ABSTRACT
Introduction
Early Exercise Intolerance (EEI) is associated with delayed recovery and longer time to Return To Play (RTP), but this has not been established.
Participants; (n = 52, male n = 30) UK university-aged rugby-union student-athletes.
Methods
Student-athletes completed baseline screening (July–October 2021 and 2022). The test battery was repeated within 48 h, 4, 8 and 14 days after a Sports-Related Concussion (SRC) with the Buffalo Concussion Bike or Treadmill Test to set sub-symptom heart rate threshold. Student-athletes then completed a controlled early exercise protocol in-between reassessment (days 3, 5–7 and 9–13). Those with EEI were compared to those with early-exercise tolerance.
Outcome measures
Post-Concussion Symptom Scale, Immediate Post-Concussion and Cognitive Test, Vestibular-Ocular Motor Screening Tool and the Revised Perceived Academic Impact Tool.
Results
EEI was seen throughout the initial 14-days post-SRC (23.8%, 22.4%, 25.5%. 25.0%). EEI was associated with a slower reaction time within 48 h (−0.01 (−0.030–0.043) Vs 0.06 (0.033–0.24), p = 0.004) and greater VOMS scores within 48 h; (0.00 (0.00–4.00) Vs 5.50 (2.75–9.00), p = 0.016) and 4 days (0.00 (0.00–2.00) Vs 5.00 (0.00–6.00), p = 0.044). RTP was 12.5 days longer in those with EEI at 14-days post-SRC.
Conclusion
EEI is prevalent following an SRC in university-aged student-athletes and was associated with delayed recovery and RTP.
Introduction
Sports-Related Concussion (SRC), from direct or indirect impact to the head or body, results in the transmission of forces to the head, resulting in shear and strain to the brain (Citation1,Citation2). It is thought this results in a neurometabolic cascade and energy crisis, causing an interruption to neural connections and coupling between neural networks (Citation3). Reduced cerebral blood flow coupling reduces the ability for glycolysis (Citation4), further worsening the energy crisis. This impairs signaling between regions of the brain and the diffuse nature of impairment is thought to be the reason many SRC’s present with a plethora of symptoms and dysfunctions, which may include dysfunction of the Autonomic Nervous System (ANS) (Citation5). It has been postulated that the ANS is unable to effectively detect or respond to the demands of exercise, due to the uncoupling of the parasympathetic and sympathetic nervous system that controls ANS functions (Citation6). Hence, when resuming exercise changes in cerebral blood flow, heart rate and blood pressure are not adequate enough to meet the demands of activity and one can experience exacerbation of symptoms, or the onset of other SRC-related symptoms such as headaches and dizziness (Citation7,Citation8). Exercise tolerance testing gradually increases exertion and demand upon the ANS, and the examinee is monitored for the onset or worsening of SRC-related symptoms. The onset or worsening of SRC-related symptoms indirectly indicates the functioning of the ANS and is considered an indicator of physiological severity and recovery (Citation9,Citation10).
Early exercise tolerance testing is safe and can be conducted within 24 h of an SRC (Citation11) although Early Exercise Intolerance (EEI) has been associated with: abnormal cerebral blood (Citation12–14), variability in heart rates (Citation15–18), low blood pressure (Citation14,Citation19,Citation20), increased symptom burden (Citation9) and delayed recovery (Citation21–23). EEI is defined as terminating exercise tolerance testing due to the onset or exacerbation of symptoms, whereas Early Exercise Tolerance (EET) is the ability to achieve 90% age predicted heart rate maximum (220-age) or reach fatigue without exacerbation or onset of SRC-related symptoms (Citation9). Previous studies assessing the effects of EEI (within the initial 10 days) on time to recovery have recruited mainly adolescents (Citation9,Citation21–23) with very limited studies recruiting university-aged participants (18–25 years of age) (Citation24,Citation25). Studies have not tested all participants within the same time frame, rather, assessing between 3 and 8 days (Citation21), 5 and 7 days (Citation23), or within 10 days (Citation9,Citation26) of an SRC. This may have resulted in some participants having more time to recover and, therefore, may no longer experience EEI. Furthermore, all studies recruited participants from concussion clinics or emergency departments, where greater severity of injury may have driven participants to seek assistance, and thus results may not be applicable to minor SRCs. Additionally, no study has been conducted that tracks early exercise tolerance throughout the acute period following an SRC.
Due to the diffuse nature of SRC injury, student-athletes may present with other dysfunctions such as neurocognitive impairment, sleep disorders, altered mood and Vestibular-Ocular-Motor (VOM) dysfunction (Citation27). VOM dysfunction may present as headache, dizziness or nausea and can be assessed with the Vestibular Ocular-Motor Screening Tool (VOMS) (Citation28). A study found those with EEI had greater VOMS impairment (Citation23) and when another study accounted for VOM rehabilitation in their statistical analysis, there was also an association of delayed recovery in those with exercise intolerance (Citation22). In both studies, the exercise tolerance testing was completed on a treadmill, where the participants head and body will have been moving, demanding processing and responses from the VOM system. It is possible results presented in previous studies reflect not only exercise intolerance but also VOM impairment.
Therefore, the primary aim of this study was to establish the effects of EEI throughout the initial 14 days following an SRC on recovery and time to Return To Play (RTP) in a university-aged student-athletes. Secondary aims were to explore if VOM dysfunction and self-reported neck pain influenced recovery in those with EEI and EET. Furthermore, the study aimed to explore the effect of confounding factors (sex, history of SRC, learning disability, mental health disorder, number of previous SRCs), treatment compliance (the percentage of sessions in between reassessments completed) and severity of dysfunction on recovery of those with and without EEI after an SRC.
Material and methods
Participants and design
Ethical approval for this study was granted by the Loughborough University Ethics Review Sub-Committee (approval numbers 2020–2992–2383 and 2020–1273–1511). This prospective, observational study recruited university-aged student-athletes who participated in rugby union domestic and university leagues and were enrolled in a full-time higher education course at Loughborough University. Student-athletes were invited to enroll following presentations delivered during pre-season (July–October 2021 and 2022) sport team meetings. Student-athletes participated in either National weekend leagues (UK rugby union Tier 1 or 3, or British University Sport Competition Tier 1 and 2). After informed consent was obtained, student-athletes underwent baseline testing (VOMS, Post-Concussion Symptom Score (PCSS), Immediate Post-Concussion and Cognitive Assessment Test (ImPACT), and the Revised Perceived Academic Impact Tool (PAIT2)). In the event, an SRC was sustained, the assessing clinician (physiotherapist) referred the student-athlete to the lead researcher.
