at the cortical and subcortical level (Citation8). The mechanisms targeted by the studies included in this review focused on approaches that work by supporting biochemical events that help restore function in neural tissue (Citation12,Citation13). Timing of restitutive intervention initiation after mTBI was variable across the studies with most of the studies applying the intervention one year or more after mTBI (Citation14,Citation15,Citation32–34,Citation36–41). One rational was to ensure that the ‘natural healing process’ had occurred. However, more recent evidence and guidelines for mTBI rehabilitation recommend that early intervention, which is associated with better outcomes (Citation16,Citation72).
Outcome measures
The included studies used a comprehensive set of outcomes measures not limited to vergence, accommodation, reading rate, and symptoms such as those suggestive of convergence insufficiency, to evaluate the effectiveness of oculomotor rehabilitation. Variation in the choice of outcomes is reasonable, given that depending on the specific deficits presented in each participant in the sample, not all outcomes will be relevant (Citation36). However, the main issue, as indicated by our meta-analysis, is that few rehabilitation studies had a sufficient sample size to detect meaningful outcomes for individual persons and group differences in each outcome concurrently. There is a recognized need for research reporting individual person data to enhance the precision and depth of understanding the efficacy of oculomotor rehabilitation (Citation73).
Currently, knowledge of psychometric properties for self-reported outcomes such as those measured by the convergence insufficiency symptoms survey relevant to the utility to detect change in response to intervention in persons with mTBI is limited, which raised the question whether the reported improvement reflects a clinically meaningful difference for this specific population (Citation21). Furthermore, while one study looked at the persistence of improvement following oculomotor rehabilitation, the results were mixed with insufficient reporting of data, leaving uncertainty of whether the level of improvement experienced after oculomotor rehabilitation will remain stable over time.
Safety and adverse effect
Our review found that none of the studies explicitly reported the presence of any adverse events, and one study reported ‘no adverse events.’ However, there was a lack of transparency regarding drop out and loss to follow up. It remains unknown whether participants experiencing a limited or negative response to intervention chose not to continue.
Potential limitations in the review process
There are several limitations in this systematic review. We excluded unpublished research, thesis (Citation74), conference abstracts (Citation75), gray literature and limited inclusion to studies published in the English language, which may have led to missing relevant evidence. The meta-analysis we conducted required statistical assumptions of homogeneity, consistency and an absence of publication bias that were not met, and therefore the results of the meta-analysis we presented should be interpreted with caution. Furthermore, the use of pre-post SMD for meta-analysis is discouraged by some authors as they are not independent of one another and may result in biased results (Citation76).
The meta-analysis for NPC, CISS, PFV, VF, reading rate, and VSAT was performed with very few studies, as only two studies for each outcome provided sufficient data for the analysis. The use of fixed-effects models restricts the applicability of results solely to the included studies. Furthermore, we observed high study heterogeneity in one measured outcome (visual search and attention) but were unable to perform random-effects analysis due to large within-study variance (Citation35). Despite statistically significant differences for all reported outcomes, the clinical meaningfulness of these findings remains uncertain (Citation35).
In addition, recognizing the need for a comprehensive approach to evaluate rehabilitation outcomes in clinical settings, our rationale for emphasizing individual measures in the meta-analysis centers on three critical considerations. First, analyzing diverse oculomotor rehabilitation measures, enriches our understanding of visual function in mTBI. Second, despite potential questions, focusing on individual markers is crucial for comprehending overall impact, particularly given challenges in standardizing diagnostic criteria for mTBI-related visual impairments. Lastly, our goal was to explore trends across diverse interventions and populations and provide a robust synthesis of evidence, recognizing the inherent complexity of a comprehensive clinical diagnosis.
