ABSTRACT
Individual differences in fear generalisation have been proposed to play a role in the aetiology and/or maintenance of anxiety disorders, but few data are available to directly support that claim. The research that is available has focused mostly on generalisation of peripheral and central physiological fear responses. Far less is known about the generalisation of avoidance, the behavioural component of fear. In two experiments, we evaluated how neuroticism, a known vulnerability factor for anxiety, modulates an array of fear responses, including avoidance tendencies, towards generalisation stimuli (GS). Participants underwent differential fear conditioning, in which one conditioned stimulus (CS+) was repeatedly paired with an aversive outcome (shock; unconditioned stimulus, US), whereas another was not (CS−). Fear generalisation was observed across measures in Experiment 1 (US expectancy and evaluative ratings) and Experiment 2 (US expectancy, evaluative ratings, skin conductance, startle responses, safety behaviours), with overall highest responding to the CS+, lowest to the CS− and intermediate responding to the GSs. Neuroticism had very little impact on fear generalisation (but did affect GS recognition rates in Experiment 1), in line with the idea that fear generalisation is largely an adaptive process.
Both excessive fear generalisation and excessive threat avoidance are increasingly receiving attention as potential vulnerability factors for anxiety disorders. However, little attention has been paid to individual differences in the interaction between both, that is the overgeneralisation of avoidance (van Meurs, Wiggert, Wicker, & Lissek, Citation2014) or the excessive tendency to avoid stimuli resembling danger cues (generalisation stimuli, GS), as a pathway to pathological anxiety. In two experiments, we examined the effects of neuroticism (N), a known predisposing factor for clinical anxiety (e.g. Watson, Gamez, & Simms, Citation2005), on avoidance and other fear responses towards GSs.
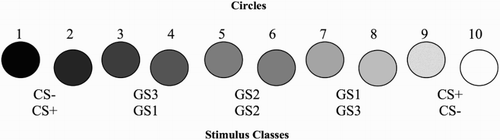
Fear tends to generalise from threat stimuli towards stimuli that are conceptually (e.g. Dunsmoor, White, & LaBar, Citation2011) or perceptually similar (e.g. Lissek et al., Citation2008; for a review, see Dymond, Dunsmoor, Vervliet, Roche, & Hermans, Citation2015). In a prototypical generalisation experiment, individuals may be exposed to circles of different sizes (see Lissek et al., Citation2008). During conditioning, one circle (e.g. the smallest) is repeatedly followed by an aversive consequence (e.g. shock; unconditioned stimulus, US) and becomes a conditioned threat stimulus (CS+), eliciting a fear response. Another circle (e.g. the largest; CS−), on the contrary, is repeatedly presented without the US. At test, fear responses typically generalise to a different extent to the various circles, in accordance with their position on the continuum between the CS+ and the CS−.
Overgeneralisation refers to excessive fear responding towards stimuli that are rather dissimilar to the CS+. Relative to controls, such overgeneralisation is observed in individuals with panic disorder (Lissek et al., Citation2010) and generalised anxiety disorder (Lissek et al., Citation2014; but see Tinoco-González et al., Citation2015), indicating that excessive generalisation is a potential marker for at least some anxiety disorders. However, establishing differences between anxiety patients and controls does not allow discerning whether overgeneralisation predisposes individuals to pathology or rather is a consequence of pathology (Beckers, Krypotos, Boddez, Effting, & Kindt, Citation2013). Research in people at risk for the development of pathological anxiety can help to establish whether individual differences in generalisation are present before the onset of anxiety pathology. Whereas a few previous studies have examined the effect of other individual difference factors on generalisation (see below), none of them has focused on neuroticism, as we do here. Neuroticism has historically and empirically been associated with anxiety pathology (e.g. Watson et al., Citation2005). Of importance, neuroticism is a trait disposition characterised by a general tendency to react with increased negative emotions to a variety of situations (Clark, Watson, & Mineka, Citation1994; Watson & Clark, Citation1984) and might therefore be particularly relevant to overgeneralisation.
As indicated, only a few generalisation studies have examined people at risk, with varying results. Torrents-Rodas et al. (Citation2013) found differences between low trait-anxious and other participants in self-reported risk ratings towards the CS− and GSs most similar to the CS−, but not in fear-potentiated startle (FPS) or skin conductance responses (SCR) to GSs. Another study (Kaczkurkin & Lissek, Citation2013) found increased generalisation only on FPS in participants who scored high on threat estimation. Using conditioned safety stimuli either similar or dissimilar to a conditioned threat stimulus, Haddad, Pritchett, Lissek and Lau (Citation2012) found differences in generalisation between high and low trait-anxious individuals on FPS, but not on SCR. Given the limited attention that the effect of vulnerability factors on perceptual stimulus generalisation has received so far, more research in at-risk individuals is clearly warranted.
Most studies on fear generalisation have moreover disregarded a critical component of fear responding, avoidance, although excessive avoidance is a purported risk factor for anxiety pathology (Beckers et al., Citation2013). One exception is a study by Lommen, Engelhard, and van den Hout (Citation2010), who found that individuals high on neuroticism performed more (button press) avoidance responses to GSs than individuals low on neuroticism under some conditions. In another experiment (van Meurs et al., Citation2014), participants could guide a symbolic “farmer” to his “garden” through either a short or longer route on a computer screen in the presence of GSs. Results indicated that individuals who were low on distress endurance showed more avoidance (choosing the longer route when GSs were presented) than those high on distress endurance. Taken together, these studies suggest that clinically relevant individual traits might moderate the degree of avoidance generalisation.
