ABSTRACT
Background
Detecting cognitive impairments early after stroke is essential for appropriate referrals. Although recommended in stroke guidelines, early cognitive screening is not always implemented. We assessed whether the Montreal Cognitive Assessment (MoCA) adds diagnostic value compared to clinical observation alone. In addition, discharge destinations for stroke patients with and without cognitive deficits detected with the screening tool or the treatment team were explored.
Methods
Forty-four stroke patients were screened with the MoCA during stroke unit admission. Their charts were studied for cognitive impairments reported by the stroke care team, who were blinded to screening scores. Proportions of detected cognitive deficits were compared between screening (score <26) and patient charts. Discharge destination distribution (home vs. rehabilitation) was explored.
Results
The proportion of cognitively impaired patients indicated by the MoCA (84%) and reported in patients’ charts (25%) differed significantly (p < 0.001). The distribution of discharge destination did not suggest an association with the detection of cognitive deficits by the treatment team or the cognitive screening.
Conclusions
The MoCA detects more cognitive deficits than clinical impression alone, emphasizing the importance of standard screening for cognitive impairments in acute stroke patients. Ultimately, systematic screening may enhance discharge planning and improve long-term outcomes.
Introduction
Early cognitive impairments after stroke, such as memory, attention, and executive impairments predict cognitive and functional deficits in the years thereafter (Citation1,Citation2). Detection of early cognitive impairments is important for minimizing the impact of cognitive deficits by appropriate referral to aftercare (Citation3). However, cognitive deficits are easily missed upon clinical observation by the treatment team during hospital stay when patients do not have to perform cognitively demanding tasks (Citation4).
In clinical practice guidelines, there is consensus that cognitive assessment should be part of routine stroke care (Citation5). Screening tools are quick and easy to administer (Citation4), which is why guidelines recommend screening all stroke patients in the (sub)acute phase with a sensitive screening instrument (Citation6) However, guidelines are often not specific in how to screen and which instrument to use (Citation5). Additionally, guideline adherence is low, causing cognitive deficits to be missed by the treatment team during hospital stay in mild stroke patients who are discharged early under the assumption of not having any cognitive deficits. In patients suffering from moderate or severe stroke, management of motor or neurological impairments is often prioritized (Citation3,Citation7).
Several systematic reviews recommend the Montreal Cognitive Assessment (MoCA) as the most acceptable cognitive screening instrument for stroke patients in terms of sensitivity and specificity (Citation8–10). Its diagnostic value is determined by comparison to a gold standard such as a neuropsychological test battery consisting of domain-specific cognitive tests. Since extensive testing is often not feasible or necessary in the acute phase post stroke, cognitive screening is recommended as alternative. Given the low adherence to this recommendation, clinicians may believe they can detect cognitive deficits as often as the MoCA can. Investigating the diagnostic value of clinical observations against the MoCA as gold standard has, however, not been conducted yet.
In the national Dutch stroke guidelines, the MoCa is recommended as standard instrument for routine cognitive assessment (Citation6). This study therefore evaluated whether the MoCA detects cognitive deficits in the acute phase after stroke that may be overlooked based on clinical impressions alone. We hypothesized that the MoCA has a higher diagnostic value than clinical judgment (according to patients’ charts). Discharge destinations were explored for differences between patients whose deficits were detected versus those whose deficits were missed.
Materials & methods
Design
This prospective observational study was conducted between August and December 2021 and February to December 2022 at the Maastricht University Medical Centre (MUMC+) stroke unit in the Netherlands. Data of fifty-seven participants was required to detect a 15% difference between proportions of cognitive deficits detected by the MoCA and those reported in patient charts (power 80%, alpha 0.05). The study received approval from the medical ethics committee of MUMC+ (METC 2021–2692).
Participants
Included were adults who had sustained a stroke (hemorrhagic/ischemic; first or recurrent) confirmed by a neurologist, were admitted to the stroke unit of our hospital, were proficient in Dutch and were able to utilize a pen/pencil. Patients with (severe) aphasia (based on clinical judgment) or previously diagnosed with dementia (as noted in the medical file) were excluded. The treatment team of the stroke unit judged aphasia as severe in case a patient was not able to follow verbal instructions during daily nursing routines.