Implications for clinical practice
This systematic review demonstrated only low certainty evidence for the efficacy of restitutive interventions for managing oculomotor deficits in patients with mTBI. However, the meta-analysis found a consistent trend of improvement, there were no reported adverse events, and patients reported the interventions helped them understand their visual issues after mTBI (Citation36). Given the nuance of mTBI, and the rapidly evolving field of rehabilitation, the variation in protocols between studies included in this review is reasonable, and, this aligns with the perspective that, in the presence of some evidence of benefit and low risk to the patient, the use of said intervention, such as restitutive oculomotor rehabilitation, with patient-centered goals, may be clinically beneficial in adults with oculomotor deficits and mTBI. Furthermore, emphasizing the importance of personalized interventions that meet the needs of people with diverse cognitive profiles, exploring adaptive strategies to address sensory processing, attentional challenges, and executive functions, collaborative efforts with healthcare providers to manage both mTBI and other symptoms concurrently (i.e., migraine, fatigue, etc.) are crucial.
Implications for future research
The limited number of studies with variation in timing, frequency, total number of sessions for the intervention, inconsistency in the outcome data, and the lack of attention to sex and gender highlights the need for the development of robust rehabilitation trial protocols for oculomotor interventions in mTBI. Comparative studies against the current standard of care to ascertain the relative effectiveness of interventions.
In addition, the timing of application of oculomotor rehabilitation initiation in patients with mTBI needs to be determined. Investigating whether earlier commencement of interventions yields superior results, as suggested in research by Peters and Price (Citation15), and if this differs between age groups or sex and/or gender warrants further exploration (Citation36). Standardization is another critical aspect. Establishing a consistent set of outcome measures for the most frequently occurring deficits and for evaluating the efficacy of interventions is greatly needed. This standardization will not only promote consistency across studies but also facilitate evidence synthesis in future systematic reviews and meta-analyses.
Transparency in intervention study protocols is essential for future research, with the availability of study protocols in open access form, along with a priori statistical analysis plans, to ensure research rigor. In cases where multiple comparisons are involved, it is advisable to apply appropriate statistical corrections, such as Bonferroni adjustments, to mitigate the risk of type I errors (Citation77). However, this is rarely feasible due to the limited sample size in rehabilitation trials (Citation77). Furthermore, there is a need for more long-term studies to assess if the benefits of restitutive-oculomotor rehabilitation are stable over time. Only, one study in our review examined the long-term persistence of benefit of oculomotor rehabilitation in individuals with mTBI. Clarifying if timing, duration, and frequency of the intervention, or other parameters such as home-based reinforcement, influence the long-term effects is also needed to determine efficacy and inform best practices.
Sex and gender-based considerations should be integrated early into the development of hypotheses, data collection, analysis, interpretation, and reporting of results. This would account for potential biological and sociocultural differences in response to rehabilitation interventions. Finally, researchers should ensure that study results are reported with the necessary data for the potential of conducting a meta-analysis of individual patient data.
Conclusions
Findings from this systematic review and meta-analysis of non-randomized studies indicate that oculomotor rehabilitation in individuals with mTBI may improve a variety of oculomotor deficits, such as convergence insufficiency, and patient-centered outcomes, such as occupational performance measures. However, there continues to be a lack of studies overall and a lack of controlled studies with an appropriate comparison group, and existing studies suffer from high RoB, conferring uncertainty on the efficacy of these interventions. A randomized controlled trial comparing oculomotor rehabilitation to usual care is needed to strengthen the level of evidence. In addition, future research should aim to resolve uncertainties around 1) optimal timing of intervention, 2) if specific domains of oculomotor deficits respond better to rehabilitation, and 3) if treatment effect varies based on biological (i.e., age, sex) and sociocultural (i.e., gender) characteristics of the individual with mTBI.
