ABSTRACT
Objectives
To establish the prevalence of tinnitus in adults who have sustained non-blast related traumatic brain injury (TBI), as well as the prevalence of tinnitus following TBI in the absence of hearing loss.
Methods
A systematic search was carried out using MEDLINE, EMBASE, PsycINFO, CINAHL from January 1st 1990 to August 14th 2023. TBI, tinnitus and auditory findings were extracted from all eligible studies, and a descriptive synthesis performed. This systematic review was registered with PROSPERO (Registration number: CRD42022377637).
Results
Based on the Oxford Centre of Evidence-Based Medicine (OCEBM) (2011) criteria, the highest quality evidence identified was at Level 2b, with the bulk of the included studies predominantly populating the lower evidence tiers. While there was a substantial variability in the methods used to establish and report the presence of tinnitus, its occurrence following TBI was evident in adults with and without hearing loss.
Conclusion
The need for prospective, longitudinal research into tinnitus following non-blast related TBI is evident. Such comprehensive studies hold the potential to inform and enhance the clinical diagnosis and management of this patient population.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide with an estimated annual incidence of 150–300 people per 100,000 (Citation1,Citation2). Approximately half of the world’s population sustains at least one TBI over their lifetime (Citation3–5) accounting for 30–40% of all injury-related deaths across all ages (Citation5,Citation6). Such injuries not only lead to a spectrum of physical, neurocognitive and sensory deficits (Citation3,Citation5,Citation7,Citation8) but also impose a substantial economic burden, with costs estimated at AUD$8.6 billion in Australia (2009) (Citation9,Citation10), €33 billion in Europe (2010) (Citation11,Citation12) and USD$40.6 billion (2016) in the United States of America (Citation13). TBI results from primary injuries, including penetrating (open-head, e.g., bullet wounds), non-penetrating (closed-head, e.g., blunt force trauma), and blast injuries, which directly impact the brain (Citation14,Citation15). Secondary injuries, manifesting through neuropathological processes such as axonal swelling and apoptosis, occur in the weeks and months following the primary trauma, exacerbating the initial damage (Citation15,Citation16). Given the effects of both primary and secondary TBI-related damage, the patterns of post-TBI disease are markedly variable, leading to distinct prognoses and outcomes. In particular, the occurrence of physical and sensory deficits can manifest as motor function loss, compromised balance and coordination, heightened fatigue, episodes of dizziness, visual impairments, hearing deficits and tinnitus (Citation5,Citation16,Citation17).
Tinnitus, defined as the subjective perception of sound in the absence of an external source (Citation18,Citation19), occurs in around 10–15% of the general adult population (Citation20). While the etiology of tinnitus remains uncertain, it is commonly associated with hearing loss (Citation21–25). However, tinnitus can also manifest in individuals without detectable hearing impairment (Citation26–30). Various risk factors have been associated with tinnitus, including otological and psychological conditions, medications, noise exposure, hearing loss, and head and neck trauma (Citation31–33). To comprehensively assess tinnitus, guidelines recommend an examination to exclude physical causes, a complete audiological assessment, and use of a validated tinnitus questionnaire (Citation34). Validated questionnaires provide a consistent and standardized measure for the assessment of tinnitus in the absence of any objective measures (Citation35,Citation36). While the use of validated tinnitus questionnaires is recommended for the assessment of tinnitus (Citation34,Citation37), in many cases the collection of tinnitus information occurs through a variety of methods, such as non-validated questionnaires or participant (e.g. single item) self-reports including anamnesis, semi-structured interviews or retrospective review of medical data (Citation38–40). This variability in assessment methods emphasizes the complexity of accurately characterizing tinnitus.
The issue of tinnitus becomes particularly salient in the context of TBI, where auditory deficits are well-documented (Citation39,Citation41,Citation42), and given the notable comorbidity with hearing loss (Citation21–25), its impact is further magnified. Auditory deficits following TBI have been reported at significant rates, with up to 44.4% (Citation43) of individuals experiencing these issues after a moderate/severe TBI and up to 66% (Citation44) after a mild TBI (mTBI). Furthermore, while there is strong evidence linking tinnitus to blast-related TBI (Citation45–47), indicating a well-established concern in such cases, the characterization of tinnitus following non-blast related TBI remains underexplored. This holds notable significance as non-blast related TBI reflects the more common type of TBI encountered in non-specialized clinical audiological settings (Citation48), emphasizing the importance of investigating its association with tinnitus and auditory deficits. This gap in understanding highlights the need for comprehensive research into tinnitus across all forms of TBI, not only to improve identification and management of tinnitus but also to guide the development of effective diagnostic criteria and targeted interventions for post-TBI tinnitus.
Hence, the aim of this systematic review was to determine the prevalence of tinnitus following non-blast related TBI in adults and determine the methods (i.e. participant [e.g. single item] self-report, validated questionnaire and non-validated questionnaire) used to report tinnitus. A systematic search of literature was conducted to address the following research questions:
In adults who have sustained non-blast related TBI, what is the prevalence of tinnitus as reported through validated and non-validated measures?
In adults who have sustained non-blast related TBI, what is the prevalence of tinnitus in the absence of measurable hearing loss?
Methods
Search strategy
A systematic search of English language literature was performed from January 1st 1990 to August 14th 2023 using Medline, CINAHL, PubMed, EMBASE and PsycINFO databases. Additional records were acquired through a manual search of reference lists and SCOPUS author search. The concept map outlines the search terms, keywords and subject headings uniquely crafted to guide the search process across the selected databases, see . In this context, ‘concepts’ refer to the fundamental themes or elements extracted from the research questions, which dictate the thematic scope articles included in the review must cover. The term ‘mapped terms’ pertains to the process of associating these concepts with specific search terms, keywords, and subject headings. This mapping ensures that the search strategy is both targeted and broad enough to encapsulate all relevant literature, facilitating a thorough exploration of the predefined concepts within the selected databases. In the structuring of the search strategy, within each individual concept, terms were amalgamated using the Boolean operator ‘OR’ while ‘AND’ was utilized to link between concepts. Depending on the database, subject headings were used as either ‘mapped terms’ or as ‘keywords.’
Table 1. Concept map.
