?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective
Older adults (OA) after mild traumatic brain injury (mTBI) have a high risk of developing persistent post-injury cognitive impairments. Lower pre-morbid cognitive reserve (CR) is increasingly investigated as a risk factor for cognitive dysfunction in OA. However, how CR protects against effects of mTBI at the brain level remains largely understudied.
Methods
We examined 22 OA who sustained mTBI (mean 67.69 years, SD 5.11) in the sub-acute phase and 15 age- and CR-matched healthy OA (mean 68 years, SD 5.55) performing a three-level visual N-back task using electroencephalography. We calculated inverse efficiency scores of performance from accuracy and reaction times. Event-related potentials served as neurocognitive correlates of attentional (P2) and working memory (P3) processing.
Results
Overall, mTBI OA performed worse than healthy OA (p = 0.031). Lower CR generally decreased performance (p < 0.001). Furthermore, with increasing task difficulty, task performance was more affected by CR (p = 0.004). At the brain level, P2 amplitude was lower in mTBI OA than in healthy OA (p = 0.05). There was no clear effect of CR on P2 or P3 measures.
Conclusion
As mTBI OA with lower CR performed worse on a working-memory task, lower CR may be a risk factor for worse recovery after mTBI in this group.
Introduction
Mild traumatic brain injury (mTBI) affects millions of people worldwide and accounts for 80% to 90% of all traumatic brain injury cases, with higher incidence in children and older adults (Citation1,Citation2). Although most mTBI patients will functionally recover, approximately 20% will not show full recovery after six months (Citation3–6), experiencing impairing cognitive and emotional sequelae that can last for years (Citation7). One in four mTBI patients is 60 years or older (Citation8) and older adults comprise one of the most vulnerable subcategories for cognitive dysfunction due to the accumulative neurodegenerative effect of aging (Citation9–11). Furthermore, older adults are less likely to fully recover after sustaining a mild TBI (Citation3–5,Citation12). For these reasons, and given the steeply increasing number of older adults worldwide, it is of utmost importance to determine which factors affect the recovery process of older patients after mTBI.
The additional cognitive impact of mTBI in older adults might be diminished by cognitive reserve (CR). CR refers to ‘the adaptability of cognitive processes that helps to explain differential susceptibility of cognitive abilities or day-to-day function to brain aging, pathology or brain insult’ (Citation13). In this view of CR, efficient usage of remaining functional brain resources – also referred to as neural reserve – in combination with neural compensatory mechanisms masks the effect of age- or brain-related pathology (Citation14,Citation15). Here, neural compensation refers to the increased recruitment of brain networks after pathology, or normal aging disrupted those typically recruited for a particular task. It has also been proposed that higher CR may protect patients with head injury from the increased risk of cognitive decline due to their trauma (Citation16). CR is considered a theoretical construct that can be indirectly measured by proxies such as pre-morbid IQ, years of education, leisure activities or occupation. Recently, questionnaires that combine CR proxies into a composite score of CR, such as the Cognitive Reserve Index Questionnaire (CRIq (Citation17);), have been developed and methodologically evaluated (Citation18). Epidemiological studies suggest a modest effect of higher pre-morbid CR on enhancing recovery after mTBI and predicting higher functional outcomes (Citation19–21). In the case of mTBI, lower pre-morbid CR may thus serve as a potential risk factor for incomplete recovery.
Cognitive complaints in the sub-acute phase after mTBI are often related to impaired working memory (WM), speed of information processing, attention and executive functions (Citation22–26). When determining the relation between CR and outcome of patients with mTBI, WM is of particular interest, as it has been suggested to play a fundamental role in cognitive performance after TBI linking long-term memory impairments to CR (Citation27–31).
The current neural model of CR is based primarily on studies using functional magnetic resonance imaging (fMRI) in patients with Alzheimer’s disease reporting brain activations that correlate with CR proxies, mainly in the anterior cingulate cortex (Citation15,Citation32–35). However, despite the growing literature on CR and mTBI, studies on the neural basis of the protective effects of CR after mTBI remain scarce (Citation36).
In the last decade, brain functioning related to CR has also been studied with other neuroimaging techniques such as electroencephalography (EEG) and magnetoencephalography (MEG), using both resting-state and event-related potential (ERP) paradigms (Citation36). These techniques have a higher (millisecond) temporal resolution than fMRI, which is in the order of seconds (Citation37), making them potentially more suitable to measure the temporal changes in brain activity that may underlie CR (Citation38). In healthy younger and older adults, first EEG evidence of the modulatory effect of CR on WM brain networks was found in an ERP study, indicating that higher CR leads to smaller changes in P3 component amplitude and latency when increasing WM task difficulty (Citation39). In this study, P3 component characteristics were used as neural indexes of WM capacity. This association between CR and P3 component characteristics was replicated in healthy older adults performing an N-back task (Citation40). As WM and attention are generally thought to be related (Citation41), and the P2 component has been used to establish the modulating effect of CR on attention (Citation42,Citation43), to assess the modulating effect of CR on attention and WM performance by means of the P2 and P3 ERP components in older adults with mTBI, might be of interest.
