ABSTRACT
Aim
To systematically review the prevalence, risk factors and timing of onset of hip displacement in children with a post-neonatal (PNN) brain injury with regards to hip surveillance recommendations.
Method
A search of PubMed, MEDLINE, Embase, CINAHL and Web of Science was conducted on 22nd February 2022. Studies were included if they reported presence of, and risk factors for, hip displacement in children with PNN brain injury. Data was extracted on patient characteristics, and analyzed in terms of risk factors of interest and timing of development of hip displacement.
Results
Six studies met the inclusion criteria (n = 408 participants). All were cohort studies: five retrospective and one prospective. Rates of hip displacement ranged from 1% to 100%, and were higher in children with diffuse brain injury at an early age, who were non-ambulant and had spastic quadriplegia. Hip displacement and hip dislocation were first identified at one and three months respectively following PNN brain injury.
Interpretation
Evidence on hip displacement in children with PNN brain injury is sparse and low quality. Children who remain non-ambulant after diffuse PNN brain injury before five years of age appear most at risk of developing progressive hip displacement and earlier hip surveillance is recommended.
What this paper adds
As for children with cerebral palsy (CP), children with a post-neonatal (PNN) brain injury who are non-ambulant are most at risk of progressive hip displacement.
Children with a diffuse brain injury before five years of age appear to be at greater risk.
Hip displacement can occur very early and progress rapidly following PNN brain injury.
Introduction
Cerebral palsy (CP) and acquired brain injury (ABI) are heterogenous neuromotor disorders with many etiologies and are the two leading causes of disability in childhood (Citation1,Citation2). Cerebral palsy refers to conditions resulting from a diverse range of non-progressive disturbances in the fetal or infant brain (Citation3). Most cases of CP are attributed to a pre- or perinatal brain injury or disturbance (Citation4,Citation5). For around 6% of children with CP in high-income countries, their CP is attributable to a brain injury acquired in the post-neonatal (PNN) period; that is, after 28 days post birth (Citation4,Citation6). The upper age limit for a diagnosis of PNN-CP is not explicit (Citation7), but most commonly two years of age (Citation8). A recent study identified the most frequent proximal causes of PNN-CP to be, in order, cerebrovascular accidents, infection and non-accidental injuries (NAI) (Citation4). Acquired brain injury also refers to the multiple disabilities arising from damage to the brain from the PNN period onwards (Citation9), with common causes including trauma, infection, stroke and brain tumors (Citation10). Children with an ABI after 28 days post birth fit under the clinically descriptive term of CP or PNN-CP (Citation11). Children older than two years of age at time of brain insult are most commonly classified as ABI, however some CP surveillance programs have an upper age limit for PNN brain injury of five or eight years of age (Citation8).
Regardless of this heterogeneity in terminology, children with CP and those with a permanent motor disorder following a PNN brain injury may have similar movement difficulties and activity limitations. Both groups may develop secondary musculo-skeletal deformity (Citation3,Citation10,Citation12), including progressive hip displacement (Citation13). Progressive hip displacement, defined as Reimers migration percentage (Citation14) (MP) >30%, is the second most common orthopedic deformity seen in children with CP (Citation15). Children with progressive hip displacement are at risk of progressing to complete hip dislocation (a MP of 100%) if not managed (Citation16). Children with severe hip displacement often experience pain (Citation17,Citation18), decreased functional skills (Citation19), and reduced quality of life (Citation20,Citation21). In children with CP, the risk of hip displacement is directly related to gross motor functional ability as classified with the Gross Motor Function Classification System (GMFCS) (Citation24). A linear relationship between progressive hip displacement and GMFCS level has been identified, with those children who have very little or no ability to walk (GMFCS level IV and V) most at risk (Citation22). The overall prevalence of hip displacement in children with CP has been reported as approximately 35%, with a prevalence of 0% in children with GMFCS level I and up to 90% for children with GMFCS level V (Citation22). Little is known about hip displacement specifically in children with PNN-CP and ABI. Expert opinion suggests that in some children with PNN brain injury hip displacement, when present, can progress quickly (Citation23).
Hip surveillance guidelines for children with CP in Australia have been available since 2008 and are reviewed every five years (Citation23). Current guidelines recommend children with a PNN brain injury in the first two years of life be included in hip surveillance programs for children with CP, and should have ‘early and frequent surveillance’ (Citation23). Guidance for hip surveillance in children with an ABI after the age of two years has not been established. It is difficult to generalize hip surveillance guidelines for children with CP directly to children with PNN-CP and ABI for several reasons. Firstly, the risk of hip displacement in CP is directly related to GMFCS, a classification system specifically validated for children with CP (Citation24), but not, however, validated for children with ABI. Secondly, the natural history of hip displacement in CP has been identified from studies on long term surveillance of hip radiographs of children with CP. Children with CP attributed to pre- or perinatal brain injury are thought to have normal hip joints at birth, which are then exposed to reduced motor capacity and physical activity in weightbearing (Citation25,Citation26), especially for children classified as GMFCS level V. Most children with a PNN brain injury have had a period of typical motor development, and therefore hip development, prior to their injury or illness. It is unknown how this period of typical development, followed by sudden alteration to muscle tone and interruption to motor development, may influence the development of hip displacement following brain injury. Finally, for children with PNN-CP and ABI it is unknown whether risk factors such as age at injury, etiology of injury, abnormal motor posturing following injury, severity of hypertonia, type and topographical distribution of motor disorder, level of gross motor function and levels of physical activity affect the development of hip displacement.
The purpose of this systematic review was to identify and appraise the available evidence on the prevalence, risk factors, and development of hip displacement in children with PNN-CP and ABI. Specifically, the following questions would be reviewed: 1) What is the prevalence of hip displacement in children with PNN-CP and ABI? 2) What are the potential risk factors for development of hip displacement in children with PNN-CP and ABI? 3). What is the timing of onset of hip displacement in children with PNN-CP and ABI? It is hoped this information will provide preliminary evidence regarding the natural history of hip displacement in children with PNN brain injury, and assist with recommendations for hip surveillance in children with PNN-CP and ABI.