Glossary and description of complex terms
Abbreviations
| AA | = | Amplitude of accommodation |
| CD | = | Cannot determine |
| CI | = | Confidence interval |
| CISS | = | Convergence insufficiency symptom survey |
| CITT | = | Convergence insufficiency treatment trial |
| GRADE | = | Grading of recommendations, assessment, development and evaluations |
| MD | = | Mean difference |
| mTBI | = | Mild traumatic brain injury |
| N | = | No |
| NA | = | Not applicable |
| NFV | = | Negative fusional vergence |
| NPC | = | Near point of convergence |
| NR | = | Not reported |
| PFV | = | Positive fusional vergence |
| RoB | = | Risk of Bias |
| RR | = | Reading rate |
| SESMD | = | Standard error of the SMD |
| SMD | = | Standardized mean differences |
| VF | = | Vergence facility |
| VSAT | = | Visual search and attention test |
| Y | = | Yes |
Authors contributions
MBi proposed the review question, co-ordinated the review, organized retrieval of the papers and wrote to trial authors for additional information. MBi and ZG screened search results, screened retrieved papers against inclusion criteria, appraised quality of papers, and extracted data from papers with ongoing guidance from TM. AC, MB provided editorial feedback during protocol preparation and publication, and editorial feedback for the final manuscript. TM provided extensive methodological expertise and editorial feedback for the protocol and final manuscript.
Supplemental Material
Download MS Word (29.6 KB)Acknowledgments
We would like to acknowledge Jessica Babineau (Information Specialist) for her assistance with developing the search strategy, Professor Kevin Thorpe for his feedback on methods. This work was undertaken in partial fulfillment of M. Biscardi PhD degree with Professors Angela Colantonio and Tatyana Mollayeva as co-supervisors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data used for the completion of this review is available on request from the corresponding author (MBi). Database search strategies are available at: https://figshare.com/s/0026079cac43d2cc17fd.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02699052.2024.2320163
Additional information
Funding
References
- Langer L, Levy C, Bayley M. Increasing incidence of concussion: true epidemic or better recognition? J Head Trauma Rehabil. 2020;35(1):E60–E66. doi:10.1097/HTR.0000000000000503.
- Pierpoint LA, Collins C. Epidemiology of sport-related concussion. Clin Sports Med. 2021;40(1):1–18. doi:10.1016/j.csm.2020.08.013.
- Hiploylee C, Dufort PA, Davis HS, Wennberg RA, Tartaglia MC, Mikulis D, Hazrati LN, Tator CH. Longitudinal study of Post concussion syndrome: not everyone recovers. J Neurotrauma. 2017;34(8):1511–23. doi:10.1089/neu.2016.4677. Epub 2016 Nov 29. PMID: 27784191; PMCID: PMC5397249.
- Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, Grady MF. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260–67. doi:10.1177/0009922815594367.
- Scheiman M, Grady MF, Jenewein E, Shoge R, Podolak OE, Howell DH, Master CL. Frequency of oculomotor disorders in adolescents 11 to 17 years of age with concussion, 4 to 12 weeks post injury. Vision Res. 2021;183:73–80. doi:10.1016/j.visres.2020.09.011.
- Ventura RE, Jancuska JM, Balcer LJ, Galetta SL. Diagnostic tests for concussion: is vision part of the puzzle? J Neuroophthalmol. 2015;35(1):73–81. doi:10.1097/WNO.0000000000000223.
- Thiagarajan P, Ciuffreda KJ, Ludlam DP. Vergence dysfunction in mild traumatic brain injury (mTBI): a review. Ophthal Physl Opt. 2011;31(5):456–68. doi:10.1111/j.1475-1313.2011.00831.x.
- Tyler CW, Likova LT, Mineff KN, Nicholas SC. Deficits in the activation of human oculomotor nuclei in chronic traumatic brain injury. Front Neurol. 2015 Aug 25; 6: 173. doi:10.3389/fneur.2015.00173. PMID: 26379615; PMCID: PMC4548181.
- Simpson-Jones ME, Hunt AW. Vision rehabilitation interventions following mild traumatic brain injury: a scoping review. Disabil Rehabil. 2019;41(18):2206–2222. doi:10.1080/09638288.2018.1460407.
- Rowe FJ, Hanna K, Evans JR, Noonan CP, Garcia-Finana M, Dodridge CS, Howard C, Jarvis KA, MacDiarmid SL, Maan T, et al. Interventions for eye movement disorders due to acquired brain injury. Cochrane Database Syst Rev. 2018;2018(3):CD011290. doi:10.1002/14651858.CD011290.pub2.