Therefore, we investigated the effect of CR on WM performance in older adults with mTBI as well as the effect of CR on the neural correlates of attention and WM reflected in the P2 and P3 ERP components in the EEG collected during a WM task. We hypothesized that older adults with lower CR would adapt worse to mTBI injury compared to older adults with higher CR as measured during a WM N-back task and compared results with healthy age-matched controls.
Methods
Participants
This study is part of an ongoing prospective cohort study on long-term outcome in older adults after mTBI (‘Recovery and CONNectivity in Elderly after Cerebral Trauma’ or ReCONNECT Study). Patients aged 60 years or older admitted with mTBI to the Emergency Department of the University Medical Center Groningen (UMCG) between January 2019 and October 2020 were included in the EEG part of the ReCONNECT study if they fulfilled the following criteria: 1) Glasgow Coma Scale (GCS) score between 13 and 15 in the first 24 hours after the injury, 2) loss of consciousness or post-traumatic amnesia, 3) understanding of Dutch language, 4) corrected-to-normal or unimpaired vision, and 5) ability to perform motor tasks while sitting behind a laptop. Healthy older adults were age- and CR-matched. Exclusion criteria for all participants were psychiatric disorders, drug or alcohol addiction, or previous admittance to a hospital due to TBI. The ReCONNECT study was approved by the UMCG medical ethical committee and was executed in accordance with the Declaration of Helsinki (Citation44). All participants provided written informed consent.
Experimental task paradigm
We employed a visual N-back task with three levels of memory load (0-, 1- and 2-Back), adapted from a previous fMRI experiment from our group on TBI patients (Citation25). Individual letters were presented for 500 ms with inter-stimulus intervals of 1000 ms. During the 0-Back condition, participants had to press a button with the index finger of their dominant hand when the letter ‘X’ (target stimulus) appeared on the screen. They pressed this button during the 1-Back condition when the presented letter was the same as the previous one, and during the 2-Back condition when the letter was the same as the letter two letters back. The entire task consisted of twelve blocks (four blocks per condition) with a fixation period between conditions.
The task was built using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA, United States) and presented on a computer screen at 60 cm distance from the participant’s eyes. The triggers of both target and nontarget stimuli and responses were transmitted to the EEG recording software Brain Vision Recorder v.2 through a TriggerBox (both: Brain Products GmbH, Gilching, Germany).
Behavioural analysis
The triggers associated with stimulus presentation and button presses were used to determine reaction time and accuracy. Task accuracy was defined as the percentage of correct responses that were given between 200 and 1200 ms after stimulus onset. As there is a trade-off between accuracy and speed (Citation45), we used the inverse efficiency score (IES) which calculates the ratio between mean reaction time of correct responses and accuracy (Citation45–47), as a measure of performance:
EEG acquisition
The EEG signal was recorded using a cap with 64 Ag/AgCl active electrodes (ActiCAP/ActiCHamp; Brain Products GmbH, Gilching, Germany) placed according to the international 10–20 system (Citation48) and referenced to Fz with ground at Fpz using a sampling frequency of 250 Hz. Impedances were kept below 10 kΩ.
EEG analysis
Raw EEG data were preprocessed using EEGLab (Citation49) version 2019.1 (sccn.ucsd.edu/eeglab) and ERPLAB (Citation50) version 7.0 (erpinfo.org/erplab) in MATLAB version 2019b (MathWorks Inc, Massachusetts, USA). Raw EEG was first inspected semi-automatically for artifacts (voltage exceeding ±200μV). When a channel had more than 10% of artifact time points, it was interpolated, while allowing not more than six interpolated channels (10% of the total number of channels) per recording. Otherwise, the whole recording was excluded from further analysis. EEG data were then re-referenced to the average of the 64 scalp channels and filtered using a 12 dB/oct bandpass Butterworth filter between 0.1–45 Hz and a 50 Hz notch filter. Independent Component Analysis (ICA) was used for semi-automatic ocular correction. Segmentation of epochs was then performed from 200 ms before to 1000 ms after stimulus onset. To ensure that we only included trials in the ERP in which the working memory process was actually invoked, we only used segments with correct responses for each condition. Segments were subsequently baseline corrected and averaged per condition.
We then pooled the data by averaging across channels for two regions of interest (ROIs): a fronto-central ROI (FC1, FC2, F1, F2, Fz) to assess the P2 component and a centro-parietal ROI (CPz, CP1, CP2, P1, P2, Pz) to assess the P3 component. From each participant’s ERP we obtained P2 and P3 peak voltage latency using the fronto-central and centro-parietal ROI, respectively, employing a semi-automatic algorithm in ERPLAB. We determined P2 and P3 amplitudes by averaging the ERP amplitude in a 40 ms symmetric interval around peak latency.