Method
A systematic review was designed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) (Citation27). Details of the protocol for this systematic review were specified in advance and registered on the International Prospective Register of Systematic Reviews, PROSPERO (Registration number CRD42022328913).
For the purposes of this systematic review children were classified into three groups: PNN-CP (children with a brain injury after 28 days of life, and before the age of 25 months); ABI ‘early’ (children with a brain injury from 25 months to 5 years of age); and ABI ‘late’ (children with a brain injury after 5 years of age and up to 16 years of age). This was to investigate the potential association between age at time of brain injury and development of hip displacement.
Identification of studies
A comprehensive search strategy was developed with the assistance of a research librarian and used to conduct a systematic literature search of the electronic databases PubMed, MEDLINE, Embase, CINAHL and Web of Science. Each database was searched by one reviewer (EB) from their individual dates of inception through to 22nd February 2022 with no language restriction. A search strategy was developed for PubMed using keywords for ‘acquired brain injury,’ ‘cerebral palsy’ and ‘hip displacement,’ and a list of potential ‘factors of interest’ along with MeSH headings and synonyms. This search strategy was then adapted for the other databases (Table SI, online supporting information). References from the database searches were prepared in EndNoteTM 20 reference management software and then uploaded to the online Research Screener machine learning tool (Citation28). Research Screener semi-automates abstract screening for systematic reviews so the most relevant abstracts are presented first for review, and has been shown to be a valid and sensitive tool for abstract screening if 4–40% of the identified abstracts are screened (Citation28). To capture all relevant articles, two of the authors (EB, NG) independently screened the titles and abstracts presented in Research Screener based on the eligibility criteria for inclusion in the review until 50% of all eligible abstracts had been reviewed. Where it was not possible to determine eligibility based on the title and abstract alone, the full text article was obtained and reviewed. Disagreements about eligibility were resolved by consensus. Titles and abstracts of any references removed in manual and automated dataset cleaning for Research Screener were also screened against the eligibility criteria. Relevant articles identified from the screening process were exported to Covidence (Covidence systematic review software; Veritas Health Innovation, Melbourne, Australia; www.covidence.org), an online systematic review management software. An independent full text review was completed for all studies that met the inclusion criteria by the same two reviewers. Disagreements about eligibility were again resolved by consensus. Reference lists of the selected articles were manually searched for any additional articles. When multiple articles reporting data from the same study population were encountered, the most comprehensive study was selected. Where it was not possible to ascertain separate data for PNN-CP and ABI from pre- or perinatal CP in potentially relevant studies, attempt was made to contact one of the original authors to determine if separate data for the population of interest was collected in the original study and available for inclusion in our systematic review.
Eligibility criteria
Studies were included if they: 1) involved participants aged 0 to 18 years of age with PNN-CP and/or ABI; 2) reported on presence of hip displacement and/or hip dislocation; 3) reported on potential risk factors for the development of hip displacement and/or hip dislocation; and 4) included separate data for children with PNN-CP and/or ABI in the study, or authors could be contacted and separate data obtained. Where a radiological measure of MP was available this data was collected. To enable a comprehensive review of the literature all languages and study methodologies were permitted, providing authors could be contacted for additional data if required.
Studies were excluded if they involved: 1) only children with pre- or perinatal CP; 2) children with hip displacement or hip dislocation due to congenital hip pathology or traumatic injury, as these involve different underlying causes and mechanisms of displacement; 3) reported only on the outcome of intervention to specifically prevent or treat hip displacement; 4) reported on techniques or reliability of techniques to measure hip displacement; and 5) the full-text article was a narrative review, opinion piece or editorial.
Data extraction and quality appraisal
A data extraction template specific to the purpose of this review was developed and piloted on three of the identified studies independently by two of the authors (EB, NG). The finalized form was used to independently extract and record data from all identified studies including publication year, geographical location of the study, study design, recruitment method, sample size, participant demographics, age at brain injury, etiology of brain injury, gross motor function, motor type and distribution, presence of hip displacement and/or hip dislocation, MP if reported, and time from brain injury to development of hip displacement and/or hip dislocation. For patients with hip displacement and dislocation, patients were analyzed according to the worst hip outcome, i.e., hip dislocation. For studies published prior to development of the GMFCS, participants were assigned a GMFCS level (for children with PNN-CP), and GMFCS-equivalent level (for children with ABI) based on reported ambulatory ability by an experienced physiotherapist (EB) to allow clearer comparison of gross motor function across study participants. Consensus on content of tables was achieved through discussion. Each included paper was assigned a level of evidence based on the guidelines of the Oxford Center for Evidence-Based Medicine (OCEBM) (Citation29). Two reviewers (EB, NG) independently assessed risk of bias at the study level using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Cohort Studies (Citation30). This checklist contains 11 questions that assess specific study domains to determine potential risk of bias and could be answered with ‘yes,’ ‘no,’ ‘unclear’ or ‘not applicable’ (). Consensus on scoring between reviewers was achieved through discussion. An overall risk of bias judgment was not made, and all studies were included in the review to discover all that has currently been documented about hip displacement in PNN-CP and ABI.
Table 1. Level of evidence and JBI risk of bias assessment for cohort studies.