- Watabe T, Suzuki H, Abe M, Sasaki S, Nagashima J, Kawate N. Systematic review of visual rehabilitation interventions for oculomotor deficits in patients with brain injury. Brain Inj. 2019;33(13–14):1592–96. doi:10.1080/02699052.2019.1658225.
- Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P, Livingstone K, Munro FA, Orr H, Rowe FJ, et al. Interventions for visual field defects in patients with stroke. Cochrane Database Syst Rev. 2011;10: CD008388. doi:10.1002/14651858.CD008388.pub2.
- Kerkhoff G. Neurovisual rehabilitation: recent developments and future directions. J Neurol Neurosurg Psychiatry. 2000 Jun;68(6):691–706. doi:10.1136/jnnp.68.6.691. PMID: 10811691; PMCID: PMC1736971.
- Scheiman MM, Talasan H, Mitchell GL, Alvarez TL. Objective assessment of vergence after treatment of concussion-related CI: a pilot study. Optom Vis Sci. 2017 Jan;94(1):74–88. doi:10.1097/OPX.0000000000000936. PMID: 27464574; PMCID: PMC5182092.
- Smaakjaer P, Wachner LG, Rasmussen RS. Vision therapy improves binocular visual dysfunction in patients with mild traumatic brain injury. Neurol Res. 2022;44(5):439–45. doi:10.1080/01616412.2021.2000825.
- Peters M, Price J. The peters/price (see to play) vision concussion protocol: diagnosis and treatment. Optom Vis Perform. 2015;3(2):126–38.
- Valera EM, Joseph AC, Snedaker K, Breiding MJ, Robertson CL, Colantonio A, Levin H, Pugh MJ, Yurgelun-Todd D, Mannix R, et al. Understanding traumatic brain injury in females: a state-of-the-art summary and future directions. Journal Of Head Trauma Rehabilitation. 2021 Jan 1;36(1):E1–17. doi:10.1097/HTR.0000000000000652.
- Niemeier JP. Biological sex/gender and biopsychosocial determinants of traumatic brain injury recovery trajectories. Curr Phys Med Rehabil Rep. 2019;7(4):297–304. doi:10.1007/s40141-019-00238-3.
- Mollayeva T, El-Khechen-Richandi G, Colantonio A. Sex & gender considerations in concussion research. Concussion. 2018 Jan 18;3(1). doi:10.2217/cnc-2017-0015.
- Mikolić A, van Klaveren D, Groeniger JO, Wiegers EJA, Lingsma HF, van Klaveren D, Polinder S, Åkerlund C, Amrein K, Andreassen L, et al. CENTER-TBI participants and investigators. Differences between men and women in treatment and outcome after traumatic brain injury. J Neurotrauma. 2021 Jan 15;38(2):235–51. doi:10.1089/neu.2020.7228. Epub 2020 Oct 19. PMID: 32838645. CNC51 PMID: 30202593; PMCID: PMC6094024.
- Subramanian PS, Barton JJS, Ranalli P, Smith C, Francis CE, Frishberg B. Consensus statement on visual rehabilitation in mild traumatic brain injury. Neurol Clin Pract. 2022;12(6):422–28. doi:10.1212/CPJ.0000000000200071.
- Eapen BC, Bowles AO, Sall J, Lang AE, Hoppes CW, Stout KC, Kretzmer T, Cifu DX. The management and rehabilitation of post-acute mild traumatic brain injury. Brain Inj. 2022;36(5):693–702. doi:10.1080/02699052.2022.2033848.
- Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, Tugwell P. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J Clin Epidemiol. 2015;14(1). doi:10.1186/s12939-015-0219-2. Elsevier; Sep10.
- Biscardi M, Grossinger Z, Colantonio A, Bayley M, Mollayeva T. Rehabilitation interventions for oculomotor deficits in adults with mild traumatic brain injury: a systematic review protocol. BMJ Open. 2023 Sep 15;13(9):e072786. doi:10.1136/bmjopen-2023-072786. PMID: 37714680.