Test battery
The test battery was always completed in the following order: the VOMS, ImPACT and PAIT2. The VOMS required student-athletes to rate exacerbation of headache, dizziness, nausea and fogginess on a 0–10 verbal analogue scale (0=no symptoms, 10=worst you can imagine) on six tests (smooth pursuits, horizontal saccades, vertical saccades, horizontal vestibular-ocular reflex (VOR), vertical VOR, visual motion sensitivity test (VMST), and near point convergence (NPC)) (Citation28). The scores were totaled across all six tests and the total score provided the total VOMS score. A high internal consistency (α = 0.92) for VOMS has been reported (Citation28).
The ImPACT was completed in a large room with adequate spacing and with no more than six people in the room. The test provides a composite score for verbal memory, visual memory, motor processing speed and reaction time. The sensitivity and specificity is deemed to be good (81.9% and 89.4%) (Citation29), as its reliability and validity (Citation30,Citation31). In the event, an invalid test was recorded (Xs and Os total incorrect and impulse control > 30, word memory learning correct < 69%, design memory learning percentage correct < 50% or three letter total letter correct < 8); student-athletes repeated the test no sooner than 48 h later. The PCSS within the ImPACT software was used to indicate symptom burden. The PCSS asks student-athletes to rate themselves on 22 symptoms associated with SRC (7-item Likert Scale; 0=no symptoms, 6=severe, total score 0–132).
Finally, the PAIT2 is a novel questionnaire tool (Citation32). The PAIT2 has been developed through previous studies (Citation33,Citation34) based on the initial work of Wasserman et al. (Citation35). PAIT2 can detect 96.00%, 92.00%, 85.71% and 92.00% of concussed student-athletes with academic impairment at within 48 h, 4, 8 and 14-days post-SRC, with a reliability of 88.89%, 75.00%, 25.00% and 50.00% (Citation32). Student-athletes are required to rate their perceived academic ability on 23 different academic activities according to a worded response, that provided a score on a 0–6 Likert Scale. A score of 0 indicates no academic impairment and 6 the worst, and all questions are summed to provide the PAIT2 score. A score increase of 5 points or greater increase indicates worsening academic ability and is used as a detection threshold (Citation32).
Post sport-related concussion assessments
Student-athletes were assessed within 48 h, at 4, 8 and 14-days post-SRC and at RTP. These re-assessment time points were selected to ensure reassessments would be before and after the expected time to recovery for university-aged student-athletes (Citation33). Decision on RTP was done solely by the assessing clinician and was based upon completion of the Graded Return to Play (GRTP) protocol without any symptoms. At each reassessment, student-athletes repeated the test battery in the same order with the addition of reporting what percentage of academic activity was missed specifically due to their SRC. This was recorded as non-contact academic time loss (any self-study, preparation, essay writing etc.) and contact academic time loss (seminars, lectures, laboratories, workshops, tutorials). Academic attendance was separated into non-contact and contact academic time loss as the graded return to learn protocols specify non-contact academic activities can be commenced prior to contact activities. Additionally, the environment of contact academic activities may exacerbate symptoms in some cases, such as bright lights or noise exacerbating headaches and affecting attendance of these activities. Following this, as described by Leddy et al. (Citation36), student-athletes completed either the Buffalo Concussion Bike Test (BCBT) or the Buffalo Concussion Treadmill Test (BCTT). To minimize VOM dysfunction interfering with exercise tolerance testing if student-athletes had symptoms on VOMS, they completed the BCBT and if not, the BCTT. If student-athletes reported any symptom on the PCSS at a 5 or higher, they did not complete the exercise tolerance testing and physically rested until the next reassessment.
Sport-related concussion management
Student-athletes recruited to this study followed the Rugby Football Union Community Pathway (Citation37) whereby the GRTP cannot be commenced prior to day 15 and RTP is no sooner than 19 days. During the initial 14 days, rather than the traditional physical rest protocol, student-athletes completed a controlled early exercise program, the Sub-Symptom Heart Rate Threshold (SSHeRT) protocol. Therefore, early exercise tolerance is considered as intolerance to exercise within the traditional 14-day physical rest period. A timeline from initial assessment to starting the Graded Return To Play (GRTP) is detailed in .
Figure 1. Study timeline (VOMS; Vestibular Ocular Motor Screening, BCBT; Buffalo Concussion Bike Test, BCTT; Buffalo Concussion Treadmill Test, SSHeRT; Sub-Symptom Heart Rate Threshold Protocol).
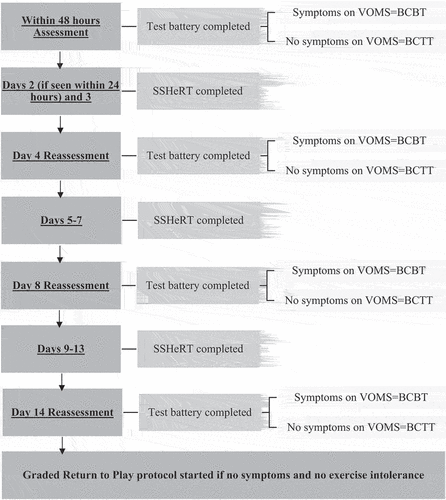
To mitigate the possible effects of VOM dysfunction on exercise tolerance testing, the sub-symptom heart rate threshold (80% of HR achieved on testing; Heart Rate Threshold (HRt)) was established on either the BCBT or BCTT depending upon the presence or not of symptoms on VOMS testing. On the other days (day 3, 5–7, 9–13), student-athletes completed the SSHeRT, totaling a maximum of nine sessions. The SSHeRT started with a 5-min warm up, where student-athletes gradually raised their heart rate to the HRt, maintained it at the HRt for 15 min and then did a 5-min cool down. This SSHeRT was completed on the bike if the student-athlete was tested on the BCBT and the treadmill if assessed on the BCTT. Following this, a small rehabilitation program was completed (maximum 10 min), consisting of six low-level resistance exercises (supplementary file 1). If student-athletes reported neck pain at any reassessment when asked ‘do you have any neck pain?,’ they were referred back to their assessing clinician who provided treatment (which may have consisted of exercise, manual therapy and strengthening) as deemed appropriate. If symptoms were present on VOMS, the assessing clinician provided rehabilitation and was encouraged to use the exercises presented in the supplementary file to assure consistency (supplementary file 1).