Inclusion and exclusion criteria
Studies were included if: (1) tinnitus was assessed by any method following non-blast related TBI; (2) their publication date was between January 1st 1990 to August 14th 2023; (3) participants were adults (≥18 years old); (4) they were published in English and were peer-reviewed. No restrictions were imposed on the design of the studies, owing to the limited availability of relevant literature. Studies were excluded if: (1) they focused exclusively on blast-related TBI; (2) participants were <18 years of age; (3) participants were from a military population, given the possibility of blast-related injury; (4) the primary focus was outside the realm of tinnitus dysfunction (e.g., cognitive or psychological aspects of injury); (5) participants experienced tinnitus following concussion, acknowledging the inconsistent definitions and interchangeable use of concussion/mTBI in existing literature; (6) where mTBI qualifiers (e.g., loss of consciousness, PTA, GCS) as outlined by the World Health Organization Collaborating Task Force and the Centers for Disease Control Prevention (CDC) (Citation49) were not applied as part of the mTBI diagnosis; (7) where tinnitus onset was attributed to focal brain injury; (8) participants were minimally conscious at the time of testing; (9) participants experienced tinnitus following open head injury; (10) the primary focus was on other disturbances secondary to TBI, rather than those relating to tinnitus; (11) where tinnitus onset was attributed to whiplash, due to the differences in mechanism of injury; (12) they were unrelated to TBI or tinnitus; and; (13) the studies focused on other topics unrelated to the current review.
Data extraction and analysis
Data extraction was streamlined through Covidence (Citation50), a digital platform designed for systematic review management. Covidence facilitated the efficient retrieval of references and aided in the screening and categorization of studies for analysis. To ensure rigor and reliability of extraction, a comprehensive quality assurance was employed. Initially, data extraction was conducted by the primary reviewer to maintain consistency in the evaluation of studies. Subsequently, the second independent reviewer verified a randomly selected 10% of the publications to check for accuracy and reliability. This dual-reviewer approach was complemented by regular team meetings to discuss any discrepancies and achieve consensus, thereby safeguarding the integrity of the data extraction process. Additionally, any conflicts that arose during the verification process were resolved through discussion with the third reviewer, ensuring a robust methodological framework underpinned the review.
Further, for this review, data were extracted from studies that evaluated audiological outcomes in individuals experiencing tinnitus following non-blast related TBI. Key audiological findings included pure tone audiometry, immittance measures (tympanometry and acoustic reflexes), auditory brainstem response (ABR), and otoacoustic emissions (OAE). These measures were selected based on their relevance to detecting auditory dysfunction with which tinnitus is frequently associated, although tinnitus can also occur in individuals without any detectable hearing impairment. Findings were categorized as normal or outside of normal limits, primarily guided by pure-tone audiometry results to align with established associations between tinnitus and auditory sensitivity. This approach was critical for identifying potential auditory dysfunction in TBI participants, even in cases presenting normal audiometry results, reflecting the nuanced relationship between tinnitus and auditory pathway impairments. In Table S5, normal hearing was defined as 20 dB or less for all studies. Substantial variations in defining hearing loss for pure-tone audiometry is evident in the literature, resulting from the use of different reference guidelines (Citation51,Citation52). Given the noted variability in defining hearing loss and for the purpose of our analysis, the World Health Organization (WHO) criteria for classifying degrees of hearing loss was adopted. This approach utilizes the average thresholds at 500, 1000, 2000, and 4000 Hz in the better ear in determining the degree of hearing loss, which was classified into the following categories: mild (20–34 dB), moderate (35–49 dB), moderately severe (50–64 dB), severe (65–79 dB), and profound (80–94 dB) hearing loss levels (Citation53,Citation54).
Notably, the extraction of data for the prevalence analysis of tinnitus in participants with TBI, as shown in Table S5, necessitated the inclusion of studies with precise reporting on the co-occurrence of tinnitus and hearing loss. Studies that did not distinctly segregate data for TBI participants with tinnitus from those without or failed to provide specific information on tinnitus-related hearing loss, were not included in this targeted analysis. This selection criterion was essential to accurately delineate the audiological profile of tinnitus within the TBI cohort, thus preserving the integrity of our results. It is important to note that while these studies did not contribute to the prevalence analysis in Table S5, they were not omitted from the systematic review in its entirety; their exclusion was a consequence of the requirement for detailed participant data for this aspect of the study.
Inter-rater reliability
Inter-rater reliability was employed to ensure consistency in the screening process of titles and abstracts of included articles. Four hundred and seventy-nine articles were independently evaluated by two authors to determine eligibility based on the inclusion/exclusion criteria. Where there was uncertainty, full text articles were reviewed in detail to make informed decisions on their inclusion or exclusion. The Cohen’s Kappa statistic was utilized to quantify agreement between the two reviewers during the initial screening phase, resulting in a Kappa coefficient (κ) of 0.58. According to Landis and Koch (Citation55) guidelines, this coefficient signifies moderate agreement between the reviewers. Discrepancies at any stage were resolved with the involvement of the third author, who assisted in the final decision.
Study selection
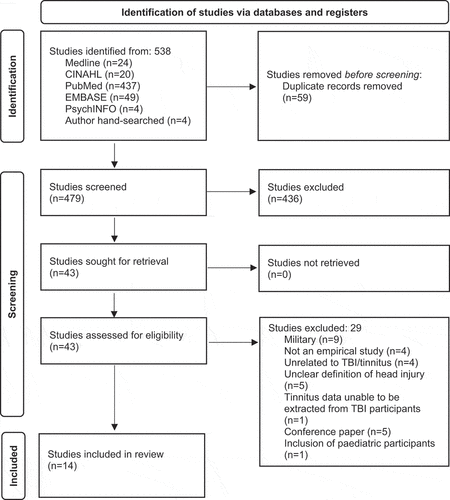
A total of 538 articles were identified across the five databases and imported into Covidence (Citation50). Following the removal of 59 duplicates, a total of 479 articles were retained for screening. These articles underwent title and abstract screening by two independent reviewers, resulting in the exclusion of 436 articles based on the exclusion criteria. The remaining 43 articles underwent full-text screening of which 29 were excluded for the following reasons: military (n = 9), not an empirical study (n = 4), unrelated to TBI/tinnitus (n = 4), unclear definition of head injury (n = 5), tinnitus data unable to be extracted from TBI participants (n = 1), conference paper (n = 5), and inclusion of pediatric participants (n = 1). This resulted in a total of 14 articles retained for systematic review, see .