Measures
Demographic and stroke-related data were derived from patient charts; education information was collected during the MoCA screening. The MoCA is a one-page cognitive screening tool used to assess attention and concentration, executive functioning, memory, language, and visuo-constructional skills, conceptual thinking, calculations, and orientation in approximately 10 minutes (Citation11). The maximum score is 30 points. One point is added for participants with less than 12 years of education; a score below 26 indicates cognitive impairment (Citation11). During the study, the use of the MoCA was not standard clinical care on the stroke unit. Clinical judgment of cognitive functioning was based on information in the patients’ medical chart. Any mentioning of cognitive impairments was considered relevant. This could be a report by a certain discipline based on their assessments (e.g. occupational therapist) or an observation of a cognitive failure by a nurse on the stroke unit, or a conclusion written in the report of the multidisciplinary team meeting, or mentioning of cognitive impairments in the discharge letter by the treating neurologist. The review of the charts was done by several medical students (LV, RH) as part of their scientific internship and checked by the treating neurologist (JS) and supervised by the first author (AS).
Procedures
Hospital staff informed eligible stroke patients about the study (convenience series) and notified the research team of their interest. A MoCA-trained member of the research team obtained written consent before administering the MoCA at the bedside or a low stimulus office. After discharge, patient charts were reviewed for cognitive impairment as noted by the doctor, nurses or rehabilitation team of the stroke unit, and demographics. Attending clinical staff and research team members other than the assessors were blinded to MoCA scores.
Statistical analysis
Descriptive analyzes were applied to demographics. McNemar’s test was utilized to compare the proportion of people with a MoCA score below 26 to the proportion of people with cognitive deficits documented in their patient chart using IBM SPSS statistics version 27.
Results
Participants
Out of 329 admitted stroke patients, 71 were ineligible due to severe neurological deficits or language barriers. Ninety-nine were discharged before recruitment. For 94 patients, exclusion criteria were not indicated, but their charts were not forwarded to the research team. Of the remaining 65 patients, 12 were discharged before assessment, 7 met exclusion criteria, and 2 had already been assessed with a Montreal Cognitive Assessment. Ultimately, 44 patients completed the MoCA (see ).
Table 1. Demographics and stroke-related characteristics.
Outcomes
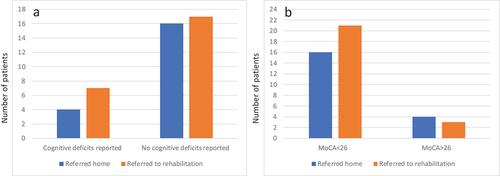
Thirty-seven patients (84.1%) scored below 26 points with a mean score of 20.9 (SD 4.7). Reports of cognitive deficits were found in 11 patient charts (25.0%). The proportion of cognitively impaired patients as detected by the MoCA and patient charts differed significantly (p < 0.001, ). The distribution of discharge destination is depicted in .
Table 2. Proportion of cognitive deficits detected by the MONTREAL COGNITIVE ASSESSMENT and documented in patient charts.
Discussion
This study demonstrates that the Montreal Cognitive Assessment has a higher diagnostic value than clinical judgment because it indicates cognitive impairments in patients in the acute phase after stroke that go unnoticed by the treatment team, which is in line with previous findings involving more lengthy screening tools (Citation4). The identification of cognitive impairments by either the Montreal Cognitive Assessment or the treatment team does not appear to affect discharge destination, but the sample size is insufficient to test this association.
The MoCA indicated cognitive impairments in 84.1% of stroke patients, which is higher compared to previous studies (43% (Citation12) and 73% (Citation13) involving slightly younger patients with possibly milder strokes. Some studies have used lower cutoffs to prevent overestimation of cognitive impairments, but the cutoff score that best balances the risk of false negatives (possibly having detrimental effects on a person’s life) and false positives (causing unnecessary worry or burden) is yet to be determined (Citation8).