Statistical analysis
Descriptive data have been presented as median and quartile ranges due to the non-parametric nature of the data. Individual change from baseline to each reassessment time point for all outcome measures was calculated and used in all the following analyses (score change = score at reassessment time point–baseline). To address the primary aim of the effect of exercise intolerance, student-athletes were grouped into either EEI or EET at each reassessment time point. Student-athletes that were unable to achieve 90% Heart Rate Maximum HRmax (HRmax) (220 age) due to the exacerbation or onset of new symptoms were grouped into the EEI group. Student-athletes who either stopped the BCBT or BCTT due to fatigue or reached their 90% HRmax were grouped into the EET group. A sub-group of student-athletes were not able to complete exercise tolerance testing due to rating a symptom on the PCSS at a 5 or higher and were grouped into ‘unable to test’ for that re-assessment time point. If their symptom burden improved by the next reassessment time point, they were grouped accordingly. Change in outcome measures were calculated for each group (medians and interquartile ranges) and analyzed for significant difference using the Mann–Whitney U-test or the Kruskal–Wallis as appropriate. A Mann–Whitney test was used to compare EEI and EET to determine the sole effect of exercise intolerance. Correlations with the series of measures from the BCBT and BCTT and number of days to RTP were completed as described above using a Spearman’s rank correlation. Additionally, a Kaplan–Meier survival analysis with log-rank tests of significance was used to evaluate the effect of EEI on time taken to RTP at each reassessment time point. To explore the effect of severity of impairment on the outcome measures on time RTP between EEI and EET, a Quade’s ANOVA was performed. To further explore the effect of EEI on recovery, a series of measures from the BCBT or BCTT were correlated with the outcome measures: overall symptom score at the start, end and the change in score on the BCBT and BCBT (measured with the Visual Analogue Scale (VAS); 0–10, 0=no symptoms, 10=worst you could imagine), maximum Heart Rate (HR) achieved, 80% HRt (80% of the maximum HR achieved on the BCBT or BCTT) and HRmax.
To address the secondary aim of assessing if any confounding factor affected time to recovery or RTP a Mann–Whitney U-test, or a Kruskal–Wallis H-test if there were three groups or more. Confounding factors were sex, history of SRC learning disability, mental health disorder, number of previous SRCs. If any were found to be significant interaction with difference in scores on the outcome measures between students-athletes with EEI and EET using a Friedmans two-way ANOVA.
To assess the effect of neck pain and presence of VOM dysfunction on outcome measures, a Mann–Whitney U-test was used. Student-athletes were considered to have VOM dysfunction if they reported any symptoms with the VOMS assessment. Compliance to the SSHeRT was measured by calculating the percentage of sessions the student-athlete completed (maximum of 10 sessions). Those completing less than 60% of the sessions were grouped as not compliant and differences in time to RTP between the group was assessed for using a Mann–Whitney U-test. To establish the effect of severity of symptoms, difference in RTP time was calculated for all groups (EET, EEI and unable to test). All three groups were analyzed with a Kruskal–Wallis to determine how all three scenarios affected time to RTP.
Results
During baseline screening, 256 student-athletes were recruited and 58 SRCs were sustained. Due to student-athletes returning home (n = 2), not being referred within 48 h (n = 2) and student-athletes not wishing to commit to the reassessments (n = 2), 52 SRCs were included in the analysis. The student-athletes’ demographics are presented in .
Table 1. Demographic data for student-athletes recruited at baseline and concussed student-athletes (n = number, BMI = body mass index, SRC = sports-related concussion).
Primary aim; effect of EET on recovery and time to RTP
EEI was seen throughout, including assessments up to 14 days post-SRC (). Student-athletes with EEI took longer for performance to return to baseline levels on PCSS, ImPACT’s visual memory, motor processing speed and reaction time, PAIT2, NPC and VOMS. However, there were significantly worse performances for those with EEI on ImPACT’s reaction time (U = 55.000, z = −3.106, p = 0.004, r = −0.431) and VOMS (U = 64.000, z = −2.919, p = 0.016, r = −0.405) at 48 h post-SRC. Performance on VOMS remained significantly worse for those with EEI at 4 days also (U = 117.500, z = −2.433, p = 0.044, r = −0.337) ().
Table 2. Descriptives for outcome measures for student-athletes with Early Exercise Intolerance (EEI) and Early Exercise Tolerance (EET) presented as medians and inter-quartile ranges (unless stated).
When comparing student-athletes with EET, EEI and those unable to exercise, significant differences within 48 h were also seen (symptom burden; H (Citation2) = 11.605, z = −0.962, p = 0.003, r = −0.133, NPC; H (Citation2) = 14.271, z = −0.857, p = 0.001, r = −0.119, non-contact academic time loss; H (Citation2) = 11.919, z = −1.604, p = 0.003, r = −0.222, contact academic time loss; H (Citation2) = 8.857, z = −1.894, p = 0.014, r = −0.263) ().
Student-athletes with EEI at 14 days post-SRC took 12.5 days longer to RTP (intolerant; 35.50 (27.25–45.75), tolerant; 23.00 (20.00–26.50), U = 134.500, z = −2.528, p = 0.001, r = −0.351). Descriptives for the series of exercise tolerance testing measures (initial, end and difference in overall symptom burden, maximum heart rate achieved, age-predicted HRmax and HRt) can be seen in . Positive correlations indicate there was an association of longer RTP time with greater VAS ratings at the end of the exercise tolerance testing at 4 (rs (Citation38) = 0.364, p = 0.003, r2 = 0.132) and 8 days post-SRC (rs (Citation39) = 0.280, p = 0.046, r2 = 0.078) and greater change in VAS scores at 4 days post-SRC (rs (Citation38) = 0.364, p = 0.003, r2 = 0.132). The same correlations were also seen between time to RTP and VAS ratings at the beginning of the exercise tolerance testing and (rs (Citation39) = 0.309, p = 0.027, r2 = 0.095) at the end of testing (rs (Citation39) = 0.280, p = 0.046, r2 = 0.78) at 8 days post-SRC. Negative correlations indicate higher HRt were associated with a shorter RTP at 8 (rs (Citation39)=-0.277, p = 0.031, r2 = 0.073) and 14-days post-SRC (rs (Citation39)=-0.294, p = 0.036, r2 = 0.086).