- Singman E, Quaid P. Chapter 15: vision disorders in mild traumatic brain injury. In: Hoffer ME, and Balaban CD, editors. Neurosensory disorders in mild traumatic brain injury, 2019 pp. 223–44. London: Elsevier.
- Dawson D. (2023).University of Manitoba, Canada. Personal communication.
- National Heart, Lung, and Blood Institute. (2019). Study Quality Assessment Tools. https://www. nhlbi. nih.gov/health-topics/study-quality-assessment-tools
- Higgins JPT, Li T, Sterne J. Revised Cochrane risk of bias tool for randomized trials (RoB 2) additional considerations for crossover trials. Cochrane Methods. Cochrane Database Syst Rev. 2021. https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-crossover-trials.
- Slavin RE. Best evidence synthesis: an intelligent alternative to meta-analysis. J Clin Epidemiol. 1995;48(1):9–18. doi:10.1016/0895-4356(94)00097-A.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi:10.18637/jss.v036.i03.
- Higgins JP. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–60. doi:10.1136/bmj.327.7414.557. P MID: 12958120; PMCID: PMC192859.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed). 2008;336(7650):924–26. doi:10.1136/bmj.39489.470347.AD.
- Thiagarajan P, Ciuffreda KJ. Short-term persistence of oculomotor rehabilitative changes in mild traumatic brain injury (mTBI): a pilot study of clinical effects. Brain Inj. 2015;29(12):1475–1479. doi:10.3109/02699052.2015.1070905.
- Gallaway M, Scheiman M, Mitchell GL. Vision therapy for post-concussion vision disorders. Optom Vis Sci. 2017;94(1):68–73. doi:10.1097/OPX.0000000000000935.
- Yadav NK, Thiagarajan P, Ciuffreda KJ. Effect of oculomotor vision rehabilitation on the visual-evoked potential and visual attention in mild traumatic brain injury. Brain Inj. 2014;28(7):922–929. doi:10.3109/02699052.2014.887227.
- Möller ML, Melkas S, Johansson J. Improving visual function after mild traumatic brain injury using a vision therapy program: case reports. Brain Sci. 2020;10(12):947. doi:10.3390/brainsci10120947.
- Thiagarajan P, Ciuffreda KJ. Effect of oculomotor rehabilitation on vergence responsivity in mild traumatic brain injury. J Rehabil Res Dev. 2013;50(9):1223–1240. doi:10.1682/JRRD.2012.12.0235.
- Thiagarajan P, Ciuffreda KJ, Capo-Aponte JE, Ludlam DP, Kapoor N, Parente R, Parente R. Oculomotor neurorehabilitation for reading in mild traumatic brain injury (mTBI): an integrative approach. NeuroRehabilitation. 2014;34(1):129–46. doi:10.3233/NRE-131025.
- Thiagarajan P, Ciuffreda KJ. Accommodative and vergence dysfunction in mTBI: treatment effects and systems correlations. Optom Vis Perform Sci. 2014;2(6):280–88.
- Thiagarajan P, Ciuffreda KJ. Versional eye tracking in mild traumatic brain injury (mTBI): effects of oculomotor training (OMT). Brain Inj. 2014;28(7):930–943. doi:10.3109/02699052.2014.888761.
- Thiagarajan P, Ciuffreda KJ. Effect of oculomotor rehabilitation on accommodative responsivity in mild traumatic brain injury. J Rehabil Res Dev. 2014;51(2):175–91. doi:10.1682/JRRD.2013.01.0027.
- Ciuffreda KJ, Yadav NK, Thiagarajan P, Ludlam DP. A novel computer oculomotor rehabilitation (COR) program for mild traumatic brain injury (mTBI). Brain Sci. 2017;7(8):99. doi:10.3390/brainsci7080099.
- Conrad JS, Mitchell GL, Kulp MT. Vision therapy for binocular dysfunction post brain injury. Optom Vis Sci. 2017;94(1):101–107. doi:10.1097/OPX.0000000000000937.