Additional measures
CR was assessed with the CRIq Dutch version (Citation51) scoring three domains independently: education, working activity, and leisure time. These results were then combined with a composite CR score, the cognitive reserve index (CRI (Citation17)), Handedness preference was assessed with the Annett Handedness Scale (Citation52), ranging from 0 (extremely left-handed) to 32 (extremely right-handed).
Statistical analysis
SPSS version 26 (IBM Corp., Armonk, NY) was used for statistical analysis. Normality of data distributions was tested using the Kolmogorov–Smirnov test. T-tests were used for group comparisons of demographical data in case of normality. Otherwise, Mann-Whitney U tests were used. For categorical data, Chi-square tests were used.
To investigate the effect of CR on performance and P2 and P3 component characteristics, we performed five separate mixed model repeated measures analyses of covariance (RM-ANCOVA) of the performance measure (IES) and amplitude and latency for each component (P2 and P3), with CR as covariate. Note that the mTBI and healthy older adult groups were CR-matched, thereby allowing the inclusion of CR as a covariate. To assess the assumption of homogeneity of regression slopes for each outcome variable, we first built five RM-ANCOVA models that included the interaction between the group variable and the CR covariate. If the group × CR interaction was not significant, implying similar regression slopes, we continued to build new full-factorial RM-ANCOVA models without this interaction term to investigate our main research question. In all RM-ANCOVA models, the between-subject factor was group (older adults with mTBI and healthy older adults), while the within-subject factor was task condition (0-, 1-, & 2-Back). To verify sphericity of the dependent variables in the RM-ANCOVA, we employed Mauchly’s test. In case sphericity was violated, we applied the Greenhouse–Geisser correction. Levene’s test was used to test for equality of error variances across task conditions. We used an F-test to analyze the effect of group, based on pairwise comparisons among the estimated marginal means. To control for multiple comparisons, we used Bonferroni correction, and as a measure of effect size, we calculated η2. Additionally, Pearson correlation analysis was employed to investigate the relationship between CR and the P2/P3 component characteristics as well as task performance, for each group and task condition, separately. A significance level of alpha = 0.05 was assumed for all statistical tests.
Results
Participant characteristics
We included 22 older adults with mTBI and 15 healthy older adults. Demographics and CR estimates are provided in . Fourteen (63%) of the older adults with mTBI and eight (53%) of the healthy older adults were male and there was no difference in sex distribution (X2 (1,37) = .393, p = 0.531) between groups. Age and CR estimates did not differ between groups either, as expected because of matching for age and CR. Both groups had mainly right-handed individuals and did not differ in handedness distribution (X2 (1,37) = .070, p = 0.791). Other characteristics specific to the mTBI group are summarized in .
Table 1. Demographic characteristics for mild traumatic brain injury older adult and healthy older adult groups.
Homogeneity of regression slopes
The assumption of homogeneity of regression slopes was not violated for any of the outcome variables, as the Group×CR interaction effect was never significant (see ).
Table 2. Statistical results for testing homogeneity of regression slopes.
Task performance
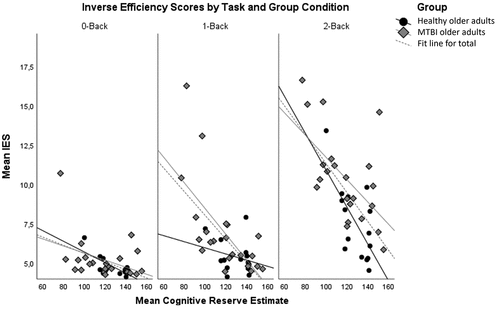
Task performance results are illustrated in . We found a significant main effect of group on the performance of older adults (F (1,34) = 5.060, p = 0.031, η2=.13) reflecting that older adults with mTBI (M = 7.299, SE = .287) generally perform worse than healthy older adults (M = 6.264, SE = .35). Furthermore, there was a main effect of task condition on the IES score (F (2,68) = 13.118, p < 0.001, η2=.278), indicating that increased task difficulty decreased overall performance. Also, CR as a covariate was significant (F (1,34) = 26.809, p < 0.001, η2=.441), showing that participants with higher CR had better performance than those with lower CR. Moreover, there was a significant task condition × CR interaction (F (2,68) = 5.971, p = 0.004, η2=.149), reflecting that with increasing task difficulty, task performance was more affected by CR. In other words, the more difficult the task, the more higher CR helped to perform better. Finally, we found a significant task condition × group interaction (F (2,68) = 3.527, p = 0.035, η2=.094), showing that the difference in performance between older adults with mTBI and healthy older adults increased with task difficulty. This shows that there is a stronger effect of task difficulty on performance in older adults with mTBI than in healthy older adults.
