ABSTRACT
Objective: This study aimed to establish a permanent middle cerebral artery occlusion (pMCAO) model in rats to simulate the pathological process of stroke patients with no reperfusion. And screen highly sensitive items that could be used to detect long-term behavioral abilities in rat of intraluminal suture models. Method: Established the pMCAO model then tested the rats for the bilateral asymmetry, modified neurological severity score, grid-walking, cylinder, rotating, and water maze test from week 1 to week 16. Results: The infarct volume of the model rats was stable (26.72% ±1.86%). The sensorimotor test of bilateral asymmetry, grid-walking, cylinder, and mNSS test showed significant differences from week 1 to week 16 after injury. The water maze test at week 16 showed significant differences in spatial exploration and learning ability between the two groups. We confirmed that there was no significant difference between MRI and TTC staining in detecting the degree of brain injury, which facilitated the diversity of subsequent detection methods. We also confirmed that at multiple time points, grid, cylinder and water maze test were significantly positively correlated with rat brain infarct volume. Conclusion: They are suitable for the long-term observation of behaviors in the sequela stage of stroke in rat.
Introduction
Cerebral stroke, also known as ‘stroke’ or ‘cerebral vascular accident,’ is caused by the rupture of blood vessels in the brain or vascular obstruction caused by brain tissue damage, including ischemic and hemorrhagic stroke. Among them, ischemic stroke accounts for 60–70% of the total stroke cases (Citation1,Citation2). Stroke is the fifth leading cause of death in the United States, and its incidence is much higher in China than in most Western countries, causing one million deaths annually. With an overall prevalence of approximately 2.5% worldwide, stroke is the second leading cause of death and the leading cause of acquired long-term disability worldwide (Citation3,Citation4).
Cerebral apoplexy is divided into the acute, recover, and sequela stage according to time. Generally, the acute period, recovery period, and sequelae refer to the stroke from 0 h to 1 week, 1 week to 6 months, and 6 months or more (Citation5). At present, most researchers and doctors focus on the research field of the acute or subacute stage. Tissue plasminogen activator (tPA) is the only FDA-approved treatment for thrombotic stroke, but it only works within a few hours of stroke onset (Citation6). In reality, patients can barely receive treatment in time, and they are very easy relapse, leading the majority of them into sequelae. The tPA can also increase the risk of intracerebral hemorrhage (ICH) (Citation7). Therefore, the proportion of patients with stroke in the sequelae stage is high.
Approximately 80% of stroke cases occur in the middle cerebral artery (MCA) (Citation8). Thus, the MCA occlusion (MCAO) model is widely used in the study of focal cerebral ischemia in rats (Citation9–12). The intraluminal suture model method has advantages of the non-requirement for craniotomy, little injury, easy operation, good repeatability, and accurate control of the time of ischemia reperfusion (Citation13). Permanent MCAO (pMCAO) models can better reflect emergent large-vessel occlusion (ELVO) stroke without thrombolytic therapy or mechanical thrombectomy, and clinical data suggest that more than half of patients with ischemic stroke do not experience spontaneous reperfusion (Citation14). However, in 2014 and 2015, 88% of published fundamental science studies on ELVO used the transient MCAO (tMCAO) models. The model represents only 2.5%–11.3% of patients with stroke. Therefore, for the study of the stroke sequelae period, the permanent cerebral infarction model is superior to the cerebral ischemia–reperfusion model both in the degree of injury and simulating actual conditions of clinical patients (Citation15,Citation16). The STAIR conference also promotes the use of pMCAO (Citation17).
According to our previous studies, the success rate of the tMCAO/R model in rats was affected by ischemic time, body weight, and diameter of the suture embolus. Rats weighing 300 g survived better with the same success rate (Citation5). In order to facilitate the study of stroke sequelae, it is necessary to explore several detection items with high sensitivity, which can reflect the changes in the behavior of the experimental animals for a long time.
Materials and methods
Animals
Male Sprague Dawley (SD) rats, weighing 280–310 g, were provided by Beijing Vitonlihua Experimental Animal Technology Co., Ltd. All rats were fed at a temperature of 20–22 °C for 12 h with dark and light cycles and given drinking water freely. All the experimental procedures were performed with the approval of the Ethical Committee of the Langfang 4th Hospital (202001) and followed the Institutional Animal Care and the Use of Laboratory Animals and the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines.
Establishment of the pMCAO model and postoperative nursing
The 60 SD rats weighing 280–310 g were randomly divided into two groups using a random table, with 15 in the control group and 45 (12 rats were used for 24-hour data analysis: pMCAO group rat modeling success rate and infarct volume statistics) in the pMCAO group. And they were placed in the anesthetic box of the isoflurane (RWD, Shenzhen, China, https://www.rwdstco.com) anesthesia machine. The oxygen flow rate was adjusted to 1 L/min, and the isoflurane flow rate was 0.5 L/min. After the rat was anesthetized, the isoflurane flow rate was adjusted to 0.3 L/min for continuous anesthesia.
Cut the skin vertically in the middle of the rat’s neck for 3 cm. Separate the skin and subcutaneous muscles until the common carotid artery is visible. Separating arteries and ligating the common carotid artery (CCA, slipknot), far end of external carotid artery (ECA, dead knot), and internal carotid artery (ICA, slipknot). Use micro scissors to cut a notch in the ECA, insert an intraluminal suture (soak it in physiological saline before use to make it softer), when the intraluminal suture is successfully inserted into the ECA, tie a knot on the proximal end of ECA with suture to prevent intraluminal suture from falling off, and cut off the ECA. Loosen the knot of ICA, and adjust the suture of ECA to a degree that can just prevent bleeding (the looser the suture line at ECA, the clearer you can feel slight resistance when intraluminal suture reaches anterior end of cerebral artery). Continue to insert intraluminal suture until there is slight resistance (18–22 mm), stop inserting intraluminal suture, and ligation it with suture (Citation18). intraluminal suture information is shown in .