According to dual-process models, overt behaviour results from the interplay of automatic action tendencies and controlled decision-making processes (e.g. Strack & Deutsch, Citation2004). So far in the fear-conditioning literature, mostly controlled avoidance behaviours (button presses) with a clear instrumental component (terminating shock) have been measured (e.g. Lommen et al., Citation2010). However, a recent study from our lab shows that through Pavlovian fear conditioning, participants also acquire automatic avoidance tendencies to conditioned fear cues, which do not have an instrumental component (Krypotos, Effting, Arnaudova, Kindt, & Beckers, Citation2014). These avoidance tendencies can be measured through a symbolic approach–avoidance reaction time task (AAT; De Houwer, Crombez, Baeyens, & Hermans, Citation2001). Using similar tasks, faster initiation of away than towards responses (avoidance tendencies) has also been observed for a variety of non-conditioned negative stimuli (Phaf, Mohr, Rotteveel, & Wicherts, Citation2014) and individuals with anxiety show increased avoidance tendencies towards objects related to their fear (e.g. spiders in arachnophobia; Klein, Becker, & Rinck, Citation2011; Rinck & Becker, Citation2007). In addition to overgeneralisation, such increased avoidance tendencies might contribute to the development of clinically severe anxiety. It is yet unclear whether those tendencies generalise to perceptually similar GSs.
We are also not aware of any studies that examined the effect of vulnerability factors for anxiety on avoidance tendencies, despite their purported role in overt avoidance and fear. Previous studies focusing on approach tendencies and their contribution to psychopathology have found increased approach tendencies in individuals at risk for alcoholism, where maladaptive approach to alcohol is a key symptom (Wiers, Rinck, Dictus, & van den Wildenberg, Citation2009), lending some support to the notion that conversely, in individuals at risk for anxiety, avoidance tendencies to threat cues might be enhanced.
In two experiments, we tested the generalisation of fear responding towards perceptually similar GSs following fear conditioning, using the paradigm introduced by Lommen et al. (Citation2010). No individual differences during conditioning were expected, but we hypothesised that individual differences would emerge in responding to the more ambiguous GSs (Lissek, Pine, & Grillon, Citation2006). We measured controlled (e.g. US expectancy ratings) as well as more automatic (e.g. speeded responding in an approach-avoidance reaction time task, AAT) indices of fear for the CSs and GSs, as it has been argued that individual differences are more likely to influence less controlled response systems (Beckers et al., Citation2013). So, we hypothesised that conditioned avoidance tendencies would generalise more widely in individuals high rather than those low on neuroticism. In order to explore whether participants were able to discriminate between the different stimuli, we also added a forced-choice recognition task in Experiment 1. In Experiment 2, we additionally measured FPS and SCR responses to the CSs and GSs, to evaluate whether automatic psychophysiological fear responses would be affected in a similar way as self-reported US expectancies. Finally, in order to examine whether overt behaviour mirrors differences in avoidance tendencies and to conceptually replicate the findings of Lommen et al. (Citation2010), we also included a safety behaviour test in Experiment 2, in which participants could prevent an expected US in the presence of a CS or GS by pressing a button (an instrumental avoidance response).
Experiment 1
Materials and methods
Participants
Participants were pre-screened for the following exclusion criteria: (1) age under 18; (2) a history of psychiatric disorders, heart problems or epilepsy; (3) use of medication affecting memory, attention or reaction times; (4) current pregnancy and (5) colour blindness (Lommen et al., Citation2010). In order to achieve similar groups sizes as Lommen et al., we recruited 70 participants who received financial compensation (€10) or research credits for participating. One participant was excluded due to technical problems, five participants for having participated in similar experiments before, one participant for not following experimental instructions and three participants for having used illegal substances in the 24 hours preceding participation.Footnote1 Two participants terminated the experiment prematurely. We used the criteria from Lommen et al. (Citation2010) to divide the remaining sample (N = 58; 19 males; MAGE = 21.91, SDAGE = 2.66) into Low (score ≤ 4, n = 18), Moderate (score > 4 and <11, n = 23) and High (score ≥ 11, n = 17) neuroticism (N) groups, based on their total scores on the neuroticism scale of the Eysenck Personality Questionnaire (EPQ; Eysenck & Eysenck, Citation1975).
Materials
Two pairs of stimuli at both ends of the black–white continuum were used as CSs with stimulus assignment counterbalanced across participants (). Six intermediate grey stimuli served as GSs; for data analysis, stimuli were grouped in classes of two, following the convention established by Lissek and colleagues (Lissek et al., Citation2008, Citation2014; van Meurs et al., Citation2014). For more information about the stimuli, see supplementary material (Section S1.1). Circles were superimposed on white square frames and presented against a black background. White rectangular frames with horizontal or vertical orientation were used for the AAT.
The US, a 2-ms electric stimulus, was administered 7.5 s after CS+ onset to the dorsal side of the wrist of the participant’s non-dominant hand (Effting & Kindt, Citation2007). The US was delivered through two Ag electrodes, covered with conductive gel (Signa Gel, Parker Laboratories Inc., Fairfield, NJ) and connected to a DS7A Constant Current Stimulator (Digitimer Ltd., Hertfordshire, UK).
Subjective measures
Online US expectancies were measured on an 11-point computerised Likert scale, ranging from −5 (certainly not expecting an electric stimulus) to 5 (certainly expecting an electric stimulus). The cursor was located at 0 (uncertain) at the beginning of each trial, regardless of the response given on the previous trial (Arnaudova et al., Citation2013). Participants were given 5 s to move the cursor and confirm their response with a mouse click. Otherwise, the cursor’s last position was recorded.
Evaluative ratings of CSs, GSs and the US were collected on an 11-point Likert scale ranging from −5 (unpleasant) to 5 (pleasant). The US was also evaluated on intensity (light, moderate, intense, enormous and unbearable) and startlingness (not, light, moderate, strong and very strong).
