ABSTRACT
Background
We investigated the extent of literature and findings on relationships between vestibular issues, noise sensitivity (NS), and anxiety. We were interested in how relationships among these factors impacted adults’ recovery three months or more after mild traumatic brain injury (mTBI).
Methods
We conducted a scoping review to evaluate the extent of evidence linking relationships between vestibular issues, NS and anxiety with recovery after mTBI. Data relating to study characteristics and key findings were extracted and used to inform a critical narrative synthesis of findings.
Results
After screening and full-text review, we included two studies. Both studies considered the combination of vestibular issues, NS and anxiety and mTBI recovery. Vestibular issues, NS and anxiety were all significantly associated with one another and their presence was the strongest indicator that symptoms would extend beyond three-months after mTBI.
Conclusion
Few studies have focused on the relationships that vestibular issues, NS and anxiety have with one another and recovery after mTBI. Given the apparent strong relationships between these factors and prolonged recovery, we highlight this as an area warranting further investigation.
IMPLICATIONS FOR REHABILITATION
Vestibular issues, noise sensitivity and anxiety all appear to impact on recovery from mild traumatic brain injury.
There appear to be quite strong relationships between vestibular, noise sensitivity and anxiety symptoms following mild traumatic brain injury.
More work exploring these key symptoms and how they impact recovery from mild traumatic brain injury using a wide range of study methods and approaches are needed to advance the field.
Introduction
Mild traumatic brain injury (mTBI) is a significant public health concern. Dewan and colleagues (Citation1) estimated that 69 million (95% CI 64–74 million) individuals worldwide sustain traumatic brain injuries from all causes each year, with the Southeast Asian and Western Pacific regions experiencing the highest rates of injuries. The World Health Organisation task force estimates that between 70% and 90% of traumatic brain injuries that receive treatment are in the mild range of severity (Citation2).
Until recently, it was expected that substantial recovery would typically occur within the first three months after mTBI (Citation3). However, there is now growing evidence suggesting that recovery after mTBI can have a longer trajectory for some, with studies reporting that between 11% and 48% of people experience symptoms for more than 12 months (Citation4–7). Individuals with persistent issues typically present with complex and clinically challenging symptoms and functional difficulties. In addition, the long-term issues experienced can impact on quality of life due to psychological, physical, and social-functioning limitations that affect daily living activities.
Identifying predictive factors for recovery extending beyond the three-month period after mTBI is clinically important. Predicting those likely to recover slowly, soon after an injury, will facilitate targeted treatment for people who are most at risk of a poor outcome (Citation8,Citation9). Previous research has pointed toward symptoms indicative of vestibular issues, noise sensitivity (NS), and anxiety, as separately being factors amongst those who develop persistent symptoms after mTBI (Citation10–12). Recent discussion in the literature suggests that these issues may be interrelated and mutually reinforcing (Citation8). However, there appears to be very little published evidence demonstrating relationships between this triumvirate of issues and impact on mTBI recovery. The information below forms the rationale for looking at associations between these factors.
In a systematic review of 101 human studies by Iverson and colleagues (Citation13), acute characteristics (demographics, presenting signs and symptoms) associated with clinical recovery after mTBI were examined. In the review they found conflicting results with four studies finding vestibular issues, including acute dizziness, were predictive of poor recovery, but six studies found no relationship. In addition, one study reported poor initial balance to be predictive of poor recovery, but four studies did not. However, the quality of the studies included in this review was variable, and the methodologies varied considerably, potentially influencing the strength of evidence. Since this review was published, other researchers (Citation14,Citation15) have found that vestibular issues following mTBI are predictors of increased time to recover in college athletes.
In another study, presence of NS and vestibular issues were predictive of increased time to recover in college athletes with mTBI (Citation16). In this study, the duration of symptoms correlated with dizziness and NS, while return to play times correlated with imbalance and NS. Shepherd and Faulkner along with their colleagues (Citation17,Citation18) found that NS and anxiety occurred at heightened levels in adults with mTBI, whether these symptoms were correlated with recovery was not discussed.
Within non-mTBI populations, researchers demonstrate that certain peripheral and central vestibular disorders are associated with increased anxiety (Citation19,Citation20), in the same way, researchers of mTBI populations have demonstrated increased prevalence of both vestibular issues and anxiety (Citation21,Citation22). The influence of these factors on prolonged recovery could not be determined in these studies as researchers did not assess participants beyond three months after injury.
Considerable effort has been put into defining clusters of associated mTBI symptoms or subtypes over recent years (Citation23). Vestibular and anxiety/mood are well-accepted subtypes, while NS is not commonly acknowledged, there is increasing recognition of its impact on other symptoms and outcomes (Citation10,Citation23). Researchers accept that multiple subtypes may contribute to a patient’s clinical presentation after injury (Citation24). However, little empirical attention appears to have been paid to the roles the relationships between symptoms and subtypes play in prolonging recovery amongst adults with mTBI. Therefore, we aimed to use scoping review methods to a) investigate the extent of literature examining the relationships between vestibular issues, NS and anxiety among adults recovering from mTBI and b) synthesize the findings within the literature regarding these relationships.
Methods
Type of review
Scoping reviews are a popular approach to reviewing health research evidence, commonly used to map evidence on a topic allowing the identification of the main concepts, theories and sources (Citation25,Citation26). They are used to determine the scope of literature; examining emerging evidence or identifying knowledge gaps, investigating research conduct and in many cases informing systematic reviews (Citation27,Citation28). We chose to conduct a scoping review to answer our research aims given the novel focus of this study on the relationships between vestibular issues, NS and anxiety among adults recovering from mTBI. This scoping review was conducted in accordance with the JBI methodology for scoping reviews (Citation29), the population, concept, and context (PCC) framework guided the overall design and reporting of this scoping review (Citation30). We used the PRISMA Extension for Scoping Reviews (PRISMA-ScR) framework to guide the steps and rationale for our approach (Citation25). Once the research question was defined, we undertook a search of the literature to identify relevant studies, extract pertinent data and synthesize findings.
Study selection
Both analytical and descriptive observational studies including prospective and retrospective cohort, case–control, and cross-sectional studies were considered for inclusion. Only English language articles were included. Keywords used in database searches are shown in , an example of our Medline Ovid search can be found in Appendix I.
Table 1. Keywords used in database search.1
Eligibility criteria
Population
We included human studies whose participants were adults and youth over the age of 13, who met diagnostic criteria for mTBI or concussion.
Concussion is considered a form of mTBI (Citation31,Citation32), with the terms used interchangeably (Citation33). Studies were included if concussion/mTBI definitions described were consistent with the American Congress of Rehabilitation Medicine criteria for mTBI (Citation33). This definition requires evidence of a biomechanically plausible mechanism of injury and one or more of the following criteria are met. 1) One or more clinical signs attributable to brain injury (loss of consciousness, alteration of mental status, complete or partial amnesia for events immediately following the injury, other acute neurological sign(s)). 2) At least two acute symptoms and at least one clinical or laboratory finding (cognitive, balance or oculomotor impairment on acute clinical examination; elevated blood biomarker(s) indicative of intracranial injury) attributable to brain injury. 3) Neuroimaging evidence of TBI. 4) Not better accounted for by confounding factors (Citation33,Citation34).