Screening instruments are often used as the sole method for assessing cognition after stroke, but they merely inform about the risk of cognitive impairments (Citation8). Further or repeated testing is recommended for patients with cognitive impairments indicated by the MoCA in the acute phase, as cognition typically improves in the first weeks after stroke (Citation14). Several cognitive domains should be assessed during early follow-up to guide treatment planning. In case of more complex rehabilitation goals, extensive assessment including emotional, behavioral and psychological factors should be performed from three months onwards (Citation14).
A previous study found no differences in cognitive functioning between patients who were discharged home and those who were referred to rehabilitation, suggesting that cognition may not play a role in discharge decisions (Citation15). Our findings support this notion, as cognitive deficits often go unnoticed by the treatment team. Cognitive rehabilitation interventions have shown to benefit functioning, so considering cognition during treatment planning potentially improves long-term outcomes (Citation3,Citation15).
Study limitations include the low participation rate, which was partly due to short hospital admissions. This resulted in patients being missed when recruiting staff was not working (Fridays-Sundays) or when MoCA assessors were not readily available. The calculated sample size was not reached, but inclusion was stopped after a year to allow MoCA use in clinical practice. COVID-19 restrictions further complicated recruitment because during lock downs researchers were not allowed to visit the stroke unit. The single-site nature of the study limits generalizability. Despite these limitations, the characteristics of our sample show that we included a regular ischemic stroke population (i.e. low number of hemorrhagic stroke patients) and our results seem rather robust because we compared the screening results to any kind of note on cognitive functioning in the medical records. Therefore, we believe that these initial results underpin stroke guidelines recommending cognitive screening.
We used the MoCA because it is recommended in our national stroke guidelines (Citation6) even though other national guidelines are not that explicit in the use of a specific tool (Citation5). This decision is based on several systematic reviews concluding that the MoCA is the most sensitive instrument for post-stroke cognitive impairment. However, the MoCA has also been criticized because it is not suitable for many stroke patients and may not be sufficiently sensitive for impairments in processing speed (Citation16). Other screening instruments have been developed to overcome these problems, such as the (Dutch version of the) Oxford Cognitive Screen (OCS-NL) (Citation16) and Cognitive assessment scale for stroke patients (CASP) (Citation17). Future studies may need to confirm the added value of standard cognitive screening using those instruments. The CASP is further recommended because it minimizes the use of verbal materials, which is important in case stroke patients have mild or moderate aphasia (Citation18). The CASP score is less affected by language comprehension impairments than the MoCA score.
To conclude, the results suggest that the Montreal Cognitive Assessment can highlight otherwise unnoticed cognitive deficits and alert the treatment team of possible functional impairments that may develop as a result. The findings, although with limited power, emphasize the importance of following clinical guidelines and screening all stroke patients with the Montreal Cognitive Assessment to improve discharge planning and long-term outcomes.
Acknowledgments
The authors thank Roos Roberts, Indy Ezra Hol, Rani Knops, Laudy Dikken, and Pauline van Gils for recruitment and data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dong Y, Slavin MJ, Chan BPL, Venketasubramanian N, Sharma VK, Crawford JD, Collinson SL, Sachdev P, Chen CLH. Cognitive screening improves the predictive value of stroke severity scores for functional outcome 3–6 months after mild stroke and transient ischaemic attack: an observational study. BMJ Open. 2013;3(9):1–6. doi:10.1136/bmjopen-2013-003105.
- Salvadori E, Pasi M, Poggesi A, Chiti G, Inzitari D, Pantoni L. Predictive value of MoCA in the acute phase of stroke on the diagnosis of mid-term cognitive impairment. J Neurol. 2013;260(9):2220–27. doi:10.1007/s00415-013-6962-7.
- Stolwyk RJ, Mihaljcic T, Wong DK, Chapman JE, Rogers JM. Poststroke cognitive impairment negatively impacts activity and participation outcomes. Stroke. 2021;52(2):748–60. doi:10.1161/STROKEAHA.120.032215.