During a forced-choice recognition test participants had to report on each trial whether or not a specific stimulus had been presented during conditioning, while electrodes were attached. They did this by pressing a keyboard button (1 = yes, it was presented, 2 = no, it was not presented) upon the presentation of each stimulus. Participants also reported both their retrospective (for the CSs, encountered during acquisition: how much they would have expected an electric stimulus, had they seen the stimulus when electrodes were attached) and hypothetical (for the GSs, not encountered during acquisition: how much they would have expected an electric stimulus, had the stimulus been presented when electrodes were attached) US expectancies on the same scale as their online US expectancies. These ratings were added to examine the generalisation of US expectancies for the GSs. US expectancy ratings for the GSs could not be measured online, because GSs were not presented during acquisition. Ratings for the CSs were included in this task to confirm that participants were still aware of the CS-US contingencies at the end of the experiment. Similar retrospective ratings have been used as an outcome measure in fear-conditioning experiments before (e.g. Soeter & Kindt, Citation2012; Vansteenwegen et al., Citation2006).
Questionnaires
Neuroticism and extraversion were measured using the respective subscales (EPQ-N and EPQ-E) of the EPQ (Dutch translation by Sanderman, Arrindell, Ranchor, Eysenck, & Eysenck, Citation2012). Negative affects were examined with the Depression Anxiety Stress Scales ( Lovibond & Lovibond, Citation1995; Dutch translation by de Beurs, Van Dyck, Marquenie, Lange, & Blonk, Citation2001). Trait worry was assessed with the Penn State Worry Questionnaire (Meyer, Miller, Metzger, & Borkovec, Citation1990; Dutch translation by van Rijsoort, Emmelkamp, & Vervaeke, Citation1999). Other than EPQ-N, these questionnaires were included in the experiment for exploratory purposes. They are not reported here.
Procedure
After reading an information brochure and signing for informed consent, participants were pre-screened and a colour blindness test (Ishihara & Ishihara, Citation1970) was administered. Participants then underwent an electric stimulus work-up procedure where the level of the electric stimulus was increased incrementally to an uncomfortable, but non-painful level (e.g. Orr et al., Citation2000).
At the start of acquisition, participants were instructed that objects presented on the screen would either be followed by a US or not and that their task was to predict the US and report online US expectancies. Participants received 10 CS+ and 10 CS− trials (5 of each CS circle) during this acquisition phase. CS pictures were presented centred on the screen, with the US expectancy scale centred underneath. Presentation order was block-randomised so that no more than two consecutive trials of the same type could occur. Each CS trial had an 8-s duration and all CS+ trials ended with US presentation. Trials were separated by an inter-trial interval (ITI) with an average duration of 20 s (15, 20 or 25 s). During ITIs, the US expectancy scale was inactive.
After a 3-min pause, the electric stimulation electrodes were removed and participants were then introduced to the AAT as in Krypotos et al. (Citation2014).
The AAT consisted of 2 blocks of 10 practice trials and 40 target trials. On each AAT trial, participants were requested to move a small stick figure that appeared centred at the bottom or top half of the screen either towards or away from a stimulus picture presented 1500 ms later on the other half of the screen. Before each practice or target AAT block, participants were instructed to make their response by pressing a key on the keyboard (B, marked with ↓ or Y, marked with ↑) based on the orientation of the stimulus picture’s frame (horizontal or vertical). Instructions to approach or avoid horizontal or vertical pictures were reversed before the second training block; the starting instructions were counterbalanced across participants. Reaction time (RT) was recorded.
During the practice trials, pictures of every stimulus were presented once, so that one stimulus of each CS or GS class was presented superimposed on a vertical and one superimposed on a horizontal white frame. During the target trials, each stimulus was presented four times, twice in a horizontal and twice in a vertical frame, so that each picture had to be approached and avoided twice in each block. Trials were separated by 2000 ms ITIs and semi-randomised, so that no more than two consecutive trials of the same type could occur. Incorrect responses were marked by the 500-ms presentation of a red cross at the manikin’s starting position. After correct responses, the manikin remained at its end location for 500 ms. The AAT trial set-up has been described in more detail elsewhere (Krypotos et al., Citation2014).
The experiment was concluded after participants gave their evaluative ratings, performed the forced-choice recognition test, reported their retrospective or hypothetical US expectancies, and filled in the computerised questionnaires. The University of Amsterdam Ethics Committee approved the experiment.
Data analysis
Demographic and US evaluation comparisons between the neuroticism groups were analysed with one-way analyses of variance (ANOVA). Mixed repeated-measures ANOVA was used for the US-expectancy data (mean US-expectancy ratings across 10 acquisition trials, averaged across the 2 stimuli in each class) with Stimulus Class (CS+, CS−) as a within-subjects variable and N Group (Low, Moderate, High) as between-subjects variable. When the assumption of sphericity was violated, Greenhouse–Geisser correction was applied.
For the AAT, median RTs (RTmd) per stimulus class and response (approach and avoid) were calculated from the raw RT data. All practice trials as well as all trials with incorrect responses and trials with RTs exceeding 3000 ms were removed (n = 139, 2.96% of all trials). Mean RTmd were analysed with a mixed ANOVA, focusing on the Response (Approach, Avoid) × Stimulus Class (CS+, CS− or GS1, GS3) interaction and the Response (Approach, Avoid) × Stimulus Class (CS+, CS− or GS1, GS3) × N Group (Low, Moderate, High) interaction. The GS2 class was omitted from the analyses for lack of a comparison class.
Evaluative ratings and retrospective/hypothetical US expectancies were analysed in the same way as the online US-expectancy data, but with all five Stimulus Classes as within-subject variables. Alpha levels for post hoc multiple pairwise comparisons per Stimulus Class were Bonferroni-corrected.