Table 1. Intraluminal suture information.
In the control group, rats underwent the same surgical procedures without occlusion of the right MCA. We assessed regional cerebral blood flow (rCBF) through the MCA using a transcranial laser Doppler (RWD, Shenzhen, China, https://www.rwdstco.com); Severely reduced rCBF (80%) was established after successful MCAO. We monitored the rat’s heart rate using a biological signal acquisition system (Zhenghua, Anhui, China, www.6062307.com) during the surgery to confirm that there were no abnormal changes in the rat’s heart rate.
After the operation, only two rats were placed in each cage, and the jellies for the experimental animals were placed into the cage in the first three days after the completion of the model so that the animals could replenish energy and water. The animals were given penicillin 80,000 U intramuscularly and meloxicam 3 mg/kg subcutaneously for three consecutive days. During the period, if the animals were in poor condition, 37 °C heating pads or electric blankets were used for approximately 2 h to maintain their body temperature.
Longa’s score
Behavioral deficit was assessed using Longa’s 5-point scale at 2 h and 24 h after pMCAO (Citation19). The scores were as follows: 0, no neurological deficit. 1, failure to fully extend the left forepaw; 2, circling to the left; 3, falling to the left; 4, no spontaneous walking and decreased awareness. pMCAO group selection criteria: 18 rats (12 rats were tested for long-term behavior, and 6 rats were tested for pathology in the early phase of the experiment) were scored 2–3 points in 2 h and 1–3 points in 24 h after surgery. Screening pMCAO rats twice can effectively remove rats with damaged nerve bundles and loose sutures during surgery. Control group selection criteria: 12 rats with longa’s score of 0 at 2 h and 24 h after surgery.
Detection of cerebral infarction
At 24 h after brain injury, the rats were examined by magnetic resonance imaging (MRI) and 2,3,5-triphenyl tetrazolium chloride (TTC) staining.
Magnetic Resonance Imaging for small animals (PharmaScan70/16US, Bruker, Germany), the infarct volume of cerebral ischemia rats was quantitatively analyzed in the region. The rats were anesthetized with a mixture of isoflurane and oxygen and placed in a prone position on the scanning bed with their heads fixed. T2-weighted imaging (T2WI) was performed using a fast spin echo (TSE) sequence. The Image J software was used to analyze and process the T2WI images. The volume of cerebral infarction was calculated as follows: Infarct volume (mm3) = Sum of infarct areas on each section (mm2) × Layer thickness (mm) (Citation20).
In the TTC staining, rat brains were taken after cardiac perfusion and frozen at − 20 °C for 20 min (Short-term freezing facilitates tissue formation without damaging their activity.) (Citation21). The brains were cut into six equal parts (approximately 2 mm) using a rat brain model. Then, 2% TTC was used for staining at 37 °C constant-temperature shaking bed for 30 min, and the brain tissue was turned over once every 10 min (Citation22).
The percentage of the infarct volume was calculated using Image Pro Plus software (excluding edema): infarct volume = infarct volume × (contralateral hemisphere/ipsilateral hemisphere) (Citation23)
Assessment of sensorimotor function
The behavioral function of rats in control and pMCAO groups was detected at weeks 1, 4, 6, 8, 10, 12, 14, and 16 after modeling. The evaluation included the modified neurological severity score (mNSS), bilateral asymmetry, grid-walking, rotarod, cylinder, and water maze test, in which the water maze test was only used to test the endpoint of the experiment. Moreover, the body weight of each group was measured every weeks.
mNSS
The mNSS score included movement, sensation, balance beam, and reflex test (Citation24). Each detection item has a corresponding score. Record the corresponding score according to the rat’s response. 0 points represent rats with completely healthy functions, and the highest score is 18 points.
Rotarod test
For this test, an accelerating rotarod (Anhui Zhenghua Biologic Apparatus Facilities Co., Ltd.) was used. Rats were placed on the rotarod rungs (90 mm in diameter), which accelerated at a speed of 13 rpm for 5 min. A trial ended if the rat fell off the rungs or gripped and spun around for a complete revolution. Rats were acclimatized to the rotarod for three trials, of which the longest time was used as the final result of this rat, with an intertrial interval of 30 min (Citation25).
Bilateral asymmetry test
This is a test of tactile extinction probing sensory neglect (Citation26). In this test, 0.8-cm squares of sterilization instruction tape was placed on the rat’s palm at the same pressure, and the position was unified. The time that the rats were feeling (the rats touching the tape with their whiskers, mouths, or their forelimbs shaking) the tape and the time that the rats were tearing the tape off were recorded. The longest time was 180 s, and each rat was tested three times, 10 min apart. The mean was taken as the final result.
Grid-walking test
Animals were placed on an elevated grid surface platform (area, 1 m2; height, 40 cm) with grid openings of 2.5 cm2. Rats were encouraged to traverse the grid surface for 2 min. The number of foot faults made by the unaffected (right) and affected (left) limbs and the total number of steps was counted (Citation27). Error times (%) = [unaffected (right) limb – affected (left) limb]/total step number × 100. Cases with a total number of steps below 20 were removed.
Cylinder test
Transparent plexiglass cylinders (18 cm in diameter; 30 cm in height) were prepared (Citation28). Record the number of times a rat’s left, right, or both forelimbs touch the side of the cylinder within 5 minutes. And 75% alcohol was used to disinfect and remove odors after each replacement. Uncertain and difficult actions were not included in the record range. The preference, asymmetry, and deviation of the forelimbs were analyzed as follows:
Predilection for forelimb use (%) = (number of right forelimb contacts-number of left forelimb contacts)/number of left forelimb contacts + number of right forelimb contacts + number of double forelimb contacts).