- Edwards DF, Hahn MG, Baum CM, Perlmutter MS, Sheedy C, Dromerick AW. Screening patients with stroke for rehabilitation needs: validation of the post-stroke rehabilitation guidelines. J Neurol Rehabil. 2006;20(1):42–48. doi:10.1177/1545968305283038.
- McMahon D, Micallef C, Quinn TJ. Review of clinical practice guidelines relating to cognitive assessment in stroke. Disabil Rehabil. 2022;44(24):7632–40. doi:10.1080/09638288.2021.1980122.
- Abzhandadze T, Buvarp D, Å L-N, Sunnerhagen KS. Barriers to cognitive screening in acute stroke units. Sci Rep. 2021;11(1):19621. doi:10.1038/s41598-021-98853-5.
- Stolwyk RJ, O’Neill MH, McKay AJD, Wong DK, O’Neill MH, McKay AJD, Wong DK. Are cognitive screening tools sensitive and specific enough for use after stroke? A systematic literature review. Stroke. 2014;45(10):3129–34. doi:10.1161/STROKEAHA.114.004232.
- Van Heugten CM, Walton L, Hentschel U. Can we forget the Mini-Mental State Examination? A systematic review of the validity of cognitive screening instruments within one month after stroke. Clin Rehabil. 2015;297:694–704. doi:10.1177/0269215514553012.
- Burton L, Tyson SF. Screening for cognitive impairment after stroke: a systematic review of psychometric properties and clinical utility. J Rehabil Med. 2015;47(3):193–203. doi:10.2340/16501977-1930.
- Nederlandse Vereniging voor Neurologie. Herseninfarct en hersenbloeding. 2017.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–99. doi:10.1111/j.1532-5415.2005.53221.x.
- Zietemann V, Georgakis MK, Dondaine T, Müller C, Mendyk A-M, Kopczak A, Hénon H, Bombois S, Wollenweber FA, Bordet R, et al. Early MoCA predicts long-term cognitive and functional outcome and mortality after stroke. Neurology. 2018;91(20):e1838–50. doi:10.1212/WNL.0000000000006506.
- Horstmann S, Rizos T, Rauch G, Arden C, Veltkamp R. Feasibility of the Montreal cognitive assessment in acute stroke patients. Eur J Neurol. 2014;21(11):1387–93. doi:10.1111/ene.12505.
- Bos AY, Vloothuis JD, Visser-Meily A, van Heugten CM. Cognitieve screening en diagnostiek na een beroerte: het kan beter. Ned Tijdschr voor Revalidatiegeneeskd. 2022;5:55–59.
- Slenders JPL, Verberne DPJ, Visser-Meily JMA, Van den Berg-Vos RM, Kwa VIH, van Heugten CM. Early cognitive and emotional outcome after stroke is independent of discharge destination. J Neurol. 2020;267(11):3354–61. doi:10.1007/s00415-020-09999-7.
- Huygelier H, Schraepen B, Demeyere N, Gillebert CR. The Dutch version of the Oxford Cognitive Screen (OCS-NL): normative data and their association with age and socio-economic status. Aging, Neuropsychol Cogn. 2020;27(5):765–86. doi:10.1080/13825585.2019.1680598.
- Benaim C, Wauquiez G, Pérennou D, Piscicelli C, Lucas-Pineau B, Bonnin-Koang H-Y, Vuadens P, Binquet C, Bourredjem A, Devilliers H. Cognitive assessment scale for stroke patients (CASP): a multicentric validation study. Ann Phys Rehabil Med. [Internet]. 2022;65(3):101594. doi: 10.1016/j.rehab.2021.101594.
- Crivelli D, Spinosa C, Angelillo MT, Balconi M. The influence of language comprehension proficiency on assessment of global cognitive impairment following acquired brain injury: a comparison between MMSE, MoCA and CASP batteries. Appl Neuropsychol Adult. [Internet]. 2023;30(5):546–51. doi:10.1080/23279095.2021.1966430.