ABSTRACT
It is well known that relative to neutral stimuli, attention is biased towards processing stimuli that convey threat. In a previous study in which a particular stimulus (e.g. a blue diamond) was associated with the delivery of an electrical shock, the presence of the fear-conditioned stimulus interfered with the execution of voluntary eye movements to other locations. Here, we show that this effect not only occurs early in time, but remains present long after the fear-conditioned stimulus was removed from the screen. In a subsequent experiment, we associated the presence of a particular stimulus with safety, that is, when this stimulus was present it was certain that no electrical shock would be delivered. The presence of the safety signalling stimulus also interfered with the execution of voluntary saccades, but only when the time between stimulus and cue presentation was relatively long. The results indicate that both signals of threat and signals of safety interfere with execution of a saccade long after the source of threat or safety has been removed. However, only threatening stimuli affect saccade execution early in time, suggesting that threatening stimuli drive selection exogenously.
KEYWORDS:
Fear is an adaptive behavioural response, allowing us to react and adjust to potential threat. Fast identification of potentially threatening cues may have several evolutionary advantages (LeDoux, Citation1996). Since a quick detection of threat is assumed to reduce the risk of being harmed, automatic orienting to threat seems highly significant for an organism’s survival. Quick detection of threat is suggested to prepare the organism to take action and to cope with a potentially dangerous situation. It has been argued that the visual system is biased towards quick and relatively automatic processing of threat (Esteves, Dimberg, & Öhman, Citation1994; LeDoux, Citation1996; Öhman, Citation1986). For example, it has been shown that pictures of threatening events, such as spiders and angry faces, are usually detected more quickly compared to neutral pictures (e.g. Calvo, Avero, & Lundqvist, Citation2006; Fox et al., Citation2000; Pitica, Susa, Benga, & Miclea, Citation2012; Öhman, Flykt, & Esteves, Citation2001; Soares, Esteves, Lundqvist, & Öhman, Citation2009).
Potentially threatening situations are often signalled by cues, for example, a sound that announces the presence of a predator. A state of fear can be elicited by a cue when there is a contingency between the cue and a potentially dangerous outcome (e.g. Rescorla & Solomon, Citation1967). When the same conditioned cues are encountered in the future, defensive responses in anticipation of the predator’s attack could help an organism to increase chances of survival. Associative learning between a cue and an outcome has been widely used to induce fear in laboratory animals (e.g. Bekhterev, Citation1913; Kim & Jung, Citation2006; Pape & Pare, Citation2010; Pape & Stork, Citation2003), and has also long been proven effective in humans (e.g. Fendt & Fanselow, Citation1999; LaBar, Gatenby, Gore, LeDoux, & Phelps, Citation1998; Sehlmeyer et al., Citation2009; Watson & Rayner, Citation1920). In a classic fear-conditioning paradigm, an initially neutral stimulus (CS) such as a sound is associated with an aversive unconditioned stimulus (US) such as an electrical shock, while another neutral stimulus remains unpaired (CS−) (Maren, Citation2001; Pavlov, Citation1927). After conditioning, the CS acquires aversive properties and will elicit responses that are usually caused by threatening stimuli, such as increased heart rate, and becomes a conditioned stimulus (CS+).
Several studies have used classical fear conditioning to examine if threatening stimuli affect allocation of spatial attention. Relative to pictorial threatening stimuli, associating neutral stimuli with fear has the advantage that threatening and non-threatening stimuli can be perfectly matched for differences in low-level features such as luminance and complexity. Most studies involving fear conditioning have used variations of Posner’s (Citation1980) exogenous cueing task. In this task, a non-predictive peripheral stimulus (cue) is followed by the presentation of a target displayed either at the same location as the cue (validly cued) or at the opposite location (invalidly cued). When a location is cued, manual responses to targets appearing at the cued location are faster than responses to targets presented at non-cued locations. This occurs because attention is drawn to the location of the cue, thereby facilitating processing of subsequent information appearing at the same location. In studies that combine fear conditioning with spatial cueing one of the cues is usually paired with an aversive outcome, while the other is neutral. It has been shown that reaction times to targets preceded by a fear-conditioned cue (e.g. a visual stimulus paired with an aversive noise) are faster compared to a neutral cue, indicating facilitated engagement of attention to fear-conditioned cues (Koster, Crombez, Van Damme, Verschuere, & De Houwer, Citation2005). Moreover, response times to invalidly cued targets were slower for fear-conditioned compared to neutral cues, indicating that threatening cues delay disengagement of attention (Koster, Crombez, Van Damme, Verschuere, & De Houwer, Citation2004). Facilitation and delayed disengagement of attention to threatening cues were also demonstrated by Van Damme, Crombez, Hermans, Koster, and Eccleston (Citation2006), who used an electrical shock as US. In addition, it has been shown that threat cues facilitate directing attention to their locations compared to neutral signals (Van Damme et al., Citation2004). However, in that particular study, threat signals did not impair disengagement of attention from their location in comparison to neutral cues. Note that other studies failed to find facilitation of attention by threatening cues, but only found disengagement of attention from threat instead (Massar, Mol, Kenemans, & Baas, Citation2011; Van Damme, Crombez, & Notebaert, Citation2008).
Since the manual spatial cueing tasks described earlier presented only one cue at a time, the abrupt visual onset of the cue may have attracted attention due to its visual properties (e.g. a visual transient) and irrespective of its valence (see Yantis & Jonides, Citation1984). Simultaneous presentation of two equally salient cues may help overcome this problem (e.g. Schmidt, Belopolsky, & Theeuwes, Citation2012, Citation2015). So far, one study investigated the effects of fear-conditioned cues on attention when two cues of equal perceptual salience were presented simultaneously in a manual spatial cueing task (Armony & Dolan, Citation2002). Similar to studies using one cue, subjects were significantly slower in responding to the fear-conditioned target in invalidly cued trials than in validly cued ones.
In addition to measuring covert attention by registering manual response time, eye tracking has been often used to study the allocation of (overt) attention (Kowler, Anderson, Dosher, & Blaser, Citation1995; McPeek, Maljkovic, & Nakayama, Citation1999; Shepherd, Findlay, & Hockey, Citation1986). Indeed, the eyes usually follow the allocation of attention, as it has been claimed that an attentional shift is nothing more than a byproduct of saccade planning (Belopolsky & Theeuwes, Citation2012). As such, eye movements are considered a direct behavioural measure of the allocation of spatial attention (Henderson, Citation2003). The largest advantage of measuring eye movements is that it allows to track the allocation of attention as it unfolds over time on every trial. With eye movements it is not only possible to determine when and which object was attended, but also how long attention dwelled on it. All of this is difficult, or even impossible, when measuring only manual response times as this provides only one cumulative measure of all processes leading up to the button press. In addition, it might be that other factors rather than attention (e.g. response competition) are responsible for the effects in some studies using manual response times (Hunt, Cooper, Hungr, Kingstone, Citation2007). Overall, relative to manual responses, eye movements may provide higher temporal and spatial resolution for measuring visual selection. Quick attentional selection of a threatening object when presented along with a neutral object would be directly reflected in saccadic behaviour. If the threatening object wins the competition for selection over the neutral object, one would predict that saccades are executed more quickly towards threatening than towards neutral object.