P2 component
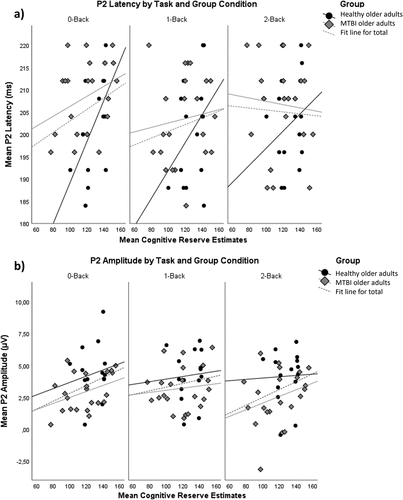
provides P2 latency and amplitude values across task conditions and group. There was a borderline significant main effect of group on P2 latency (F (1,34) = 3.558, p = 0.068, η2=.095), indicating a slightly increased latency in older adults with mTBI (M = 206.565, SE = 1.743) compared to healthy older adults (M = 201.305, SE = 2.123). We found a main effect of group for P2 amplitude (F (1,34) = 4.12, p = 0.05, η2=.108), reflecting an overall higher P2 amplitude in healthy (M = 4.143, SE = .442) than in mTBI older adults (M = 2.966, SE = .362). No other main or interaction effects were found for P2 component characteristics.
P3 component
There was a borderline significant main effect of CR on P3 amplitude (F (1,34) = 3.744, p = 0.061, η2 = .099), suggesting an increasing P3 amplitude with higher CR in older adults. There were no significant main or interaction effects of task condition, group or CR on the latency of the P3 component.
Correlations
provides correlations of CR estimates with performance and P2 and P3 component characteristics, for each group. We expected CR to correlate more strongly with performance and P2 and P3 component characteristics in older adults with mTBI than in healthy older adults. Yet, no statistically significant correlations were found between CR and P2 or P3 component characteristics for older adults with mTBI. However, higher CR corresponded with better performance in older adults with mTBI, for the two more difficult task conditions (1-back: r = −.663, p < 0.001, 2-back: r = −.581, p = 0.005). For healthy older adults, there was a significant correlation between CR estimates and P2 latency for the 0-back condition (r = .569, p = 0.027), indicating that P2 latency increased with higher CR estimates. Furthermore, in healthy older adults, higher CR also corresponded with better performance for the 0-back (r = −.739, p = 0.002) and 2-back (r = −.667, p = 0.007) task conditions.
Table 3. Pearson correlations for performance and P2 component measures with the CRIq score.
Discussion
We investigated the effect of cognitive reserve on WM performance in older adults in the subacute phase after mTBI by determining the neural correlates of attention and WM reflected in EEG- derived P2 and P3 ERP components, respectively. To our knowledge, this is the first ERP study trying to elucidate the relation between pre-morbid CR and the neural correlates of working memory during an N-back task in older adults after mTBI. We found that older adults with mTBI generally performed worse than healthy older adults and lower CR decreased WM performance across both groups. Particularly, when considering task conditions separately, lower CR correlated with worse performance for older adults with mTBI, for the two more difficult 1- and 2-back task conditions. On a neural level, lower P2 amplitude was found in older adults with mTBI compared to healthy older adults. As older mTBI patients with lower CR performed worse on the WM task, overall our findings suggest that lower CR may be a risk factor for worse recovery after mTBI.
Our results on a behavioral level corroborate the role of CR in mediating WM performance after mTBI (Citation22–24), and hence, support that CR may have a prognostic role in mTBI recovery (Citation19–21). In this study, we have confirmed these previous findings particularly in older adult patients who have sustained an mTBI. The effect of task difficulty on performance was similar to previous research (Citation53,Citation54); we found lower accuracy and longer reaction times, resulting in higher IES, with increasing task difficulty. Our behavioral results also illustrate that CR not only comes into play in older adults after mTBI but is also important for cognitive performance of healthy older adults (Citation13). Our findings could be interpreted in the context of the Compensation-Related Utilization of Neural Circuits Hypothesis (CRUNCH (Citation55)), in which, beyond a certain level of task demand, older brains fall short of sufficient activation levels, and performance declines relative to younger brains. We here observe a similar role for CR within older adults after mTBI: for higher task demands lower CR brains apparently can no longer compensate and performance declines. The benefits from higher CR are lacking in the older adults with mTBI with lower CR, which may make them more vulnerable to develop long-term cognitive post-concussive complaints (Citation7,Citation10,Citation11,Citation21). Furthermore, our results support the use of IES as a compound performance index for cognitive aging studies, as it has allowed to find the relation between performance and CR in our older adults sample. What our findings also illustrate is that matching and controlling for CR is important when comparing older healthy and patient samples on cognitive performance.