Results
Study selection
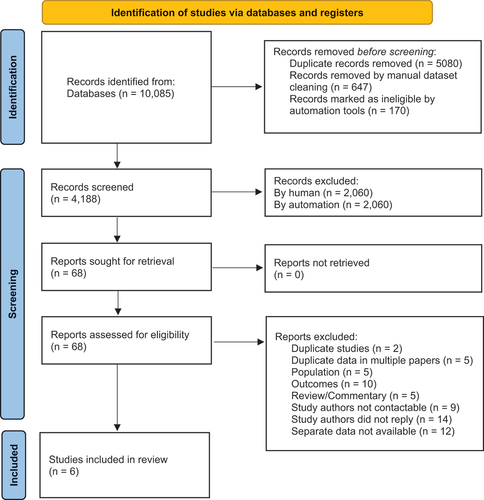
The initial search yielded 5,005 unique references (), of which 817 were removed in dataset cleaning manually and using Research Screener. Final screening in Research Screener included 4,188 titles and abstracts, of which 68 records were selected for full text review. This included four non-English publications; of which three were translated to English using online translation tools, and were excluded as they did not meet the inclusion criteria. One article in Japanese was translated by a native speaking Japanese Physiotherapy colleague, and met criteria for inclusion, with additional separate data obtained from the first author (Citation32). Thirty-five potentially eligible studies, pending availability of separate data for PNN-CP and/or ABI, were excluded because the authors could not be contacted (n = 9), the authors did not reply to correspondence (n = 14), or the authors indicated separate data for children with PNN-CP and/or ABI was not available (n = 12). Following full-text review, six studies were included for descriptive analysis (Citation13,Citation31–35). Reference list screening of these articles did not identify any additional studies for inclusion.
The level of evidence, as assessed according to the OCEBM and the JBI quality assessment, is included in . summarizes the participant characteristics and length of follow-up of the included studies. The hip displacement outcomes in relation to potential risk factors of interest, and timing of onset of hip displacement following brain injury, are summarized in and respectively.
Table 2. Summary of studies: participant characteristics and length of follow-up.
Table 3. Characteristics of children who developed hip displacement.
Table 4. Summary of studies: hip displacement outcome and timing of onset.
Study design and quality
Of the six included studies, two were published in the last 10 years (Citation13,Citation32), with the remaining studies published in the 1970s (Citation33), 1980s (Citation34) and early 1990s (Citation31,Citation35). All included studies were cohort studies. The study by Kentish et al. (Citation13) was the only study conducted prospectively, using data from a hip surveillance database, and the only study to include a comparison group. Four studies involved retrospective hospital clinical record review (Citation31,Citation32,Citation34,Citation35), and one of these studies also completed physical examination and new radiographs of some study participants (Citation31). The remaining retrospective study involved hospital clinic patients and did not include its data sourcing method (Citation33).
The level of evidence for the included studies was low (), with one graded level 3 (Citation13), and the remaining five at level 4 (Citation31–35). Most studies had small sample sizes (n = 15–58) (Citation13,Citation31,Citation32,Citation34,Citation35), with the exception of the study by Hoffer and Brink (Citation33) (n = 241). Most studies included only participants with adequate records for analysis, did not provide complete data on participants, and lacked clear documentation regarding methods used to measure hip displacement, contributing to high risk of bias ().
Due to the heterogenous nature of the study participants and outcome measures used meta-analysis was not possible.
Participant characteristics
The six studies incorporated a total of 408 participants. Participant demographics are listed in , and were variable across age and etiology of brain injury, and description of gross motor function. Two of the studies reported on only children with PNN-CP (Citation13,Citation35), and the remaining four reported on children with PNN-CP, ABI ‘early’ and ABI ‘late’ (Citation31–34). Three studies included children with only a single etiology of brain injury, namely central nervous system (CNS) infection (n = 15) (Citation35), hypoxia/anoxia following near-drowning (n = 36) (Citation31), and traumatic brain injury (TBI) (n = 16) (Citation34). One study included children with hypoxic-ischemic encephalopathy (HIE) from various causes (n = 42) (Citation32). Hoffer and Brink (Citation33) included children with TBI (n = 221) and anoxic brain injury from various causes (n = 20). Kentish et al. (Citation13) reported the etiology of brain injury only for children with PNN-CP who developed MP ≥ 30% prior to two years of age, which was due to hypoxia/anoxia (n = 3), CNS infection (n = 3) and NAI (n = 1).
Measurement of hip displacement
Two studies used MP to measure hip displacement, with the presence of marked hip displacement defined as MP ≥ 30%, and hip dislocation as MP = 100%, on pelvic radiograph (Citation13,Citation32). Abrams and Mubarak (Citation31) scored severity of radiographic changes of center edge angle, acetabular index, femoral head shape, sourcil and teardrop, however the scoring criteria for a definition of hip displacement and dislocation were not reported. The remaining three studies did not report the radiological measure, or definition used, to determine presence of hip displacement (Citation33–35).
Prevalence of hip displacement in children with post-neonatal cerebral palsy
Prevalence was not reported in any studies. Only rates within cohorts and descriptive analysis from numbers reported in the studies were possible. The highest rate of hip displacement reported in subjects with PNN-CP was 61% (14/23 children), including seven participants with hip dislocation (Citation32). Kentish et al. (Citation13) identified 12% (7/58 children) with PNN-CP developed hip displacement (MP ≥ 30%) prior to two years of age. Of these children, two developed hip dislocation (MP = 100%). All five patients with PNN-CP investigated by Blasier and Letts (Citation34) developed hip displacement (n = 3) or hip dislocation (n = 2). Abrams and Mubarak (Citation31) documented 15 of the 36 (42%) children studied developed hip displacement. Seven of these children had PNN-CP, and developed either hip displacement (n = 6) or hip dislocation (n = 1). Loder (Citation35) reported development of ‘hip deformity’ in 67% of children with PNN-CP, with at least one child developing hip dislocation, but hip displacement outcomes could not be determined from the data provided. One study did not provide separate data for children with PNN-CP and ABI (Citation33).
Prevalence of hip displacement in children with acquired brain injury
Again, prevalence was not reported in any studies. The highest rate of hip displacement, including dislocation, in children with an ABI ‘early’ (brain injury between 25 months and 5 years of age) was 36% (4/11 children) (Citation32). In the study by Blasier and Letts (Citation34), two of the five children with an ABI ‘early’ developed hip displacement. In their study of 36 children, Abrams and Mubarak (Citation31) reported that 8 of the 15 children who developed hip displacement had an ABI between two and five years of age. Of the three studies who included children with an ABI after 5 years of age and reported age at brain injury for those with hip displacement (Citation31,Citation32,Citation34), only one case of hip displacement was reported in one study (Citation32).