- Facchin A, Figliano G, Daini R. Prism adaptation and optokinetic stimulation comparison in the rehabilitation of unilateral spatial neglect. Brain Sci. 2021 Nov 11;11(11):1488. doi:10.3390/brainsci11111488. PMID: 34827487; PMCID: PMC8615435.
- Johansson J, Berthold Lindstedt M, Borg K. Vision therapy as part of neurorehabilitation after acquired brain injury - a clinical study in an outpatient setting. Brain Inj. 2021;35(1):82–89. doi:10.1080/02699052.2020.1858495.
- Murray NP, Hunfalvay M, Roberts CM, Tyagi A, Whittaker J, Noel C. Oculomotor training for poor saccades improves functional vision scores and neurobehavioral symptoms. Arch Rehabil Res Clin Transl. 2021;3(2):100126. doi:10.1016/j.arrct.2021.100126.
- Waldorf GR. Treatment of traumatic brain injury-induced oculomotor and binocular vision dysfunctions with vision therapy. Optom Vis Perform. 2015;3(6):324–330.
- Watabe T, Suzuki H, Sako R, Abe M, Aoki K, Yoda M. Effect of an oculomotor rehabilitation program for subacute brain injury patients with ophthalmoplegia: a case-control study. Disabil Rehabil. 2022;44(22):6642–6648. doi:10.1080/09638288.2021.1970249.
- Carrick FR, Clark JF, Pagnacco G, Antonucci MM, Hankir A, Zaman R, Oggero E. Head–eye vestibular motion therapy affects the mental and physical health of severe chronic postconcussion patients. Front Neurol. 2017;8:414. doi:10.3389/fneur.2017.00414.
- Johansson J, Nygren De Boussard C, Öqvist Seimyr G, Pansell T. The effect of spectacle treatment in patients with mild traumatic brain injury: a pilot study. Clin Exp Optom. 2017;100(3):234–242. doi:10.1111/cxo.12458.
- Kontos AP, Collins MW, Holland CL, Reeves VL, Edelman K, Benso S, Schneider W, Okonkwo D. Preliminary evidence for improvement in symptoms, cognitive, vestibular, and oculomotor outcomes following targeted intervention with chronic mTBI patients. Mil Med. 2018;183(suppl_1):333–338. doi:10.1093/milmed/usx172.
- Rosner MS, Feinberg DL, Doble JE, Rosner AJ. Treatment of vertical heterophoria ameliorates persistent post-concussive symptoms: a retrospective analysis utilizing a multi-faceted assessment battery. Brain Inj. 2016;30(3):311–317. doi:10.3109/02699052.2015.1113564.
- Weissberg E, Lyons SA, Richman JE. Fixation dysfunction with intermittent saccadic intrusions managed by yoked prisms: a case report. Optometry (St Louis, Mo). 2000;71(3):183–88.
- Wetzel PA, Lindblad AS, Mulatya C, Kannan MA, Villamar Z, Citchel GT, Weaver LK. Eye tracker outcomes in a randomized trial of 40 sessions of hyperbaric oxygen or sham in participants with persistent post concussive symptoms. Undersea Hyperb Med. 2019;46(3):299–311. doi:10.22462/13.15.2019.8.
- Chang A, Cohen AH, Kapoor N. Top-down visual framework for optometric vision therapy for those with traumatic brain injury. Optom Vis Perform. 2013;1(2):48–53.
- Fimreite V, Willeford KT, Ciuffreda KJ. Effect of chromatic filters on visual performance in individuals with mild traumatic brain injury (mTBI): a pilot study. J Optom. 2016;9(4):231–239. doi:10.1016/j.optom.2016.04.004.
- Ciuffreda KJ, Kapoor N, Rutner D, Suchoff IB, Han ME, Craig S. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optom J Am Optom Assoc. 2007;78(4):155–61. doi:10.1016/j.optm.2006.11.011.
- Stelmack J. Measuring Outcomes of Neuro-Optometric Care in Traumatic Brain Injury. J Behavioural Optom. 2007;18(3):67–71.
- Valenti C, COVD Committee on Rehabilitative Optometry. Rehabilitation of persons with visual sequelae resulting from traumatic brain injury. J Optome Vis Development. 2003;34(3):105–10.