Excluded studies were those examining mTBI in children younger than 13 years and those where data could not be extracted for youth and adults. Children below the age of 13 were excluded as there is consensus that they do not follow the same recovery time frames as adults (Citation35–37). While there are conflicting results when comparing high school and college aged athletes, there is some evidence noting no differences across these age groups, therefore, we include studies examining youth over the age of 13 (Citation38–40).
Concept
An attempt was made to explore the relationship between the key factors; vestibular issues, NS and anxiety and their effect on prolonged recovery after mTBI. Therefore, included studies must have assessed two or more of the key factors to be included. We were most interested in studies which contained all three factors compared to combinations of two, and the factors must have been able to be related to persistence of symptoms lasting three months or more after mTBI. In line with JBI scoping review methods, we clearly define the conditions of interest for this review below.
Vestibular issues are defined as signs and symptoms associated with disturbance of the body’s vestibular system and could come from central causes within the brain or peripheral causes such as damage to the labyrinth, the eighth cranial nerve, or conditions such as benign paroxysmal positional vertigo (Citation41). Symptoms may include dizziness, vertigo, nausea and vomiting, intolerance to head motion, nystagmus, unsteady gait, and postural instability (Citation42). Some of the vaguer symptoms of dizziness reported in the included studies may not be unique to vestibular disorders and could indicate pituitary (Citation43,Citation44), autonomic nervous system (ANS) (Citation45) or cervicogenic dysfunction (Citation46).
To date, the research on NS in mTBI has used a single item from concussion measures such as the Neurobehavioral Symptom Inventory used by Storzbach et al. (Citation47). or the Rivermead Post Concussion Questionnaire as used by Shepherd et al. (Citation48). It is unclear whether these items are measuring NS as defined by Job (Citation49) which ‘refers to the internal states (be they physiological, psychological [including attitudinal], or related to life style or activities conducted) of any individual which increase their degree of reactivity to noise in general’ (p59), or hyperacusis as defined by Fackrell and colleagues (Citation50) which is ‘increased sensitivity to ordinary environmental sounds that would not usually be bothersome to most individuals’ (p1). For this scoping review the term NS incorporated all forms of noise intolerance whether it be NS or hyperacusis.
Anxiety is an excessive worry about a number of activities or events in one’s daily life in addition to symptoms such as muscle tension, restlessness, sleep disturbance or concentration difficulties (Citation51). Gray and McNaughton (Citation52) postulate that activity in a behavioral inhibition system within the brain produces anxious symptoms. The range and intensity of anxiety disorders represent different types of reactivity within the system.
Context
In line with JBI scoping methods, the context defines the timeframe for including studies. The use of the term prolonged recovery in this study implies persistent symptoms for three months or more. Prolonged recovery following mTBI is a difficult concept to define as, in sporting contexts, anything beyond 7–10 days would be considered prolonged (Citation53) and there is limited agreement regarding what recovery means. However, for this study, persistence of signs and symptoms associated with mTBI beyond three months or 90 days was considered prolonged (Citation54). This aligns closely with the Diagnostic and Statistical Manual of Mental Disorders Four (DSM-IV) definition of postconcussional disorder (Citation54). We acknowledge that post-concussion syndromes or disorders are controversial concepts and are not included in more recent iterations of the DSM. There is little consensus on criteria for diagnosis, with great variability in presentation, and symptoms lacking specificity to mTBI (Citation55,Citation56). There is also a great variety of definitions and assessment methods used to evaluate post-concussion symptoms (Citation57). Studies that included participants with injuries less than three months prior to study completion were excluded.
Data sources and search strategy
In keeping with JBI scoping review methods, in May 2022, RM with assistance from a medical subject librarian conducted a pilot search of MEDLINE and CINAHL to identify articles on the topic. The text words and index terms contained in the titles and abstracts of relevant articles identified by the pilot, were used to develop a full search strategy. Once finalized, search terms were then translated into formats for each of the following databases: CINAHL, EMBASE, MEDLINE, PsycINFO, Pubmed and SPORTDiscus, Scopus, Web of Science and The Cochrane Library. A search of gray literature (Google, Google Scholar, Mednar, and Trip) was conducted using a modified version of the search strategy with the same keywords used in database searching. Search terms are shown in .
Table 2. Inclusion and exclusion criteria.
To ensure that this scoping review gathered a broad range of articles, we firstly looked at the literature that included all three symptom descriptors; vestibular issues, NS and anxiety, as well as mTBI and recovery. Then we searched combinations of two of the symptom descriptors as well as mTBI and recovery.
Following the search, all identified citations were uploaded into EndNoteX9 (Clarivate Analytics, PA, USA) and duplicates removed. The review protocol (unpublished) dictated that all authors meet prior to undertaking the screening process; we clarified the inclusion criteria, and jointly screened a selection of five titles and abstracts until 100% agreement in included/excluded studies was reached. Then title screening took place to eliminate any articles which clearly did not meet the inclusion criteria.
All articles were then uploaded into Rayyan (Citation58) and RM and JD separately examined the title and abstracts to determine if they met the inclusion criteria. If agreement could not be reached, then the article went to author DS for final determination.
The full text of selected articles was assessed in detail by RM against the inclusion criteria, and we recorded reasons for exclusion of full-text sources of evidence. Any uncertainty that arose at this stage of the selection process was resolved through discussion with an additional reviewer (JD).
Data extraction
Based on recommendations of Levac et al. (Citation26), we developed a data charting system. We extracted data on participant demographics (e.g., age, gender, sports/non-sports related mTBI, military, or civilian), outcome measurement (symptom report, specific vestibular measures, specific noise sensitivity measures, specific anxiety measures, recovery/disability measures), results of each assessment (statistical significance of change in scores and themes for qualitative data). Based on scoping review methodology (Citation29,Citation59), critical appraisal of individual sources of evidence was not indicated and hence was not performed.
Data synthesis and analysis
Narrative synthesis (an approach often used in systematic reviews) is the synthesis of findings from multiple studies that relies primarily on the use of text to summarize and explain the findings of the synthesis (Citation60). It enabled us to compare studies of diverse designs, explore any potential heterogeneity and highlight any similarities or differences in the findings within the review (Citation61).