Regarding the role of CR in modulating neural activity in older adults after mTBI, we only found borderline significantly increasing P3 amplitude with higher CR, even though the N-back WM task worked as expected at performance level. This could suggest a small protective effect of CR on the P3 amplitude during a WM task in older adults; however, caution should be taken when interpreting this result. So far, effects of CR on P3 component characteristics have only been found in healthy adult and older adult populations (Citation39,Citation40,Citation42). Another possibility to investigate neural correlates of the effect of CR on WM processes may be to study brain oscillations related to the P3 component. In a study of the 2-back M3 component (Citation56), the magnetoencephalographic (MEG) equivalent of the P3 for MEG event-related fields, adults with higher CR showed higher beta intensity in parietal and occipital regions during a 2-back WM task. Future research on brain oscillations underlying the CR effect on the WM P3 component might therefore be of interest.
We found no effects of CR on earlier attentional processing, as reflected in P2 component characteristics. We did observe, however, that sustaining an mTBI decreased the P2 amplitude of older adults, an effect that has been reported in TBI veterans, during an oddball task, as well (Citation57). This decrease in P2 amplitude likely reflects the disrupting effect of mTBI on the allocation of neural resources to attentional processing. We also found a borderline effect of mTBI to increase P2 latency, supporting this disrupting effect of mTBI.
The protective effect of CR may depend on TBI severity, being more pronounced for mTBI than for moderate or severe TBI in adults (Citation14). The question thus remains whether our results are limited to mTBI, as there apparently is a neural threshold at which the axonal and neural injury due to TBI makes pre-morbid CR no longer beneficial for recovery from TBI (Citation58). It might therefore be interesting to include populations of mild as well as moderate to severe TBI in future studies.
Despite the interesting findings of our study, some limitations have to be mentioned. Although being comparable in size to several other ERP studies of the effect of CR in patients (Citation36), it is possible that our study lacked power, leading to some borderline significant results and an increased risk of type II errors. There is, however, a scarcity of older adult mTBI patients in EEG studies, as they often have comorbidities and may be physically too harmed (e.g. broken limbs) due to their accident, to come to the EEG lab at 4–6 weeks after injury.
Conclusion
To summarize, older adults with mTBI generally performed worse than healthy older adults on a 2-back WM task, while lower CR decreased WM performance in general, across both groups. This means that some older adult mTBI patients with higher CR are still able to perform on the level of healthy older adults on this WM task. More specifically, lower CR might be a risk factor for worse outcome in older adults after sustaining an mTBI, but this needs to be investigated in future studies. These findings were, however, not accompanied by a clear effect of CR on P2 or P3 component characteristics. Further electrophysiological studies are, therefore, needed to assess the effect of CR on ERP brain correlates of attentional and WM processing.
Acknowledgments
The authors would like to thank Brigit Klever for her support in the collection of data acquisition for the ReCONNECT study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14:506–17. doi:10.1016/S1474-4422(15)00002-2.
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;0:1–18. doi:10.3171/2017.10.JNS17352. Cited: in: PMID: 29701556.
- Hume CH, Wright BJ, Kinsella GJ. Systematic Review and meta-analysis of outcome after mild traumatic brain injury in older people. J Int Neuropsychol Soc. 2022[cited 2024 Feb 26];28(7):736–55. doi:10.1017/S1355617721000795. Cited: in: PMID: 34313210.
- Kristman VL, Brison RJ, Bédard M, Reguly P, Chisholm S. Prognostic markers for poor recovery after mild traumatic brain injury in older adults: a Pilot cohort study. J Head Trauma Rehabil. 2016[cited 2024 Feb 26];31: E33–43. doi:10.1097/HTR.0000000000000226. Cited: in: PMID: 27022959.
- Karr JE, Iverson GL, Berghem K, Kotilainen A-K, Terry DP, Luoto TM. Complicated mild traumatic brain injury in older adults: Post-concussion symptoms and functional outcome at one week post injury. Brain Inj. 2020 [cited 2024 Feb 26];34: 26–33. doi:10.1080/02699052.2019.1669825. Cited: in: PMID: 31550173.
- de Koning ME, Scheenen ME, van der Horn HJ, Hageman G, Roks G, Spikman JM, van der Naalt J. Non-hospitalized patients with mild traumatic brain injury: the forgotten minority. J Neurotrauma. 2017 [cited 2024 Jan 23];34(1):257–61. doi:10.1089/neu.2015.4377.
- Kamins J, Giza CC. Concussion—mild traumatic brain injury. Neurosurg Clin N Am. 2016 [cited 2020 Nov 12];27: 441–52. doi:10.1016/j.nec.2016.05.005. Cited: in: PMID: 27637394.