- Rouse MW, Borsting EJ, Mitchell GL, Scheiman M, Cotter SA, Cooper J, Kulp MT, London R, Wensveen J, Convergence Insufficiency Treatment Trial Group. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalm Physiol Opt. 2004 Sep;24(5):384–90. 10.1111/j.1475-1313.2004.00202.x PMID: 15315652
- Scheiman M, Gallaway M, Frantz KA, Peters RJ, Hatch S, Cuff M, MITCHELL GL. Near point of convergence: test procedure, target selection, and normative data. Optom Vis Sci. 2003 Mar;80(3):214–25. doi:10.1097/00006324-200303000-00011. PMID: 12637833.
- von Hippel PT.The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15(35):1–8. doi: 10.1186/s12874-015-0024-z.
- Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57(2):82–87. doi:10.1177/000841749005700207.
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt G. Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M Welch V editors. Cochrane handbook for systematic reviews of interventions version 6.3. 2022 February. Cochrane
- Kirmayr M, Quilodran C, Valente B, Loezar C, Garegani L, Franco JVA. The GRADE approach: how to access he certainty of the evidence. Medwave. 2021;21(2):e8109–8109. doi:10.5867/medwave.2021.02.8109.
- Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med. Feb 15 2021;31(1):010502. doi:10.11613/BM.2021.010502. Epub 2020 Dec 15. PMID: 33380887.
- Berryman A, Rasavage K, Politzer T, Gerber D. Oculomotor treatment in traumatic brain injury rehabilitation: a randomized controlled pilot trial. Am J Occup Ther. 2020;74(1):p74011850501–07. doi:10.5014/ajot.2020.026880.
- Canadian Institute of Health Research. Sex and gender-based analysis policy. 2023. https://cihr-irsc.gc.ca/e/50833.html.
- Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integrity Peer Rev. 2016;1(1):1–9. doi:10.1186/s41073-016-0007-6.
- Musko PA, Demetriades AK. Are sex differences in collegiate and high school sports-related concussion reflected in the guidelines? A Scoping Rev Brain Sci. 2023 Sep 12;13(9):1310. doi:10.3390/brainsci13091310. PMID: 37759911; PMCID: PMC10526868.
- Stergiou-Kita M, Mansfield E, Sokoloff S, Colantonio A. Gender influences on return to work after mild traumatic brain injury. Arch Phys Med Rehabil. 2016 Feb;97(2 Suppl):S40–5. doi:10.1016/j.apmr.2015.04.008. Epub 2015 Apr 25. PMID: 25921979.
- Patricios JS, Schneider KJ, Dvorak J, Ahmed OH, Blauwet C, Cantu RC, Davis GA, Echemendia RJ, Makdissi M, McNamee M, et al. Consensus statement on concussion in sport: the 6th international conference on concussion in sport–Amsterdam, October 2022. Br J Sports Med. 2023;57(11):695–711. doi:10.1136/bjsports-2023-106898.
- Hopkins C, Sydes M, Murray G, Woolfall K, Clarke M, Williamson P, Smith CT. UK publicly funded clinical trials units supported a controlled access approach to share individual participant data but highlighted concerns. J Clin Epidemiol. 2016;70:17–25.
- Richards D. The clinical value of oculomotor assessments across the continuum of concussion. Electronic Thesis And Dissertation Repository. 2022:9051. https://ir.lib.uwo.ca/etd/9051.
- Quaid P, Cunningham DJ. Effect of optometric visual therapy on a cohort of mTBI patients with oculomotor (OM) and visual information processing (VIP) deficits. J College Optom Vis Development. 2021;7(1). https://www.researchgate.net/publication/359400806.
- Cuijpers P, Weitz E, Cristea IA, Twisk J. Pre-post effect sizes should be avoided in meta-analyses. Epidemiol Psychiatr Sci. 2017 Aug;26(4):364–368. doi:10.1017/S2045796016000809.
- Chen SY, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis. 2017;9(6):1725–29. doi:10.21037/jtd.2017.05.34.