Risk factors for development of hip displacement in children with post-neonatal cerebral palsy and acquired brain injury
Descriptive analysis of possible factors of interest are reported below.
Age at brain injury
Information on age at brain injury for children who developed hip displacement was reported in 5 studies for a total of 51 participants (Citation13,Citation31,Citation32,Citation34,Citation35). When combining the studies for children with PNN-CP who developed hip displacement (n = 36), the median age at brain injury was 0 years, 6 months (range 0 years, 1 month-2 years, 0 months). For children with an ABI ‘early’ (brain injury between 25 months and 5 years of age) who developed hip displacement (n = 14), the median age at brain injury was 3 years, 4.5 months (range 2 years, 5 months − 5 years, 0 months). Across the studies, for children with an ABI ‘late’ (brain injury after the age of five years), only one case of hip displacement was identified, with age at brain injury 5 years, 2 months (Citation32).
Etiology of brain injury
Three studies investigated a single etiology of brain injury and orthopedic complications (Citation31,Citation34,Citation35). In children with brain injury following near-drowning, Abrams and Mubarak (Citation31) identified 42% of children developed hip displacement in one or both hips. Blasier and Letts (Citation34) reported 44% (7/16 children) with TBI developed hip displacement following MVA (n = 4), NAI (n = 2) and injury of unknown cause (n = 1). In children with PNN-CP following CNS infections, Loder (Citation35) identified ‘hip deformity’ in 78% (7/9 children) with bacterial infections and 50% (3/6 children) with meningitis of unknown organism. Kitai et al. (Citation32) identified 45% (19/42 children) developed hip displacement following HIE of various causes including cardiogenic, asphyxia and near-drowning. In the study by Hoffer and Brink (Citation33) hip dislocation was identified in 20% (4/20 children) with brain injury following anoxia, and less than 1% of children with TBI. Kentish et al. (Citation13) reported atiology of brain injury for 7 of 58 children with hip displacement, which included hypoxia/anoxia (n = 3), CNS infection (n = 3) and NAI (n = 1).
Severity of injury
Three of the five studies provided details on severity of brain injury (Citation32–34). One study used the extent of brain lesions on MRI, classified as diffuse or focal, to investigate long-term outcomes following HIE, and reported 68% (13/19 children) who developed hip displacement had sustained diffuse brain damage (Citation32). Blasier and Letts (Citation34) reported length of coma for those who developed hip displacement or hip dislocation ranged from three weeks to eight weeks. Hoffer and Brink (Citation33) used length of coma and levels of cognitive functioning, with the two children who developed hip dislocation following TBI ‘responsive to noxious stimuli in a purposeful manner’ for 20 weeks or more post brain injury, and the four children with hip dislocation following anoxia in a ‘deep coma’ for over 24 hours.
Gross motor function
Information regarding the gross motor function of children with hip displacement was provided in five of the six studies for 54 children (Citation13,Citation31–34), with two studies reporting GMFCS level (Citation13,Citation32). Where GMFCS level was reported, 77% (20/26 children) who developed hip displacement were GMFCS level V, and 15% (4/26 children) were GMFCS level IV (Citation13,Citation32). For the remaining three studies that predate the GMFCS (Citation31,Citation33,Citation34), a GMFCS or equivalent level was assigned based on reported ambulatory ability. In these studies, 83%-100% of the children who developed hip displacement were GMFCS or equivalent level IV or V.
Motor disorder type
Motor disorder type for the children who developed hip displacement was recorded for 42 children across five studies (Citation13,Citation32–35). Spasticity was the predominant motor disorder type (≥81%), with small numbers each of mixed, dyskinetic and hypotonic motor types, and one case of ‘rigidity,’ reported. Abrams and Mubarak (Citation31) did not provide specific details on motor disorder type, although 94% of children had hypertonia, with 69% of children described as having decorticate or decerebrate posturing. No study reported a scale or measure used to quantify the level of spasticity in its subjects.
Topographical distribution of motor disorder
Motor distribution for children who developed hip displacement was reported for 51 children across five studies (Citation13,Citation31,Citation32,Citation34,Citation35). Across four of these studies, involving 44 participants, 98% had quadriplegia (Citation31,Citation32,Citation34,Citation35). Kentish et al. (Citation13) documented six subjects had bilateral involvement, and one subject had unilateral involvement, whose maximum MP was 31%.
Timing of onset of hip displacement following brain injury
Only two studies reported frequency of hip surveillance, with Kentish et al. (Citation13) conducted according to the Australian ‘Standards of Care (Citation23) and Blasier and Letts (Citation34) completing yearly pelvic radiographs.
For children with PNN-CP, separate data on timing of onset of hip displacement and hip dislocation following brain injury was provided for 33 children across five studies (Citation13,Citation31,Citation32,Citation34,Citation35). For children who developed hip displacement, where reported (n = 20), the median time from brain injury to when hip displacement was first detected was 1 year, 3.5 months, with a range of 0 years, 1 month-9 years, 2 months. When present, hip displacement was first detected in 40% of children in the first 12 months after brain injury, and for 75% of children in the first 2 years after brain injury. For children who developed hip dislocation, where reported (n = 13), the median time from brain injury to detection of hip dislocation was 0 years, 11 months, with a range of 0 years, 4 months −7 years, 7 months. More than half of the dislocations (54%, 7/13 children) were identified in the first year post brain injury.