Morris water maze test
The water maze test was conducted 16 weeks after modeling, and the experimenters were blinded to the rat groups. Each rat was trained four times per day for the first 4 days. The platform was hidden 1 cm below the water surface. Rats were randomly placed into the water from four entry points (two times near and two times far from the platform), and the escape latency (time needed for the rats to board the platform) was recorded. If the rats did not find the platform after 120 s, they were guided to the platform and stayed there for 15 s.
On the fifth day, the rats were removed from the platform and placed into the water from the opposite quadrant of the platform. The time needed to pass the platform for the first time, times of passing the platform, time of staying in the destination quadrant within 120 s, and distance were recorded. A 1-h interval was needed between two experiments (Citation29).
Pathological observation
After modeling, three rats were randomly selected at three different time points: 7 days, 28 days, and 16 weeks. Their brains were extracted after perfusion. The extracted brains were dehydrated using a pathological tissue dehydrator (ASP200S, Leica, Germany). The damaged area of the brain tissue was sliced using a paraffin slicer (RM2235, Leica, Germany) with a slice thickness of 4 μm. Drying all the slices for later user.
The HE staining process involved several steps:
Dewaxing: the slices were placed in xylene I for 10 minutes, xylene II for 5 minutes, and xylene III for another 5 minutes. Dehydration: slices were then dehydrated with anhydrous ethanol I for 5 minutes, with anhydrous ethanol II for 2 minutes, followed by a series of ethanol washes: 90% ethanol (5 minutes), 80% ethanol (5 minutes), 70% ethanol (5 minutes), and finally pure water (5 minutes). Hematoxylin-eosin (HE) staining: The paraffin sections were placed in hematoxylin for 4 minutes, followed by distilled water (3 minutes), 1% hydrochloric acid ethanol (15 seconds), distilled water again (20 seconds), and eosin for 1 minute. Dehydration and sealing: the sections were then dehydrated with a series of ethanol washes: 75% ethanol (2 minutes) and 85% ethanol (2 minutes) before being placed twice in anhydrous ethanol (5 minutes each) and finally in xylene (5 minutes) to complete the dehydration process. Finally, the sections were sealed with neutral gum and observed under a Leica inverted fluorescence microscope (DM18, Leica, Germany) under bright field conditions with objectives of 1.3× and 20 × . Photographs were taken.
Statistical analysis
Spss17 Quantile-Quantile Plots were used to confirm that the data matched the normal distribution. And data between the different groups were analyzed using a one-way analysis of variance or a two-tailed t-test. Data were analyzed using GraphPad Prism Version 6.0 software. Values shown represent mean ± S.E.M. Differences were considered significant at p < 0.05. The sample size of behavioral tests was calculated using G*Power software. Effect size d = 1.41, α = 0.05,1-β = 0.95, the required sample size was calculated to be 12. Double-blinding was used during all experimental operations, outcome assessments, and data analysis.
Results
After 24 h of pMCAO, brain damage, success rate, and mortality were observed
The longa’s score was 2.25 ± 0.46 at 2 hours after operation and 2.00 ± 0.53 at 24 hours after operation. The survival rate at 24 hours post-surgery was 66.67% (with 8 surviving rats). The volume of whole-brain infarction was 26.72% ± 1.86% as observed in the TTC staining image (). Additionally, to confirm whether there were any differences in assessing the extent of brain injury using different detection methods and to facilitate diverse choices for assessing brain damage in later stages, we performed both magnetic resonance imaging (MRI) and TTC staining on the same group of rats after modeling. According to , there were no significant differences between the results of MRI and TTC staining.
Figure 1. Assessment of brain injury 24 hours later. (a) TTC staining results 24 hours after model establishment (n = 8). (b, c) No significant difference was found between the results of MRI and TTC 24 h after modeling in the same group (p > 0.05). (d) The changes in body weight following the establishment of the permanent cerebral ischemia model (n = 10–12). Error bars represent S.E.M., *p < 0.05, **p < 0.01, ***p < 0.001 denote pMCAO group vs. control group rat body weight. pMCAO, permanent middle cerebral artery occlusion.
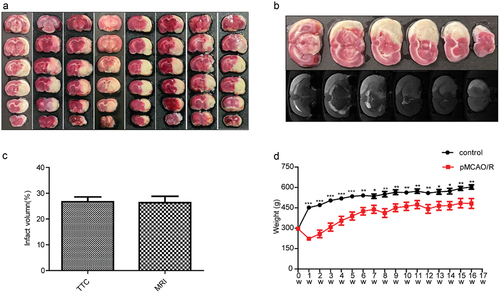
Comparison of the body weight and behavior ability of experimental animals 16 weeks after modeling
The weekly weight of the rats is shown in , the body weight of the pMCAO group was significantly lower than that of the control group 1 week after injury (p < 0.001). One week later, the rats began to gain weight, and a significant difference was still found between the two groups until the end of the experiment.
Behavioral tests were performed one week after modeling. As shown in , significant differences were found between the pMCAO group and the control group in the bilateral asymmetry(perception part), grid-walking, cylinder, and mNSS score test during the experiment period (p < 0.05). In the bilateral asymmetry test (remove part), significant differences between the pMCAO group and the control group were found only at weeks 6, 8, and 14. This may be related to the adhesive force of the adhesive tape; the control group could not remove the tape after repeated attempts, although they had quickly sensed its presence. At week 8 of the rotarod test, no significant difference was found between the two groups because of the heavy body weight and insufficient power of the instrument. After maintenance, the difference between the two groups could still be detected at week 14.