Table 3. Descriptives for measures taken during either the Buffalo Concussion Bike Test (BCBT) or the Buffalo Concussion Treadmill Test (BCTT) presented as medians and inter-quartile ranges (unless stated).
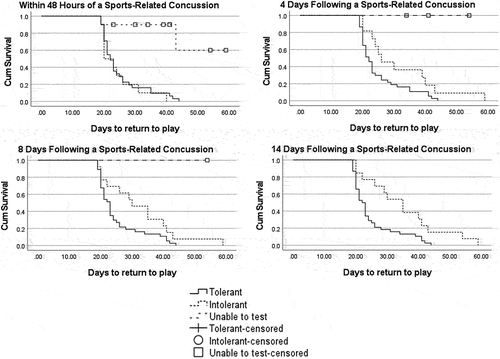
Log rank tests indicated there was a significant difference in time to RTP between student-athletes who were EEI, EET and those that were unable to test at all time points (within 48 h; χ2 (2) = 19.584, p = 0.001, 4 days; χ2 (2) = 11.617, p = 0.003, 8 days; χ2 (2) = 7.786, p = 0.020 and 14-days; χ2 (2) = 7.685, p = 0.006) (). RTP was significantly different between student-athletes that were either EEI or EET at 8 (χ2 (Citation2) = 3.951, p = 0.047) and 14-days post-SRC (χ2 (Citation1) = 7.685, p = 0.006) ().
Figure 2. Kaplan–Meier survival analysis plots for time (days) taken to return to play depending on being exercise tolerant or exercise intolerant and being able to undergo exercise tolerance testing.
Performance on ImPACT, symptom burden and VOM dysfunction interacted with RTP between EEI and EET student-athletes at many time points. Symptom burden severity significantly affected time to RTP, between EEI and EET student-athletes at 14-days post-SRC (f (2) = 7.467, p = 0.009, eta2 = 0.050). Verbal memory performance had significant interactions on RTP between EEI and EET student-athletes at 4 (f (2) 4.517, p = 0.039, eta2 = 0.015) and 14-days post-SRC (f (2) 8.991, p = 0.004, eta2 = 0.013). Performance on visual memory had significant effect at 4 (f (2) 4.366, p = 0.042, eta2 = 0.015), 8 (f (2) 4.125, p = 0.048, eta2 = 0.027) and 14-days post-SRC (f (2) 9.196, p = 0.004, eta2 = 0.014), as did reaction time (f (2) = 8.334, p = 0.006, eta2 = 0.042) and motor processing speed (f (2) 9.081, p = 0.004, eta2 = 0.024) at 14-days post-SRC. Academic ability and attendance also influenced RTP time between EEI and EET student-athletes at 4 (PAIT2; 5.864, p = 0.019, eta2 = 0.041, non-contact academic time loss; 4.624, p = 0.037, eta2 = 0.107, contact academic time loss; 5.197, p = 0.027, eta2 = 0.154) and 14-days post-SRC (PAIT2; 8.638, p = 0.005, eta2 = 0.081, non-contact academic time loss; 6.892, p = 0.012, eta2 = 0.050, contact academic time loss; 8.273, p = 0.006, eta2 = 0.054). Performance on VOMS and NPC at day 14 also interacted with RTP time between EEI and EET student-athletes (NPC; f (Citation2)8.135, p = 0.006, eta2 = 0.058, VOMS; f (Citation2)6.823, p = 0.012, eta2 = 0.096).
Secondary aims; effect of confounding factors
Females reported greater symptom burdens within 48 h (U = 178.00, z = −2.868, p = 0.016, r = 0.398), 4 (U = 196.500, z = −2.530, p = 0.044, r = 0.351) and 8 days post-SRC (U = 185.500, z = −2.791, p = 0.020, r = 0.388) (). Males were more compliant with the exercise protocol (male; 90.00 (70.00–100.00), female; 72.73 (54.55–80.00), U = 195.500, z = −2.563, p = 0.010, r = 0.355).
Table 4. Change in scores on battery tests for males and females presented as medians and inter-quartile ranges (male n = 30, female n = 22).
When considering the influence of sex on recovery between EEI and EET, there was a general trend for females and those with exercise intolerance to perform worse or report more symptoms on the PCSS and VOMS, except for visual memory within 48 h where males performed worse. Significant interaction was seen on PCSS within 48 h (χ2 (2) = 43.403, p < 0.001) and at 14-days post-SRC (χ2 (2) = 18.221, p < 0.001). Significant interaction was seen on neurocognitive testing (verbal memory; within 48 h χ2 (2) = 3.954, p = 0.007 and 14 days; χ2 (2) = 9.648, p = 0.008, visual memory; within 48 h; χ2 (Citation2) = 5.091, p = 0.002, motor processing speed; 8 days; χ2 (2) = 15.116, p < 0.001 and 14-days; χ2 (2) = 9.045, p = 0.011, reaction time; 4 days; χ2 (2) = 10.155, p = 0.006, 8 days; χ2 (2) = 17.398, p < 0.001 and 14-days; χ2 (2) = 22.682, p < 0.001). Academic ability and attendance between those with and without EET was also affected by sex within 48 h (PAIT2; χ2 (2) = 9.511, p = 0.009, non-contact academic ability; χ2 (2) = 9.551, p = 0.008, contact academic ability; χ2 (2) = 8.083, p = 0.018) and 4 days post-SRC (non-contact academic ability; χ2 (2) = 14.486, p < 0.001, contact academic ability; χ2 (2) = 8.481, p = 0.014). Interactions of sex were also seen on VOM function within 48 hours (NPC; χ2 (2) = 8.302, p = 0.016, VOMS; χ2 (2) = 19.733, p < 0.001), 4 (NPC; χ2 (2) = 8.709, p = 0.013, VOMS; χ2 (2) = 7.701, p = 0.021) and 14-days post-SRC (NPC; χ2 (2) = 29.360, p < 0.001, VOMS; χ2 (2) = 16.938, p < 0.001).