In order to maximise between-group differences, the analyses were repeated including the Low and High Neuroticism groups only. For the latter analysis, only significant effects that did not emerge in the analyses including all three groups are reported.
Results
US evaluation
No significant differences between the N groups were observed in actual US intensity or US evaluation (all ps > .30). As a whole, participants found the US unpleasant (M = −3.19, SD = 1.13), intense (M = 2.79, SD = 0.49) and startling (M = 3.24, SD = .84).
Acquisition
Acquisition was successful, with higher US expectancies for the CS+ (M = 3.93, SD = .56) than the CS− Stimulus Class (M = −3.84, SD = .81), yielding a main effect of Stimulus Class, F(1, 55) = 2399.96, p < .001, p η2 = .98. There was no significant Stimulus Class × N Group interaction (F < 1).
AAT
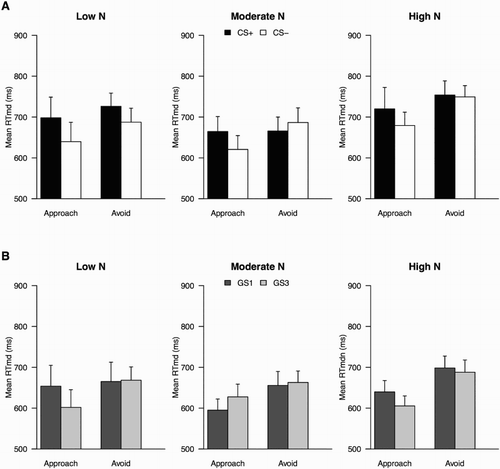
AAT results for the CSs showed that overall, no conditioned avoidance tendencies were present, Response × Stimulus Class interaction, F < 1, with no significant interaction with N groups, F(2, 55) = 1.75, p = .18, p η2 = .06 ((a)). Planned analyses per N group showed that only in the High N group the Response × Stimulus Class interaction approached significance, F(1, 16) = 3.83, p = .07, p η2 = .19.
Figure 2. Experiment 1: mean median RTs in the AAT for CSs (a) and GSs (b), by N group. Note: + p < .10.
Two participants had very long mean RTs (more than 2SD away from the sample mean) across AAT trials. When they were excluded from the analyses, the F-value for the Response × Stimulus Class × N Group interaction increased, F(2, 53) = 2.82, p = .07, p η2 = .10 and the pattern for the High N group became significant (p = .02).
The Response × Stimulus Class interaction was not significant for the GS stimuli either, F < 1, with no significant difference between groups, F < 1 ((b)). Planned analyses showed that the pattern was significant for none of the groups (largest F = 1.76 for Low N Group).
Recognition
Participants indicated on average for 3.24 of the GS circles (SD = 1.48) that they had seen them during acquisition. No significant difference between the three N groups was observed, F(2, 55) = 1.67, p = .20.
Visual examination of the data ((a)) suggested that recognition patterns might be predicted from the perceptual distance of the GS to the CSs and N group membership. In order to validate this impression, we fitted a logistic regression model to the data with Circle, N group and their interaction as independent variables and the individual binary response to each circle as a dependent variable. We used the High N group as reference within the model. The model was a good fit to the data (see ) and showed that the High N group differed significantly in their recognition pattern from both the Low and Moderate N group. So, surprisingly, the high neuroticism group showed better recognition accuracy (higher correct recognition rates for the actual CS+ and CS− stimuli and lower false recognition rates for the GSs most dissimilar to the actual CS+ and CS− stimuli) than the other groups.
Figure 3. Experiment 1: percentage of participants who reported to have seen a particular circle during acquisition (a), (retrospective/hypothetical) US expectancies (b) and pleasantness ratings (c), by N group and stimulus. Data points represent mean responding across each set of two stimuli, per class.
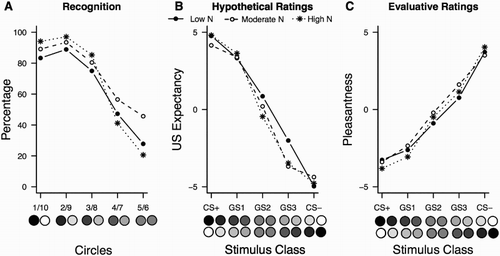
Table 1. Results of logistic regression analysis with recognition (yes/no) as outcome and Circle (1–10, 2–9, 3–8, 4–7, 5–6) and N group (Low, Moderate, High) and their interaction (Circle × N group) as predictor variables.
Retrospective/hypothetical US expectancies
Retrospective/hypothetical US expectancies differed as a function of Stimulus Class, F(3.37, 185.30) = 215.16, p < .001, p η2 = .80 (all pairwise comparisons were highly significant, p < .001), but the interaction with N group did not reach significance, F(6.74, 185.30) = 1.30, p = .25, p η2 = .05. This suggests that all groups showed similar generalisation patterns ((b)).
Evaluative ratings
All stimuli were evaluated differently in terms of pleasantness, F(2.17, 119.48) = 120.86, p < .001, p η2 = .69, with no significant differences between the N groups (Stimulus Class × Group interaction, F < 1). Pairwise comparisons of the stimuli were also significant (all ps < .01, (c)).
Discussion
Following successful acquisition, a clear downward generalisation gradient for stimuli on the continuum between the CS+ and CS− was observed in retrospective/hypothetical US expectancies. There was no evidence for an effect of individual differences in neuroticism on this gradient. We did find that high N individuals had more accurate recognition responses than those low or moderate on neuroticism. This might suggest that high N individuals are more vigilant during the experiment and more aware of perceptual differences between stimuli.