So far, a few studies have used eye tracking to investigate the effects of fear-conditioned stimuli on attention. In a study by Mulckhuyse, Crombez, and Van der Stigchel (Citation2013), an initially neutral stimulus was paired with aversive noise during a classical fear-conditioning procedure. Afterwards, the fear-conditioned or neutral stimulus was presented as a task-irrelevant distractor together with a target stimulus. Participants had to make quick saccades to the target, while ignoring the distractor stimulus. The results showed that saccades with a long latency deviated more strongly away from the fear-conditioned than from the neutral distractor. Saccades with a short latency deviated more strongly towards the fear-conditioned than towards the neutral distractor. Since saccade trajectories have been shown to indicate the amount of spatial attention that is allocated to a certain location (Van der Stigchel, Meeter, & Theeuwes, Citation2006), the results suggest a stronger attentional capture by fear-conditioned stimuli as compared to neutral ones. In a recent study, Mulckhuyse and Dalmaijer (Citation2015) also demonstrated oculomotor capture by distractors that were previously associated with threat. Moreover, the presence of such distractors increased the latency of saccades to the target, suggesting that these distractors captured covert attention.
In a spatial cueing study by Schmidt et al. (Citation2015), two cues were briefly presented left and right from fixation. After 50 ms, an arrow appeared at fixation, indicating the direction in which a speeded saccade had to be executed. On some trials, a potentially threatening cue (CS+) was present, and these trials were sometimes followed by an electrical shock (US). Results showed that saccadic latencies to locations previously occupied by the CS+ were faster than saccades in the opposite direction. When CS+ trials were compared to trials with a neutral baseline, saccades towards the CS+ were faster than saccades on neutral trials, and saccades away from the CS+ were slower than saccades on neutral trials. The results indicated that the presence of a potentially threatening stimulus interfered with execution of a voluntary saccade, indicating that conditioned fear has a direct and fast influence on visual attention.
The goal of the present study was to replicate and extend the previous findings by investigating the time course of attentional allocation to signals of potential threat. Since detecting potential threat is of high behavioural relevance, it may be important to maintain attention at the location of the signal of threat, even after the source of threat is no longer present. In that case, saccades to locations previously occupied by a threatening stimulus may also be facilitated at longer stimulus onset asynchronies (SOAs). On the other hand, attention might be quickly withdrawn from the location of the signal of threat since there is no more useful information present at that location. Furthermore, it is also feasible that participants might avoid attending to locations occupied by the signals of threat (e.g. Mogg, Bradley, DeBono, & Painter, Citation1997; Mogg, Bradley, Miles, & Dixon, Citation2004).
In addition to cues that can be relevant to observers because of their threat value, cues may also be relevant because they convey safety information. In this sense, safety signals can be considered behaviourally relevant and may also produce an attentional orienting effect. Such an orienting response would require the allocation of attention both to identify the potential safety stimulus and to initiate the appropriate response. The visual system may process safety signals in a preferential way, since avoiding aversive outcomes is highly relevant for an organism that is confronted with a threatening situation. A high motivation to escape from threat may be reflected in an attentional bias to signals of safety, compared to signals conveying more ambiguous or no information. Therefore, another goal of the present study was to determine whether signals of safety have similar effects on attention as threatening stimuli.
Similar to Schmidt et al. (Citation2015), two cues were presented left and right from fixation. Before the experiment started, participants were informed that the presence of one of the cues could be followed by an electrical shock. A centrally presented arrow indicated the direction in which the participants had to make a speeded saccade. To map out the time course of the allocation of attention, in Experiment 1 we included a condition in which the arrow was presented 600 ms after cue offset. In Experiment 2, we extended this period up to 1000 ms. In Experiment 3, we examined whether signals of safety modulate attention in a similar way as signals of threat. Instead of being informed about the aversive outcome of one of the cues, participants were informed that the presence of one of the cues was never followed by a US. They were informed that the shock was randomly delivered on trials in which any other pair of cues was present.
In Schmidt et al. (Citation2015) we only employed a 50 ms interval between the cue and the arrow instructing participants to make a saccade. In that study, we observed fast orienting towards a fear-conditioned cue. The question in the present study was whether attention remained at the location of the fear-conditioned cue at longer intervals, even when the stimulus conveying this information (i.e. the fear-conditioned cue) is no longer visible. Alternatively, it is possible that following orienting towards the fear-conditioned cue, participants immediately disengage attention from that location. In that case, only at the 50 ms interval participants should be faster to make a saccade to the fear-conditioned cue than to the other location (i.e. faster saccade to validly cued location versus invalidly cued location) but not at the longer intervals of 600 and 1000 ms SOA. Indeed, as there is ample time to disengage attention from the fear-conditioned cue one expects no performance benefits for validly over invalidly cued locations.
Experiment 1
Method
Ethics statement
Written consent was obtained from each participant prior to the experiments. The experiments were approved by the ethics committee of the VU University.
Participants
Sixteen naïve students from VU University Amsterdam (11 females, mean age 23 ± 3) participated in return for course credits or cash. All participants reported having normal or corrected-to-normal vision.
US calibration
Conditioning started with calibration of the US. The US consisted of a 400 V electrical stimulus with duration of 2 ms and was delivered to the left ankle. Two electrocardiogram-electrodes were placed over the tibial nerve at the medial malleolus of the left ankle of participants. Electrodes were connected to a Digitimer DS7A constant current stimulator (Hertfordshire, UK), which is devised for percutaneous electrical stimulation of subjects in clinical and biomedical research settings. The intensity of the current was calibrated to an “unpleasant but painless” level for each participant individually. Starting at 16 mA, the current was increased stepwise with 4 mA, each time checking with the participant whether the “unpleasant, but painless” level had been reached. When the participant indicated that the stimulus was painful, the current was regulated down. The maximum amperage was 45 mA and calibration ended when this maximum was reached.
Eye tracking calibration
Participants were seated 75 cm from a computer screen with their head positioned on a chin rest. Eye movements of the right eye were recorded with an EyeLink 1000 tracker (SR Research Ltd, Canada), with 1000 Hz temporal and 0.2° spatial resolution. An automatic algorithm detected saccades using minimum velocity and acceleration criteria of 35°/s and 9500°/s2, respectively. During calibration, participants had to fixate nine calibration dots, presented randomly in a 3 × 3 grid across the monitor.
Stimuli, design and procedure
Stimuli were green (CIE (International Commission on Illumination)): x = .300, y = .600; 22.39 cd/m2), blue (CIE: x = .176, y = .155; 23.05 cd/m2), orange (CIE: x = .542, y = .407; 22.75 cd/m2) and magenta (CIE: x = .321, y = .154; 23.19 cd/m2) outline diamonds, with sides of 1.74°. Either the blue or orange diamond served as the CS+ and was paired with the US during the experiment (see ). Stimulus-US contingencies were counterbalanced across participants.