Secondary aims; effect of neck pain and vestibular-ocular-motor dysfunction
Student-athletes with VOM dysfunction at 4 days post-SRC (therefore assessed on the BCBT vs the BCTT) reported greater symptom burdens (BCBT; 8.00 (0.25–15.00), BCTT; 0.00 (−3.50–5.00), U = 135.500,z = −2.747, p = 0.024, r = 0.381) and RTP was slower (BCBT; 30.00 (21.00–42.00), BCTT; 22.00 (20.00–24.50), U = 129.000,z = −2.654, p = 0.032, r = 0.368) (). Presence of VOM dysfunction at 8 days was also associated with slower RTP time (BCBT; 30.00 (22.00–41.50), BCTT; 22.00 (20.0–24.00), U = 23.000, z = −3.274, p < 0.001, r = 0.454) (). The presence of neck pain did not significantly affect RTP or any outcome measure.
Secondary aims; effect of exercise compliance
Compliance to the SSHeRT was 78.05 ± 17.45% (median; 78.89 (70.00–90.91), with 7.94 ± 1.92 (median; 8.00 (7.00–9.00) sessions completed (maximum number of nine sessions). Those that were compliant had better performance on NPC within 48 h (compliant; −0.60 (−2.10–0.50), not compliant; 3.40 (0.70–4.40), U = 46.000,z = −2.992, p = 0.018). Compliance had no effect on change in performance on any other outcome measure at any time point.
Secondary aims; effect of severity of symptoms
Analysis was only conducted for within 48 h and day 4 as all but one participant was able to undergo exercise tolerance testing by day 8. Of the nine unable to initially undergo exercise tolerance testing by day 4, three were still unable to test, four were EEI and two were EET. By day 8, one remained unable to test, one was EEI and one was EET. The student-athlete unable to exercise test at day 8 was EEI at day 14 (). Those who were unable to undergo exercise testing within 48 h took 17.5 days longer to RTP (unable; 40.00 (31.00–50.75), able; 22.50 (20.00–27.50), U = 53.500,z = −3.124, p = 0.001) and 14-days longer to RTP if unable to test at day 4 (unable; 34.00 (21.00–41.00), able; 20.00 (20.00–29.50), U = 16.00,z = −2.255, p = 0.020) (). Inability to exercise test was associated with a greater symptom burden (within 48 h; unable; 38.50 (30.25–51.00), able; 5.50 (2.00–12.00), U = 20.500,z = −3.948, p < 0.001, day 4; unable; 19.00 (0.00–23.00), able; 0.00 (−2.00–9.00), U = 8.00,z = −2.576, p < 0.024) (). Worse performance was seen on NPC within 48 h (unable; 2.35 (0.10–4.23), able; −0.65 (−2.10–0.50) U = 62.000,z = −2.893, p = 0.003) and greater VOMS scores within 48 h and 4 days (within 48 h; unable; 14.00 (6.50–24.50), able; 2.00 (0.00–5.75), U = 59.000,z = −3.023, p = 0.002, day 4; unable; 3.00 (0.00–14.00), able; −0.35 (−2.00–1.48), U = 9.000,z = −2.757, p = 0.012) ().
Discussion
EEI was seen in university-aged student-athletes throughout the 14-day post-SRC reassessment period, with 25% remaining exercise intolerant at 14-days post-SRC. Because of this and due to the association of EEI with a delayed recovery of symptom burden, neurocognitive function, academic ability, VOM function and RTP seen in this current study, management of concussed student-athletes should include an assessment of exercise tolerance. Furthermore, student-athletes with EEI reported poorer perceived academic ability at 8 and 14 days on PAIT2 compared to scores at 4 days post-SRC. This suggests student-athletes with EEI still at 8 and 14 days may have greater academic impairment. It has been suggested exercise may be associated with improvements in cognitive function (Citation36) and therefore, controlled early exercise may be an effective way to improve recovery of academic ability. A longer RTP time was associated with inability to exercise at higher intensities at 8 and 14 days post-SRC and greater symptoms burdens when undergoing exercise intolerance testing at day 4 and 8. These factors may help clinicians identify student-athletes who may take longer to RTP and assist with setting expectations of athletes and coaches. The results of this study suggest EEI is associated with delayed recovery and time to RTP, therefore, clinicians should consider adding exercise tolerance testing to their assessments prior to commencing the GRTP and to identify university-aged student-athletes who may take longer to recover and RTP. Previous studies have demonstrated controlled early exercise may improve recovery following an SRC (Citation11). Therefore, clinicians may consider using the BCBT and BCTT to identify student-athletes with EEI, and use the SSHeRT to manage EEI student-athletes to possibly improve the time taken to recover and RTP.
Other studies assessing the effects of EEI on recovery and time to RTP have recruited adolescents (Citation9,Citation20,Citation22,Citation23,Citation40). All studies have found EEI, or a measure reflecting exercise tolerance testing, is associated with delayed recovery and RTP. Orr et al. (Citation23) are the only other study to assess the association of greater symptom burden and EEI. Orr et al. (Citation23) reported those with EEI reported greater symptom burdens when assessed between 5 and 7 days post-SRC. The current study did not find a significant difference in symptom burden between the EEI and EET group but did at 48 h when comparing all three groups (EET, EEI and those unable to undergo exercise tolerance testing). Student-athletes in the unable to undergo exercise tolerate testing group had a symptom burden severity which meant it was not safe to assess their exercise tolerance and this was associated with a median of 17.5 days longer to RTP. Therefore, the results of this study corroborate the findings of Orr et al. (Citation23). The study by Orr et al. (Citation23) also found RTP was much longer in those with EEI (45.5 days). Orr et al. (Citation23) recruited participants from a hospital, where 38% had been admitted for observation overnight and 54% required brain imaging. No student-athletes in this study were admitted to hospital or required brain imaging. It is likely participants in Orr et al. (Citation23) had more severe brain injuries than those included in this study. The results from the current study indicate EEI and an inability to undergo exercise tolerance could be used by clinicians to detect student-athletes who may take longer to RTP. Other factors that may indicate a longer RTP could be, greater overall symptoms at the start and end of, and greater symptom score increases, during exercise tolerance testing, as well as lower maximal heart rates at the end of testing. These factors may help clinicians to recognize those who may be slower to recover and provide realistic expectations to athletes.