Results
Study characteristics
Characteristics of TBI and audiological/tinnitus findings are synthesized in Tables S2 and S3 respectively. For details pertaining to authors’ country of origin and study design, refer to columns one and two in Table S2.
Audiological findings included the following: pure tone audiometry, a behavioral test measuring hearing thresholds from 250 to 8000 Hz, essential for assessing auditory sensitivity (Citation56); immittance measures, comprising tympanometry and acoustic reflexes, providing objective evaluations of middle ear function by assessing acoustic admittance changes in relation to air pressure, and bilateral reflexive contraction of the stapedius muscle in response to sound (Citation56); OAE, an objective test measuring energy generated in the outer hair cells of the cochlea (Citation56); and ABR, an auditory evoked potential that measures auditory pathway function within the auditory nerve and brainstem (Citation56).
Quality appraisal
Evidence was appraised using the Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Citation57). Given the specialized nature of our study area and the inherent challenges in sourcing extensive research, we did not exclude any studies based on their quality. They ranged from one nonconsecutive cohort study (Level 2b) (Citation58), one case control study (Level 3b) (Citation40), one nonconsecutive case control series (Level 3b) (Citation59), one retrospective case control series (Level 3b) (Citation60), one retrospective nonconsecutive case control series (Level 3b) (Citation44), three consecutive case series (Level 4) (Citation43,Citation61,Citation62), three retrospective nonconsecutive case series (Level 4) (Citation25,Citation39,Citation63), and three single case reports (Level 5) (Citation64–66), translating to Level C grade of recommendation, see Tables S2 and S3. This diversity in study designs and significant heterogeneity prompted a descriptive synthesis of the data, as opposed to a meta-analysis.
Participant characteristics
Table S2 provides an overview of participant characteristics across the 14 included studies, comprising a cumulative total of 1003 participants in the non-blast TBI related category. Participant’s age was inconsistently reported across the studies, with nine studies reporting both mean and range (Citation39,Citation40,Citation43,Citation44,Citation58,), two studies reporting mean only (Citation25,Citation61) and three case studies reporting the participant’s actual age (Citation64–66). Participant’s mean age across the studies ranged from 18 (Citation65) to 69.8 years of age (Citation25) (M = 39.4, SD = 12.7), calculated from studies that offered mean age data.
Results of individual studies
Traumatic brain injury findings
Table S2 outlines a detailed summary of the TBI findings. The most frequently reported cause of injury was motor vehicle accidents (MVA), which was reported in eight studies (Citation39,Citation40,Citation43,Citation44,Citation60,Citation63–65). Four studies did not specify the cause of injury (Citation25,Citation58,Citation59,Citation62).
Further, assessment time post-TBI showed considerable variation across the studies, ranging from less than 72 hours (Citation62) to 27 years (Citation63) (M = 4.1 years, SD = 3.8 years), from those reporting mean times since injury or a specific time since injury. Four studies reported the mean and the range of assessment time since injury (Citation44,Citation59,Citation61,Citation63), two reported the range only (Citation57,Citation61), two reported the mean only (Citation43,Citation60), and three case studies reported the precise assessment time since injury (Citation63–65). Meanwhile, three studies did not report on assessment time since injury (Citation25,Citation39,Citation40). Notably, three studies (Citation62,Citation64,Citation66) reported some form of follow-up (1 or 3) assessments from time of injury ranging from three months (Citation62) to three years (Citation66) whereas the remaining 11 primary studies did not report any follow-up assessment.
Building on the variability in assessment time post injury, the studies also varied in their approaches to measuring TBI severity. Seven studies reported specific measures of TBI severity, employing a range of tools including the Glasgow Coma Scale (GCS) (Citation61), Reaction Level Scale (RLS) scores (Citation57), length of Post Traumatic Amnesia (PTA) (Citation40,Citation63), the Mayo TBI Severity Classification System (Citation43) and the American Congress of Rehabilitation Medicine (ACRM) criteria (Citation44,Citation60). In contrast, the remaining studies did not report using established TBI severity classification measures or criteria to determine injury severity.
In terms of TBI severity among participants, four studies reported focusing on participants with mild TBI (Citation44,Citation60–62) while two studies included participants with severe TBI (Citation25,Citation66). A combination of mild, moderate and severe TBI severity was reported by two studies (Citation40,Citation43) and six studies did not report the severity of injuries (Citation39,Citation58,Citation59,Citation63–65). Additionally, radiological findings, such as MRI or CT scans, to support TBI diagnoses were reported in eight studies (Citation43,Citation44,Citation58,Citation59,Citation61,Citation62,Citation64,Citation65), while six studies (Citation25,Citation39,Citation40,Citation60,Citation63,Citation66) did not report such findings.
Tinnitus findings
A total of 1003 participants across 14 studies were assessed for tinnitus at their initial assessment following TBI. Tinnitus was identified using validated tinnitus questionnaires (Citation43,Citation60,Citation66), non-validated questionnaires (Citation25) or were participant (e.g., single item) self-reported (Citation39,Citation40,Citation44,Citation58,Citation59,Citation61–65), see Table S3. A total of 84 participants across three studies were assessed for tinnitus using validated questionnaires such as Tinnitus Handicap Inventory (THI) (Citation43,Citation60) and Tinnitus Questionnaire (TQ) (Citation66). In contrast, a non-validated questionnaire was used on 495 participants in one study (Citation25). Further, self-reported measures of tinnitus, ranging from single-item self-reports, including anamnesis (Citation40,Citation58,Citation59,Citation61,Citation62,Citation64,Citation65), semi-structured interviews (Citation44,Citation63) and retrospective electronic medical records reviews (Citation39), were utilized across 10 studies, accounting for 424 participants.