Figure 1. Experiment 1: Example of a trial in the experimental session. Two diamonds were presented left and right from fixation for 100 ms. In this example, the presence of an orange diamond indicated that a shock could be delivered. All colours could appear on the left or on the right with equal probability. After a 50 or 600 ms SOA, a centrally presented arrow pointed to the left or to the right. Participants had to make a speeded eye movement in that direction. On 7 of the CS+ trials, the US was presented after 1000 ms.
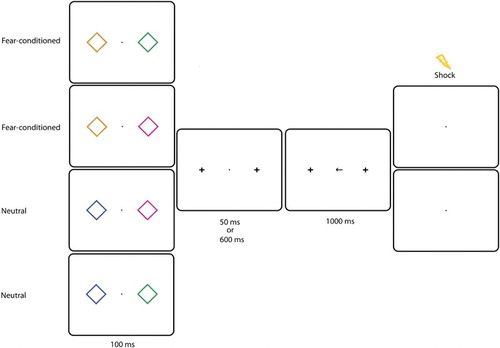
Each trial started with the presentation of a central fixation cross (0.5° × 0.5°) for a variable time (800–1300 ms), followed by presentation of two diamonds on the horizontal plane at 7° from fixation (see ). On half of the trials one diamond had the colour of the CS+ (e.g. orange), the other was green or magenta with an equal probability. On the other half of the trials two diamonds with a neutral colour (e.g. blue and green or blue and magenta) were presented. All colours could appear on the left or on the right with equal probability. After 100 ms, the diamonds were replaced by crosses (0.5° × 0.5°), which stayed on the screen throughout the trial. After a random SOA of 50 or 600 ms, the central fixation cross was replaced by an arrow which was pointing to the left or to the right with an equal probability (see ). Participants were instructed to make an eye movement as fast as possible to the location indicated by the arrow. If the saccadic latency exceeded 500 ms, or if the saccadic latency exceeded the average of the previous 10 trials, the text “too slow!” appeared on the screen (0.4° × 1.0°) for 300 ms. In addition, on seven trials in which the CS+ was present and the saccadic latency was too slow, the US was delivered together with the termination of the text. The probability of receiving a US on those trials was 80%, given that the maximum number of shocks had not been reached. All participants received a total number of seven shocks during the experiment. After the last shock had been delivered, there was no chance of receiving a shock anymore. To make sure that fear extinction did not occur directly after the maximum number of shocks had been reached, participants were not informed of the maximum number of shocks in the experiment.
The experiment started with a practice block of 36 trials, in which stimuli with different colours (red, turquoise and purple) were used. During the practice block, no USs were delivered. After the practice block, participants received verbal instructions about the experimental blocks. They were informed that they only had a chance of receiving a shock when a stimulus of a certain colour (i.e. the CS+) was present on the screen. It was emphasised that the shock could be delivered regardless of the location (left or right) of the CS+. Finally, it was emphasised that participants should be fast in order to avoid shocks. Moreover, participants were informed that they could only get a shock when the eye movement latency was slower than the average latency of their previous 10 eye movements. This manipulation ensured that the participants had to make fast saccades on all trials, irrespective of the types of stimuli that were present.
The experimental session consisted of 4 blocks of 36 trials. Half of the trials contained the fear-conditioned stimulus, the other half contained neutral stimuli (see ). Each block was followed by feedback on speed and accuracy.
After finishing the experimental session, each stimulus was centrally presented on the screen, and participants had to indicate for each stimulus how fearful they were when that stimulus was presented during the experiment. A scale that was labelled from “not at all fearful” (1) to “very fearful” (7) was presented at the bottom of the screen. Participants rated each of the four stimuli by pressing a number from 1 to 7 on the keyboard.
Results and discussion
Trials with saccades faster than 80 ms and slower than 500 ms and saccades that did not start within 1° from the fixation point were excluded from further analyses. This resulted in an average loss of 5.2% of the trials. Saccades that landed within 15° of arc from the centre of the location indicated by the arrow were classified as correct. Saccades that landed within 15° of arc from the centre of the location opposite from the location indicated by the arrow were classified as a landing on the wrong location. All other trials were classified as incorrect. Incorrect trials and trials that landed on the wrong location were excluded from analyses. This resulted in an average loss of 0.9% of the trials. Planned comparisons indicated that participants did not make more errors on invalid (0.6%) than on valid (0.8%) fear-conditioned trials in the 50 ms (t(15) = 0.324, p = .75, d = .13) nor in the 600 ms condition (2.0% vs. 1.0%; t(15) = 0.845, p = .41, d = .30).
Mean saccadic latencies for each condition are shown in . For the fear-conditioned condition, a 2 × 2 repeated measurements analysis of variance (ANOVA) on saccadic latencies with SOA (50 and 600 ms) and validity (valid and invalid) as factors revealed that there was a main effect of validity, F(1,15) = 9.941, p < .001, = .40, as well as a main effect of SOA, F(1,15) = 50.260, p < .001,
= .77. Saccade latencies to validly cued locations were faster than saccades to invalidly cued locations (253 vs. 267 ms; t(15) = 3.153, p < .01, d = .44). Saccadic latencies in the 600 ms condition were faster than in the 50 ms condition (245 vs. 275 ms; t(15) = 7.089, p < .01, d = .95), which can be explained by a better response preparation when the time between stimulus and cue is long (foreperiod effect, e.g. Näätänen, Citation1971). There was no interaction effect of validity × SOA, F(1,15) = 0.989, p = .34,
= .062. Planned comparisons showed that there was a validity effect in the 50 ms condition: saccadic latencies to locations previously occupied by the CS+ were faster (267 ms) than saccadic latencies in the opposite direction (282 ms; t(15) = 3.208, p < .001, d = 0.44). There was also a validity effect in the 600 ms condition, (239 vs. 251 ms; t(15) = 2.551, p < .05, d = 0.37).
Figure 2. Experiment 1: Saccadic latencies per condition. In both the 50 and 600 ms SOA conditions, latencies on invalid (i.e. away from the CS+) fear-conditioned trials were slower compared to valid (i.e. towards the CS+) trials. Error bars reflect within-subject normalised standard errors (Loftus & Masson, Citation1994).