Previous studies investigating the effects of exercise tolerance on recovery from SRC have not considered the influence other dysfunctions may have (Citation41). The presence of neck pain had no significant effect in the current study but VOM dysfunction did affect some results. This is the first study to use the BCBT in an attempt to limit VOM dysfunction affecting results of early exercise tolerance testing. The results presented in the current study may be more conservative than previously published (Citation20,Citation23), as greater symptoms exacerbation on the exercise tolerance testing may have been seen if those with VOM dysfunction were assessed on the BCTT. Initial results demonstrate using VOMS to decide whether to use either the BCBT or BCTT to assess exercise tolerance could be utilized by clinicians to more appropriately indirectly assess ANS dysfunction and manage student-athletes. Furthermore, not identifying VOM dysfunction and initiating rehabilitation has been shown to delay recovery (Citation42). Assessing exercise tolerance testing on a bike may dampen the effects of VOM dysfunction on exercise tolerance testing but clinicians should consider that those not conditioned to exercising on a bike may not tolerate the testing procedure as well as those accustomed to it (Citation43).
No previous studies have analyzed the possible effect of sex on recovery of those with and without EEI. Previous research has shown females may take longer to recover from SRC (Citation44). Results from this study suggest sex interacted with recovery on all outcome measures at some time point. The reasons for this are not yet known, though factors such as reduced neck strength (Citation45) resulting in increased injury severity from similar levels of force to the head (Citation46,Citation47) may contribute. Additionally, females may be more likely to report symptoms than males (Citation38,Citation46,Citation48–50) and the current study found females reported more symptoms than males. Results from the current study suggest clinicians may want to consider how sex may affect recovery and alter management and rehabilitation planning accordingly.
The current study found only ImPACT’s reaction time was significantly different between those with and without EET at 4 days post-SRC. No other study has compared neurocognitive function between those with and without EEI. The current study found there was a significant interactions between change on many of the ImPACT’s composites and time to RTP when comparing student-athletes with EEI and EET. This association indicates neurocognitive impairment may be associated with exercise intolerance, and interaction between this may delay RTP. This association may help clinicians to identify student-athletes who may take longer to RTP. The reliability and sensitivity of neurocognitive tests remains a contentious topic within the field (Citation51). One should consider current neurocognitive testing methods may not be sensitive enough to detect subtle changes and many other factors such as stress and lack of sleep can influence performance (Citation39). Therefore, the results in this study may also reflect other factors.
Compliance with completing the exercise program on non-reassessment days (day 3, 5, 6, 7, 9, 10, 11, 12 and 13 post-SRC) was associated with better performance on NPC within 48 h. A possible explanation is that those with NPC felt less confident to exercise following an SRC. The current guidelines have recommended physical rest for 14 days following as SRC to avoid worsening symptoms and delaying recovery (Citation37). It is possible this novel approach to recovery is unfamiliar to the current cohort and not widely accepted. Additionally, females were less compliant than males (females; 72.73 (54.55–80.00), males; 90.00 (70.00–100.00)). Student-athletes recruited to this study would have previously been managed with 14-days physical rest. Studies have shown females are less likely to take risks (Citation52) and it is possible that they may not have wanted to participate in novel treatment methods.
Limitations
The degree of exercise compliance varied between student-athletes, and this may have resulted in some student-athletes gaining greater possible benefits from a controlled early exercise program. The sample sizes for student-athletes with EEI and EET were small; however, the sample size met the number needed to treat which indicates the results were reliable (Citation53,Citation54). It cannot be assumed VOM function was not required when using the bike, looking around, focusing on the WattBike screen will have required processing from the VOM system, therefore, using the BCBT can only be assumed to minimize VOM demands. Steps were taken to ensure VOM rehabilitation was standardized, but specifics of this and any cervicogenic treatment were not recorded. VOM rehabilitation and manual therapy (Citation55) has been shown to improve recovery and may have affected the results on this study. The PAIT2 is novel and has limited ability to detect academic impairment beyond 4 days. Therefore, some student-athletes may have had academic impairment that was not detected.
Conclusions
EEI was prevalent amongst university-aged student-athletes up to and including assessment at 14-days post-SRC. Results from this study indicate clinicians may be able to use exercise intolerance testing to identify student-athletes who may take longer to recover based upon if EEI is present or exercise tolerance cannot be assessed due to a symptom on the PCSS rated at a 5 or higher. If EEI is still present at 14 days post-SRC, results from this study suggest RTP may take significantly longer, and this may help with setting expectations for the athlete and the wider support team. Therefore, clinicians should consider implementing exercise tolerance testing into their assessments to identify and appropriately managed concussed university-aged student-athletes.
Supplemental Material
Download MS Word (1.6 MB)Acknowledgments
The authors would like to thank the Musculoskeletal Association of Chartered Physiotherapists and Association of Chartered Physiotherapist in Sports and Exercise Medicine for their kind research grants which have supported the undertaking of this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02699052.2024.2367477
Additional information
Funding
References
- Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014 [cited 2022 Jun 15];75(Supplement 4):S24–33. doi:10.1227/NEU.0000000000000505.
- Patricios JS, Schneider KJ, Dvorak J, Ahmed OH, Blauwet C, Cantu RC, Davis GA, Echemendia RJ, Makdissi M, McNamee M, et al. Consensus statement on concussion in sport: the 6th international conference on concussion in sport–Amsterdam, October 2022. Br J Sports Med. 2023;57(11):695–711. doi:10.1136/bjsports-2023-106898.
- Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi:10.1016/j.expneurol.2012.01.013.
- Ellis MJ, Leddy JJ, Willer B. Physiological, vestibulo-ocular and cervicogenic post-concussion disorders: an evidence-based classification system with directions for treatment. Brain Inj. 2015;29(2):238–48. doi:10.3109/02699052.2014.965207.
- Giza C, Greco T, Prins ML. Concussion: pathophysiology and clinical translation. Handb Clin Neurol. 2018;158:51–61.
- Pertab JL, Merkley TL, Cramond AJ, Cramond K, Paxton H, Wu T. Concussion and the autonomic nervous system: an introduction to the field and the results of a systematic review. NeuroRehabilitation. 2018;42(4):397–427. doi:10.3233/NRE-172298.
- Laskowski RA, Creed JA, Raghupathi R. Brain Neurotrauma: Mol Neuropsychological Rehabil Aspects, Kobeissy FH, editor. Boca Raton (FL): CRC Press/Taylor & Francis. 2015:35–42. ( Frontiers in Neuroengineering).
- La Fountaine MF, Hohn AN, Testa AJ, Weir JP. Attenuation of spontaneous baroreceptor sensitivity after concussion. Med Sci Sports Exccise. [2019 Apr 1];51(4):792–97. doi:10.1249/MSS.0000000000001833.