Figure 2. Changes in motor function 1–16 weeks after injury in different behavioral items. (a, b) bilateral asymmetry test of animals’ perception: time the tape was removed. (c) Grid-walking error times in the grid-walking test. (d) Cylinder test: the degree of predilection for forelimb use within the cylinder. (e) Length of time in the rotarod test. (f) Modified neurological severity score. Error bars represent S.E.M., n = 10–12. *P < 0.05,**P < 0.01,***P < 0.001 showed motor function compared with the control group. pMCAO, permanent middle cerebral artery occlusion.
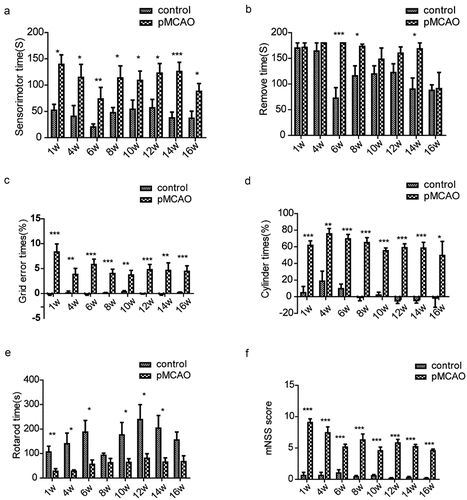
In the water maze test (), a significant difference in the time to find the platform was found between the two groups during the whole acquisition phase (p < 0.05). After training (), the probe trial showed significant differences in the time of reaching the platform, time of passing the platform, and time of platform quadrant between the two groups (p < 0.05). However, no significant difference was found between the two groups over the target quadrant distance ().
Figure 3. The water maze test was performed at 16 weeks after pMCAO. (a) Time of finding the platform in different stages of acquired training. (b) The time for the rats to find the platform was investigated during the training. (c) Number of times the rats passed the platform during the exploration training. (d) The time that the rats stayed in the quadrant of the platform was investigated during the training. Error bars represent S.E.M., n = 10–12. *p < 0.05, **p < 0.01, *** p < 0.001 vs. The control group. pMCAO, permanent middle cerebral artery occlusion.
Correlation analysis of brain injury severity with other indicators
Both MRI and TTC staining have certain limitations for determining the extent of brain damage in rats. Rats have certain external manifestations after injury, such as continuous weight loss and weakened behavioral ability, if we can confirm its correlation with the degree of brain injury, we can preliminarily judge the degree of injury of rats through these results. Based on this, we carried out the correlation analysis between six experimental items and the MRI results of rats after injury, and found that the grid-walking and cylinder tests not only had high degree of sensitivity in distinguishing normal rats from injured rats, but also had significant correlation with the degree of injury of rats at multiple time points (p < 0.05): 4 time points of 8 time points in the grid-walking test and 3 time points of 8 time points in the cylinder test were statistically significant. The result of Correlation analysis is shown in . In the water maze test, in the results of the probe trial after acquisition phase, the time for injured rats to reach the platform was significantly positively correlated with the degree of injury. At the same time, we also analyzed the correlation between brain injury volume and rat weight change during one week, but there was no significant correlation between the two (p = 0.236).
Table 2. Correlation analysis information of each item and cerebral infarction volume.
Pathological changes of the injured site 16 weeks after the establishment of the PMCAO model
The permanent MCAO model frequently involves regions of the neocortex and striatum in the infarct area (Citation30).
In the control group, the shape of the brain was intact, the neurons were arranged regularly, the cytoplasm was abundant and transparent, the nucleus was round, and the nucleolus was clear. No obvious pathological changes were found in the brain tissue.
Seven days after the establishment of the pMCAO model, the brain contours of the rats remained intact, most of the neurons in the infarction area were necrotic and disintegrated, the infarction center was necrotic, and the edge was clear. Inflammatory cells and glial infiltration increased significantly. The density of neurons in the ischemic perifocal area decreased, and the pericellular space was enlarged with mild gliosis.
At 28 days after the establishment of the pMCAO model, the contours of the rat brain were damaged remarkably, and the number of glial cells increased evidently. Scar tissue gradually formed.
At week 16 after the establishment of the pMCAO model, the cerebral contours of the rats were severely damaged, no inflammatory cells were found, and the neurons in the infarcted area were disorganized, denatured, necrotic, and dissolved. Glial scar formation occurred ().
Figure 4. Pathological changes in rat brains at different time points. (a) Pathological examination of the brain in normal rats (hematoxylin and eosin [HE] staining, ×1.3). (b) Pathological examination of the hippocampus and cortex in normal rats (HE, ×20). (c) Brain pathology of rats seven days after pMCAO (HE, ×1.3). (d) Area of the injured brain in 7 days after pMCAO (HE, ×20). (e) Brain pathology of rats 28 days after pMCAO (HE, ×1.3). (f) Area of the injured brain 28 days after pMCAO (HE, ×20). (g) Brain pathology of rats 16 weeks after pMCAO (HE, ×1.3). (h) Area of the injured brain 16 weeks after pMCAO (HE, ×20).