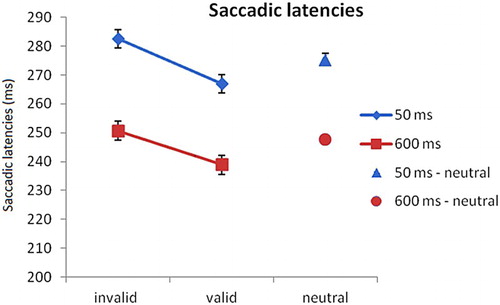
To examine whether the validity effect for the CS+ was driven by faster responses to valid locations, slower responses to invalid locations, or both, we compared saccadic latencies in the fear-conditioned condition to latencies in the neutral condition. For the 50 ms condition, results showed that saccades on valid CS+ trials were faster than saccades on neutral trials (267 vs. 275 ms; t(15) = 2.683, p < .05, d = 0.26). In addition, saccades on invalid CS+ trials (282 ms) were slower than saccades on neutral trials (t(15) = 2.375, p < .05, d = 0.21). For the 600 ms condition, trials in the valid condition were faster than in the neutral condition (239 vs. 248 ms, t(15) = 2.967, p < .01, d = .34). The trials in the invalid condition (250 ms) did not differ from the neutral condition, t(15) = 0.897, p = .38, d = .09.
Paired-samples t-tests on fear ratings showed that the CS+ was rated as more fearful than the neutral stimuli (all p’s < .001).
The results of the 50 ms SOA condition fully replicated our previous study (Schmidt et al., Citation2015). Importantly, the longer SOA 600 ms interval basically did not alter the effects as participants were still faster to execute a saccade to the location associated with the CS+ than to the other location. This may imply that attention remains engaged at the location that previously contained the stimulus that was associated with the CS+. Even though the cue associated with the CS+ was not visible for an interval of 600 ms, saccades to its location were executed faster compared to neutral stimuli. This implies that threatening stimuli not only elicit an immediate orienting of attention, but attention remains engaged at the location previously occupied by a threatening stimulus. To test whether these attentional effects were still present after an even longer interval, we used SOAs of 50 and 1000 ms in Experiment 2.
Experiment 2
Method
Participants
Sixteen naïve students from VU University Amsterdam (10 females, mean age 23 ± 3) participated in return for course credits or cash. None of the participants participated in Experiment 1. All participants reported having normal or corrected-to-normal vision.
Stimuli and procedure
US calibration, eye tracking calibration and stimuli were identical to Experiment 1. The procedure was also identical to Experiment 1, except that the SOA between termination of stimulus presentation and arrow presentation was now 50 or 1000 ms, with an equal probability. In Experiment 1, all neutral stimuli were presented together with the CS+ on half of the trials and could therefore have acquired fear value themselves (see Schmidt et al., Citation2015). To create a completely neutral baseline, in Experiment 2 the neutral condition consisted of two stimuli that were never presented together with the CS+ (see ).
Figure 3. Experiment 2: Example of a trial in the experimental session. Two diamonds were presented left and right from fixation for 100 ms. In this example, the presence of an orange diamond indicated that a shock could be delivered. All colours could appear on the left or on the right with equal probability. After a 50 or 1000 ms SOA, a centrally presented arrow pointed to the left or to the right. Participants had to make a speeded eye movement in that direction. On 7 of the CS+ trials, the US was presented after 1000 ms.
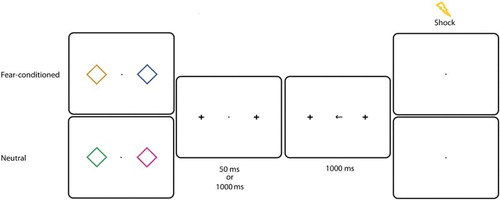
The experiment started with a practice block of 36 trials, followed by an experimental session consisting of 4 blocks of 36 trials. Half of the trials contained the fear-conditioned stimulus, the other half contained neutral stimuli. Each block was followed by feedback on speed and accuracy.
Results and discussion
Based on the same exclusion criteria as in Experiment 1, 3.1% of the trials were removed from further analyses. Planned comparisons indicated that participants did not make more errors on invalid (2.2%) than on valid (2.0%) fear-conditioned trials in the 50 ms condition (t(15) = 0.230, p = 0.82, d = .05), nor in the 1000 ms condition (1.5% vs. 0.6%; t(15) = 1.4286, p = 0.17, d = .47).
Mean saccadic latencies for each condition are shown in . For the fear-conditioned condition, a 2 × 2 repeated measurements ANOVA on saccadic latencies with SOA (50 and 1000 ms) and validity (valid and invalid) as factors revealed that there were main effects of validity (F(1,15) = 9.398, p < .01, = 0.39) and SOA (F(1,15) = 6.254, p < .05,
= 0.29). Saccades to validly cued locations were faster than saccades to invalidly cued locations (239 vs. 246 ms; t(15) = 3.066, p < .01, d = .18). Saccadic latencies in the 1000 ms condition were faster than in the 50 ms condition, (235 vs. 251 ms), t(15) = 2.500, p < .05, d = .39 (foreperiod effect, e.g. Näätänen, Citation1971). There was no interaction effect of validity x SOA, F(1,15) = 1.983, p = .18,
= .12. Replicating Experiment 1, planned comparisons showed that there was a validity effect in the 50 ms condition, with faster saccadic latencies to locations previously occupied by the CS+ (246 ms) compared to the opposite location (255 ms; t(15) = 3.166, p < .01, d = 0.20). In contrast, there was no validity effect in the 1000 ms condition (233 vs. 237 ms, t(15) = 1.418, p = .18, d = 0.11). For the 50 ms condition, trials in the valid condition (246 ms) did not differ from the neutral condition, t(15) = 0.597, p = .56, d = .05. Trials in the invalid condition were slower than in the neutral condition (255 vs. 248 ms, t(15) = 2.319, p < .05, d = .16). For the 1000 ms condition, trials in the valid condition (233 ms) were faster than the neutral condition, t(15) = 3.084, p < .01, d = .25. Trials in the invalid condition did not differ from the neutral condition (237 vs. 241 ms, t(15) = 1.126, p = .28, d = .11).
Figure 4. Experiment 2: Saccadic latencies per condition. In the 50 ms SOA condition, latencies on invalid (i.e. away from the CS+) fear-conditioned trials were slower compared to valid (i.e. towards the CS+) trials. Error bars reflect within-subject normalised standard errors (Loftus & Masson, Citation1994).
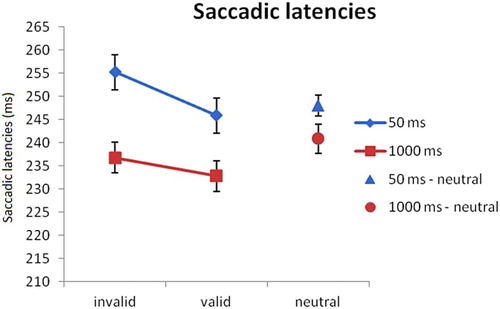
Similar to Experiment 1, we show a main effect of validity, indicating faster saccadic latencies towards locations previously occupied by the CS+ compared to the opposite location. The absence of the interaction indicates that the validity effect in the SOA 1000 ms was equally strong as in the SOA 50 ms condition.
Paired-samples t-tests on fear ratings showed that the CS+ was rated as more fearful than the neutral stimuli (all p’s < .001). The stimulus that was always presented together with the CS+ was rated as more fearful than the neutral stimuli (both p’s < .05).