- Leddy JJ, Hinds AL, Miecznikowski J, Darling S, Matuszak J, Baker JG, Picano, J. Willer, B. Safety and prognostic utility of provocative exercise testing in acutely concussed adolescents: a randomized trial. Clin J Sport Med. 2018;28(1):13–20. doi:10.1097/JSM.0000000000000431.
- Leddy JJ, Baker JG, Haider MN, Hinds A, Willer B. A physiological approach to prolonged recovery from sport-related concussion. J Athl Train. 2017;52(3):299–308. doi:10.4085/1062-6050-51.11.08.
- Leddy JJ, Burma JS, Toomey CM, Hayden A, Davis GA, Babl FE, Gagnon I, Giza CC, Kurowski BG, Silverberg ND, et al. Rest and exercise early after sport-related concussion: a systematic review and meta-analysis. Br J Sports Med. 2023;57(12):762–70. doi:10.1136/bjsports-2022-106676.
- Leddy JJ, Cox JL, Baker JG, Wack DS, Pendergast DR, Zivadinov R, Willer, B. Exercise treatment for postconcussion syndrome: a pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. J Head Trauma Rehabil. 2013 Jul;28(4):241–49. doi:10.1097/HTR.0b013e31826da964.
- Miranda NA, Boris JR, Kouvel KM, Stiles L. Activity and exercise intolerance after concussion: identification and management of postural orthostatic tachycardia syndrome. J Neurol Phys Ther. 2018;42(3):163–71. doi:10.1097/NPT.0000000000000231.
- Clausen M, Pendergast DR, Willer B, Leddy JJ. Cerebral blood flow during treadmill exercise is a marker of physiological postconcussion syndrome in female athletes. J Head Trauma Rehabil. 2016;31(3):215–24. doi:10.1097/HTR.0000000000000145.
- Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exccise. 2004;36(8):1269–74. doi:10.1249/01.MSS.0000135787.73757.4D.
- Gall B, Parkhouse W, Goodman D. Exercise following a sport induced concussion. Br J Sports Med. 2004;38(6):773–77. doi:10.1136/bjsm.2003.009530.
- Harrison A, Lane-Cordova A, La Fountaine MF, Moore RD. Concussion history and heart rate variability during bouts of acute stress. J Athl Train. 2022;57(8):741–47. doi:10.4085/1062-6050-0314.21.
- Howell DR, Hunt DL, Aaron SE, Hamner JW, Meehan WP, Tan CO. Association of hemodynamic and cerebrovascular responses to exercise with symptom severity in adolescents and young adults with concussion. Neurology. 2021 30;97(22):e2204–12. doi:10.1212/WNL.0000000000012929.
- Kozlowski KF, Graham J, Leddy JJ, Devinney-Boymel L, Willer BS. Exercise intolerance in individuals with postconcussion syndrome. J Athl Train. 2013 Sep;48(5):627–35. doi:10.4085/1062-6050-48.5.02.
- Worts PR, Mason JR, Burkhart SO, Sanchez-Gonzalez MA, Kim J-S. The acute, systemic effects of aerobic exercise in recently concussed adolescent student-athletes: preliminary findings. Eur J Appl Physiol. 2022 Jun 18;122(6):1441–57. doi:10.1007/s00421-022-04932-4.
- Haider MN, Johnson SL, Mannix R, Macfarlane AJ, Constantino D, Johnson BD, Willer B, Leddy J. The buffalo concussion bike test for concussion assessment in adolescents. Sport Heal A Multidiscip Approach. 2019 Nov 5;11(6):492–97. doi:10.1177/1941738119870189.
- Lalji R, Hincapié CA, Macpherson A, Howitt S, Marshall C, Tamim H. Association between first attempt buffalo concussion treadmill test and days to recovery in 855 children with sport-related concussion: a historical cohort study and prognostic factors analysis. Clin J Sport Med. 2023 Sep 7;33(5):505–11. doi:10.1097/JSM.0000000000001134.
- Orr R, Bogg T, Fyffe A, Lam LT, Browne GJ. Graded exercise testing predicts recovery trajectory of concussion in children and adolescents. Clin J Sport Med Off J Can Acad Sport Med. 2018;31(1):23–30. doi:10.1097/JSM.0000000000000683.
- Polak P, Leddy JJ, Dwyer MG, Willer B, Zivadinov R. Diffusion tensor imaging alterations in patients with postconcussion syndrome undergoing exercise treatment: A pilot longitudinal study. J Head Trauma Rehabil. 2015 Mar 1;30(2):E32–42. doi:10.1097/HTR.0000000000000037.
- Leddy JJ, Wilber CG, Willer BS. Active recovery from concussion. Curr Opin Neurol. 2018;31(September):1. doi:10.1097/WCO.0000000000000611.
- Tahouni Y, Cheng T, Lajewski S, Benz J, Bonten C, Wood D, Menges A. Codesign of biobased cellulose-filled filaments and mesostructures for 4d printing humidity responsive smart structures. 3D Print Addit Manuf. 2023 Feb 1;10(1):1–14. doi:10.1089/3dp.2022.0061.
- Schneider KJ. Concussion – part I: the need for a multifaceted assessment. Musculoskelet Sci Pract. 2019 Jul 1;42:140–50. doi:10.1016/j.msksp.2019.05.007.
- Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, DeWolf RM, Marchetti G, Kontos AP. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479–86. doi:10.1177/0363546514543775.
- Schatz P, Pardini JE, Lovell MR, Collins MW, Podell K. Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91‐99. doi:10.1016/j.acn.2005.08.001.
- Iverson GL, Lovell MR, Collins MW, Lovell MR, C MW, Iverson GL. Interpreting change on ImPACT following sport concussion. Clin Neuropsychol. 2003 Nov 9 [cited 2019 Mar 19];17(4):460–67. doi:10.1076/clin.17.4.460.27934.
- Lovell MR, Iverson GL, Collins MW. Validity of ImPACT for measuring processing speed following sports-related concussion. J Clin Exp Neuropsychol. 2005 Aug;27(6):683–89. doi:10.1081/13803390490918435.
- Glendon K, Pain MTG, Belli A, Blenkinsop G. The revised perceived academic impact tool (PAIT2): a tool to assess academic dysfunction in university-aged student-athletes with Sports-related concussion (SRC). J Eur Sport Sci. 2023; 24(5):537–48. ahead of p. doi:10.1002/ejsc.12051.