Results
Search results
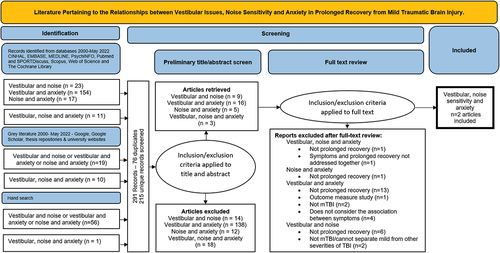
The first objective of this scoping review was to determine the extent of the literature pertaining to the relationships between vestibular issues, NS and anxiety among adults recovering from mTBI (see ). When considering the relationships between two or more of the factors (vestibular issues, NS and anxiety) and prolonged recovery we identified a total of 205 articles through initial database searches. We discovered 57 additional articles via hand searching the reference lists of systematic reviews and metanalyses and 29 articles from gray literature searches. This resulted in a total of 291 studies, which after deduplication, was reduced to 215 unique records to screen. Of these, we determined that 182 articles did not meet the inclusion criteria based on their titles or abstracts leaving 33 articles for full text review. During full text review, we excluded an additional 31 articles for several reasons: Twenty-one articles were excluded because they did not meet our definition of prolonged recovery, four were excluded as the participants within the studies had a range of severities of traumatic brain injury, one article was excluded as it did not address symptoms and prolonged recovery together, four articles did not address the relationships between the factors of interest and one article was an outcome measure study. This left us with two articles, both articles considered the relationship of all three factors and prolonged recovery from mTBI (Citation9,Citation48).
Figure 1. PRISMA Flow Chart (Citation62) - Extent of the literature available when considering the role of vestibular issues, noise sensitivity and anxiety in prolonged recovery from mild traumatic brain injury.
Synthesis of findings
The second objective was to synthesize the findings within the literature regarding the relationships between vestibular issues, NS and anxiety among adults recovering from mTBI. The population, concept and context contained within the included studies is detailed in .
Table 3. Included studies evaluating relationships between vestibular issues and/or noise sensitivity and anxiety and prolonged recovery from mild traumatic brain injury (n = 2).
Population
The two studies included in this review were conducted in the United States of America and New Zealand (Citation9,Citation48). The study undertaken in the United States of America was a prospective observational cohort study of 180 adults presenting to a level one trauma center with mTBI from blunt trauma (Citation9). The other study was a secondary analysis of data from 341 people over the age of 16 as part of the BIONIC project, a New Zealand population-based TBI incidence and outcome study (Citation48).
Concept – relationships between the factors of interest
In the two studies that looked at the relationship between vestibular issues, NS and anxiety amongst people with mTBI, Dischinger et al. (Citation9) noted a strong correlation between anxiety, and NS and objective tests of balance dysfunction BESS (ascertained at 3 months postinjury) as seen in . Shepherd et al. (Citation48) demonstrated relationships between NS and vestibular issues, with a moderate correlation between both NS and dizziness and NS and nausea as seen in .
Context – vestibular issues, noise sensitivity, anxiety, and prolonged recovery
Dischinger et al. (Citation9) reported that anxiety and NS as tested three to ten days after injury, were amongst the strongest baseline predictors of four or more symptoms being present at three months after mTBI; NS increased the odds by a factor of 3.1 while anxiety increased the odds by a factor of 3.8. Similar findings were obtained by Shepherd et al. (Citation48) who found that elevated NS at six months increased the odds of having post-concussive symptoms at 12 months post mTBI by a factor of 3.8. Furthermore, Nausea at one month predicted four or more symptoms being present at three months after mTBI at 12 months by a factor of 3.9 (Citation48).
Outcomes and measures
Prolonged recovery
Both studies included in this review used the definition that four or more symptoms lasting longer than three months was indicative of prolonged recovery (Citation9,Citation48). This is not indicative of consistency within the literature but was rather a choice made by Shepherd et al. (Citation48) to allow direct comparison with findings by Dischinger et al. (Citation9).
Vestibular issues
Vestibular issues, which may include dizziness and/or balance problems, were measured in a variety of ways. Dizziness was measured by both Dischinger et al. and Shepherd et al. with a single item on the Concussion Symptoms Checklist (Citation63) and the Rivermead Post-concussion Symptom Questionnaire (Citation64) respectively (Citation9,Citation48). Shepherd et al. (Citation48) did not measure balance despite their study being informed by the Dischinger et al. study (Citation9). Dischinger et al. (Citation9) measured balance using the Balance Error Scoring System (Citation65) and the sensory organization test on dynamic posturography.
Noise sensitivity
Noise sensitivity was measured with a single question from a generic concussion symptom measure in both included studies. Dischinger et al. (Citation9) used the single item asking about NS in the Concussion Symptom Checklist (Citation63), while Shepherd et al. (Citation48) used a single item asking about NS from the Rivermead Post-concussion Symptom Questionnaire (Citation64).
Anxiety
The measurement of anxiety between the included studies differed. Dischinger et al. (Citation9) used a single item on the Concussion Symptoms Checklist (Citation63), while Shepherd et al. (Citation48), used the anxiety subscale of the Hospital Anxiety and Depression Scale (Citation66).
Discussion
This scoping review aimed to determine the extent of literature and summarize the findings within the literature regarding the relationships between vestibular issues, NS and anxiety among adults recovering from mTBI. When relationships between combinations of these factors were explored, a total of 215 unique records were found indicating this is a growing area of interest within research. However, after careful assessment against our inclusion criteria this number was reduced to two. This is considered a very small number, given that a scoping review of other scoping reviews found the median number of articles included per review was 35, with a range from 1 to 1763 (Citation67).
Despite only two studies being included in this review (Citation9,Citation48), relationships were evident between vestibular issues, NS, anxiety and prolonged mTBI recovery. Finding such interrelationships is consistent with the network analysis hypothesis proposed by Iverson (Citation8) where such symptoms can be mutually reinforcing and, together, potentially predictive of prolonged recovery after mTBI. Within TBI populations there is evidence of a bidirectional relationship between NS and mental health issues, while anxiety is thought to maintain sensory sensitivity (Citation68). In a military population with mTBI, significant correlations between vestibular issues, post-concussive symptoms, and anxiety have been found, mediation analysis demonstrated that vestibular symptoms directly impacted mTBI symptoms and there was also a significant association between vestibular symptoms and psychiatric mediators including anxiety (Citation69,Citation70). These studies (Citation68–70) alongside the two included studies (Citation9,Citation48) provide further support for the complex interrelationships of symptoms and their impact on recovery.
Further evidence supporting the complex interrelationships of symptoms and recovery is demonstrated when researchers have considered relationships between vestibular symptoms and other mTBI symptoms. For example, visual and verbal memory were moderately correlated with balance and gait measures among adolescents who had sustained an mTBI (Citation71). Within the same age group, other researchers have found an association between health-related quality of life and balance (Citation72).