Of the 14 included studies, five studies reported the assessment of tinnitus following TBI as their primary objective (Citation25,Citation40,Citation43,Citation64,Citation66), whereas the other nine treated it as a secondary objective (Citation39,Citation44,Citation58–63,Citation65). Of the studies with a primary focus on tinnitus, the most common methods for reporting post-TBI tinnitus were validated questionnaires employed in two studies (Citation43,Citation66) encompassing 32 participants, and self-reported measures (e.g., single-item reports) used in two others (Citation40,Citation64), involving 33 participants.
Validated tinnitus questionnaires
The assessment of tinnitus through validated questionnaires not only reveals insights into the condition’s impact on individuals but also plays a critical role in quantifying the prevalence of tinnitus following TBI. Among these instruments, the Tinnitus Handicap Inventory (THI) and the Tinnitus Questionnaire (TQ) stand out for their application and utility in clinical research.
The THI emerged as the most commonly utilized validated questionnaire for assessing tinnitus, employed in two studies (Citation43,Citation60), to evaluate the severity of tinnitus across three subscales: functional, emotional, and catastrophic. This 25-item measure categorizes tinnitus severity into five levels, ranging from very mild (0–16) to catastrophic (78–100), with scores above 18 reflecting greater distress (Citation67). In this context, two studies reporting on the THI identified a mean severity score of 22.3 (SD = 7.3) across 83 participants, aligning with a mild classification of tinnitus impact. The TQ was employed in one study (Citation66) involving one participant and offers a comprehensive 52-item evaluation across multiple domains, including emotional distress and cognitive impact (Citation68,Citation69). Although it doesn’t classify tinnitus severity in the same manner as the THI, an initial TQ score of 67 out of 84 was noted, with higher scores corresponding to increased tinnitus distress. Though comprehensive data on classifying tinnitus severity utilizing the TQ is limited, the German version of the TQ designated scores above 47 as indicative of decompensated tinnitus (Citation70), characterized by distress of notable magnitude and lack of habituation (Citation71,Citation72). See Table S4 for an overview of the reported prevalence of tinnitus following TBI, broken down by assessment method – whether through participant (e.g. single item) self-reports, non-validated questionnaires, or validated questionnaires. This breakdown not only illuminates the prevalence rates obtained through various methods but also reinforces the importance of diverse assessment strategies in capturing the full scope of tinnitus experiences within the TBI patient population.
Tinnitus and audiological findings
Building upon our findings regarding the prevalence of tinnitus, we turned our attention to the specific audiological outcomes in participants who had reported tinnitus following TBI. Table S5 outlines the audiological findings for participants that reported tinnitus following TBI in three studies (Citation43,Citation63,Citation66). The prevalence of tinnitus, as influenced by different audiological results, is detailed in column five, providing insights into the relationship between tinnitus and auditory function within this population.
Discussion
This systematic review revealed inconsistencies in the design of studies addressing the presence of tinnitus post non-blast related TBI. This finding mirrors that of a recent review on post-TBI peripheral audiological deficits, which similarly underscored research insufficiencies in this area (Citation41). Of the 14 studies included in this review, only one met the criteria for Level 2b evidence (Citation57). The remainder fell under Level 3b, Level 4, or Level 5, cumulating in a Grade C recommendation. The prevalence of lower-tier evidence, combined with considerable heterogeneity in study designs, rendered a meta-analysis unfeasible, instead necessitating a descriptive synthesis of the data. Notably, there was a marked absence of prospective study designs in acute settings, with the majority of included studies gathering data from tertiary institutions using retrospective methodologies. While retrospective designs are cost-effective by leveraging existing clinical records, they are inherently susceptible to several threats to both internal and external validity. Specifically, these designs encounter threats to internal validity through reliance on documented records and individuals’ recollections, which introduces the risk of recall bias. The absence of crucial data and the challenge of conclusively establishing causation further complicates the accurate assessment of tinnitus post-TBI. Among the 14 studies retained for this review, eight utilized retrospective designs, including three case series, two case-control series, and three case reports. Retrospective designs do not account for maturation effects, such as neuroplastic changes following the acute phase of TBI, which could influence the long-term maintenance of tinnitus (Citation71). Such critical aspects of tinnitus progression could be more accurately monitored through prospective study designs initiated in acute settings. The retrospective approach, while practical for initial hypothesis generation given its cost-effectiveness and ability to utilize existing records, is hampered by high attrition rates, history effects, and selection bias. Despite these challenges, retrospective studies play a valuable role in exploring rare occurrences and laying the groundwork for future research directions. Nonetheless, the insights garnered from these retrospective analyses should pave the way for designing prospective studies, which can offer more definitive conclusions about the natural history, treatment efficacy, and recovery patterns of tinnitus following TBI (Citation73). Prospective longitudinal studies, particularly those following a consecutive admission approach shortly after injury, are crucial for providing a comprehensive view of tinnitus development, enabling researchers to observe its onset, progression, and potential resolution or persistence over time. This methodological shift is critical for advancing the field and ensuring that future research can offer actionable insights for clinical practice, patient management, and the development of targeted interventions.
Moreover, there was a pronounced inconsistency in the methods (e.g., case history reviews, patient files, questionnaires) used to establish and report the presence of tinnitus. This resonates with concerns expressed within the broader literature, where variability in tinnitus assessment and documentation is frequently attributed to the absence of a standardized approach (Citation18,Citation74). The majority of the studies included in this review relied on participant (e.g., single item) self-reports of tinnitus, often through the use of a single question. This aligns with trends observed in other systematic reviews, where participant-reported tinnitus data, particularly within case-control and retrospective study designs, are more frequently utilized in comparison to other validated methodologies (Citation18,Citation33). However, this approach for assessing tinnitus oversimplifies the complex nature of tinnitus and neglects its multi-faceted dimensions (Citation18,Citation73). Hence, there is now a growing consensus in the field about the necessity of employing validated questionnaires for a more precise and comprehensive assessment of tinnitus (Citation34,Citation36,Citation74). In the current review, three studies used validated questionnaires to assess tinnitus, with the THI being the most frequently employed questionnaire. The use of the THI is particularly advocated due to its proven reliability and validity in capturing the multifaceted impact of tinnitus, positioning it as the preferred benchmark for tinnitus assessment (Citation34,Citation67,Citation75). By offering a more detailed exploration of tinnitus’ impact across several domains, the THI exemplifies the shift toward adopting standardized, validated instruments for tinnitus assessment. Such multi-item tools not only enhance the reliability of tinnitus identification but also facilitate a deeper understanding of its consequences, supporting the development of more targeted and effective interventions (Citation34,Citation36,Citation74). Although validated tinnitus questionnaires rely to some degree on self-reporting, they provide a standardized approach across studies to enable a more effective evaluation of tinnitus identification and outcomes post-TBI (Citation63,Citation64). Therefore, to refine our understanding and derive a more accurate representation of tinnitus following non-blast related TBI, future investigations should prioritize the incorporation of validated tinnitus questionnaires, thus laying the foundation for more rigorous and uniform research outcomes in this domain.