- Hartholt KA, Van Lieshout EMM, Polinder S, Panneman MJM, Der Cammen TJM V, Patka P. Rapid increase in hospitalizations resulting from fall-related traumatic head injury in older adults in the Netherlands 1986–2008. J Neurotrauma. 2011[cited 2021 Aug 2];28(5):739–44. doi:10.1089/neu.2010.1488. Cited: in: PMID: 21355818.
- Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015 [cited 2020 Nov 12];66:75–80. doi:10.1016/j.mcn.2015.03.001. Cited: in: PMID: 25748121.
- Susman M, SM D, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma Inj Infect Crit Care. 2002 [cited 2020 Nov 12];53(2):219–24. doi:10.1097/00005373-200208000-00004.
- Eman Abdulle A, van der Naalt J. The role of mood, post-traumatic stress, post-concussive symptoms and coping on outcome after MTBI in elderly patients. Int Rev Psychiatry. 2020 [cited 2020 Nov 12];32(1):3–11. doi:10.1080/09540261.2019.1664421.
- Thompson HJ, WC M, Kagan SH. Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications [Internet]. J Am Geriatr Soc NIH Public Access. 2006 [cited 2024 Feb 7];54(10):1590–95. doi:10.1111/j.1532-5415.2006.00894.x. Available from:/pmc/articles/PMC2367127/.
- Stern Y, Arenaza‐Urquijo EM, Bartrés‐Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020 [cited 2020 Nov 12];16(9):1305–11. doi:10.1016/j.jalz.2018.07.219.
- Jeon IC, Kim OL, Kim MS, Kim SH, Chang CH, Bai DS. The effect of premorbid demographic factors on the recovery of neurocognitive function in traumatic brain injury patients. J Korean Neurosurg Soc. 2008 [cited 2020 Nov 12];44(5):295–302. doi:10.3340/jkns.2008.44.5.295.
- Bosch B, Bartrés-Faz D, Rami L, Arenaza-Urquijo EM, Fernández-Espejo D, Junqué C, Solé-Padullés C, Sánchez-Valle R, Bargalló N, Falcón C, et al. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2010;46(4):451–61. doi:10.1016/j.cortex.2009.05.006. Cited: in: PMID: 19560134.
- Smith C. Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathol Appl Neurobiol. 2013 [cited 2020 Nov 12];39(1):35–44. doi:10.1111/nan.12006. Cited: in: PMID: 23206160.
- Nucci M, Mapelli D, Mondini S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin Exp Res. 2012 [cited 2020 Nov 13];24(3):218–26. doi:10.3275/7800. Cited: in: PMID: 21691143.
- Kartschmit N, Mikolajczyk R, Schubert T, Lacruz ME.Measuring Cognitive Reserve (CR) – a systematic review of measurement properties of CR questionnaires for the adult population. In: Fragkos Keditor. PLoS one [internet]Vol. 14. 2019 [cited 2020 Nov 12]. p. e0219851. doi:10.1371/journal.pone.0219851.
- Oldenburg C, Lundin A, Edman G, Nygren-de Boussard C, Bartfai A Cognitive reserve and persistent post-concussion symptoms—A prospective mild traumatic brain injury (mTBI) cohort study. Brain Inj. 2016 [cited 2020 Nov 12];30:146–55. doi: 10.3109/02699052.2015.1089598.
- Donders J, Stout J. The influence of Cognitive Reserve on recovery from traumatic brain injury. Arch Clin Neuropsychol. 2018[cited 2020 Nov 12];34(2):206–13. doi:10.1093/arclin/acy035. Cited: in: PMID: 29659665.
- Stenberg J, Håberg AK, Follestad T, Olsen A, Iverson GL, Terry DP, Karlsen RH, Saksvik SB, Karaliute M, Ek JAN, et al. Cognitive reserve moderates cognitive outcome after mild traumatic brain injury. Arch Phys Med Rehabil. 2020;101:72–80. doi:10.1016/j.apmr.2019.08.477. Cited: in: PMID: 31562876.
- Ulam F, Shelton C, Richards L, Davis L, Hunter B, Fregni F, Higgins K. Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin Neurophysiol. 2015;126:486–96. doi:10.1016/j.clinph.2014.05.015. Cited: in: PMID: 24947595.
- Karlsen RH, Saksvik SB, Stenberg J, Lundervold AJ, Olsen A, Rautio I, Folvik L, Håberg AK, Vik A, Karr JE, et al. Examining the subacute effects of mild traumatic brain injury using a traditional and computerized neuropsychological test battery. J Neurotrauma. 2021 [cited 2021 Jan 29];38(1):74–85. doi:10.1089/neu.2019.6922.
- Mcallister TW, Flashman LA, Mcdonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma. 2006 [cited 2021 Jan 29];23(10):1450–1467. doi:10.1089/neu.2006.23.1450.