We were unable to show conditioned avoidance tendencies towards the CS+ as in Krypotos et al. (Citation2014), but we found some indication for such conditioned avoidance tendencies in individuals high in neuroticism. Our AAT included five stimulus classes, while so far AAT tasks have included only two (Krieglmeyer & Deutsch, Citation2010; Krypotos et al., Citation2014). This difference in task characteristics might potentially explain our inability to find robust conditioned avoidance tendencies. In Experiment 2, we split the AAT task in two tasks that each included only two stimulus classes, to make the procedure more similar to the original cue-irrelevant version of the AAT used in Krypotos et al. (Citation2014) and elsewhere.
To corroborate the observation of similar generalisation gradients across neuroticism groups, we replicated Experiment 1 with a few modifications. We included physiological measurements (FPS and SCR) and a test phase to evaluate differences between neuroticism groups on other fear measures. This would allow us to examine whether neuroticism affects all fear responses similarly or not (Beckers et al., Citation2013). A reminder phase was included before the final test phase, to counter any extinction that could have resulted from the AAT procedure. Differential responding between the CS+ and CS− was expected across all measures in this stage, and generalisation gradients across all measures, with increased generalisation for those high on neuroticism. We also included a variation of the avoidance task used by Lommen et al. (Citation2010) to measure overt avoidance responses. In this safety behaviours task (SBT), participants could press a response button (safety behaviour) to prevent the occurrence of a US whenever they expected one. We expected more overt avoidance responses to GSs more dissimilar from the CS+ in High N individuals relative to Moderate or Low N individuals, in replication of Lommen et al. (Citation2010).
Experiment 2
Materials and methods
Participants
Participants in Experiment 2 were compensated financially (€20) or through research credits. Additional exclusion criteria to those of Experiment 1 were age above 50 and hearing problems. Three participants were excluded due to technical problems and five participants for having used drugs within 24 hours before participation. The remaining 58 participants (19 males, MAGE = 21.95, SDAGE = 4.02) were divided into Low (n = 15), Moderate (n = 21) and High (n = 22) N groups, as in Experiment 1.
Materials
The same CSs, GSs and US were used as in Experiment 1. The computer screen (ASUS VW222U, 22″, 1680 × 1050) was calibrated to a linear gamma of 2.2 with a maximum stimulus luminance of 40 cd/m2 (CalMan 5, C3 Colorimeter, Spectracal, Shoreline, WA).
FPS was assessed by measuring the strength of the reflexive eye blink response to an acoustic startle probe with two electrodes (BME-175 6-mm sintered Ag/AgCl, BioMed Products Inc., Fair Oaks, CA) filled with conductive gel (Signa Gel, Parker Laboratories Inc., Fairfield, NJ) and attached to the participant’s left orbicularis oculi muscle and a ground electrode placed below the hairline in the centre of the forehead (Blumenthal et al., Citation2005). The 40-ms startle probe (104 dB) was delivered at the 7th second after stimulus onset, binaurally, through headphones (Senheiser HD 280 pro 64Ω, Wedemark, Germany).
SCR was assessed by measuring the electrodermal activity on the skin of the index and ring finger of participants’ non-dominant hand with two curved Ag/AgCl electrodes (20 × 16 mm). For more technical details on FPS and SCR, see the supplementary material (Section S2.1).
Procedure
After reading an information brochure and signing for informed consent, participants directly underwent the electric stimulus work-up procedure (Orr et al., Citation2000). The electrodes for the physiological measurements were attached thereafter.
A schematic representation of the experimental procedure can be found in . Trial set-up during all phases was the same as in Experiment 1. Following 10 habituation trials, where the startle probe was presented alone (noise alone (NA) trials), participants received 8 CS+, 8 CS− and 8 NA trials during the acquisition stage. NA trials had the duration of the startle probe (40 ms), separated by ITIs.
Table 2. Schematic representation of the procedure of Experiment 2.
After a 3-min pause, all electrodes were removed and participants were introduced to the AAT as in Krypotos et al. (Citation2014). In this experiment, participants received four blocks of 4 practice trials and 16 target trials. In the first two blocks, pictures of one stimulus from the CS+ class (circle 10 or 1) and one stimulus from the CS− class (circle 1 or 10) were presented centred to the top or bottom half of the screen. In the last two blocks, pictures of one stimulus from the GS1 class (circle 7 or 4) and one from the GS3 class (circle 4 or 7) were used.
Before starting the SBT, electric stimulus electrodes were reattached, to allow for the possibility of US administration. The electrodes for the physiological measurements were not attached, in order to keep this stage as similar as possible to the task used in Lommen et al. (Citation2010). Participants were instructed that a written message presented underneath a stimulus picture would indicate the possibility to prevent the US by pressing the space bar on the keyboard and were encouraged to press the button only when they expected a US. The exact instructions provided to participants can be found in the supplementary material (Section S2.2). CSs and GSs were presented centred on the computer screen, in random order, and the message appeared underneath all GS and CS− presentations. GSs were never followed by the US regardless of whether a response was executed or not. CS+ presentations were always followed by the US and no message was present; participants could nonetheless press the button at that time, but the button press did not prevent US occurrence. Stimulus duration was 8 s and ITI was 1 s. Total button presses were recorded for all stimuli.
FPS and SCR electrodes were then reattached and participants were instructed to use the knowledge gained during acquisition to predict the US. Crucially, having all electrodes attached assured that the context was the same as during acquisition. Following a second habituation phase, participants were presented six reminder trials (two CS+, two CS−, two NA). During acquisition and reminder, trials were semi-randomised as in Experiment 1. The following test phase was semi-randomised into 2 blocks of 12 trials, so that every half of each block included 1 CS+, GS1, GS2, GS3, CS− and NA trial. Thus, each circle of every stimulus class was presented twice during the test phase.