![Figure 4. Pathological changes in rat brains at different time points. (a) Pathological examination of the brain in normal rats (hematoxylin and eosin [HE] staining, ×1.3). (b) Pathological examination of the hippocampus and cortex in normal rats (HE, ×20). (c) Brain pathology of rats seven days after pMCAO (HE, ×1.3). (d) Area of the injured brain in 7 days after pMCAO (HE, ×20). (e) Brain pathology of rats 28 days after pMCAO (HE, ×1.3). (f) Area of the injured brain 28 days after pMCAO (HE, ×20). (g) Brain pathology of rats 16 weeks after pMCAO (HE, ×1.3). (h) Area of the injured brain 16 weeks after pMCAO (HE, ×20).](/cms/asset/7e802eb2-8bcd-40e0-988d-f93eced851be/ibij_a_2346804_f0004_oc.jpg)
Discussion
Stroke is a brain vascular disease that can induce behavioral and cognitive disorders, even cause paralysis or death (Citation31). Unlike reperfusion models, the pMCAO model best represents the natural history of ELVO stroke untreated by thrombolytic therapy or mechanical thrombectomy and represents > 80% of patients with LVO (Citation15). The permanent cerebral infarction model has relatively few influencing factors (no reperfusion process) and high stability and is a replicable model. In the long-standing thread occlusion therapy, drugs enter the ischemic tissue through the blood vessel, increasing its clinical relevance (Citation32,Citation33). In our previous study, we found that in tMCAO model, when the rat weighs around 300 g, using the 3040A4 intraluminal suture and a 3-hour ischemic time, the brain injury effect is more pronounced, and rats with higher body weight have a higher survival rate (Citation5). Therefore, the above intraluminal sutures and rats were still used in this study. The results showed that the infarct volume of the pMCAO model rats was larger than that of the reperfusion rats, which could slow the recovery of the rats and facilitate long-term observation (Citation34–37). Meanwhile we found some key techniques for establishing the pMCAO model in this study. For example, confirming the specific depth of intraluminal suture entry into the blood vessel. Most of the literature only writes 18-20 mm (Citation38), but there are differences in blood vessels between different rats, and the depth of insertion of intraluminal suture cannot be completely consistent. We found that when the intraluminal suture reaches the appropriate position, the operator will feel slight resistance. To more clearly feel the presence of resistance, the operator can relax the suture that fixes the intraluminal suture to a state that does not cause bleeding, and soften intraluminal suture s in physiological saline one hour before using them.
The volume of cerebral infarction in rats is an important indicator to confirm the extent of damage. We can use this as a basis for grouping to ensure consistency the groups and facilitate analysis of their prognosis later. The commonly used detection methods are TTC staining and MRI. TTC staining is simple and fast, and the reagents used are cheap, but it requires animal sacrifice. MRI detection can be carried out on live rats, and the results are more refined, but it requires professional equipment and technicians, and is more expensive (Citation39). The key to stroke treatment is to promote recovery of the ischemic penumbra near the infarct area. MRI can be used to confirm the location of the ischemic penumbra, which facilitates more accurate drug transplantation (Citation40). MRI and TTC staining have their own characteristics in detecting the volume of cerebral infarction. Moreover, after our comparison, the results of both methods are relatively consistent. Therefore, in subsequent research, we can choose different detection methods according to different actual situations, greatly improving the flexibility of the experiment.
The recovery of gripping and locomotion are the main treatment goals for patients with stroke (Citation41). Thus, a screening index that can evaluate the behavior of experimental animals is very important. At present (Citation42–44), most studies on stroke focus on the acute and recovery phases of stroke, which require less sensitivity to behavioral test items. To identify which items can be used for long-term behavioral testing, this research conducted long-term observation and analysis on several behavioral items, and finally confirmed that the bilateral asymmetry (perception), grid-walking, cylinder, mNSS, and water maze test had high sensitivity within 16 weeks after modeling. Among them, the grid-walking, cylinder, and water maze test were significantly positively correlated with the volume of rat cerebral infarction at multiple time points(p < 0.05). Of course, we also made a lot of adjustments in the pre-experimental process. For example, there was an anomaly in the rotarod test during the later stages of the experiment, we found that it was due to the rats being overweight, which resulted in insufficient power to the instrument. We resolved the issue by increasing the instrument’s power. In the bilateral asymmetry test, the size and stickiness of the tape are the most important factors. If the selected tape size is too small or the stickiness is too high, rats often ignore the presence of the tape or all rats are unable to remove the tape. Conversely, if the selected tape is opposite to the above, it will lead to another extreme. We compared multiple models of tape and found that steam indicator tape(1322, 3 M, America) cut into 0.8 cm squares has suitable stickiness and size. The size of grid openings is crucial in grid-walking test. In pre-experiments, we adjusted the size of grid openings based on the response of rats. For instance, when rats move on the mesh, if the grid openings are too large, the limbs of the rats will frequently fall off, which may hinder their smooth movement and result in a significant reduction in activity. On the other hand, if the mesh is too small, it will be difficult for the injured forelimbs to fall off. All these issues can affect the analysis of differences in forelimb drop between the two groups. In the mNSS test, the results of the balance beam test account for a larger proportion of the total score. The focus of the balance beam test is to determine the critical point at which injury and normal rats can barely maintain stability on the balance beam (ensuring they do not fall off). In contrast, injured rats cannot maintain a stable state and will fall off. In the pre-experiment, compared to balance beams with widths of 2 cm and 3 cm, the 2.5 cm balance beam can better demonstrate the differences between the two groups of rats. For the Rotarod test, we attempted to use the mean and maximum values as the final experimental results for the Rotarod test. However, there was not much difference between the two. The experimental method of the Rotarod test is to force rats to move on the rotarod rung. However, rats do not receive rewards for this, and falling off does not cause harm to them. Therefore, it may be the reason why the rats stopped moving voluntarily in the later stage. Nevertheless, the Rotarod test still has high sensitivity in short-term experiments.