For children with an ABI ‘early’, data on timing of onset of hip displacement and hip dislocation following brain injury was available for 14 children across three studies (Citation31,Citation32,Citation34). Hip displacement was first detected in children (n = 9) at a median age of 0 years, 9 months following brain injury, with a range of 0 years, 1 month-4 years, 2 months. Hip dislocation (n = 5) was first detected at a median age of 1 year, 1 month following injury, with a range of 0 years, 3 months-4 years,1 month. For children with an ABI ‘early’, hip displacement and/or dislocation, when present, was first detected in 50% of children in the first year following brain injury (Citation31,Citation32,Citation34).
Separate data on timing of onset of hip displacement for children with PNN-CP and ABI was not provided in the study by Hoffer and Brink (Citation33), however these authors noted that 6 patients developed hip dislocation, with time from brain injury to identification of hip dislocation range of 0 years, 2 months-3 years, 0 months. For the four patients in this group with anoxic brain injury all hip dislocations occurred in the first two to six months following injury.
Discussion
The need for a greater understanding of the natural history of hip displacement in children with PNN brain injury to help guide hip surveillance programs for this cohort has been identified by experts (Citation23). To our knowledge, this is the first systematic review on hip displacement in children with PNN-CP and ABI. To thoroughly examine all that has been reported about hip displacement in PNN brain injury, the search strategy and eligibility criteria for this systematic review were deliberately broad to locate and include all relevant original research. We identified only six studies, all predominantly low level and quality of evidence.
To optimize hip surveillance programs for children with PNN brain injury it is important to know which children are most at risk of developing progressive hip displacement. This review identified several potential risk factors for development of hip displacement in children with PNN brain injury up to five years of age. We considered reduced gross motor function, particularly limited ambulatory ability, would be an important risk factor for development of hip displacement in children with PNN brain injury, given its known association for risk of developing hip displacement in children with CP GMFCS level IV and V (Citation22,Citation36–39). Results from studies included in this review suggest children with PNN brain injury up to five years of age who do not ambulate are also at greater risk of developing hip displacement than children who are ambulant.
Whilst the GMFCS is not validated for children with a PNN brain injury who do not meet the clinical criteria for a diagnosis of CP, clinicians caring for children with PNN brain injury have used GMFCS-equivalent levels to help to classify the motor function of these children. Across the six studies most children who developed hip displacement were GMFCS level IV or V or equivalent. This is in keeping with findings reporting the linear relationship between GMFCS level and risk of hip displacement in children with CP of all-causes (Citation22). Use of GMFCS for children with PNN-CP, and GMFCS-equivalent for children with ABI after two years of age, in the absence of other validated measures of gross motor function, should be used to stratify risk in these cohorts of children until further data on the natural history of hip displacement in PNN brain injury are available.
In our results, spastic quadriplegia was found to be the predominant motor type and topographical distribution of children with PNN brain injury who developed hip displacement. Children with CP from pre- and perinatal causes classified as spastic quadriplegia have also been reported to have the highest risk of hip displacement (Citation22,Citation36,Citation39). Most children with CP who have spastic quadriplegia do not walk (Citation22). As noted by Soo et al. (Citation22) motor type and topographical distribution may be surrogates for gross motor functional ability; however, they are less reliable than use of GMFCS level to stratify risk of hip displacement for children with CP. Results from this review suggest children with spastic quadriplegia who do not ambulate following PNN brain injury in the first five years of life should be considered high risk for developing hip displacement.
Information from the studies included in our review suggests that children with more severe neurologic involvement following diffuse PNN brain injury may be at greatest risk of developing early and progressive hip displacement. Early literature investigating hip displacement in children with CP of all-causes correlated the risk of hip displacement with severity of involvement (Citation40). More recently the link between severity of involvement in children with CP of all causes and the risk of hip displacement was related to the lack of functional weight bearing, as quantified with GMFCS (Citation22). For children with PNN-CP, some links between etiology of injury and severity of gross motor functional difficulties have been identified (Citation4,Citation41). Children with PNN-CP from infections, near-drowning and near-miss cot deaths are more likely to have spastic bilateral motor types and higher levels of walking disability than children with PNN-CP from TBI and vascular episodes (Citation41). In a study by Waight et al. (Citation4) children with PNN-CP from NAI and infections were positively associated with greater limitations in gross motor function (GMFCS levels III-V). Until more data on hip displacement in PNN brain injury are available, children with diffuse PNN brain injury in the first five years of life who remain non-ambulant should be considered at high risk for progressive hip displacement.
Earlier age at brain injury appears to be a risk factor for developing hip displacement. Hip displacement and hip dislocation were identified in children with a PNN brain injury in the first two years of life (Citation13,Citation31–35), and those with a brain injury between two and five years of age (Citation31–34). Where reported, hip displacement was rare in children with an ABI after five years of age (Citation32). This should not be taken as evidence that hip displacement in children who have an ABI after the age of five years does not occur, rather that there is currently an absence of published evidence in this age group.
Whilst there is no evidence that spasticity contributes to hip displacement in CP (Citation42) and all hypertonia motor types have similar incidence of hip displacement (Citation22), in PNN brain injury this information is missing. Children with severe PNN brain injury can exhibit abnormal motor posturing, manifesting as decorticate, decerebrate or opisthotonic posturing (Citation43,Citation44). These abnormal motor postures are characterized by generalized trunk extension, lower limb extension and internal rotation, and increased muscle tone (Citation45). The effect of this sudden onset severe hypertonia and abnormal motor posturing, and any contribution to development of hip displacement, remains unknown. Small numbers of children identified in this review developed hip displacement in the first one to six months following brain injury (Citation13,Citation31,Citation33,Citation34). Abrams and Mubarak (Citation31) considered the severity of hip adductor spasticity and abnormal motor posturing, combined with young age at injury when the acetabulum was incompletely developed, to be factors in the development of very early hip displacement and hip dislocation seen in their cohort of children. Presence of significant hypertonia and abnormal motor posturing in children in the acute phase following brain injury, who are expected to have ongoing significant neuromotor sequelae, should be a consideration for early and more frequent review of hip displacement in children with a PNN brain injury before five years of age until further research evidence is available.