Despite the substantial variability in study designs, the current review has revealed important insights into the prevalence of tinnitus following non-blast related TBI. Our findings indicated that around one-third of participants reported tinnitus post-TBI, as evidenced by data from both questionnaires and single-item self-reporting measures. This finding differs from previous studies where prevalence rates of 61.5%-66% have been reported for TBI-induced tinnitus in similar contexts (Citation43,Citation44,Citation60). Such discrepancies in reported prevalence rates could be attributed to a combination of factors including study design heterogeneity, varied tinnitus reporting methodologies, and the primary objectives of the included studies. Given that 10 out of the 14 studies in our review relied on participant (e.g., single item) self-reported data and acknowledging the intricate nature of tinnitus, there is a possibility that this reliance may have influenced the reported prevalence rates. In contrast, among the four studies that used questionnaires to investigate tinnitus following TBI, three utilized validated questionnaires. Notably, in these studies, 63.1% of participants reported tinnitus post-TBI, a figure that aligns more closely with the prevalence rates of 61.5%-66% cited in other research (Citation43,Citation44,Citation60). This disparity underscores the potential for self-report single-item methodologies to affect the true prevalence of tinnitus post-TBI and highlights the value of utilizing validated questionnaires for more accurate epidemiological assessments.
The marked difference in reported prevalence rates (63.1% using validated questionnaires; 33.3% using participant [e.g., single item] self-reports; and 25.5% using non-validated questionnaires) highlights the critical need for methodological consistency in future tinnitus research post-TBI. Notably, while this systematic review combined data from multiple sources, the prevalence rates cited are frequently derived from individual studies. This aggregation of data introduces variability in reported rates, as systematic reviews afford a broader, integrated view that may differ from the more singular perspectives offered by individual studies. Moreover, differences in recruitment strategies such as whether participants were sourced from tinnitus clinics, referred post-TBI, or recruited from the general population can further contribute to the variability observed in these prevalence rates. Consequently, the prevalence rates presented herein should be considered within the broader context of their aggregated nature, illustrating the complexities of deriving a unified prevalence rate for tinnitus post-TBI.
Further, our findings indicate that the majority of participants with post-TBI tinnitus had normal audiological findings, which is notably higher than prevalence rates within the general population (Citation76). It is important to clarify that the insights from this prevalence analysis were primarily driven by a few studies that explicitly detailed the relationship between tinnitus and hearing status. Eleven studies from the review were excluded from this analysis due to ambiguity in presenting audiological data, which made it difficult to distinguish between individuals with normal hearing and those with hearing loss among tinnitus sufferers with and without TBI. Additionally, the prevalence analysis was further affected by two studies that incorporated normal hearing as a part of their inclusion criteria. This potential bias makes it challenging to draw definitive conclusions from the aggregated data. Despite these caveats, our findings highlight the importance of further exploring the interplay between TBI, hearing loss and tinnitus. Notably, the existing literature presents varied perspectives on the co-occurrence of tinnitus and hearing loss following non-blast related TBI, as evidenced by discrepancies across several studies (Citation25,Citation58,Citation59,Citation61,Citation63). These variations could be attributed to differences in assessment methodologies, criteria for defining hearing loss, tinnitus assessment tools and individual factors such as the severity and cause of the TBI, as well as the time of assessment post injury. However, some studies have also reported evidence of disruptions in auditory function among TBI patients in the absence of hearing loss (Citation77–79). Future research should prioritize a comprehensive and standardized test battery through prospective consecutive cohort study designs. This approach will facilitate a thorough exploration of post-TBI auditory deficits, even in cases where no hearing loss is reported.
Limitations
Caution should be exercised when interpreting the findings of this review due to several limitations. Firstly, the validity of our results is influenced by the substantial study design heterogeneity, further exacerbated by small sample sizes and evidence levels that did not surpass Level 2b. The substantial heterogeneity in the included studies not only presented challenges in terms of comparing and synthesizing the results but also made it unfeasible to conduct a meta-analysis. Consequently, our findings relied on a descriptive synthesis, which, while providing valuable insights, might not capture the nuances and potential quantitative patterns observed in meta-analytic reviews. The low levels of evidence (according to the OCEBM criteria) presented in the included studies, particularly regarding tinnitus following non-blast related TBI, limited the scope of our review. Secondly, the variability in tinnitus assessment tools, especially the use of single-item self-reports by participants, introduces inconsistencies in the evaluation of tinnitus post-TBI. The use of single-item self-reporting methods may not fully encapsulate the diverse range of tinnitus experiences post-TBI, highlighting the need for a more comprehensive diagnostic approach to assess tinnitus in this population. Lastly, by restricting our inclusion criteria to studies published in English, we may have omitted relevant research published in other languages.
Conclusions and future directions
Despite the limited findings that emerged from this review, it is evident that there is much to be understood about tinnitus following non-blast related TBI. The majority of the studies included in this review yielded low levels of evidence. Insufficient data regarding TBI severity, tinnitus and audiological findings stemmed from many studies focusing on tinnitus as a secondary aim. A substantial need for prospective, longitudinal research investigating tinnitus following non-blast related TBI exists. Additional information regarding tinnitus chronicity and TBI severity may provide further insight into future prevalence studies. These comprehensive studies have the potential to provide a more accurate depiction of incidence rates and to offer invaluable insights into the prognosis of post-TBI tinnitus. The findings of this review should encourage researchers to pursue a more focused and comprehensive approach to studying tinnitus post-TBI, acknowledging its potential impact on patient care. The imperative for future research lies not only in enhancing methodological rigor but also in deepening our understanding of tinnitus as a significant consequence of TBI. Such efforts are crucial for advancing clinical practice and improving outcomes for individuals affected by post-TBI tinnitus.