At the end of the experiment, participants reported their evaluative ratings, filled in the computerised questionnaires and provided demographic information. The University of Amsterdam Ethics Committee approved the experiment.
Data analysis
Data were analysed as for Experiment 1. For the AAT, 163 trials in total were excluded (4.39% of all trials). Absolute raw SCR were square-root transformed as in Milad et al. (Citation2006) with the negative sign re-applied where the raw SCR was lower than 0. Raw FPS and SCR were analysed in the same way as US expectancies in Experiment 1. Mean values per stimulus class across trials were used for the analyses of fear responses on all measures during all phases.
Data from the SBT were analysed with one-way ANOVAs, using number of GSs and maximum colour of GS to which a safety behaviour was executed as dependent variables, in keeping with Lommen et al. (Citation2010). Since participants were not informed about the possibility to perform safety behaviours during the presentation of the CS+ and some did not perform a safety behaviour to any GSs, we set the value for the maximum colour for these participants at 2, similar to Lommen et al. (Citation2010). Maximum colour values thus ranged from 2 to 10 (when the response key was pressed to the CS−). For further information about this analysis, see Lommen et al. (Citation2010).
Results
US evaluation
The difference in strength of the US selected by the three neuroticism groups approached significance (p = .07). However, subjective US ratings did not differ between groups; participants rated the US as unpleasant (M = −3.33, SD = 1.33), intense (M = 2.91, SD = 0.43) and startling (M = 3.52, SD = .80).
Acquisition
Participants reported higher US expectancies for the CS+ (M = 3.72, SD = .78) than the CS− (M = −3.54, SD = .86), yielding a main effect of Stimulus Class, F(1, 55) = 1317.05, p < .001, p η2 = .96, which indicates that acquisition was successful. The Stimulus Class × N group interaction was not significant, F(2, 55) = 1.39, p = .26, p η2 = .05.
Results were similar for physiological measures, with higher responding for the CS+ (FPS, M = 203.01, SD = 97.42; and SCR, M = 0.62, SD = 0.54) than the CS− (FPS, M = 185.35, SD = 103.18; and SCR, M = 0.35, SD = 0.35). The analyses yielded main effects of Stimulus Class for FPS, F(1, 55) = 8.55, p = .005, p η2 = .14, and SCR, F(1, 55) = 34.44, p < .001, p η2 = .39, in the absence of Stimulus Class × N group interactions, F(2, 55) = 1.00, p = .37, p η2 = .04 for FPS and F(2, 55) = 1.29, p = .28, p η2 = .05 for SCR.
Reminder
Conditioning effects remained significant on the reminder trials: main effect of Stimulus Class for US expectancies, F(1, 55) = 2267.23, p < .001, p η2 = .98, for FPS, F(1, 55) = 11.58, p = .001, p η2 = .17, and for SCR, F(1, 55) = 9.82, p = .003, p η2 = .15. This suggests that being exposed to the CSs during the AAT and SBT did not produce extinction. No between-groups differences in fear responding were observed.
Test
Generalisation was observed across all measures, with clear downward gradients from CS+ to CS−. There was a significant main effect of Stimulus Class for US expectancies, F(1.70, 93.71) = 428.31, p < .001, p η2 = .89, with all classes being different from each other (all ps ≤ .001, (a)); for FPS, F(4, 220) = 10.63, p < .001, p η2 = .16, with CS+ and GS1 being significantly different from GS2, GS3 and CS− ((b)); and for SCR, F(3.21, 176.74) = 2.99, p = .03, p η2 = .05, with only GS1 differing from GS3 ((c)).
Figure 4. Experiment 2: US expectancies (a), FPS (b) and SCR (c) during the test phase; evaluative ratings for all stimuli (d); number (e) and maximum colour (f) of GSs responded to during the SBT test. Data points represent mean responding across each set of two stimuli, per class.
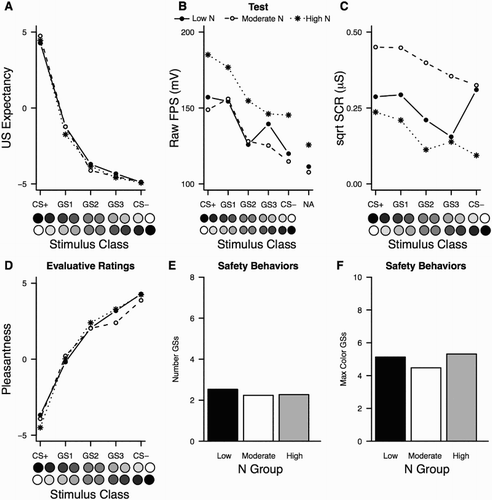
No significant differences in the effect of Stimulus Class were observed between the three N groups (all ps > .6) for FPS and US expectancies, which suggests that generalisation was similar across groups. There were no main effects of N group on FPS or US expectancies either, both F < 1. The main effect of N group on SCR responding approached significance, F(2, 55) = 2.88, p = .07, p η2 = .10. This was driven by heightened responding for the Moderate N group as compared to the High N group (main effect of group in pairwise comparison, p = .03). One-way ANOVAs showed significant differences in SCR between the three groups for GS2 (p = .02). The group comparisons did not reach significance for GS3 (p = .07) or the CS− (p = .08).