Understanding the timing of onset of hip displacement following PNN brain injury is important to help guide the commencement and frequency of hip surveillance for these children, to allow for early identification of hip displacement and timely intervention. The earliest cases of hip displacement in the studies reviewed occurred at one month post brain injury (Citation31,Citation34). All six studies included in this review identified children who had progressed to complete hip dislocation in the first 12 months following brain injury, with three studies reporting hip dislocation within four months post brain injury (Citation13,Citation31,Citation33). When comparing PNN-CP to CP of pre- or perinatal causes, Kentish et al. (Citation13) identified 12% of the population with PNN-CP developed progressive hip displacement by two years of age, compared to 2.7% of the population studied with CP from pre- or perinatal causes. Population-based studies of children with CP of all-causes have identified cases of hip displacement and hip dislocation by two years of age (Citation37,Citation39,Citation46). The most common reported age of development of hip displacement for children of GMFCS level V is two to three years of age (Citation37), although some cases of hip displacement have been reported as young as eight months (Citation47), and 12 months (Citation36,Citation48). The generally later age of identification of hip displacement in children with CP of all causes compared to children with PNN brain injury may be because the initial radiograph in hip surveillance population studies occurs after the diagnosis of CP, which may not be until two years of age. Population-based studies include small numbers of subjects with PNN-CP, who commonly have a more severe phenotype of physical impairment than CP from pre- or perinatal causes (Citation4,Citation41). It is possible that the children identified to have early progressive hip displacement in population-based studies are those with PNN-CP, and CP causality should be included in decision-making around timing of first pelvic radiograph for children with PNN-CP.
Implementation of hip surveillance programs have been shown to prevent hip dislocation in children with CP (Citation46,Citation49,Citation50). The Australian Hip Surveillance Guidelines recommend children with CP classified as GMFCS level IV or V have an initial pelvic radiograph at 12 to 24 months of age, continued 6 monthly until MP stability is established (Citation23). For children with PNN brain injury, the guidelines recommend ‘early and frequent surveillance’ (Citation23). More recently, for children with CP, it has been recommended that hip surveillance commence as soon as CP is identified (Citation51). Like for children with CP, our findings suggest hip surveillance for children with PNN brain injury, expected to be classified as GMFCS level IV or V or equivalent, should start within one to three months following brain injury. How frequently to conduct hip surveillance for children with a PNN brain injury remains unknown. Until further data on the natural history of hip displacement in PNN brain injury is available, it would appear prudent for frequency of hip surveillance in children with a PNN brain injury in the first five years of life to follow the recommended guidelines for children with CP based on GMFCS or equivalent gross motor function (Citation23). Until more data on hip displacement in children with a brain injury after five years of age is available, we suggest clinicians should consider monitoring the hips of children who are GMFCS-equivalent level IV or V according to the hip surveillance guidelines for children with CP.
Limitations of the review
This study has several limitations. We did not identify any studies reporting on prevalence in children with PNN-CP and ABI in order to compare to children with CP attributed to pre- and perinatal causes. The limited number of studies identified, very small sample sizes, study design, high levels of missing and incomplete data, lack of valid and reliable outcome measures used to measure hypertonia and hip displacement, heterogenous populations and change to hip surveillance protocols since most of the studies were published limit the analysis of data and ability to draw firm conclusions. Use of GMFCS is now well established to describe children with PNN-CP and was not available at the time when most of these studies were published. The broad search strategy allowed inclusion of all existing evidence available but had the disadvantage of including papers of predominantly poor study quality. Many studies investigating hip displacement in children with CP due to pre- or perinatal causes would likely include children with PNN-CP. Attempt to obtain separate data on children with PNN-CP and ABI with hip displacement from high quality population-based studies on CP for a more comprehensive review of the literature was unsuccessful.
Although our systematic review was able to identify that hip displacement has been reported in children with PNN-CP and ABI for at least the last 50 years, only two studies have been published in the last decade. Changes to the medical, surgical and therapeutic management of children with PNN brain injury since the studies included in this review were undertaken may change the course of hip displacement and hip dislocation for children with PNN brain injury today.
Recommendations for future research
The prevalence of hip displacement in PNN-CP and ABI remains unknown and is an important area for future research. Attention needs to be given to prospective population-based studies of children with PNN brain injury up to 16 years of age, with adequate sample size and length of follow up. Studies should consider stratifying for several potential risk factors including age at injury, etiology of injury, and GMFCS level or equivalent or clear description of ambulatory status. Outcome measures need to include MP and valid and reliable measures of hypertonia. The timing of initial hip imaging following brain injury and how frequently hip surveillance should occur for children with PNN brain injury of all ages needs to be investigated in longitudinal studies. Identifying the subset of children with PNN brain injury most at risk of hip displacement is essential to assist with planning hip surveillance programs and to balance risk versus benefit for these children; that is, ensuring timely hip surveillance to minimize risk of hip dislocation and less successful management pathways, whilst minimizing unnecessary exposure to radiation. Such information will allow clearer hip surveillance guidelines for children with a PNN brain injury to be established.
Conclusion
The results of this systematic review indicate that children with PNN-CP and ABI up to five years of age with spastic quadriplegia who are non-ambulant are at risk of progressive hip displacement, and this may occur early following brain injury and progress rapidly. Information about hip displacement in children with ABI after the age of five years is scarce.
Abbreviations
| ABI | = | Acquired brain injury |
| CNS | = | Central nervous system |
| CP | = | Cerebral Palsy |
| GMFCS | = | Gross Motor Function Classification System |
| HIE | = | Hypoxic-ischemic encephalopathy |
| MP | = | Migration percentage |
| NAI | = | Non-accidental injury |
| PNN | = | Post-neonatal |
| TBI | = | Traumatic brain injury |
Supplemental Material
Download MS Word (19.5 KB)Acknowledgments
We are thankful to David Honeyman, research librarian, for assisting us with the database search strategies. We thank Miah Okamoto for her assistance with translation, and Dr. Yukihiro Kitai for sharing his study data for this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02699052.2024.2350049.