The results of the long SOA conditions in Experiments 1 and 2 indicate that long after the source of threat has been removed, threat still interferes with execution of a saccade in the opposite direction. This suggests that the threatening location remains relevant to the observer after the initial automatic orienting towards that location. The continued engagement of attention at that location should be considered endogenous (top-down) in origin as there are no stimuli present anymore that can drive attention in an exogenous manner.
However, it is possible that other behaviourally relevant information can also affects eye movements. For example, avoiding an aversive outcome is crucial for an organism that is confronted with a threatening situation. A high motivation to avoid threat may be reflected in an attentional bias to signals of safety. Similar to this view, prior evidence has shown that a high motivation to avoid disgust-evoking stimuli causes attentional bias to stimuli conveying cleanliness (Vogt, Lozo, Koster, & De Houwer, Citation2011). Experiment 3 was designed to investigate whether stimuli that signal safety attract attention in a similar way as threatening stimuli do. We used a task that was similar to that of Experiment 1, except that the presence of one of the cues indicated that there was no chance of receiving an electrical shock. If the safety cue contains behaviourally relevant information comparable to the threatening cue in Experiments 1 and 2, we expect the safety cue to interfere with the voluntary execution of saccades.
Experiment 3
Method
Participants
Sixteen naïve students from VU University Amsterdam (12 females, mean age 21 ± 3) participated in return for course credits or cash. None of the participants participated in Experiment 1 or 2. All participants reported having normal or corrected-to-normal vision.
Stimuli and procedure
US calibration and eye tracking calibration were identical to Experiment 1. Either the blue or orange diamond served as the safety cue and was never paired with the US during the experiment (see ). Safety cues were counterbalanced across participants.
Figure 5. Experiment 3: Example of a trial in the experimental session. Two diamonds were presented left and right from fixation for 100 ms. In this example, the presence of an orange diamond indicated that no shock could be delivered. All colours could appear on the left or on the right with equal probability. After a 50 or 60 ms SOA, a centrally presented arrow pointed to the left or to the right. Participants had to make a speeded eye movement in that direction. On 7 of the “Threat” trials, the US was presented after 1000 ms.
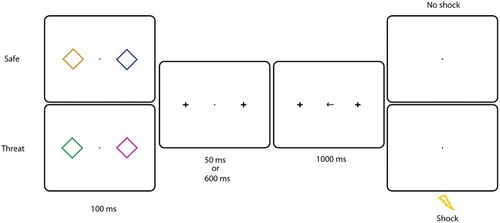
The procedure was identical to Experiment 2, except that the SOA between termination of stimulus presentation and arrow presentation was now 50 or 600 ms. There were two possible stimulus pairs: the safety cue together with a neutral stimulus (“safe” condition), and two neutral stimuli (“threat” condition, see ). All colours could appear on the left or on the right with an equal probability. In the threat condition, the US was delivered on 7 trials with a saccadic latency that was too slow. Participants were instructed that they could never receive a shock when a stimulus of a certain colour (i.e. the safety cue) was present on the screen.
The experiment started with a practice block of 36 trials, followed by an experimental session consisting of 4 blocks of 36 trials. Each condition appeared with an equal probability. Each block was followed by feedback on speed and accuracy.
Results and discussion
Based on the same exclusion criteria as in Experiment 1, 1.9% of the trials were removed from further analyses. Planned comparisons indicated that participants did not make more errors on invalid (2.9%) than on valid (2.1%) safety trials in the 50 ms condition (t(15) = 1.0742, p = 0.30, d = .17), nor in the 600 ms condition (1.0% vs. 2.0%; t(15) = 1.159, p = 0.26, d = .41).
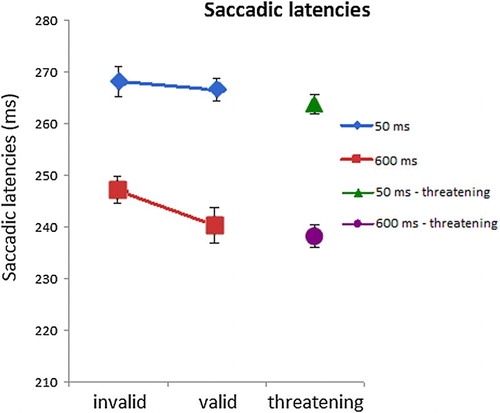
Mean saccadic latencies for each condition are shown in . For the safe condition, a 2 × 2 repeated measurements ANOVA on saccadic latencies with SOA (50 and 600 ms) and validity (valid and invalid) as factors revealed main effects of validity (F(1,15) = 10.181, p < .01, = 0.40) and SOA (F(1,15) = 25.631, p < .001, = 0.63). Moreover, there was an interaction effect of validity × SOA, F(1,15) = 9.361, p < .01,
= .38. Planned comparisons showed a validity effect in the 600 ms condition (247 vs. 240 ms; t(15) = 3.626, p < .01, d = .36) but not in the 50 ms condition (268 vs. 267 ms; t(15) = 1.339, p > .10, d = .05). Invalid safe trials in the 600 ms condition were slower than threat trials (247 vs. 238 ms, t(15) = 3.507, p < .01, d = .54), but valid safe trials (240 ms) were not faster than threat trials (t(15) = 1.099, p < .29, d = .14). Similarly, in the 50 ms condition, invalid safe trials were slower than threat trials (268 vs. 264 ms; t(15) = 2.835, p < .05, d = .13), but valid safe trials (267 ms) were not faster than threat trials (t(15)= 1.730, p = .10, d = .10).
Figure 6. Experiment 3: Saccadic latencies per condition. In the 600 ms SOA condition, latencies on invalid (i.e. away from the CS+) fear-conditioned trials were slower compared to valid (i.e. towards the CS+) trials. Error bars reflect within-subject normalised standard errors (Loftus & Masson, Citation1994).
Paired-samples t-tests on fear ratings showed that both stimuli in the safe condition were rated as less fearful than the stimuli in the threat condition (all p’s < .05). The safety stimulus was not rated as more fearful than the neutral stimulus (p = .15).
The results of Experiment 3 show that in the 50 ms SOA condition, there was no validity effect for trials in which the safety cue was present. In contrast to threatening cues, saccades towards safety cues were not faster than saccades away from safety cues. Based on these results, one could argue that safety cues did not automatically capture attention. However, a validity effect was observed for safety trials in the 600 ms SOA condition. This validity effect was caused by slower saccades away from locations previously occupied by a safety cue, compared to threatening cues. Therefore, signals safety seem to only interfere with the allocation of attention relatively late in time.
General discussion
The results of the present study indicate that stimuli that signal threat attract attention and disrupt the execution of voluntary saccades to other locations. Experiments 1 and 2 show that when a threatening stimulus is presented briefly and quickly followed by an endogenous arrow cue, saccades to locations previously occupied by the threatening stimulus are executed faster than saccades executed to the opposite direction. These results suggest that the stimulus signalling threat is quickly selected over other information. Even though the source of threat is removed (i.e. the stimulus is no longer on the screen), the quick allocation of attention to its location interferes with the execution of a voluntary eye movement. Notably, a similar validity effect was found in the 600 stimulus-cue SOA condition (Experiment 1) and the 1000 stimulus-cue SOA condition (Experiment 2), suggesting that attention remained allocated at the location that previously contained the threatening stimulus for a relatively long time. Even though there was nothing on the screen that reminded the observer that a threatening stimulus was presented at that location, attention remained allocated.