In the water maze test, a significant difference was found between the injured and normal rats. This indicates that the water maze test can still effectively evaluate the learning and memory abilities of the experimental animals by sensing the spatial position and direction 16 weeks after modeling (Citation45). Moreover, no significant difference was found between the two groups in terms of the distance of the platform quadrant pathway. After data analysis and comparison, the rats would stay in the original position after finding the platform, thus, the experimental animals had a clear memory of the platform position, and a shorter path in the platform quadrant did not mean that the rats had poor memory. We also found that the training stage has a greater impact on the final results of this experiment. In the pre-experiment, we trained the rats for 5 consecutive days, 4 times a day, and tested the time it took for them to find the platform on the 6th day, but the results were not ideal. Later, we analyzed the results of the rat training stage and found that the difference in time taken by both groups of rats to find the platform gradually increased from day 1 to day 3 but decreased from day 4 onwards. We suspect that this may be due to excessive training frequency and duration. Therefore, we reduced the training time to 4 days and conducted testing on the fifth day.
Additionally, we have tested and screened other projects. The staircase test can be used to test the ability of hungry rats to grasp food with their front paws. We followed Alexander Klein’s experimental method (Citation46). However, the experimental results were not satisfactory. Changing the feed to high sugar and adjusting the size of the instrument had no effect. Of course, the author also stated that SD rats are more difficult to train than other rats. Fasting rats will also affect other projects, and rats of different weights need to be equipped with staircases of different sizes. These issues are also why we gave up. We also conducted a lifting rope test, but as the weight of the rat increased, normal rats could no longer maintain a suspended posture with only their front paws. Of course, there may be other possibilities. In order to protect animal welfare and prevent rats from falling and being injured, we placed a soft mat under the rope. Multiple falls may make the rat no longer afraid and give up the hanging posture (Citation47). At the same time, these behavioral detection items also have their own shortcomings. For example, the mNSS score mainly uses manual detection to score. It is heavily biased toward human intervention in animals. If the detection frequency is high, it is easy to cause animal resistance and affect normal behavioral detection. It is subject to subjective judgment to a certain extent. In order to ensure the reliability of the results, it is best to be completed by the same group of personnel throughout the process.
In the acute stage of stroke, most of the neurons in the infarcted area of the brain undergo necrosis, and the number of inflammatory cells and glial infiltrates increases (Citation48,Citation49). At this time, the inflammatory environment of the brain is not conducive to the survival of in situ transplanted cells. It is better to choose intravenous or intraperitoneal injection (Citation50, Citation51). In the convalescent period (starting from day 7), brain inflammation tends to stabilize, and at this time, transplanting stem cells into the semi-dark zone can better exert their effects (Citation52). Brain inflammation completely subsides 4–5 weeks after injury, and most related studies in the sequelae period prefer drug transplantation at this time. Appropriate transplantation time and method can better exert the effect of drugs. Appropriate behavioral detection methods are also beneficial for confirming drug efficacy. In subsequent studies on the efficacy and mechanism of NSCs in treating post-stroke sequelae, we will design specific methods for drug administration and efficacy testing based on the results of this experiment, so as to confirm that the behavioral projects selected and improved by us have a positive effect on promoting preclinical research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Qin H, Chen Y, Liu G, Turnbull I, Wang Y, Li Z, Wang Y, Liu L, Zhao X, Chen Z. Management characteristics and prognosis after stroke in China: findings from a large nationwide stroke registry. Stroke Vasc Neurol. 2021;6(1):1–9. doi:10.1136/svn-2020-000340.
- Wang W, Jiang B, Sun H, Ru X, Sun D. Prevalence, incidence, and mortality of stroke in China results from a nationwide population-based survey of 480 687 adults. Circ Official J Am Heart Assoc. 2017;135(8):759–71.
- Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Lo W, Bennett DA, Barker-Collo S, Moran AE, Sacco RL, Truelsen T. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. 2015;45(3):161–76. doi:10.1159/000441085.
- Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, et al. Prevalence, incidence, and mortality of stroke in China clinical perspective: results from a nationwide population-based survey of 480687 adults. Circulation. 2017;135(8):759. doi:10.1161/CIRCULATIONAHA.116.025250.
- Liu P, Tang Y-Y, Yang X-S, Dai J, Yang M, Zhang H, Liu Y, Yan H, Song X-Y. Validation of a preclinical animal model to assess brain recovery after acute stroke. Eur J Pharmacol. 2018;835:75–81. doi:10.1016/j.ejphar.2018.07.035.
- Hamblin MH, Lee JP. Neural stem cells for early ischemic stroke. Int J Mol Sci. 2021;22(14):7703. doi:10.3390/ijms22147703.
- Flick MJ. Mechanism of ICH with tPA thrombolysis. Blood. 2021;138(1):8–9. doi:10.1182/blood.2021011268.
- Crupi R, Di Paola R, Esposito E, Cuzzocrea S. Middle cerebral artery occlusion by an intraluminal suture method. Methods Mol Biol. 2018;1727:393–401. doi:10.1007/978-1-4939-7571-6_31.
- Park JE, Jung SC, Kim HS, Suh J-Y, Baek JH, Woo C-W, Park B, Woo D-C. Amide proton transfer–weighted MRI can detect tissue acidosis and monitor recovery in a transient middle cerebral artery occlusion model compared with a permanent occlusion model in rats. Eur Radiol. 2019;29(8):4096–104. doi:10.1007/s00330-018-5964-3.
- Zemgulyte G, Tanaka S, Hide I, Sakai N, Pampuscenko K, Borutaite V, Rastenyte D. Evaluation of the effectiveness of post-stroke metformin treatment using permanent middle cerebral artery occlusion in rats. Pharmaceuticals (Basel). 2021;14(4):312. doi:10.3390/ph14040312.