- van der Horn HJ, Liemburg EJ, Scheenen ME, de Koning ME, Spikman JM, van der Naalt J. Post-concussive complaints after mild traumatic brain injury associated with altered brain networks during working memory performance. Brain Imaging Behav. 2016[cited 2020 Nov 13];10(4):1243–53. doi:10.1007/s11682-015-9489-y. Cited: in: PMID: 26667033.
- Dean PJA, Sterr A. Long-term effects of mild traumatic brain injury on cognitive performance. Front Hum Neurosci. 2013;7:29277. doi:10.3389/fnhum.2013.00030.
- Lojo-Seoane C, Facal D, Guàrdia-Olmos J, Pereiro AX, Juncos-Rabadán O. Effects of cognitive reserve on cognitive performance in a follow-up study in older adults with subjective cognitive complaints. The role of working memory. Front Aging Neurosci. 2018 [cited 2021 Jan 29];10:189. doi:10.3389/fnagi.2018.00189.
- Lojo-Seoane C, Facal D, Guàrdia-Olmos J, Pereiro AX, Campos-Magdaleno M, Mallo SC, Juncos-Rabadán O. Cognitive reserve and working memory in cognitive performance of adults with subjective cognitive complaints: longitudinal structural equation modeling. Int Psychogeriatr. 2020[cited 2021 Jan 29];32(4):515–24. doi:10.1017/S1041610219001248. Cited: in: PMID: 31547899.
- Sandry J, Deluca J, Chiaravalloti N. Working memory capacity links cognitive reserve with long-term memory in moderate to severe tbi: a translational approach. J Neurol. 2015[cited 2021 Jan 29];262(1):59–64. doi:10.1007/s00415-014-7523-4. Cited: in: PMID: 25287019.
- Thuss NS, Rakers SE, Bittencourt M, Balart-Sánchez SA, Spikman JM, van der Naalt J The cognitive profile of elderly patients with mild traumatic brain injury: a role for cognitive reserve? J Head Trauma Rehabil. 2023 [cited 2024 Jan 23]; doi: 10.1097/HTR.0000000000000911.
- Bittencourt M, van der Horn HJ, Balart-Sánchez SA, Marsman JBC, van der Naalt J, Maurits NM. Effects of mild traumatic brain injury on resting state brain network connectivity in older adults. Brain Imaging Behav. 2022[cited 2022 Nov 9];16(4):1863–72. doi:10.1007/s11682-022-00662-5. Cited: in: PMID: 35394617.
- Bartrés-Faz D, Solé-Padullés C, Junqué C, Rami L, Bosch B, Bargalló N, Falcón C, Sánchez-Valle R, Molinuevo JL. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol Psychol. 2009;80:256–59. doi:10.1016/j.biopsycho.2008.10.005. Cited: in: PMID: 19022337.
- Solé-Padullés C, Bartrés-Faz D, Junqué C, Vendrell P, Rami L, Clemente IC, Bosch B, Villar A, Bargalló N, Jurado MA, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiology Of Aging. 2009;30(7):1114–24. doi:10.1016/j.neurobiolaging.2007.10.008. Cited: in: PMID: 18053618.
- Beeri MS, Lee H, Cheng H, Wollman D, Silverman JM, Prohovnik I. Memory activation in healthy nonagenarians. Neurobiol Aging. 2011;32(3):515–23. doi: 10.1016/j.neurobiolaging.2009.02.022. Cited: in: PMID: 19342124.
- Ansado J, Monchi O, Ennabil N, Deslauriers J, Jubault T, Faure S, Joanette Y. Coping with task demand in aging using neural compensation and neural reserve triggers primarily intra-hemispheric-based neurofunctional reorganization. Neurosci Res (N Y). 2013;75(4):295–304. doi: 10.1016/j.neures.2013.01.012. Cited: in: PMID: 23453977.
- Balart-Sánchez SA, Bittencourt-Villalpando M, van der Naalt J, NM M. Electroencephalography, Magnetoencephalography, and cognitive reserve: a systematic review. Arch Clin Neuropsychol. 2021 [cited 2021 Feb 10];0:1–18. doi: 10.1093/arclin/acaa132.
- Sharon D, Hämäläinen MS, Tootell RBH, Halgren E, Belliveau JW. The advantage of combining MEG and EEG: comparison to fMRI in focally stimulated visual cortex. Neuroimage. 2007;36(4):1225–35. doi: 10.1016/j.neuroimage.2007.03.066. Cited: in: PMID: 17532230.
- Rajji TK Neurophysiology and cognitive reserve: a promising path. Clin Neurophysiol Elsevier Ireland Ltd. 2018. 286–87. 129 1 10.1016/j.clinph.2017.12.007
- Speer ME, Soldan A. Cognitive reserve modulates ERPs associated with verbal working memory in healthy younger and older adults. Neurobiol Aging. 2015;36:1424–34. doi:10.1016/j.neurobiolaging.2014.12.025.