Previous studies on fear conditioning and attention have obtained mixed results. Some studies demonstrated facilitated engagement to fear-conditioned stimuli (Koster et al., Citation2004), others found delayed disengagement of attention from fear-conditioned stimuli (Van Damme et al., Citation2008). Most studies have used a spatial cueing task with presentation of only one cue at a time. The abrupt onset of this cue could have attracted attention due to its visual properties and irrespective of its valence (Yantis & Jonides, Citation1984). In our studies however, we presented both cues simultaneously making sure that neither cue attracted attention simply on the basis of its unique transient property. Indeed previous studies have shown that presenting several stimuli simultaneously ensures that none of the stimuli attract attention (Armony & Dolan, Citation2002; Schmidt et al., Citation2012, Citation2015). In addition, our study used eye movement measurements instead of manual responses. Compared to manual response times, eye movements – as a measure of overt attention – provide a higher temporal and spatial resolution compared to manual response times. It has been argued that saccadic latencies can reveal earlier stages of processing than can manual response times (van Zoest, Hunt, & Kingstone, Citation2010).
The results of the 50 ms SOA conditions in Experiments 1 and 2 indicate that threat interferes with execution of voluntary saccades very early in time. Although there was no incentive to pay attention to any of the competing stimuli, the presence of a stimulus that was associated with threat attracted attention and interfered with the execution of an immediately following saccade. Therefore, it seems likely that processing of threatening stimuli is, at least partly, under bottom-up control. However, the validity effects observed in the longer SOA conditions (600 and 1000 ms) of Experiments 1 and 2 indicate that there might be a top-down component involved in the allocation of attention to threat. When the delay between stimulus presentation and saccade cue is long, top-down control is the crucial factor in the deployment of spatial attention to competing stimuli. In other words, the observer may have kept attention voluntarily at the location of the threatening cue since it contained behaviourally relevant information. It is therefore unlikely that the cueing effects observed at SOAs 600 and 1000 ms are the result of attentional capture, but instead reflect enduring top-down processing at the location that previously contained the threatening stimulus. This is consistent with the rating scale findings in which showed that participants consistently rated the stimulus associated with threat as more threatening than the other stimuli.
Since the participants were told that only the CS+ was predictive of the aversive outcome, it cannot be fully excluded that the participants voluntarily paid attention to the CS+ in the 50 ms conditions. It can be argued that the CS+ was not just a fear-inducing stimulus, but also had an informative value, because it provided information about the likelihood of an upcoming event. However, Experiment 3 makes this explanation less creditable as verbal instructions about safety signals in Experiment 3 did not have this effect at the early 50 ms SOA. Instructions of Experiment 3 were exactly the same as those in Experiments 1 and 2, except that the participants were now informed that they would never receive a shock after presentation of one of the stimuli. Therefore, we believe that the results in the 50 ms SOA conditions cannot be explained by the fact that the threatening stimulus was informative. Instead, as saccades were executed rapidly there may have been enough time for the processing of fear to have an effect, but not enough time for effects that are driven by the informational value of the stimulus (i.e. safety signals).
The observed attentional bias to safety stimuli for the long interval of Experiment 3 indicates that when there is enough time to process the information and select a relevant response, behaviourally relevant information other than threat can also interfere with attention. It is likely that allocation of attention towards signals of safety is evolutionary significant because it may allow for reactions that could minimise or reduce danger. Such a response would require an allocation of attention to stimuli that signal safety, in order to select an appropriate reaction. A high motivation to approach safety may be reflected in an attentional bias to safety signals, compared to signals conveying more ambiguous or no information. Therefore, when given enough time to process information about competing cues, the voluntary execution of an eye movement can be interrupted by the presence of a cue that signalled safety. Although a bottom-up component can still be involved, the lack of a validity effect in the 50 ms condition implies that the attentional bias is most likely driven by top-down motivational factors. As assumed for threatening stimuli, the attentional biases at the longer intervals are probably under top-down control.
Recently, it has been suggested that the classic top-down vs. bottom-up dichotomy may be incomplete (Belopolsky, Citation2015; Egeth & Yantis, Citation1997; Theeuwes, Olivers, & Belopolsky, Citation2010), since there is large number of experimental results that do not fit into these two categories (Awh, Belopolsky, & Theeuwes, Citation2012). Specifically, objects selected in the past and associated with positive outcomes (such as monetary reward) attract attention despite being irrelevant to current task goals and despite lacking physical salience (e.g. Anderson, Laurent, & Yantis, Citation2011, Citation2012; Failing & Theeuwes, Citation2014; Failing, Nissens, Pearson, Le Pelley, & Theeuwes, Citation2015; Mohanty, Gitelman, Small, & Mesulam, Citation2008; Theeuwes & Belopolsky, Citation2012). It is reasonable to suggest that the signals of threat and safety might also fall into this new category of attentional control referred to as “selection history”. Future studies should directly relate the present findings to other events from the same category and closely examine their time course. It is possible that the time course of selection history is different from the well-known time-courses of goal-driven and salience-driven attention. It would also be important to link the present results on attentional orienting to physiological measurements of fear (e.g. skin conductance response or startle eye blink). Finally, it may be interesting to investigate the effect of threatening and safe cues on attention when participants are not explicitly informed about the contingency between the cue and outcome.
There is a large body of evidence that suggests that the amygdala is a crucial neural system in fear conditioning (Davis, Citation1997; Fendt & Fanselow, Citation1999; Lavond, Kim, & Thompson, Citation1993; LeDoux, Citation1996). It has been argued that facilitation of attention to threatening events occurs through feedback from the amygdala to sensory cortical regions (e.g. Alpers, Ruhleder, Walz, Muhlberger, & Pauli, Citation2005). Several brain imaging studies demonstrated that amygdala activation to the presentation of fearful stimuli is enhanced compared to neutral stimuli (Breiter & Rauch, Citation1996; LaBar et al., Citation1998; Morris et al., Citation1996), and that this fast amygdala response enables modulation of subsequent attention. For example, it has been shown that an intact amygdala is necessary for guiding gaze towards emotionally relevant features of fearful face stimuli (Adolphs et al., Citation2005). In a spatial cueing study, Armony and Dolan (Citation2002) showed that facial stimuli which acquired fear value through conditioning captured attention to their location. Moreover, fear-conditioned stimuli induced enhanced responses in the amygdala, lateral orbitofrontal cortext (lOFC) and fronto-parietal network, which is thought to underlie the control of spatial attention (Corbetta, Citation1998). Armony and Dolan (Citation2002) argued that the lOFC can transfer information about the emotional value of a stimulus from the amygdala to cortical areas involved in spatial attention. Based on these findings, we speculate that the amygdala may help with rapidly guiding spatial attention towards stimuli associated with threat. This may not be the case for the safety cues, which could explain a slower time course of attentional orienting.