AAT
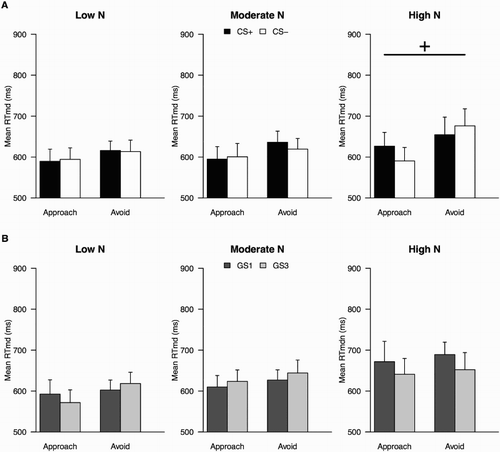
For the CSs, in a Response × Stimulus × N group ANOVA, no Response × Stimulus interaction was obtained, F(1, 55) = 2.44, p = .12, p η2 = .04, and no modulation of that interaction by N group, F < 1 ((a)). The Response × Stimulus interaction also did not reach significance when N group was removed from the model, F(1, 57) = 2.85, p = .10, p η2 = .05. One participant had very long mean RTs (more than two SD away from the sample mean) across AAT trials. When he was excluded from the analyses, the results remained the same, both when N group was included in the model (p = .10) and when it was not (p = .07).
When data for the GSs were analysed, the Response × Stimulus interaction was again not significant, F(1, 55) = 1.13, p = .29, p η2 = .02, and there was no significant modulation by N group either, F(2, 55) = 1.89, p = .16, p η2 = .06 ((b)). However, when only the Low and High N groups were included, a significant Response × Stimulus interaction was obtained, F(1, 35) = 4.87, p = .03, p η2 = .12, with no significant interaction with N group. This interaction remained significant (p = .04) when the one participant with long RTs was removed from the sample. Thus, in Experiment 2, we did not observe strong conditioned avoidance tendencies towards the CSs. We did observe avoidance tendencies towards GSs, but those seemed to be affected by individual differences.
SBT
No significant difference was observed in the number of GSs or in the maximum colour of the GS to which individuals from the three groups executed safety behaviours, both Fs < 1 ((e,f) , respectively). We failed to replicate the finding reported by Lommen et al. (Citation2010) also when Low and High N groups were compared separately.
We performed a similar logistic regression analysis as for the recognition data of Experiment 1 on the SBT data, to examine if the safety behaviour pattern differed as a function of stimulus class and N group. We used a binary dependent variable for this analysis, which represented whether or not individuals pressed the button at least once to a stimulus from a given class. We found a significant main effect of Stimulus Class, but not of N group, with no significant interaction between the two (, ). As expected, the highest responding in this task was observed for GS1, which was the cue most similar to the CS+ for which responding was available. Response rates to the CS+ were lower, because the message suggesting that the button was available to press was not presented on CS+ trials.
Figure 6. Experiment 2: Percentage of safety behaviour responses to CSs and GSs, by N group. Data points represent mean responding across each set of two stimuli, per class.
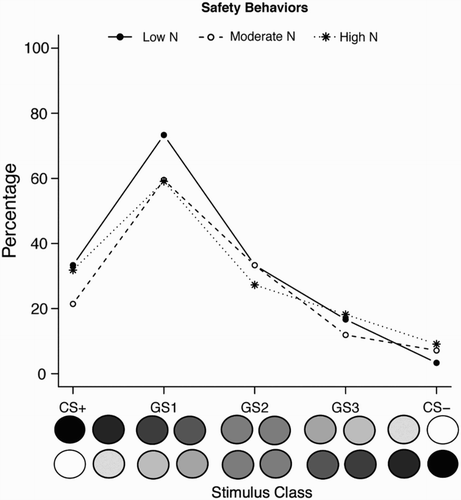
Table 3. Results from logistic regression analysis with safety behaviour (yes/no) as outcome and Stimulus Class (CS+, GS1, GS2, GS3, CS−) and N group (1: Low; 2: Moderate; 3: High) and their interaction (Stimulus Class × N group) as predictor variables.
Evaluative ratings
Similar to Experiment 1, stimuli were evaluated differently, F(2.71, 149.12) = 143.47, p < .001, p η2 = .72, without an effect of N group (p = .71). All pairwise comparisons were highly significant (all ps ≤ .001). Low and High N groups differed in their evaluative ratings for the CS+ only, F(1, 35) = 4.23, p = .05.
General discussion
In two experiments, we examined the effect of neuroticism on gradients of perceptual fear generalisation. Following successful fear acquisition, clear downward generalisation gradients were observed across automatic and controlled measures of fear responding. Very limited evidence was found for an influence of individual differences in neuroticism on those gradients. Differences were neither found in terms of US expectancies or evaluative ratings (both experiments), nor in terms of physiological responding (SCR and FPS) or safety behaviours (Experiment 2). In Experiment 1, individuals high on neuroticism did show more accurate recognition for stimuli than other individuals; this was also the only group in which some indication for conditioned avoidance tendencies was found in Experiment 1. In Experiment 2, even though results did not indicate strong conditioned avoidance tendencies towards the CS+ across the whole sample, conditioned avoidance tendencies towards the GSs were observed for some individuals (i.e. those high and low on N).
In both experiments, downward generalisation gradients were observed, with highest fear responding towards the CS+, lowest towards the CS−, and responses to the GS in between. Using this experimental paradigm, generalisation occurred across both automatic (SCR and FPS) and controlled (US expectancies, evaluative ratings and safety behaviours) response systems. Our failure to find clear individual differences in generalisation contradicts some earlier reports (Kaczkurkin & Lissek, Citation2013; Lommen et al., Citation2010), but is consistent with others (e.g. Torrents-Rodas et al., Citation2013). The findings presented here suggest that fear generalisation is often a robust phenomenon (Torrents-Rodas et al., Citation2013) that occurs across measures and across participants.