Symptoms of interest and their association with prolonged recovery from mTBI
Researchers interested in audiological issues and mTBI, found that audiological issues (tinnitus) and vestibular issues (dizziness) after mTBI due to a blast, were present in 35% and 16% a sample of 3690 military personnel three months following mTBI respectively (Citation73). The same authors found this group had poorer self-rated health (Citation74). Noise Sensitivity and tinnitus are often comorbid, with 40% to 55% of people with tinnitus experiencing hyperacusis (Citation75,Citation76) and 40% to 86% of people with hyperacusis experiencing tinnitus (Citation77,Citation78). The concurrent nature of the two symptoms suggests that the disorders share a common underlying central mechanism of sensitization (Citation76,Citation79). Limbic and auditory brain areas are thought to interact at the level of the thalamus (Citation80), messages from the limbic regions of the brain appear to be able to tune out tinnitus and if compromised, this mechanism breaks down (Citation80). Research is able to demonstrate that the thalamus gates or influences headache pain, fatigue, insomnia, and cognition in mTBI (Citation79). Damage at the level of the thalamus may result in central sensitization causing both NS and tinnitus in people with mTBI.
Vestibular issues and anxiety in those slow to recover from mTBI have been noted by researchers (Citation7,Citation69,Citation81). Two studies note a large proportion of participants reported either vestibular issues 69–85% or anxiety 76–93% (Citation69,Citation81) more than three months after injury. While, Theadom et al. (Citation7). noted correlations between vestibular issues (dizziness), anxiety and post-concussion symptoms four years after injury. In this study, dizziness significantly correlated with difficulties coping with physical demands, and both dizziness and anxiety significantly correlated with mental and interpersonal concerns in a population-based sample (Citation7).
Gard and colleagues' case–control study found, 21 athletes with sports related concussion reported balance problems a level 85% higher than controls and dizziness was reported to be 80% higher in the athletes with sports related concussions compared with controls (Citation81). This contrasts with the findings of Buttner et al. (Citation82), in a prospective longitudinal study, they found no difference in dizziness and anxiety levels between 47 amateur athletes with mTBI and uninjured controls six and 12 months after injury.
Relationships between the symptoms of interest outside of mTBI
Outside of the field of mTBI, research has shown relationships between vestibular issues, NS and anxiety. Anxiety has been associated with vestibular issues, including dizziness, decreased balance, and instability in postural control among people with vestibular conditions such as vestibular migraine, vestibular diseases and even in healthy subjects (Citation83–87). In addition, noise sensitivity/hyperacusis has also been correlated with anxiety among people diagnosed with hyperacusis and tinnitus (Citation88,Citation89). Therefore, despite the variable underlying conditions, these studies support the idea that these factors could also be associated with one another in people with mTBI.
Populations with complex, persistent symptoms with similarities to mTBI include fibromyalgia and chronic lower back pain (Citation90–94). Like mTBI, fibromyalgia is not fully understood, and signs and symptoms overlap with many other conditions (Citation90). Since its classification by the American College of Rheumatology in 1990, increased knowledge of the interaction between stress-response systems and sensory modulation and the variable nature of psychosocial inputs and neurophysiological responses linked to fibromyalgia is well accepted (Citation91). Network analysis (Citation87) has been undertaken in the fibromyalgia population and produced a model where sensory-related, psychological/cognitive, health-related, and physical factors are inter-connected (Citation92).
As with mTBI, predicting which people with lower back pain are likely to develop persistent symptoms is very important to direct care to those who require it (Citation93). Chronic musculoskeletal pain commonly occurs concomitantly with anxiety, depression, fatigue, difficulty remembering, and difficulty concentrating (Citation94). As occurs in mTBI, these coexisting conditions can result in substantial difficulties with work participation, social interactions, and self-care practices. The risk of chronicity in individuals with lower back pain is predicted by physical, psychological, and occupational factors (Citation91). The network perspective in chronic musculoskeletal pain postulates that symptoms are causally connected rather than merely correlated. In this approach, disorders exist as systems rather than entities (Citation95). Network perspective findings from research with populations with similarities to mTBI suggest this is a promising approach for mTBI also.
Implications for practice and research
Finding predictors of prolonged recovery amongst those with mTBI would be advantageous for the purpose of prognosis and to facilitate targeted treatment and research for people most at risk of a poor outcome. Recovery from mTBI is likely to be complex, and searching for a small number of independent predictors of outcomes may not move the field forward.
There appears to be very few researchers looking at symptom clusters and their utilization in predicting outcomes. Langdon et al. (Citation96) undertook a systematic review and meta-cluster analysis and found 19 articles investigating associations between symptom clusters and clinical outcome but only included sports related concussion studies. More recently, Aderman et al. (Citation97) found specific symptoms clusters resulted in prolonged return to activity. Looking for interrelated predictors may offer options for treatments targeting one area which could impact on other symptoms.
This scoping review found that there may be relationships between the triumvirate of vestibular, NS and anxiety symptoms, with two studies (Citation9,Citation48) being able to demonstrate correlations between all three factors. These two studies were able to demonstrate that these key factors were associated with prolonged recovery (Citation9,Citation48). However, recovery is difficult to measure as those with mTBI can misperceive their preinjury functioning as better than the average person (Citation98). This means patient self-report alone can be unreliable as a measure of recovery. Iverson and colleagues' systematic review defined clinical recovery as a return to normal activities, including school/work and sports, following injury (Citation13). In practice, however, recovery often consists of a resolution of injury-related symptoms and a return to clinically normal function (Citation13). Clearer definitions of prolonged recovery from mTBI would assist future research. We found that the studies included in our review defined non-recovery as four or more symptoms lasting for more than three months. Twenty-one articles were eliminated from the PRISMA shown in at the full text review stage as they did not assess participants at least three months after injury. This inconsistency in the way prolonged recovery has been defined means the current research in this area is difficult to compare and interpret. Once consensus on what constitutes prolonged recovery has been reached, consistent measurement of functional outcome after injury should occur.
Strengths and limitations
This scoping review’s strength lies in the replicable, transparent, rigorous process that was undertaken to synthesize evidence on this emerging topic. However, limitations in the design of this review are acknowledged. In keeping with scoping review methodology (Citation29,Citation59), we have not considered the strength or quality of the evidence. This means statements about the quality of the evidence are not included and can make interpreting review findings challenging (Citation99).
The use of a narrative synthesis approach also has limitations. Data extraction relies heavily on the reviewers’ interpretation of the literature, which may introduce bias. However, this approach allowed the synthesis of results despite the fact that the included studies used a range of approaches to measure our three key symptoms.
Many studies were excluded as they did not meet our definition of prolonged recovery and/or did not assess participants at least three months after their mTBI. Defining recovery and prolonged recovery are contentious and can differ depending on the mTBI sub-population under study. It is widely reported that a majority of adults recover from sports related mTBI within seven to ten days (Citation53,Citation100), although this evidence seems to be based on earlier versions of consensus-statement definitions (Citation101). Accordingly, our choice of defining criteria for prolonged recovery could have resulted in some relevant literature being excluded. In addition, because we limited our study to literature written in English we may have missed some studies and this could introduce the potential for language bias (Citation102).