Additional information
Funding
References
- Shaklai S, Peretz Fish R, Simantov M, Groswasser Z. Prognostic factors in childhood-acquired brain injury. Brain Inj. 2018;32(5):533–39. doi:10.1080/02699052.2018.1431843
- Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55(6):509–19. doi:10.1111/dmcn.12080
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14.
- Waight E, McIntyre S, Woolfenden S, Watson L, Reid S, Scott H, Martin T, Webb A, Badawi N, Smithers‐Sheedy H, et al. Temporal trends, clinical characteristics, and sociodemographic profile of post-neonatally acquired cerebral palsy in Australia, 1973–2012: a population-based observational study. Dev Med Child Neurol. 2022;00(1):1–10. doi:10.1111/dmcn.15293
- Goldsmith S, McIntyre S, Scott H, Himmelmann K, Smithers-Sheedy H, Andersen GL, Blair E, Badawi N, Garne E. Congenital anomalies in children with postneonatally acquired cerebral palsy: an international data linkage study. Dev Med Child Neurol. 2021;63(4):421–28. doi:10.1111/dmcn.14805
- Germany L, Ehlinger V, Klapouszczak D, Delobel M, Hollódy K, Sellier E, Cruz JDL, Alberge C, Genolini C, Arnaud C, et al. Trends in prevalence and characteristics of post-neonatal cerebral palsy cases: a European registry-based study. Res Dev Disabil. 2013;34(5):1669–77. doi:10.1016/j.ridd.2013.02.016
- Cans C, Dolk H, Platt MJ, Colver A, Prasauskiene A, Krägeloh-Mann I. Recommendations from the SCPE collaborative group for defining and classifying cerebral palsy. Dev Med Child Neurol Suppl. 2007;49:35–38. doi:10.1111/j.1469-8749.2007.tb12626.x
- Goldsmith S, McIntyre S, Smithers-Sheedy H, Blair E, Cans C, Watson L, Yeargin‐Allsopp M. An international survey of cerebral palsy registers and surveillance systems. Dev Med Child Neurol. 2016;58(S2):11–17. doi:10.1111/dmcn.12999
- Australian Institute of Health and Welfare. Disability in Australia: acquired brain injury. Bulletin no. 55. Canberra: Australian Institute of Health and Welfare. 2007.
- Forsyth R, Kirkham F. Predicting outcome after childhood brain injury. CMAJ. 2012;184(11):1257–64. doi:10.1503/cmaj.111045
- Smithers-Sheedy H, Badawi N, Blair E, Cans C, Himmelmann K, Krägeloh-Mann I, McIntyre S, Slee J, Uldall P, Watson L, et al. What constitutes cerebral palsy in the twenty-first century? Dev Med Child Neurol. 2014;56(4):323–28. doi:10.1111/dmcn.12262
- Khan F, Baguley IJ, Cameron ID. Rehabilitation after traumatic brain injury. MJA. 2003;178(6):290–95. doi:10.5694/j.1326-5377.2003.tb05199.x
- Kentish M, Wynter M, Snape N, Boyd R. Five-year outcome of state-wide hip surveillance of children and adolescents with cerebral palsy. J Pediatr Rehabil Med. 2012;4(3):205–17. doi:10.3233/PRM-2011-0176
- Reimers J. The stability of the hip in children. A radiological study of the results of muscle surgery in cerebral palsy. Acta Orthop Scand Suppl. 1980;184(sup184):1–00. doi:10.3109/ort.1980.51.suppl-184.01
- Shore B, Spence D, Graham H. The role for hip surveillance in children with cerebral palsy. Curr Rev Musculoskelet Med. 2012;5(2):126–34. doi:10.1007/s12178-012-9120-4
- Cooke PH, Cole WG, Carey RPL. Dislocation of the hip in cerebral palsy. Natural history and predictability. J Bone Joint Surg Br. 1989;71(3):441–46. doi:10.1302/0301-620X.71B3.2722938
- Penner M, Xie WY, Binepal N, Switzer L, Fehlings D. Characteristics of pain in children and youth with cerebral palsy. Pediatrics. 2013;132(2):e407–13. doi:10.1542/peds.2013-0224
- Ramstad K, Terjesen T. Hip pain is more frequent in severe hip displacement: a population-based study of 77 children with cerebral palsy. J Pediatr Orthop B. 2016;25(3):217–21. doi:10.1097/BPB.0000000000000282
- Samilson RL, Tsou P, Aamoth G, Green WM. Dislocation and subluxation of the hip in cerebral palsy Pathogenesis, Natural History And Management. J Bone Joint Surg Am. 1972;54(4):863–73. doi:10.2106/00004623-197254040-00017
- Jung NH, Pereira B, Nehring I, Brix O, Bernius P, Schroeder SA, Kluger GJ, Koehler T, Beyerlein A, Weir S, et al. Does hip displacement influence health-related quality of life in children with cerebral palsy? Dev Neurorehabil. 2014;17(6):420–25. doi:10.3109/17518423.2014.941116
- Ramstad K, Jahnsen RB, Terjesen T. Severe hip displacement reduces health-related quality of life in children with cerebral palsy. Acta Orthop. 2017;88(2):205–10. doi:10.1080/17453674.2016.1262685
- Soo B, Howard JJ, Boyd RN, Reid SM, Lanigan A, Wolfe R. Hip displacement in cerebral palsy. J Bone Joint Surg Am. 2006;88(1):121–29. doi:10.2106/00004623-200601000-00015
- Australian National Hip Surveillance Working Group. Australian Hip Surveillance Guidelines for Children with Cerebral Palsy. Australian academy of cerebral palsy and developmental medicine. 2020 [accessed 2022 July 12]. https://www.ausacpdm.org.au/wp-content/uploads/2020/12/200240-Hip-survey-A5-booklet-WEB.pdf
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–23. doi:10.1111/j.1469-8749.1997.tb07414.x
- Pountney T, Green EM. Hip dislocation in cerebral palsy. BMJ. 2006;332(7544):772–75. doi:10.1136/bmj.332.7544.772
- Flynn JM, Miller F. Management of hip disorders in patients with cerebral palsy. J Am Acad Orthop Surg. 2002;10(3):198–209. doi:10.5435/00124635-200205000-00006
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Chai KEK, Lines RLJ, Gucciardi DF, Ng L. Research screener: a machine learning tool to semi-automate abstract screening for systematic reviews. Syst Rev. 2021;10(1):93. doi:10.1186/s13643-021-01635-3
- Oxford Centre for Evidence-based Medicine. The oxford levels of evidence 2. Oxford, UK; 2011 [accessed 2022 June 9]. https://www.cebm.net/index.aspx?o=5653
- Joanna Briggs Institute. Checklist for cohort studies. 2020. https://jbi.global/sites/default/files/2020-08/Checklist_for_Cohort_Studies.pdf
- Abrams RA, Mubarak S. Musculoskeletal consequences of near-drowning in children. J Pediatr Orthop. 1991;11(2):168–75. doi:10.1097/01241398-199103000-00005
- Kitai Y, Ohmura K, Hirai S, Arai H. Long-term outcome of childhood hypoxic-ischemic encephalopathy. No To Hattatsu. 2015;47(1):43–48.