In sum, the presence of a potential threatening or safe event biases our attention and interrupts the execution of voluntary saccades. Fast allocation of attention is specific for threatening events as initial execution of a saccade to a location previously occupied by threat was fast and automatic. When the interval is longer and the stimuli can be cognitively appraised, attention is still deployed to behaviourally relevant stimuli that provide information about threat and safety. Together, these results imply that humans keep monitoring not only the sources of threatening information but also the sources of safety in order optimise future behaviour.
Additional information
Funding
References
- Adolphs, R., Gosselin, F., Buchanan, T. W., Tranel, D., Schyns, P., & Damasio, A. R. (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433(7021), 68–72. doi:10.1038/Nature03086
- Alpers, G. W., Ruhleder, M., Walz, N., Muhlberger, A., & Pauli, P. (2005). Binocular rivalry between emotional and neutral stimuli: A validation using fear conditioning and EEG. International Journal of Psychophysiology, 57(1), 25–32. doi:10.1016/j.ijpsycho.2005.01.008
- Anderson, B. A., Laurent, P. A., & Yantis, S. (2011). Value-driven attentional capture. Proceedings of the National Academy of Sciences, 108(25), 10367–10371. doi: 10.1073/pnas.1104047108
- Anderson, B. A., Laurent, P. A., & Yantis, S. (2012). Generalization of value-based attentional priority. Visual Cognition, 20(6), 647–658. doi: 10.1080/13506285.2012.679711
- Armony, J. L., & Dolan, R. J. (2002). Modulation of spatial attention by fear-conditioned stimuli: An event-related fMRI study. Neuropsychologia, 40(7), 817–826. doi: 10.1016/S0028-3932(01)00178-6
- Awh, E., Belopolsky, A. V., & Theeuwes, J. (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16(8), 437–443. doi: 10.1016/j.tics.2012.06.010
- Bekhterev, V. M. (1913). Objektive Psychologie oder Psychoreflexologie: die Lehre von den Assoziationsreflexe. Leipzig: BG Teubner.
- Belopolsky, A. V. (2015). Common priority map for selection history, reward and emotion in the oculomotor system. Perception. doi:10.1177/0301006615596866
- Belopolsky, A. V., & Theeuwes, J. (2012). Updating the premotor theory: The allocation of attention is not always accompanied by saccade preparation. Journal of Experimental Psychology: Human Perception and Performance, 38(4), 902–914. doi:10.1037/A0028662
- Breiter, H. C., & Rauch, S. L. (1996). Functional MRI and the study of OCD: From symptom provocation to cognitive-behavioral probes of cortico-striatal systems and the amygdala. Neuroimage, 4(3), S127–S138. doi:10.1006/nimg.1996.0063
- Calvo, M. G., Avero, P., & Lundqvist, D. (2006). Facilitated detection of angry faces: Initial orienting and processing efficiency. Cognition and Emotion, 20(6), 785–811. doi: 10.1080/02699930500465224
- Corbetta, M. (1998). Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences of the United States of America, 95(3), 831–838. doi:10.1073/pnas.95.3.831
- Davis, M. (1997). Neurobiology of fear responses: The role of the amygdala. The Journal of Neuropsychiatry and Clinical Neurosciences, 9(3), 382–402. doi: 10.1176/jnp.9.3.382
- Egeth, H. E., & Yantis, S. (1997). Visual attention: Control, representation, and time course. Annual Review of Psychology, 48(1), 269–297. doi: 10.1146/annurev.psych.48.1.269
- Esteves, F., Dimberg, U., & Öhman, A. (1994). Automatically elicited fear: Conditioned skin-conductance responses to masked facial expressions. Cognition & Emotion, 8(5), 393–413. doi:10.1080/02699939408408949
- Failing, M., Nissens, T., Pearson, D., Le Pelley, M., & Theeuwes, J. (2015). Oculomotor capture by stimuli that signal the availability of reward. Journal of Neurophysiology, 114(4), 2316–2327. doi: 10.1152/jn.00441.2015
- Failing, M. F., & Theeuwes, J. (2014). Exogenous visual orienting by reward. Journal of Vision, 14(5), 6. doi: 10.1167/14.5.6
- Fendt, M., & Fanselow, M. S. (1999). The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and Biobehavioral Reviews, 23(5), 743–760. doi:10.1016/S0149-7634(99)00016-0
- Fox, E., Lester, V., Russo, R., Bowles, R. J., Pichler, A., & Dutton, K. (2000). Facial expressions of emotion: Are angry faces detected more efficiently? Cognition & Emotion, 14(1), 61–92. doi: 10.1080/026999300378996
- Henderson, J. M. (2003). Human gaze control during real-world scene perception. Trends in Cognitive Sciences, 7(11), 498–504. doi:10.1016/j.tics.2003.09.006
- Hunt, A. R., Cooper, R. M., Hungr, C., & Kingstone, A. (2007). The effect of emotional faces on eye movements and attention. Visual Cognition, 15(5), 513–531. doi: 10.1080/13506280600843346
- Kim, J. J., & Jung, M. W. (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews, 30(2), 188–202. doi: 10.1016/j.neubiorev.2005.06.005
- Koster, E. H., Crombez, G., Van Damme, S., Verschuere, B., & De Houwer, J. (2004). Does imminent threat capture and hold attention? Emotion, 4(3), 312–317. doi:10.1037/1528-3542.4.3.312
- Koster, E. H. W., Crombez, G., Van Damme, S., Verschuere, B., & De Houwer, J. (2005). Signals for threat modulate attentional capture and holding: Fear-conditioning and extinction during the exogenous cueing task. Cognition & Emotion, 19(5), 771–780. doi:10.1080/02699930441000418
- Kowler, E., Anderson, E., Dosher, B., & Blaser, E. (1995). The role of attention in the programming of saccades. Vision Research, 35(13), 1897–1916. doi: 10.1016/0042-6989(94)00279-U
- LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E., & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron, 20(5), 937–945. doi:10.1016/S0896-6273(00)80475-4
- Lavond, D. G., Kim, J. J., & Thompson, R. F. (1993). Mammalian brain substrates of aversive classical-conditioning. Annual Review of Psychology, 44, 317–342. doi:10.1146/annurev.ps.44.020193.001533
- LeDoux, J. E. (1996). The emotional brain. New York, NY: Simon & Schuster.