A limiting factor in this study was, that we did not consider that symptoms such as NS or those associated with vestibular issues may have been due to direct injury to the neck or the ear. Noise sensitivity has been associated with whiplash injuries (Citation103–105), therefore, cervicogenic dysfunction may be the cause of symptoms we attributed to vestibular issues (Citation46). Vestibular symptoms may be due to direct injury of the labyrinth (Citation106,Citation107) instead of purely mTBI. Symptoms that we have attributed to vestibular issues such as dizziness could also be attributed to ANS (Citation106,Citation108) or pituitary (Citation43,Citation44) dysfunction according to researchers. Systematic reviews on the topic of ANS dysfunction after mTBI conclude, mTBI may cause ANS anomalies (Citation109–111). Autonomic nervous system dysfunction is hypothesized as one of many factors producing clinical symptoms, both at the acute and sub-acute phase post-injury (Citation112). Studies have linked ANS dysfunction (measured with heart rate variation) and anxiety (Citation62,Citation112–114).
The studies included in this review both relied on self-report measures. People with mTBI can misperceive their preinjury functioning as better than the average person (Citation98). This means patient self-report alone can be unreliable as a measure of recovery.
A potential limitation of the study could have been that we included studies with youth above the age of 13 years, as there are questions about homogeneity between youth and adults with mTBI. One of the studies (Citation48) included in this scoping review involved people below the age of 18 years and limiting it would have meant we lost another study from this already very small scoping review.
Conclusion
We were interested in whether relationships between vestibular issues, NS and anxiety might impact recovery after mTBI. Only two studies were found and included in this scoping review evaluating combinations of these factors and mTBI outcome. These studies demonstrated relationships between all of the factors of interest and prolonged recovery after mTBI. Exploring the associations between inter-related symptoms and mTBI outcome may be more promising than searching for individual predictors, offering an evidence basis for prioritizing treatment targets. Given the lack of published evidence available, we highlight this as an area requiring further investigation.
Acknowledgments
Special thanks to medical librarians at the University of Otago Medical Library for their assistance in undertaking the literature review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4):1080–97. doi:10.3171/2017.10.JNS17352.
- Cassidy JD, Carroll L, Peloso P, Borg J, Von Holst H, Holm L, Kraus J, Coronado V. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;36:28–60. doi:10.1080/16501960410023732.
- McCrea M, Iverson GL, McAllister TW, Hammeke TA, Powell MR, Barr WB, Kelly JP. An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin Neuropsychol. 2009;23(8):1368–90. doi:10.1080/13854040903074652.
- Barker-Collo S, Jones K, Theadom A, Starkey N, Dowell A, McPherson K, Ameratunga S, Dudley M, Te Ao B, Feigin V, BIONIC Research Group. Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: a population-based New Zealand study. Brain Inj. 2015;29(13–14):1604–16. doi:10.3109/02699052.2015.1075143.
- Theadom A, Parag V, Dowell T, McPherson K, Starkey N, Barker-Collo S, Jones K, Ameratunga S, Feigin VL. Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br J Gen Pract. 2016;66(642):e16–23. doi:10.3399/bjgp16X683161.
- Wäljas M, Iverson GL, Lange RT, Hakulinen U, Dastidar P, Huhtala H, Liimatainen S, Hartikainen K, Öhman J. A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. J Neurotrauma. 2015;32(8):534–47. doi:10.1089/neu.2014.3339.
- Theadom A, Barker-Collo SL, Jones K, Kahan M, Te Ao B, McPherson K, Starkey N, Feigin V Kydd R, Barber PA, Parag V. Work limitations 4 years after mild traumatic brain injury: a cohort study. Arch Phys Med Rehab. 2017;98(8):1560–66. doi:10.1016/j.apmr.2017.01.010.
- Iverson GL. Network analysis and precision rehabilitation for the post-concussion syndrome. Front Neurol. 2019;10:10. doi:10.3389/fneur.2019.00489.
- Dischinger P, Ryb G, Kufera J, Auman K. A multidisciplinary evaluation of mild TBI: early predictors of persistent post concussive syndrome. J Trauma. 2009;66(2):289–96. discussion 96-7. doi:10.1097/TA.0b013e3181961da2.
- Theodoroff SM, Papesh M, Duffield T, Novak M, Gallun F, King L, Chesnutt J, Rockwood R, Palandri M, Hullar T. Concussion management guidelines neglect auditory symptoms. Clin J Sport Med. 2022;32(2):82–85. doi:10.1097/JSM.0000000000000874.
- Kontos AP, Deitrick JM, Collins MW, Mucha A. Review of vestibular and oculomotor screening and concussion rehabilitation. J Athl Training. 2017;52(3):256–61. doi:10.4085/1062-6050-51.11.05.
- Broshek DK, De Marco AP, Freeman JR. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015;29(2):228–37. doi:10.3109/02699052.2014.974674.
- Iverson GL, Gardner AJ, Terry DP, Ponsford JL, Sills AK, Broshek DK, Solomon GS. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941–48. doi:10.1136/bjsports-2017-097729.
- Anzalone AJ, Blueitt D, Case T, McGuffin T, Pollard K, Garrison JC, Jones MT, Pavur R, Turner S, Oliver JM. A positive vestibular/ocular motor screening (VOMS) is associated with increased recovery time after sports-related concussion in youth and adolescent athletes. Am J Sports Med. 2017;45(2):474–79. doi:10.1177/0363546516668624.
- Whitney SL, Eagle SR, Marchetti G, Mucha A, Collins MW, Kontos AP, CARE Consortium Investigators. Association of acute vestibular/ocular motor screening scores to prolonged recovery in collegiate athletes following sport-related concussion. Brain Inj. 2020;34(6):842–47. doi:10.1080/02699052.2020.1755055.
- Chorney SR, Suryadevara AC, Nicholas BD. Audiovestibular symptoms as predictors of prolonged sports–related concussion among NCAA athletes. Laryngoscope. 2017;127(12):2850–53. doi:10.1002/lary.26564.
- Shepherd D, Landon J, Kalloor M, Theadom A. Clinical correlates of noise sensitivity in patients with acute TBI. Brain Inj. 2019;33(8):1050–58. doi:10.1080/02699052.2019.1606443.
- Faulkner JW, Snell DL, Shepherd D, Theadom A. Turning away from sound: the role of fear avoidance in noise sensitivity following mild traumatic brain injury. J Psychosom Res. 2021;151:110664. doi:10.1016/j.jpsychores.2021.110664.
- Brandt T, Dieterich M. ‘Excess anxiety’and ‘less anxiety’: both depend on vestibular function. Curr Opin Neurol. 2020;33(1):136–41. doi:10.1097/WCO.0000000000000771.
- Padovan L, Becker-Bense S, Flanagin VL, Strobl R, Limburg K, Lahmann C, Decker J, Dieterich M. Anxiety and physical impairment in patients with central vestibular disorders. J Neurol. 2023;270(11):5589–99. doi:10.1007/s00415-023-11871-3.
- Leddy J, Baker JG, Haider MN, Hinds A, Willer B. A physiological approach to prolonged recovery from sport-related concussion. J Athl Training. 2017;52(3):299–308. doi:10.4085/1062-6050-51.11.08.