- Lee JY, Castelli V, Bonsack B, García-Sánchez J, Kingsbury C, Nguyen H, Tajiri N, Borlongan CV. Eyeballing stroke: blood flow alterations in the eye and visual impairments following transient middle cerebral artery occlusion in adult rats. Cell Transplant. 2020;29:963689720905805. doi:10.1177/0963689720905805.
- Lopez MS, Vemuganti R. Modeling transient focal ischemic stroke in rodents by intraluminal filament method of middle cerebral artery occlusion. Methods Mol Biol. 2018;1717:101–13. doi:10.1007/978-1-4939-7526-6_9.
- Qiqiang T, Han R, Xiao H, Shi L, Shen J, Lun Q, Li J. Role of suture diameter and vessel insertion position in the establishment of the middle cerebral artery occlusion rat model. Exp Ther Med. 2013;5(6):1603–08. doi:10.3892/etm.2013.1046.
- Martha SR, Collier LA, Davis SM, Goodwin SJ, Powell D, Lukins D, Fraser JF, Pennypacker KR. Early acid/base and electrolyte changes in permanent middle cerebral artery occlusion: aged male and female rats. J Neurosci Res. 2020;98(1):179–90. doi:10.1002/jnr.24422.
- Mcbride DW, Zhang JH. Precision stroke animal models: the permanent MCAO model should be the primary model, not transient MCAO. Transl Stroke Res. 2017;8(5):1–8. doi:10.1007/s12975-017-0554-2.
- Yu J, Moon J, Jang J, Choi JI, Jung J, Hwang S, Kim M. Reliability of behavioral tests in the middle cerebral artery occlusion model of rat. Lab Anim. 2019;53(5):478–90. doi:10.1177/0023677218815210.
- NA. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12): 2752–8. doi:10.1161/01.str.30.12.2752.
- Liu P, Song X-C, Yang X-S, Cao Q-L, Tang Y-Y, Liu X-D, Yang M, An W-Q, Dong B-X, Song X-Y, et al. A preclinical model to assess brain recovery after acute stroke in rats. J Vis Exp. 2019;6(153):60166. doi:10.3791/60166.
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi:10.1161/01.STR.20.1.84.
- Liu HS, Shen H, Harvey BK, Castillo P, Lu H, Yang Y, Wang Y. Post-treatment with amphetamine enhances reinnervation of the ipsilateral side cortex in stroke rats. Neuroimage. 2011;56(1):280–89. doi:10.1016/j.neuroimage.2011.02.049.
- Du L, Yang Z, Sheng H, Liu M, Sun Q, Ciobica A. Effects of long-term vagus nerve electrical stimulation therapy on acute cerebral infarction and neurological function recovery in post MCAO mice. Oxid Med Cell Longev. 2022;2022:1–9. doi:10.1155/2022/8131391.
- Wu H, Liu H, Zuo F, Zhang L. Adenoviruses-mediated RNA interference targeting cytosolic phospholipase A2α attenuates focal ischemic brain damage in mice. Mol Med Rep. 2018;17:5601–10. doi:10.3892/mmr.2018.8610.
- Nouraee C, Fisher M, Di Napoli M, Salazar P, Farr TD, Jafarli A, Divani AA. A brief review of edema-adjusted infarct volume measurement techniques for rodent focal cerebral ischemia models with practical recommendations. J Vasc Interv Neurol. 2019;10(3):38–45.
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–11. doi:10.1161/01.STR.32.4.1005.
- Li H, Wang H, Zhang L, Wang M, Li Y. Dl-3-n-butylphthalide alleviates behavioral and cognitive symptoms via modulating mitochondrial dynamics in the A53T-α-synuclein mouse model of parkinson’s disease. Front Neurosci. 2021;15:647266–647266. doi:10.3389/fnins.2021.647266.
- Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E, Stevanato L, Sinden JD, Modo M. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30(4):785–96. doi:10.1002/stem.1024.
- Wen R-X, Shen H, Huang S-X, Wang L-P, Li Z-W, Peng P, Mamtilahun M, Tang Y-H, Shen F-X, Tian H-L, et al. P2Y6 receptor inhibition aggravates ischemic brain injury by reducing microglial phagocytosis. CNS Neurosci Ther. 2020;26(4):416–29. doi:10.1111/cns.13296.
- Singh N, Bansal Y, Bhandari R, Marwaha L, Singh R, Chopra K, Kuhad A. Resveratrol protects against ICV collagenase-induced neurobehavioral and biochemical deficits. J Inflammation (London, England). 2017;14(1):14–14. doi:10.1186/s12950-017-0158-3.
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58. doi:10.1038/nprot.2006.116.
- Ling L, Shah, FA. A potent antioxidant endogenous neurohormone melatonin, rescued MCAO by attenuating oxidative stress-associated neuroinflammation. Front Pharmacol. 2020;11:1220.
- Zhang H, Zhang Z, Wang Z, Zhen Y, Yu J, Song H. Research on the changes in balance motion behavior and learning, as well as memory abilities of rats with multiple cerebral concussion-induced chronic traumatic encephalopathy and the underlying mechanism. Exp Ther Med. 2018;16:2295–302.
- Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32(7):1310–16. doi:10.1038/jcbfm.2011.186.
- Kahle MP, Bix GJ. Successfully climbing the “STAIRs”: surmounting failed translation of experimental ischemic stroke treatments. Stroke Res Treat. 2012;2012:374098. doi:10.1155/2012/374098.
- Jing SQ, Wang S-S, Zhong R-M, Zhang J-Y, Wu J-Z, Tu Y-X, Pu Y, Yan L-J. Neuroprotection of Cyperus esculentus L. orientin against cerebral ischemia/reperfusion induced brain injury. Neural Regen Res. 2020;15(3):548–56. doi:10.4103/1673-5374.266063.