Contributor-ship statement
ML, BS and RA contributed to the conception and design of this systematic review. ML conducted the literature searches and acquired the relevant articles. ML, BS and RA contributed to the manuscript. The authors agreed to accept the responsibility for the integrity of the work as a whole.
Supplemental Material
Download Zip (102.3 KB)Acknowledgments
We would like to thank Dr Denise Jones and Alesha Sayner from Barwon Health Geelong and Grampian Health Ballarat respectively, for their guidance and advice within the Stepping into Research program. We would also like to thank Helen Skoglund from the Barwon Health Library for her assistance with developing a systematic database search strategy. Finally, we would like to thank Rachael Hyder from Barwon Health Geelong for her support and encouragement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02699052.2024.2353798.
Additional information
Funding
References
- Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic Brain Injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26(7):1118–30. doi:10.1177/0963689717714102.
- Rubiano AM, Carney N, Chesnut R, Puyana JC. Global neurotrauma research challenges and opportunities. Nature. 2015;527(7578):S193–7. doi:10.1038/nature16035.
- Levin H. Understanding Traumatic Brain Injury current research and future directions. Shum D, Chan R, Levin HS, Chan RCK, Anderson V, editors. USA, Cary : Oxford University Press; 2014.
- Nguyen R, Fiest KM, McChesney J, Kwon C, Jette N, Frolkis AD, Atta C, Mah S, Dhaliwal H, Reid A, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(6):774–85. doi:10.1017/cjn.2016.290.
- Maas J, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi:10.1016/S1474-4422(17)30371-X.
- World Health Organization. Neurological disorders public health challenges. Geneva: World Health Organization; 2006.
- Šarkić B, Douglas JM, Simpson A. Auditory dysfunction in non-blast-related TBI: a guide for audiologists. Hear J. 2021;74(3):30,2,4,5. doi:10.1097/01.HJ.0000737584.09756.f6.
- Silver JM, McAllister TW, Yudofsky SC. Textbook of traumatic brain injury. 2nd ed. Arlington, VA: American Psychiatric Pub.; 2011.
- Collie A, Keating C, Pezzullo L, Gabbe B, Cooper J, Brown D, Olver J, McCartin F, Trethewey P. Brain and spinal cord injury in Australia – economic cost and burden of disease. Inj Prev. 2010;16(Suppl 1):A25–6. doi:10.1136/ip.2010.029215.92.
- Ponsford JL, Spitz G, Cromarty F, Gifford D, Attwood D. Costs of care after traumatic brain injury. J Neurotrauma. 2013;30(17):1498–505. doi:10.1089/neu.2012.2843.
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(10):718–79. doi:10.1016/j.euroneuro.2011.08.008.
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–62. doi:10.1111/j.1468-1331.2011.03590.x.
- Miller GF, Depadilla L, Xu L. Costs of nonfatal traumatic brain injury in the United States, 2016. Med Care. 2021;59(5):451–55. doi:10.1097/MLR.0000000000001511.
- National Academies of Sciences, Engineering, Medicine, Health Medicine Division, Board on Health Care Services, & Committee on the Review of the Department of Veterans Affairs Examinations for Traumatic Brain Injury. Evaluation of the disability determination process for traumatic brain injury in Veterans. 1. Washington, D.C: National Academies Press, 2019.
- de Lanerolle NC, Kim JH, Bandak FA, editors. Neuropathology of Traumatic Brain Injury: comparison of penetrating, nonpenetrating direct impact and explosive blast etiologies. Semin Neurol. 2015;35(1):12–19.
- Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, Hill S, Legrand V, Sorgner A. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): A prospective longitudinal observational study. Neurosurgery. 2015;76(1):67–80. doi:10.1227/NEU.0000000000000575.
- Maas A. Traumatic brain injury: changing concepts and approaches. Chin J Traumatol. 2016;19(1):3–6. doi:10.1016/j.cjtee.2016.01.001.
- McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70–9. doi:10.1016/j.heares.2016.05.009
- Bauer CA, Solomon CG. Tinnitus. N Engl J Med. 2018;378(13):1224–31. doi:10.1056/NEJMcp1506631.
- Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600–07. doi:10.1016/S0140-6736(13)60142-7.
- Tyler RS. Tinnitus handbook. Clifton Park, NY: Thomson Delmar Learning; 2000.
- Elmer S, Schmitt R, Giroud N, Meyer M. The neuroanatomical hallmarks of chronic tinnitus in comorbidity with pure-tone hearing loss. Brain Struct Funct. 2023;228(6):1511–34. doi:10.1007/s00429-023-02669-0.
- Savastano M. Tinnitus with or without hearing loss: are its characteristics different? Eur Arch Otorhinolaryngol. 2008;265(11):1295–300. doi:10.1007/s00405-008-0630-z.
- Norena A, Micheyl C, Chéry-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurotol. 2002;7(6):358–69. doi:10.1159/000066156.
- Sindhusake D, Golding M, Wigney D, Newall P, Jakobsen K, Mitchell P. Factors predicting severity of tinnitus: a population-based assessment. J Am Acad Audiol. 2004;15(4):269–80. doi:10.3766/jaaa.15.4.2.
- Kara E, Aydın K, Alperen Akbulut A, Karakol S, Durmaz S, Murat Yener H, Gozen ED, Kara H. Assessment of hidden hearing loss in normal hearing individuals with and without tinnitus. J Int Adv Otol. 2020;16(1):87–92. doi:10.5152/iao.2020.7062.
- Kehrle HM, Sampaio ALL, Granjeiro RC, de Oliveira TS, Oliveira CACP. Tinnitus annoyance in normal-hearing individuals: correlation with depression and anxiety. Ann Otol Rhinol Laryngol. 2016;125(3):185–94. doi:10.1177/0003489415606445.
- Ristovska L, Jachova Z. Extended high frequency hearing thresholds in tinnitus patients with normal hearing. Arch Acoust. 2022;47(4):449–55.
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31(38):13452–57. doi:10.1523/JNEUROSCI.2156-11.2011.