- Hunt DL, Oldham J, Aaron SE, Tan CO, Meehan WP, Howell DR. Dizziness Psychosocial function, and postural stability following sport-related concussion. Clin J Sport Med. 2021;9(7_suppl3):09. doi:10.1177/2325967121S00059.
- Lumba-Brown A, Teramoto M, Bloom OJ, Brody D, Chesnutt J, Clugston JR, Collins M, Gioia G, Kontos A, Lal A, Sills A. Concussion guidelines step 2: evidence for subtype classification. Neurosurgery. 2020;86(1):2–13. doi:10.1093/neuros/nyz332.
- Maruta J, Lumba-Brown A, Ghajar J. Concussion subtype identification with the rivermead post-concussion symptoms questionnaire. Front Neurol. 2018;9:413691. doi:10.3389/fneur.2018.01034.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. doi:10.7326/M18-0850.
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):1–9. doi:10.1186/1748-5908-5-69.
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evidence Implementation. 2015;13(3):141–46. doi:10.1097/XEB.0000000000000050.
- Munn Z, Peters MD, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):1–7. doi:10.1186/s12874-018-0611-x.
- Peters MD, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evidence Synth. 2020;18(10):2119–26. doi:10.11124/JBIES-20-00167.
- Pollock D, Peters MD, Khalil H, McInerney P, Alexander L, Tricco AC, Evans C, de Moraes ÉB, Godfrey CM, Pieper D, Saran A. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evidence Synth. 2023;21(3):520–32. doi:10.11124/JBIES-22-00123.
- Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline development subcommittee of the American academy of neurology. Neurology. 2013;80(24):2250–57. doi:10.1212/WNL.0b013e31828d57dd.
- Broglio SP, Cantu RC, Gioia GA, Guskiewicz KM, Kutcher J, Palm M, McLeod TCV. National athletic trainers’ association position statement: management of sport concussion. J Athletic Training. 2014;49(2):245–65. doi:10.4085/1062-6050-49.1.07.
- Silverberg ND, Iverson GL, ABISI G, Cogan A, Dams-O-Connor K, Delmonico R, Iaccarino MA, Kajankova M, Kamins J, McCulloch KL. The American congress of rehabilitation medicine diagnostic criteria for mild traumatic brain injury. Arch Phys Med Rehab. 2023;104(8):1343–55. doi:10.1016/j.apmr.2023.03.036.
- Davis GA, Patricios J, Schneider KJ, Iverson GL, Silverberg ND. Definition of sport-related concussion: the 6th international conference on concussion in sport. Br J Sports Med. 2023;57(11):617–18. doi:10.1136/bjsports-2022-106650.
- Manzanero S, Elkington LJ, Praet SF, Lovell G, Waddington G, Hughes DC. Post-concussion recovery in children and adolescents: a narrative review. J Concussion. 2017;1:2059700217726874. doi:10.1177/2059700217726874.
- Bernard CO, Ponsford JA, McKinlay A, McKenzie D, Krieser D. Predictors of post-concussive symptoms in young children: injury versus non-injury related factors. J Int Neuropsychol Soc. 2016;22(8):793–803. doi:10.1017/S1355617716000709.
- Risen SR, Reesman J, Yenokyan G, Slomine BS, Suskauer SJ. The course of concussion recovery in children 6-12 years of age: experience from an interdisciplinary rehabilitation clinic. PM&R. 2017;9(9):874–83. doi:10.1016/j.pmrj.2016.12.005.
- Nelson LD, Guskiewicz KM, Barr WB, Hammeke TA, Randolph C, Ahn KW, Wang Y, McCrea MA. Age differences in recovery after sport-related concussion: a comparison of high school and collegiate athletes. J Athl Training. 2016;51(2):142–52. doi:10.4085/1062-6050-51.4.04.
- Morgan CD, Zuckerman SL, Lee YM, King L, Beaird S, Sills AK, Solomon GS. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589–98. doi:10.3171/2014.10.PEDS14356.
- Hannah TC, Li AY, Spiera Z, Kuohn L, Dai J, McAuley F, Ali M, Durbin JR, Dreher N, Marayati NF, Gometz A. Sex-related differences in the incidence, severity, and recovery of concussion in adolescent student-athletes between 2009 and 2019. Am J Sports Med. 2021;49(7):1929–37. doi:10.1177/03635465211008596.
- Dougherty JM, Carney M, Hohman MH, Emmady PD. Vestibular dysfunction. StatPearls. US: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
- Newman-Toker DE, Hsieh YH, Camargo CA, Jr., Pelletier AJ, Butchy GT, Edlow JA. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83(7):765–75. doi:10.4065/83.7.765.
- Zaben M, El Ghoul W, Belli A. Post-traumatic head injury pituitary dysfunction. Disabil Rehabil. 2013;35(6):522–25. doi:10.3109/09638288.2012.697252.
- Tanriverdi F, Schneider HJ, Aimaretti G, Masel BE, Casanueva FF, Kelestimur F. Pituitary dysfunction after traumatic brain injury: a clinical and pathophysiological approach. Endocr Rev. 2015;36(3):305–42. doi:10.1210/er.2014-1065.
- Esterov D, Lennon RJ, Bergquist T, Brown A, Goldberg G, Eapen B, Kamen L. Predictors of neurobehavioral symptom reporting in a community based sample with mild traumatic brain injury. NeuroRehabilitation. 2020;47(1):65–77. doi:10.3233/NRE-203082.
- Cheever K, McDevitt J, Phillips J, Kawata K. The role of cervical symptoms in post-concussion management: a systematic review. Sports Med. 2021;51(9):1875–91. doi:10.1007/s40279-021-01469-y.
- Storzbach D, O’Neil ME, Roost S-M, Kowalski H, Iverson GL, Binder LM, Fann JR, Huckans M. Comparing the neuropsychological test performance of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans with and without blast exposure, mild traumatic brain injury, and posttraumatic stress symptoms. J Int Neuropsychol Soc. 2015;21(5):353–63. doi:10.1017/S1355617715000326.
- Shepherd D, Heinonen-Guzejev M, Heikkila K, Landon J, Theadom A. Sensitivity to noise following a mild traumatic brain injury: a longitudinal study. J Head Trauma Rehabil. 2021;36(5):E289–301. doi:10.1097/HTR.0000000000000645.
- Job RS. Noise sensitivity as a factor influencing human reaction to noise. Noise Health. 1999;1(3):57.
- Fackrell K, Fearnley C, Hoare DJ, Sereda M. Hyperacusis questionnaire as a tool for measuring hypersensitivity to sound in a tinnitus research population. Biomed Res Int. 2015;2015:290425. doi:10.1155/2015/290425.
- DeMartini J, Patel G, Fancher TL. Generalized anxiety disorder. Ann Intern Med. 2019;170(7):ITC49–64. doi:10.7326/AITC201904020.