- Glendon K, Desai A, Blenkinsop G, Belli A, Pain M. Recovery of symptoms, neurocognitive and vestibular-ocular-motor function and academic ability after sports-related concussion (SRC) in university-aged student-athletes: a systematic review. Brain Inj. 2022 Mar;36(4):455–68. doi:10.1080/02699052.2022.2051740.
- Glendon K, Blenkinsop G, Belli A, Pain M. Does vestibular-ocular-motor (VOM) impairment affect time to return to play, symptom severity, neurocognition and academic ability in Student-athletes following acute concussion? Brain Inj. 2021;35(7):788–97. doi:10.1080/02699052.2021.1911001.
- Wasserman EB, Bazarian JJ, Mapstone M, Block R, Van Wijngaarden E. Academic dysfunction after a concussion among us high school and college students. Am J Public Health. 2016;106(7):1247–53. http://www.ncbi.nlm.nih.gov/pubmed/27196651.
- Leddy JJ, Haider MN, Ellis MJ, Willer BS. Exercise is medicine for concussion. Curr Sports Med Rep. 2018;17(8):262–70. doi:10.1249/JSR.0000000000000505.
- England Rugby. Adult Concussion Mnagement Guidelines. Adult Concussion Management Guidelines.pdf. 2019 [cited 2020 June 18]; Available from. https://www.englandrugby.com/b60e6e31-e1cd-4d88-a268-4b6e76fb6593//dxdam/86/86c7a5b7-e65a-4f58-ae9c-3c3c1449b519/HEADCASE.
- Makdissi M, Davis G, Jordan B, Patricios J, Purcell L, Putukian M. Revisiting the modifiers: how should the evaluation and management of acute concussions differ in specific groups? Br J Sports Med. 2013 Apr 11;47(5):314–20. doi:10.1136/bjsports-2013-092256.
- Gaudet CE, Weyandt LL. Immediate post-concussion and cognitive testing (ImPACT): a systematic review of the prevalence and assessment of invalid performance. Clin Neuropsychol. 2017;31(1):43–58. doi:10.1080/13854046.2016.1220622.
- Haider MN, Leddy JJ, Wilber CG, Viera KB, Bezherano I, Wilkins KJ, Miecznikowski JC, Willer BS. The predictive capacity of the buffalo concussion treadmill test after sport-related concussion in adolescents. Front Neurol. 2019;10(APR):395. doi:10.3389/fneur.2019.00395.
- Quatman-Yates C, Bailes A, Constand S, Sroka MC, Nissen K, Kurowski B, Hugentobler J. Exertional tolerance assessments after mild traumatic brain injury: a systematic review. Archiv Phy Med Rehabil. 2018 May;99(5):994–1010. doi:10.1016/j.apmr.2017.11.012.
- Park K, Ksiazek T, Olson B, Park Tkbo K, Park K, Ksiazek T. Effectiveness of vestibular rehabilitation therapy for treatment of concussed adolescents with persistent symptoms of dizziness and imbalance. J Sport Rehabil. 2018;27(5):485–90. doi:10.1123/jsr.2016-0222.
- Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016 Jun 14;133(24):e694–711. doi:10.1161/CIR.0000000000000406.
- Dave U, Kinderknecht J, Cheng J, Santiago K, Jivanelli B, Ling DI. Systematic review and meta-analysis of sex-based differences for concussion incidence in soccer. Phys Sportsmed. 2022;50(1):11–19. doi:10.1080/00913847.2020.1868955.
- Caccese JB, Buckley TA, Tierney RT, Rose WC, Glutting JJ, Kaminski TW. Sex and age differences in head acceleration during purposeful soccer heading. Res Sport Med. 2018;26(1):64–74. doi:10.1080/15438627.2017.1393756.
- Ono KE, Burns TG, Bearden DJ, Sm M, King H, Reisner A. Sex-based differences as a predictor of recovery trajectories in young athletes after a sports-related concussion. Am J Sports Med. 2016;44(3):748–52. doi:10.1177/0363546515617746.
- Schallmo MS, Weiner JA, Hsu WK. Sport and sex-specific reporting trends in the epidemiology of concussions sustained by high school athletes. J Bone Joint Surg Am. 2017;99(15):1314–20. doi:10.2106/JBJS.16.01573.
- Covassin T, Elbin RJ. The female athlete: the role of gender in the assessment and management of sport-related concussion. Clin Sports Med. 2011;30(1):125–31. doi:10.1016/j.csm.2010.08.001.
- Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Halstead M, Herring SA, Kutcher JS, Pana A, Putukian M, Roberts WO, et al. American medical society for sports medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15–26. doi:10.1136/bjsports-2012-091941.
- Daneshvar DH, Nowinski CJ, Mckee AC, Cantu RC. The epidemiology of sport-related concussion. Clin Sports Med. 2011 Jan;30(1):1–17. doi:10.1016/j.csm.2010.08.006.
- Czerniak LL, Liebel SW, G-GPGP G, Lavieri MS, McCrea MA, McAllister TW, Broglio SP. Sensitivity and Specificity of computer-based neurocognitive tests in sport-related concussion: Findings from the NCAA-DoD CARE consortium. Sport Med. 2021;51(2):351–65. doi:10.1007/s40279-020-01393-7.
- Reniers RLEP, Murphy L, Lin A, Bartolomé SP, Wood SJ, Ginsberg SD. Risk perception and risk-taking behaviour during adolescence: the influence of personality and gender. Ginsberg SD, editor. PLOS ONE. 2016;11(4):e0153842. doi:10.1371/journal.pone.0153842.
- Lal A, Kolakowsky-Hayner SA, Ghajar J, Balamane M. The effect of physical exercise after a concussion a systematic review and meta-analysis. Am J Sports Med. 2018 Mar 1;46(3):743–52. doi:10.1177/0363546517706137.
- Kontos AP, Collins MW, Holland CL, Reeves VL, Edelman K, Benso S, Schneider W, Okonkwo D. Preliminary evidence for improvement in symptoms, cognitive, vestibular, and oculomotor outcomes following targeted intervention with chronic mTBI patients. Mil Med. 2018;183(suppl_1):333–38. doi:10.1093/milmed/usx172.
- Brown L, Camarinos J. The role of physical therapy in concussion rehabilitation. Semin Pediatr Neurol. [2019 Jul 1];30:68–78. doi:10.1016/j.spen.2019.03.011.