- Meng X, Xie W, Xu Q, Liang T, Xu X, Sun G, Sun X. Neuroprotective effects of radix scrophulariae on cerebral ischemia and reperfusion injury via MAPK pathways. Molecules. 2018;23(9):2401. doi:10.3390/molecules23092401.
- Qiu J, Yan Z, Tao K, Li Y, Li Y, Li J, Dong Y, Feng D, Chen H. Sinomenine activates astrocytic dopamine D2 receptors and alleviates neuroinflammatory injury via the CRYAB/STAT3 pathway after ischemic stroke in mice. J Neuroinflammation. 2016;13(1):263. doi:10.1186/s12974-016-0739-8.
- Yoo SJ, Cho B, Lee D, Son G, Lee Y-B, Soo Han H, Kim E, Moon C, Moon C. The erythropoietin-derived peptide MK-X and erythropoietin have neuroprotective effects against ischemic brain damage. Cell Death Disease. 2017;8(8):e3003. doi:10.1038/cddis.2017.381.
- Wu WN, Wu P-F, Chen X-L, Zhang Z, Gu J, Yang Y-J, Xiong Q-J, Ni L, Wang F, Chen J-G, et al. Sinomenine protects against ischaemic brain injury: involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels. Br J Pharmacol. 2011;164(5):1445–59. doi:10.1111/j.1476-5381.2011.01487.x.
- Oray D, Limon O, Ertan C, Aydinoglu Ugurhan A, Sahin E. Inter-observer agreement on diffusion-weighted magnetic resonance imaging interpretation for diagnosis of acute ischemic stroke among emergency physicians. Turk J Emerg Med. 2015;15(2):64–68. doi:10.5505/1304.7361.2015.32659.
- Leigh, Richard R, Knutsson L, Zhou J, van Zijl PC. Imaging the physiological evolution of the ischemic penumbra in acute ischemic stroke. J Cereb Blood Flow Metab Official J Int Soc Cereb Blood Flow Metab. 2018;38(9):1500–16. doi:10.1177/0271678X17700913.
- Rojek A, Mika A, Oleksy Ł, Stolarczyk A, Kielnar R. Effects of exoskeleton gait training on balance, load distribution, and functional status in stroke: a randomized controlled trial. Front Neurol. 2019;10:1344. doi:10.3389/fneur.2019.01344.
- Mohammadzadeh L, Latifi H, Khaksar S, Feiz M-S, Motamedi F, Asadollahi A, Ezzatpour M. Measuring the frequency-specific functional connectivity using wavelet coherence analysis in stroke rats based on intrinsic signals. Sci Rep. 2020;10(1):9429. doi:10.1038/s41598-020-66246-9.
- Gil CH, Kim Y, Lee H, Jung D, Shin H, Choi B. Aripiprazole exerts a neuroprotective effect in mouse focal cerebral ischemia. Exp Ther Med. 2017;15:745–50. doi:10.3892/etm.2018.5443.
- Mao XF, Wu H-Y, Tang X-Q, Ali U, Liu H, Wang Y-X. Activation of GPR40 produces mechanical antiallodynia via the spinal glial interleukin-10/β-endorphin pathway. J Neuroinflammation. 2019;16(1):84. doi:10.1186/s12974-019-1457-9.
- Chen D, Dixon BJ, Doycheva DM, Li B, Zhang Y, Hu Q, He Y, Guo Z, Nowrangi D, Flores J, et al. IRE1α inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic–ischemic brain injury in rats. J Neuroinflammation. 2018;15(1):32. doi:10.1186/s12974-018-1077-9.
- Alexander K, Dunnett, SB. Analysis of skilled forelimb movement in rats: the single pellet reaching test and staircase test. Curr Protoc Neurosci. 2012;58(1):8–28. Editorial Board Jacqueline N. Crawley … [et al.].
- Feng L, Han CX, Cao SY, Zhang HM, Wu GY. Deficits in motor and cognitive functions in an adult mouse model of hypoxia-ischemia induced stroke. Sci Rep. 2020;10(1):20646. doi:10.1038/s41598-020-77678-8.
- Wang M, Liu Z, Hu S, Duan X, Zhang Y, Peng C, Peng D, Han L. Taohong siwu decoction ameliorates ischemic stroke injury via suppressing pyroptosis. Front Pharmacol. 2020;11:590453.
- Neumann J, Henneberg S, von Kenne S, Nolte N, Müller AJ, Schraven B, Görtler MW, Reymann KG, Gunzer M, Riek-Burchardt M, et al. Beware the intruder: real time observation of infiltrated neutrophils and neutrophil—microglia interaction during stroke in vivo. PLOS ONE. 2018;13(3):e0193970. doi:10.1371/journal.pone.0193970.
- Ghali AA, Yousef MK, Ragab OA, ElZamarany EA. Intra-arterial infusion of autologous bone marrow mononuclear stem cells in subacute ischemic stroke patients. Front Neurol. 2016;7:228. doi:10.3389/fneur.2016.00228.
- Yu D, Cheng Z, Ali AI, Wang J, Le K, Chibaatar E, Guo Y. Chronic unexpected mild stress destroys synaptic plasticity of neurons through a glutamate transporter, GLT-1, of astrocytes in the ischemic stroke rat. Neural Plast. 2019;2019:1–13. doi:10.1155/2019/1615925.
- Agarwal S, Matys T, Marrapu, ST, Scoffings, DJ, Mitchell J, Jones, PS, Baron, JC, Warburton, EA. Is CT-Based perfusion and collateral imaging sensitive to time since stroke onset?. Front Neurol. 2015;6:70. doi:10.3389/fneur.2015.00070.