- Shim HJ, An Y, Kim DH, Yoon JE, Yoon JH, Malmierca MS. Comparisons of auditory brainstem response and sound level tolerance in tinnitus ears and non-tinnitus ears in unilateral tinnitus patients with normal audiograms. PLOS One. 2017;12(12):e0189157–e. doi:10.1371/journal.pone.0189157.
- Goderie T, Van Wier MF, Lissenberg-Witte BI, Merkus P, Smits C, Leemans CR, Kramer SE. Factors associated with the development of tinnitus and with the degree of annoyance caused by newly developed tinnitus. Ear Hear. 2022;43(6):1807–15. doi:10.1097/AUD.0000000000001250.
- Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: Characteristics, causes, mechanisms, and treatments. J Clin Neurol. 2009;5(1):11–19. doi:10.3988/jcn.2009.5.1.11.
- Biswas R, Genitsaridi E, Trpchevska N, Lugo A, Schlee W, Cederroth CR, Gallus S, Hall DA. Low evidence for tinnitus risk factors: a systematic review and Meta-analysis. J Assoc Res Otolaryngol. 2023;24(1):81–94. doi:10.1007/s10162-022-00874-y.
- Fuller TE, Haider HF, Kikidis D, Lapira A, Mazurek B, Norena A, Rabau S, Lardinois R, Cederroth CR, Edvall NK, et al. Different teams, same conclusions? A systematic review of existing clinical guidelines for the assessment and treatment of tinnitus in adults. Front Psychol. 2017;8:1–15.
- Figueiredo RR, Rates MA, de Azevedo AA, de Oliveira PM, de Navarro PBA. Correlation analysis of hearing thresholds, validated questionnaires and psychoacoustic measurements in tinnitus patients. Brazilian Journal of Otorhinolaryngology. 2010;76(4):522–6. doi:10.1590/S1808-86942010000400018.
- Zeman F, Koller M, Schecklmann M, Langguth B, Landgrebe M, Figueired R, Aazevedo A, Rates M, Binetti C, Elgoyhen AB, et al. Tinnitus assessment by means of standardized self-report questionnaires: psychometric properties of the Tinnitus Questionnaire (TQ), the Tinnitus Handicap Inventory (THI), and their short versions in an international and multi-lingual sample. Health Qual Life Outcomes. 2012;10(1):128–. doi:10.1186/1477-7525-10-128.
- Department of Health. Provision of services for adults with tinnitus. A good practice guide. London: Central Office of Information; 2009.
- Ohhashi G, Tani S, Murakami S, Kamio M, Abe T, Ohtuki J. Problems in health management of professional boxers in Japan…including commentary by Estwanik JJ. Br J Sports Med. 2002;36(5):346–53. doi:10.1136/bjsm.36.5.346.
- Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in Traumatic Brain Injury. J Rehabil Res Dev. 2007;44(7):921–8. doi:10.1682/JRRD.2007.09.0140.
- Ceranic BJ, Prasher DK, Raglan E, Luxon LM. Tinnitus after head injury: evidence from otoacoustic emissions. J Neurol Neurosurg Psychiatry. 1998;65(4):523–9. doi:10.1136/jnnp.65.4.523.
- Šarkić B, Douglas JM, Simpson A. Peripheral auditory dysfunction secondary to Traumatic Brain Injury: a systematic review of literature. Brain Inj. 2019;33(2):111–28. doi:10.1080/02699052.2018.1539868.
- Munjal SK, Panda NK, Pathak A. Relationship between severity of Traumatic Brain Injury (TBI) and extent of auditory dysfunction. Brain Inj. 2010;24(3):525–32. doi:10.3109/02699050903516872.
- Knoll RM, Lubner RJ, Brodsky JR, Wong K, Jung DH, Remenschneider AK, Herman SD, Kozin ED. Auditory quality-of-life measures in patients with Traumatic Brain Injury and normal pure tone audiometry. Otolaryngol Head Neck Surg. 2020;163(6):1250–54. doi:10.1177/0194599820933886.
- Vander Werff KR, Rieger B. Auditory and cognitive behavioral performance deficits and symptom reporting in postconcussion syndrome following mild Traumatic Brain Injury. J Speech Lang Hear Res. 2019;62(7):1–18. doi:10.1044/2019_JSLHR-H-18-0281.
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related Traumatic Brain Injury. J Rehabil Res Dev. 2009;46(6):797–810. doi:10.1682/JRRD.2008.09.0118.
- Yurgil KA, Clifford RE, Risbrough VB, Geyer MA, Huang M, Barkauskas DA, Vasterling JJ, Baker DG. Prospective associations between Traumatic Brain Injury and postdeployment tinnitus in active-duty marines. J Head Trauma Rehabil. 2016;31(1):30–39. doi:10.1097/HTR.0000000000000117.
- Clifford RE, Ryan AF. The interrelationship of tinnitus and hearing loss secondary to age, noise exposure, and Traumatic Brain Injury. Ear Hear. 2022;43(4):1114–24. doi:10.1097/AUD.0000000000001222.
- Šarkić B, Douglas JM, Simpson A. A cross-sectional survey of non-specialist Australian audio-vestibular clinical practice for Traumatic Brain Injury and rehabilitation. Brain Impairment. 2023;24(3):611–28. doi:10.1017/BrImp.2022.35.
- Gardner RC, Yaffe K. Epidemiology of mild Traumatic Brain Injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66(Pt B):75–80. doi:10.1016/j.mcn.2015.03.001.
- Veritas Health Innovation. Covidence systematic review software Melbourne, Australia 2023. www.covidence.org
- Gatlin AE, Dhar S. History and lingering impact of the arbitrary 25-db cutoff for normal hearing. Am J Audiol. 2021;30(1):231–4. doi:10.1044/2020_AJA-20-00181.
- Humes L. What is “normal hearing” for older adults and can “normal-hearing older adults” benefit from hearing care intervention? The complexity surrounding definitions of hearing loss and accessibility. The Hearing Review. 2020;27(7):12.
- Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health. 2013;23(1):146–52. doi:10.1093/eurpub/ckr176.
- Humes LE. The World Health Organization’s hearing-impairment grading system: an evaluation for unaided communication in age-related hearing loss. Int J Audiol. 2019;58(1):12–20. doi:10.1080/14992027.2018.1518598.