- Loftus, G. R., & Masson, M. E. J. (1994). Using confidence-intervals in within-subject designs. Psychonomic Bulletin & Review, 1(4), 476–490. doi: 10.3758/BF03210951
- Maren, S. (2001). Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience, 24, 897–931. doi: 10.1146/annurev.neuro.24.1.897
- Massar, S. A. A., Mol, N. M., Kenemans, J. L., & Baas, J. M. P. (2011). Attentional bias in high- and low-anxious individuals: Evidence for threat-induced effects on engagement and disengagement. Cognition & Emotion, 25(5), 805–817. doi:10.1080/02699931.2010.515065
- McPeek, R. M., Maljkovic, V., & Nakayama, K. (1999). Saccades require focal attention and are facilitated by a short-term memory system. Vision Research, 39(8), 1555–1566. doi:10.1016/S0042-6989(98)00228-4
- Mogg, K., Bradley, B. P., DeBono, J., & Painter, M. (1997). Time course of attentional bias for threat information in non-clinical anxiety. Behaviour Research and Therapy, 35(4), 297–303. doi:10.1016/S0005-7967(96)00109-X
- Mogg, K., Bradley, B. P., Miles, F., & Dixon, R. (2004). Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition & Emotion, 18(5), 689–700. doi:10.1080/02699930341000158
- Mohanty, A., Gitelman, D. R., Small, D. M., & Mesulam, M. M. (2008). The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cerebral Cortex, 18(11), 2604–2613. doi:10.1093/cercor/bhn021
- Morris, J. S., Frith, C. D., Perrett, D. I., Rowland, D., Young, A. W., Calder, A. J., & Dolan, R. J. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383(6603), 812–815. doi:10.1038/383812a0
- Mulckhuyse, M., Crombez, G., & Van der Stigchel, S. (2013). Conditioned fear modulates visual selection. Emotion, 13(3), 529–536. doi:10.1037/a0031076
- Mulckhuyse, M., & Dalmaijer, E. S. (2015). Distracted by danger: Temporal and spatial dynamics of visual selection in the presence of threat. Cognitive, Affective, & Behavioral Neuroscience, 16(2), 315–324. doi: 10.3758/s13415-015-0391-2
- Näätänen, R. (1971). Non-aging fore-periods and simple reaction time. Acta Psychologica, 35(4), 316–327. doi: 10.1016/0001-6918(71)90040-0
- Öhman, A. (1986). Unconscious origin of fear – conditioned autonomic responses to backwardly masked stimuli. Bulletin of the British Psychological Society, 39, A83–A83.
- Öhman, A., Flykt, A., & Esteves, F. (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130(3), 466–478. doi: 10.1037/0096-3445.130.3.466
- Pape, H. C., & Pare, D. (2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews, 90(2), 419–463. doi:10.1152/physrev.00037.2009
- Pape, H. C., & Stork, O. (2003). Genes and mechanisms in the amygdala involved in the formation of fear memory. Annals of the New York Academy of Sciences, 985(1), 92–105. doi: 10.1111/j.1749-6632.2003.tb07074.x
- Pavlov, I. P. (1927). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. London: Oxford University Press.
- Pitica, I., Susa, G., Benga, O., & Miclea, M. (2012). Visual search for real emotional faces: The advantage of anger. Procedia-Social and Behavioral Sciences, 33, 632–636. doi: 10.1016/j.sbspro.2012.01.198
- Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology (Hove), 32(Feb), 3–25. doi: 10.1080/00335558008248231
- Rescorla, R. A., & Solomon, R. L. (1967). 2-Process learning theory – relationships between Pavlovian conditioning and instrumental learning. Psychological Review, 74(3), 151. doi:10.1037/H0024475
- Schmidt, L. J., Belopolsky, A. V., & Theeuwes, J. (2012). The presence of threat affects saccade trajectories. Visual Cognition, 20(3), 284–299. doi: 10.1080/13506285.2012.658885
- Schmidt, L. J., Belopolsky, A. V., & Theeuwes, J. (2015). Potential threat attracts attention and interferes with voluntary saccades. Emotion, 15(3), 329–338. doi: 10.1037/emo0000041
- Sehlmeyer, C., Schöning, S., Zwitserlood, P., Pfleiderer, B., Kircher, T., Arolt, V., & Konrad, C. (2009). Human fear conditioning and extinction in neuroimaging: A systematic review. PLoS ONE, 4(6), e5865. doi:10.1371/journal.pone.0005865
- Shepherd, M., Findlay, J. M., & Hockey, R. J. (1986). The relationship between eye-movements and spatial attention. The Quarterly Journal of Experimental Psychology Section A, 38(3), 475–491. doi: 10.1080/14640748608401609
- Soares, S. C., Esteves, F., Lundqvist, D., & Öhman, A. (2009). Some animal specific fears are more specific than others: Evidence from attention and emotion measures. Behaviour Research and Therapy, 47(12), 1032–1042. doi: 10.1016/j.brat.2009.07.022
- Theeuwes, J., & Belopolsky, A. V. (2012). Reward grabs the eye: Oculomotor capture by rewarding stimuli. Vision Research, 74, 80–85. doi: 10.1016/j.visres.2012.07.024
- Theeuwes, J., Olivers, C. N., & Belopolsky, A. (2010). Stimulus-driven capture and contingent capture. Wiley Interdisciplinary Reviews: Cognitive Science, 1(6), 872–881.
- Van Damme, S., Crombez, G., Hermans, D., Koster, E. H., & Eccleston, C. (2006). The role of extinction and reinstatement in attentional bias to threat: A conditioning approach. Behaviour Research and Therapy, 44(11), 1555–1563. doi:10.1016/j.brat.2005.11.008
- Van Damme, S., Crombez, G., & Notebaert, L. (2008). Attentional bias to threat: A perceptual accuracy approach. Emotion, 8(6), 820–827. doi:10.1037/A0014149
- Van Damme, S., Lorenz, J., Eccleston, C., Koster, E. H., De Clercq, A., & Crombez, G. (2004). Fear-conditioned cues of impending pain facilitate attentional engagement. Neurophysiologie Clinique-Clinical Neurophysiology, 34(1), 33–39. doi:10.1016/j.neucli.2003.11.001
- Van der Stigchel, S., Meeter, M., & Theeuwes, J. (2006). Eye movement trajectories and what they tell us. Neuroscience and Biobehavioral Reviews, 30(5), 666–679. doi: 10.1016/j.neubiorev.2005.12.001
- Vogt, J., Lozo, L., Koster, E. H. W., & De Houwer, J. (2011). On the role of goal relevance in emotional attention: Disgust evokes early attention to cleanliness. Cognition & Emotion, 25(3), 466–477. Pii 931515755. doi:10.1080/02699931.2010.532613
- Watson, J. B., & Rayner, R. (1920). Conditioned emotional reactions. Journal of Experimental Psychology, 3, 1–14. doi:10.1037/H0069608
- Yantis, S., & Jonides, J. (1984). Abrupt visual onsets and selective attention – evidence from visual-search. Journal of Experimental Psychology: Human Perception and Performance, 10(5), 601–621. doi:10.1037/0096-1523.10.5.601
- van Zoest, W., Hunt, A. R., & Kingstone, A. (2010). Representations in visual cognition: It’s about time. Current Directions in Psychological Science, 19(2), 116–120. doi:10.1177/0963721410363895