- Hoffer MM, Brink J. Orthopedic management of acquired cerebrospasticity in childhood. Clin Orthop Relat Res. 1975;1975(110):244–48. doi:10.1097/00003086-197507000-00035
- Blasier D, Letts RM. Pediatric update #7. The orthopaedic manifestations of head injury in children. Orthop Rev. 1989;18(3):350–58.
- Loder RT. Orthopaedic aspects of children with infectious (central nervous system) postnatal cerebral palsy. J Pediatr Orthop. 1992;12(4):527–33. doi:10.1097/01241398-199207000-00022
- Hägglund G, Lauge-Pedersen H, Wagner P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet Disord. 2007;8(1):101. doi:10.1186/1471-2474-8-101
- Larnert P, Risto O, Hägglund G, Wagner P. Hip displacement in relation to age and gross motor function in children with cerebral palsy. J Child Orthop. 2014;8(2):129–34. doi:10.1007/s11832-014-0570-7
- Connelly A, Flett P, Graham HK, Oates J, Connelly A, Flett P. Hip surveillance in Tasmanian children with cerebral palsy. J Paediatr Child Health. 2009;45(7/8):437–43. doi:10.1111/j.1440-1754.2009.01534.x
- Terjesen T. The natural history of hip development in cerebral palsy. Dev Med Child Neurol. 2012;54(10):951–57. doi:10.1111/j.1469-8749.2012.04385.x
- Howard CB, McKibbin B, Williams LA, Mackie I. Factors affecting the incidence of hip dislocation in cerebral palsy. J Bone Joint Surg Br. 1985;67(4):530–32. doi:10.1302/0301-620X.67B4.4030844
- Cans C, McManus V, Crowley M, Guillem P, Platt MJ, Johnson A. Cerebral palsy of post-neonatal origin: characteristics and risk factors. Paediatr Perinat Epidemiol. 2004;18(3):214–20. doi:10.1111/j.1365-3016.2004.00559.x
- Howard J, Khot A, Graham H. The hip in cerebral palsy. In: Alshryda S, Howard J, Huntley J, and Schoenecker J, editors The pediatric and adolescent hip: essentials and evidence. Berlin: Springer International Publishing; 2019:467–530.
- Brown JK, Ingram TT, Seshia SS. Patterns of decerebration in infants and children: defects in homeostasis and sequelae. J Neurol Neurosurg Psychiatry. 1973;36(3):431–44. doi:10.1136/jnnp.36.3.431
- Berger MS, Pitts LH, Lovely M, Edwards MS, Bartkowski HM. Outcome from severe head injury in children and adolescents. J Neurosurg. 1985;62(2):194–99. doi:10.3171/jns.1985.62.2.0194
- Knight J, Decker LC Decerebrate and decorticate posturing. StatPearls [internet]. StatPearls Publishing; 2021.
- Wordie SJ, Robb JE, Hägglund G, Bugler KE, Gaston MS. Hip displacement and dislocation in a total population of children with cerebral palsy in Scotland. Bone Joint J. 2020;102-b(3):383–87. doi:10.1302/0301-620X.102B3.BJJ-2019-1203.R1
- Hermanson M, Hägglund G, Riad J, Wagner P. Head-shaft angle is a risk factor for hip displacement in children with cerebral palsy. Acta Orthop. 2015;86(2):229–32. doi:10.3109/17453674.2014.991628
- Wordie SJ, Bugler KE, Bessell PR, Robb JE, Gaston MS. Hip displacement in children with cerebral palsy. Bone Joint J. 2021;103-b(2):411–14. doi:10.1302/0301-620X.103B2.BJJ-2020-1528.R1
- Wynter M, Gibson N, Willoughby KL, Love S, Kentish M, Thomason P. Australian hip surveillance guidelines for children with cerebral palsy: 5-year review. Dev Med Child Neurol. 2015;57(9):808–20. doi:10.1111/dmcn.12754
- Hägglund G, Alriksson-Schmidt A, Lauge-Pedersen H, Rodby-Bousquet E, Wagner P, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Joint J. 2014;96-b(11):1546–52. doi:10.1302/0301-620X.96B11.34385
- Gibson N, Wynter M, Thomason P, Baker F, Burnett H, Graham HK, Kentish M, Love SC, Maloney E, Stannage K, et al. Australian hip surveillance guidelines at 10 years: new evidence and implementation. J Pediatr Rehabil Med. 2022;15(1):31–37. doi:10.3233/PRM-220017