- Landis JR, Koch GG. An application of hierarchical Kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33(2):363–74. doi:10.2307/2529786.
- Katz J, Chasin M, English K, Hood LJ, Tillery KL. Handbook of Clinical Audiology. Seventh. USA: Lippincott Williams & Wilkins; 2014.
- Bergemalm PO, Borg E. Peripheral and central audiological sequelae of closed head injury: function, activity, participation and quality of life. Audiol Med. 2005;3(3):185–98. doi:10.1080/16513860500291714.
- Bergemalm PO, Borg E. Long-term objective and subjective audiologic consequences of closed head injury. Acta Otolaryngol. 2001;121(6):724–34. doi:10.1080/00016480152583674.
- Knoll RM, Herman SD, Lubner RJ, Babu AN, Wong K, Sethi RKV, Chen JX, Rauch SD, Remenschneider AK, Jung DH, et al. Patient-reported auditory handicap measures following mild Traumatic Brain Injury. Laryngoscope. 2020;130(3):761–7. doi:10.1002/lary.28034.
- Jafarzadeh S, Pourbakht A, Bahrami E. Vestibular assessment in patients with persistent symptoms of mild Traumatic Brain Injury. Indian J Otolaryngol. 2022;74(Suppl 1):272–80. doi:10.1007/s12070-020-02043-0.
- Kisilevski V, Podoshin L, Ben-David J, Soustiel JF, Teszler CB, Hafner H, Chistyakov A. Results of otovestibular tests in mild head injuries. Int Tinnitus J. 2001;7(2):118–21.
- Jury MA, Flynn MC. Auditory and vestibular sequelae to Traumatic Brain Injury: a pilot study. NZ Med J. 2001;114(1134):286–88.
- Hegel MT, Martin JB. Behavioral treatment of pulsatile tinnitus and headache following traumatic head injury. Objective polygraphic assessment of change. Behav Modif. 1998;22(4):563–72. doi:10.1177/01454455980224007.
- Kim HS, Song JH, Oh JK, Ahn JH, Kim JH, Chang IB. Endovascular treatment of traumatic arteriovenous fistula in young adults with pulsatile tinnitus. J Korean Neurosurg Soc. 2020;63(4):532–8. doi:10.3340/jkns.2019.0233.
- Kreuzer PM, Landgrebe M, Frank E, Langguth B. Repetitive transcranial magnetic stimulation for the treatment of chronic tinnitus after Traumatic Brain Injury: a case study. J Head Trauma Rehabil. 2013;28(5):386–9. doi:10.1097/HTR.0b013e318254736e.
- Newman CW, Jacobson GP, Spitzer JB. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143–8. doi:10.1001/archotol.1996.01890140029007.
- Hallam RS, Jakes SC, Hinchcliffe R. Cognitive variables in tinnitus annoyance. Br J Clin Psychol. 1988;27(3):213–22. doi:10.1111/j.2044-8260.1988.tb00778.x.
- Jacquemin L, Mertens G, Van de Heyning P, Vanderveken OM, Topsakal V, De Hertogh W, Michiels S, Van Rompaey V, Gilles A. Sensitivity to change and convergent validity of the Tinnitus Functional Index (TFI) and the Tinnitus Questionnaire (TQ): Clinical and research perspectives. Hear Res. 2019;382:107796–. doi:10.1016/j.heares.2019.107796.
- Hofrichter NA, Brueggemann P, Goebel G, Mazurek B, Rose M. Maintaining the legacy and moving forward: the new tinnitus questionnaire short form version 2. J Psychosom Res. 2020;138:110248–. doi:10.1016/j.jpsychores.2020.110248.
- Mohebbi M, Daneshi A, Asadpour A, Mohsen S, Farhadi M, Mahmoudian S. The potential role of auditory prediction error in decompensated tinnitus: an auditory mismatch negativity study. Brain Behav. 2019;9(4):e01242–n/a. doi:10.1002/brb3.1242.
- Henry JA, Roberts LE, Caspary DM, Theodoroff SM, Salvi RJ. Underlying mechanisms of tinnitus: review and clinical implications. J Am Acad Audiol. 2014;25(1):005–22. doi:10.3766/jaaa.25.1.2.
- Šarkić B, Douglas JM, Simpson A, Vasconcelos A, Scott BR, Melitsis LM, Spehar SM. Frequency of peripheral vestibular pathology following Traumatic Brain Injury: a systematic review of literature. Int J Audiol. 2021;60(7):479–94. doi:10.1080/14992027.2020.1811905.
- Jarach CM, Lugo A, Scala M, van den Brandt PA, Cederroth CR, Odone A, Garavello W, Schlee W, Langguth B, Gallus S. Global prevalence and incidence of tinnitus: a systematic review and meta-analysis. Jama Neurol. 2022;79(9):888–900. doi:10.1001/jamaneurol.2022.2189.
- Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham ER, Archer SM, Blakley BW, Carter JM, Granieri EC, et al. American academy of otolaryngology—head and neck foundation clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(2_suppl):S1–40. doi:10.1177/0194599814538403a56.
- Sanchez TG, Medeiros IR, Levy CP, Ramalho Jda R, Bento RF. Tinnitus in normally hearing patients: clinical aspects and repercussions. Braz J Otorhinolaryngol. 2005;71(4):427–31. doi:10.1016/S1808-8694(15)31194-0.
- Nölle C, Todt I, Seidl RO, Ernst A. Pathophysiological changes of the central auditory pathway after blunt trauma of the head. J Neurotrauma. 2004;21(3):251–8. doi:10.1089/089771504322972040.
- OCEBM. The Oxford 2011 levels of evidence. 2011. http://www.cebm.net/index.aspx?o=5653.
- White M, Duquette-Laplante F, Jutras B, Bursch C, Koravand A. A retrospective study of the effects of Traumatic Brain Injury on auditory function: from a clinical perspective. Neuro Sci. 2022;3(1):52–62. doi:10.3390/neurosci3010004.
- Turgeon C, Champoux F, Lepore F, Leclerc S, Ellemberg D. Auditory processing after sport-related concussions. Ear Hear. 2011;32(5):667–70. doi:10.1097/AUD.0b013e31821209d6.