- Gray J, McNaughton N. The Neuropsychology of Anxiety: Reprise. In: Hope DA, Izard CE, editors. Perspective on anxiety, panic and fear. Lincoln, Neb.: U of Nebraska Press. 1996; p.61–134.
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on concussion in sport – the 3rd international conference on concussion in sport, held in zurich, november 2008. J Clin Neurosci. 2009;16(6):755–63. doi:10.1016/j.jocn.2009.02.002.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR 4th ed. Washinton, DC: American Psychiatric Association;2000.
- Evans RW. The Postconcussion Syndrome: 130 Years of Controversy. Seminars in Neurology. 1994; 14(1):32–39. doi:10.1055/s-2008-1041056.
- King NS. Post-concussion syndrome: clarity amid the controversy? Br J Psychiatry. 2003;183(4):276–78. doi:10.1192/bjp.183.4.276.
- Kristman VL, Borg J, Godbolt AK, Salmi LR, Cancelliere C, Carroll LJ, Holm LW, Nygren-de Boussard C, Hartvigsen J, Abara U, Donovan J. Methodological issues and research recommendations for prognosis after mild traumatic brain injury: results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehab. 2014;95(3):S265–77. doi:10.1016/j.apmr.2013.04.026.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. doi:10.1186/s13643-016-0384-4.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi:10.1080/1364557032000119616.
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, Britten N, Roen K, Duffy S. Guidance on the conduct of narrative synthesis in systematic reviews. A A product from the ESRC methods programme Version. 2006;1(1):b92.
- Ryan R, Group. CCaCR. Cochrane consumers and Communication review group: data synthesis and analysis 2013 Available from: https://cccrg.cochrane.org.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Miller LJ, Mittenberg W. Brief cognitive behavioral interventions in mild traumatic brain injury. Appl Neuropsychol. 1998;5(4):172–83. doi:10.1207/s15324826an0504_2.
- King N, Crawford S, Wenden F, Moss N, Wade D. Rivermead post-concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–92. doi:10.1007/BF00868811.
- Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Training. 2000;35(1):19.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi:10.1111/j.1600-0447.1983.tb09716.x.
- Colquhoun HL, Jesus TS, O’Brien KK, Tricco AC, Chui A, Zarin W, Lillie E, Hitzig SL, Seaton S, Engel L, Rotenberg S. Scoping review on rehabilitation scoping reviews. Arch Phys Med Rehab. 2020;101(8):1462–69. doi:10.1016/j.apmr.2020.03.015.
- Callahan ML, Lim MM. Sensory sensitivity in TBI: implications for chronic disability. Curr Neurol Neurosci Rep. 2018;18(9):1–8. doi:10.1007/s11910-018-0867-x.
- Denby E, Murphy D, Busuttil W, Sakel M, Wilkinson D. Neuropsychiatric outcomes in UK military veterans with mild traumatic brain injury and vestibular dysfunction. J Head Trauma Rehabil. 2020;35(1):57–65. doi:10.1097/HTR.0000000000000468.
- Denby E, Dempster T, White T, Brockman K, Ellis H, Dharm-Datta S, Wilkinson D, Brunger H. Dizziness directly influences postconcussion symptoms and is predictive of poorer mental health in UK military personnel: a retrospective analysis. J Head Trauma Rehabil. 2023;10.1097. doi:10.1097/HTR.0000000000000895.
- Alsalaheen BA, Whitney SL, Marchetti GF, Furman JM, Kontos AP, Collins MW, Sparto PJ. Relationship between cognitive assessment and balance measures in adolescents referred for vestibular physical therapy after concussion. Clin J Sport Med. 2016;26(1):46–52. doi:10.1097/JSM.0000000000000185.
- Houston MN, Bay RC, Valovich McLeod TC. The relationship between post-injury measures of cognition, balance, symptom reports and health-related quality-of-life in adolescent athletes with concussion. Brain Inj. 2016;30(7):891–98. doi:10.3109/02699052.2016.1146960.
- Macgregor AJ, Shannon KB, Dougherty AL. Time since injury as a factor in post-concussion symptom reporting among military service members with blast-related concussion. J Neurotrauma. 2021;38(17):2447–53. doi:10.1089/neu.2020.7334.
- MacGregor AJ, Joseph AR, Dougherty AL. Prevalence of tinnitus and association with self-rated health among military personnel injured on combat deployment. Mil Med. 2020;185(9–10):e1608–14. doi:10.1093/milmed/usaa103.
- Baguley DM. Hyperacusis. J R Soc Med. 2003;96(12):582–85. doi:10.1177/014107680309601203.
- Schecklmann M, Landgrebe M, Langguth B, Group TDS, Snyder J. Phenotypic characteristics of hyperacusis in tinnitus. PLOS ONE. 2014;9(1):e86944. doi:10.1371/journal.pone.0086944.
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound: questionnaire data, audiometry and classification. Scand Audiol. 1999;28(4):219–30. doi:10.1080/010503999424653.
- Jüris L, Andersson G, Larsen HC, Ekselius L. Psychiatric comorbidity and personality traits in patients with hyperacusis. Int J Audiol. 2013;52(4):230–35. doi:10.3109/14992027.2012.743043.
- Grossman EJ, Inglese M. The role of thalamic damage in mild traumatic brain injury. J Neurotrauma. 2016;33(2):163–67. doi:10.1089/neu.2015.3965.
- Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66(6):819–26. doi:10.1016/j.neuron.2010.04.032.
- Gard A, Al-Husseini A, Kornaropoulos EN, De Maio A, Tegner Y, Bjorkman-Burtscher I, Markenroth Bloch K, Nilsson M, Magnusson M, Marklund N. Post-concussive vestibular dysfunction is related to injury to the inferior vestibular nerve. J Neurotrauma. 2022;16(11–12):829–40. doi:10.1089/neu.2021.0447.
- Buttner F, Howell DR, Doherty C, Blake C, Ryan J, Delahunt E. Condition-specific health-related quality of life amongst amateur athletes six months and one-year following sport-related concussion: a prospective, follow-up. Phys Ther Sport. 2021;51:71–78. doi:10.1016/j.ptsp.2021.06.011.
- Ödman M, Maire R. Chronic subjective dizziness. Acta Otolaryngol. 2008;128(10):1085–88. doi:10.1080/00016480701805455.
- Hauck LJ, Carpenter MG, Frank JS. Task-specific measures of balance efficacy, anxiety, and stability and their relationship to clinical balance performance. Gait Posture. 2008;27(4):676–82. doi:10.1016/j.gaitpost.2007.09.002.
- Bayat A, Hoseinabadi R, Saki N, Sanayi R. Disability and anxiety in vestibular diseases: a cross-sectional study. Cureus. 2020;12(11). doi:10.7759/cureus.11813.
- Balcı B, Akdal G. Imbalance, motion sensitivity, anxiety and handicap in vestibular migraine and migraine only patients. Auris Nasus Larynx. 2020;47(5):747–51. doi:10.1016/j.anl.2020.02.015.