- Gu L, Chen J, Gao L, Shu H, Wang Z, Liu D, Yan Y, Li S, Zhang Z. Cognitive reserve modulates attention processes in healthy elderly and amnestic mild cognitive impairment: an event-related potential study. Clin Neurophysiol. 2018;129:198–207. doi:10.1016/j.clinph.2017.10.030.
- Oberauer K Working memory and attention – a conceptual analysis and review. J Cogn. 2019 [cited 2021 Feb 10];2:1–23. doi: 10.5334/joc.58.
- Moussard A, Bermudez P, Alain C, Tays W, Moreno S Life-long music practice and executive control in older adults: An event-related potential study. Brain Res. 2016;1642:146–53. doi: 10.1016/j.brainres.2016.03.028.
- Gajewski PD, Falkenstein M, Thönes S, Wascher E. Stroop task performance across the lifespan: high cognitive reserve in older age is associated with enhanced proactive and reactive interference control. Neuroimage. 2020;207:116430. doi:10.1016/j.neuroimage.2019.116430. Cited: in: PMID: 31805383.
- Association WM World medical association declaration of Helsinki: ethical principles for medical research involving human subjects [internet]. JAMA - J. Am. Med. Assoc. American Medical Association; 2013 [cited 2020 Nov 13]. p. 2191–94. Available from: http://www.jama.com.
- Bruyer R, Brysbaert M Combining speed and accuracy in cognitive psychology: is the inverse efficiency score (IES) a better dependent variable than the Mean Reaction Time (RT) and the Percentage of Errors (PE)? Psychol Belg. 2011 [cited 2020 Nov 13];51:5. doi: 10.5334/pb-51-1-5. 1
- Akhtar N, Enns JT. Relations between convert orienting and filtering in the development of visual attention. J Exp Child Psychol. 1989;48:315–34. doi:10.1016/0022-0965(89)90008-8. Cited: in: PMID: 2794859.
- Liesefeld HR, Janczyk M Combining speed and accuracy to control for speed-accuracy trade-offs(?). Behav res methods. 2019 [cited 2020 Nov 13];51:40–60. doi: 10.3758/s13428-018-1076-x. Cited: in: PMID: 30022459. 1
- Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the international federation. Electro encephalogr Clin Neuro physiol Suppl. 1999;52:3–6. [cited 2020 Nov 13]. Cited: in: PMID: 10590970.
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. Cited: in: PMID: 15102499.
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014 [cited 2020 Nov 13];8:213. doi:10.3389/fnhum.2014.00213.
- RPC Kessels & JM Oosterman. Cognitive Reserve Index questionnaire (CRIq) -Nederlandse versie. 2016. Available from: http://www.cognitivereserveindex.org/CRI_2.0_NL.pdf.
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970[cited 2020 Nov 13];61: 303–21. doi:10.1111/j.2044-8295.1970.tb01248.x. Cited: in: PMID: 5457503.
- Pergher V, Wittevrongel B, Tournoy J, Schoenmakers B, Van Hulle MM. Mental workload of young and older adults gauged with ERPs and spectral power during N-Back task performance. Biol Psychol. 2019;146:107726. doi:10.1016/j.biopsycho.2019.107726. Cited: in: PMID: 31276755.
- Wild-Wall N, Falkenstein M, Gajewski PD. Age-related differences in working memory performance in a 2-back task. Front Psychol. 2011 [cited 2022 Oct 23];2:186. doi:10.3389/fpsyg.2011.00186.
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008 [cited 2021 Apr 8];17(3):177–82. doi:10.1111/j.1467-8721.2008.00570.x.
- Yang CY, Lin CP. Classification of cognitive reserve in healthy older adults based on brain activity using support vector machine. Physiol Meas. 2020[cited 2020 Nov 12];41(6):065009. doi:10.1088/1361-6579/ab979e. Cited: in: PMID: 32464620.
- Turk KW, Marin A, Schiloski KA, Vives-Rodriguez AL, Uppal P, Suh C, Dwyer B, Palumbo R, Budson AE. Head injury exposure in veterans presenting to memory disorders clinic: an observational study of clinical characteristics and relationship of event-related potentials and imaging markers. Front Neurol. 2021;12:958. doi:10.3389/fneur.2021.626767.
- Steward KA, Kennedy R, Novack TA, Crowe M, Marson DC, Triebel KL. The role of cognitive reserve in recovery from traumatic brain injury. J Head Trauma Rehabil. 2018[cited 2020 Nov 12];33: E18–27. doi:10.1097/HTR.0000000000000325. Cited: in: PMID: 28520675.