- Hevey D. Network analysis: a brief overview and tutorial. Health Psychol Behav Med. 2018;6(1):301–28. doi:10.1080/21642850.2018.1521283.
- Blaesing L, Kroener-Herwig B. Self-reported and behavioral sound avoidance in tinnitus and hyperacusis subjects, and association with anxiety ratings. Int J Audiol. 2012;51(8):611–17. doi:10.3109/14992027.2012.664290.
- Jüris L, Ekselius L, Andersson G, Larsen HC. The hyperacusis questionnaire, loudness discomfort levels, and the hospital anxiety and depression scale: a cross-sectional study. Hear Balance Commun. 2013;11(2):72–79. doi:10.3109/21695717.2013.780409.
- Wierwille L. Fibromyalgia: diagnosing and managing a complex syndrome. J Am Acad Nurse Pract. 2012;24(4):184–92. doi:10.1111/j.1745-7599.2011.00671.x.
- Belanger HG, Proctor-Weber Z, Kretzmer T, Kim M, French LM, Vanderploeg RD. Symptom complaints following reports of blast versus non-blast mild TBI: does mechanism of injury matter? Clin Neuropsychol. 2011;25(5):702–15. doi:10.1080/13854046.2011.566892.
- Valera-Calero JA, Arendt-Nielsen L, Cigarán-Méndez M, Fernández-de-Las- PeñPeñAs C, Varol U. Network analysis for better understanding the complex psycho-biological mechanisms behind fibromyalgia syndrome. Diagnostics. 2022;12(8):1845. doi:10.3390/diagnostics12081845.
- Koes BW, Van Tulder M, Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332(7555):1430–34. doi:10.1136/bmj.332.7555.1430.
- Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20(2):146–60. doi:10.1016/j.jpain.2018.07.006.
- Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9(1):91–121. doi:10.1146/annurev-clinpsy-050212-185608.
- Langdon S, Königs M, Adang EAMC, Goedhart E, Oosterlaan J. Subtypes of sport-related concussion: a systematic review and meta-cluster analysis. Sports Med. 2020;50(10):1829–42. doi:10.1007/s40279-020-01321-9.
- Aderman MJ, Brett BL, Ross JD, Malvasi SR, McGinty G, Jackson JC, Estevez CA, Brodeur RM, Svoboda SJ, McCrea MA, Broglio SP. Association between symptom cluster endorsement at initiation of a graduated return-to-activity protocol and time to return to unrestricted activity after concussion in United States service academy cadets. Am J Sports Med. 2023;51(11):2996–3007. doi:10.1177/03635465231189211.
- Lange RT, Iverson GL, Rose A. Post-concussion symptom reporting and the “good-old-days” bias following mild traumatic brain injury. Arch Clin Neuropsychol. 2010;25(5):442–50. doi:10.1093/arclin/acq031.
- Brien SE, Lorenzetti DL, Lewis S, Kennedy J, Ghali WA. Overview of a formal scoping review on health system report cards. Implement Sci. 2010;5(1):1–12. doi:10.1186/1748-5908-5-2.
- McCrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvorak J, Echemendia RJ, Engebretsen L, Johnston KM, Kutcher JS, Raftery M, Sills A. Consensus statement on concussion in sport—the 4th international conference on concussion in sport held in zurich, november 2012. Pm&r. 2013;5(4):255–79. doi:10.1016/j.pmrj.2013.02.012.
- Kara S, Crosswell H, Forch K, Cavadino A, McGeown J, Fulcher M. Less than half of patients recover within 2 weeks of injury after a sports-related mild traumatic brain injury: a 2-year prospective study. Clin J Sport Med. 2020;30(2):96–101. doi:10.1097/JSM.0000000000000811.
- Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technolo Assess. 2012;28(2):138–44. doi:10.1017/S0266462312000086.
- Van Toor T, Neijenhuis K, Snik A, Blokhorst M. Evaluation of auditory processing disorders after whiplash injury. Audiol Med. 2006;4(4):191–201. doi:10.1080/16513860601011755.
- Tjell C, Tenenbaum A, Rosenhall U. Auditory function in whiplash-associated disorders. Scand Audiol. 1999;28(4):203–09. doi:10.1080/010503999424635.
- Blokhorst M, Meeldijk S, van Luijtelaar G, van Toor T, Lousberg R, Ganzevles P. Noise-intolerance and state-dependent factors in patients with whiplash associated disorder. J Whiplash Relat Disord. 2005;4(1):5–24. doi:10.3109/J180v04n01_02.
- Fife TD, Kalra D. Persistent vertigo and dizziness after mild traumatic brain injury. Ann NY Acad Sci. 2015;1343(1):97–105. doi:10.1111/nyas.12678.
- Fife TD, Giza C. Posttraumatic vertigo and dizziness. Seminars in Neurology. 2013;33(03):238–243. doi:10.1055/s-033-1354599.
- Pelo R, Gaudette E, Millsap L, Martindale C, Dibble LE, Cortez MM, Fino PC. A multimodal assessment of the heterogeneous nature of post-mTBI dizziness. medRxiv. 2022;15:07.
- Pertab JL, Merkley TL, Cramond AJ, Cramond K, Paxton H, Wu T. Concussion and the autonomic nervous system: An introduction to the field and the results of a systematic review. NeuroRehabilitation. 2018;42(4):397–427. doi:10.3233/NRE-172298.
- Mercier LJ, Batycky J, Campbell C, Schneider K, Smirl J, Debert CT. Autonomic dysfunction in adults following mild traumatic brain injury: a systematic review. NeuroRehabilitation. 2022;50(1):3–32. doi:10.3233/NRE-210243.
- Pelo R, Suttman E, Fino PC, MM M, Dibble LE, Cortez MM. Autonomic dysfunction and exercise intolerance in concussion: a scoping review. Clin Auton Res. 2023;33(2):1–15. doi:10.1007/s10286-023-00937-x.
- Purkayastha S, Stokes M, Bell KR. Autonomic nervous system dysfunction in mild traumatic brain injury: a review of related pathophysiology and symptoms. Brain Inj. 2019;33(9):1129–36. doi:10.1080/02699052.2019.1631488.
- Liao KH, Sung CW, Chu SF, Chiu WT, Chiang YH, Hoffer B, Ou JC, Chen KY, Tsai SH, Lin CM, Chen GS. Reduced power spectra of heart rate variability are correlated with anxiety in patients with mild traumatic brain injury. Psychiatry Research. 2016;243:349–56.
- Rao V, Syeda A, Roy D, Peters ME, Vaishnavi S. Neuropsychiatric aspects of concussion: acute and chronic sequelae. Concussion. 2017;2(1):CNC29. doi:10.2217/cnc-2016-0018.
Appendix I
Search strategy
Medline Ovid search strategy as an example – All factors plus mTBI and prolonged recovery.