The current experiments are the first to examine whether conditioned avoidance tendencies generalise along a perceptual dimension. These automatic tendencies represent a level of conditioned responding often overlooked in fear-conditioning research (Beckers et al., Citation2013). We did not replicate the finding of conditioned avoidance tendencies towards the CS+ (Krypotos et al., Citation2014) in Experiment 2 across the whole sample; in Experiment 1, the strongest indication for conditioned avoidance tendencies was observed for individuals high in neuroticism. The inability to find conditioned avoidance tendencies in Experiment 1 for the whole sample can be attributed to the modifications we made to the task compared to Krypotos et al. (Citation2014). In Experiment 1, we included all 10 CSs and GSs, which might have obstructed the emergence of clear avoidance tendencies due to the increased difficulty of the task.
Crucially, in Experiment 2, we observed avoidance tendencies in some individuals for the GSs closest to CS+ and CS−. This is important because it suggests that generalisation of avoidance might occur on an automatic level, rather than a controlled level (unlike what has been suggested by Lommen et al., Citation2010). Contrary to expectations, however, avoidance tendencies towards GSs perceptually similar to the CS+ were not only observed in the group high in neuroticism, but also in the group low in neuroticism. Possibly, rather than neuroticism, another individual difference factor governs this generalisation. We calculated an approach-avoidance index for the GSs (congruent trials: approach GS3 and avoid GS1, minus incongruent: avoid GS3 and approach GS1) and exploratorily correlated it with the other personality measures assessed in Experiment 2. However, no significant correlations were observed.
One explanation for the abovementioned results is that conditioned avoidance tendencies are not sufficiently robust to allow for the observation of group differences with the sample sizes used here. We had to exclude a number of participants from the data analysis in both experiments (see participants sections), which might have made it more difficult to observe differences between the three neuroticism groups especially in the RT tasks, where high power is needed to detect effects.
Differences in sample size and tasks between Lommen et al. (Citation2010) and the present study might also explain our inability to replicate their overt avoidance behaviour findings. Our failure to find differences between high and low neuroticism groups on safety behaviours/overt avoidance cannot be attributed to the sample characteristics, given that in the present experiments we used exactly the same criteria to compose the neuroticism groups as Lommen and colleagues. However, in Experiment 2, we did use a longer response window (8 s) than the ones used in Lommen et al. (1 and 5 s), which might explain the differences in results. Previous research has shown that presentation duration influences affective processing (Gélat & Chapus, Citation2015) and it cannot be excluded that it also affects avoidance responses, by reducing the threat imminence of the presented stimulus for a large portion of the trial duration. In addition, during the acquisition stage of Experiment 2, participant’s psychophysiological responding was measured through electrodes. These electrodes were then removed during the safety behaviour test, which might have served as a context switch and affected response rates. The fact that clear downward generalisation gradients were observed regardless of whether all or only some electrodes were attached, suggests that these context switches were not salient enough to affect generalisation. Still, the effect of context on (over)generalisation might be a fruitful avenue for future research.
Another notable finding from Experiment 1 is that individuals high in neuroticism seemed to have more accurate recognition memory for the different circles. This resulted in a steeper downward gradient in the recognition task in Experiment 1 for individuals high on neuroticism. This might reflect the ability of high neuroticism individuals to differentiate better between the stimuli and remember the perceptual characteristics of the stimulus material present during the fear-conditioning phase, which might in turn protect against overgeneralisation. This recognition task measures a component of emotional memory that might not be covered by other measures that are typically used in fear-conditioning research. It is unclear whether this recognition pattern is based on the perceived familiarity of the objects, better recollection or better recall of the stimulus material (Yonelinas & Ritchey, Citation2015). Future research should examine which of these processes is enhanced for emotional events in individuals high in neuroticism. Importantly, the findings of the recognition task in Experiment 1 show that the generalisation gradients observed are not the result of a failure to perceptually discriminate between the different GSs, but rather suggest that they result from a non-perceptual decision process that derives US expectations from the degree of perceptual similarity between each GS and the CS+ (Wiech, Ploner, & Tracey, Citation2008).
In conclusion, fear generalisation seems to be a robust process. Using a gradient of colours from black to white as GS, we obtained generalisation gradients of conditioned fear across controlled and automatic measures from subjective, physiological and behavioural response systems, including generalisation of avoidance tendencies. Fear generalisation seems largely unaffected by neuroticism levels, unlike we anticipated. In light of the lack of effect of neuroticism on generalisation observed here and considering recent criticisms regarding the role of neuroticism as a vulnerability factor for psychopathology (Ormel, Rosmalen, & Farmer, Citation2004), for future research, it may be better to shift focus towards other individual difference factors and their effects on generalisation, such as distress endurance (as in van Meurs et al., Citation2014) or intolerance of uncertainty (see Dunsmoor Campese, Ceceli, LeDoux, & Phelps, Citation2015). For now, despite having included measures of many levels of fear responding, we found little evidence for overgeneralisation in individuals at risk for developing clinically severe anxiety. Even though the experiments presented here have limitations (see above), overall the results suggest that differences in fear generalisation may be a consequence of anxiety pathology rather than a vulnerability factor.
Supplement_Generalization_Cogn___Emo_Revised.docx
Download MS Word (58.1 KB)Acknowledgements
We thank Chris Küstermann and Philip “Lino” von Klipstein for their help with collecting data, Bert Molenkamp for technical assistance and Miriam Lommen for providing us with her experimental instructions.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCiD
Angelos-Miltiadis Krypotos http://orcid.org/0000-0001-9704-6520
Tom Beckers http://orcid.org/0000-0002-9581-1505
Additional information
Funding
Notes
1. Technically, some of the substances used by these participants are not in fact illegal in the Netherlands (i.e. marihuana).
References
- Arnaudova, I., Krypotos, A.-M., Effting, M., Boddez, Y., Kindt, M., & Beckers, T. (2013). Individual differences in discriminatory fear learning under conditions of ambiguity: A vulnerability factor for anxiety disorders? Frontiers in Psychology, 4, 298. doi:10.3389/fpsyg.2013.00298
- Beckers, T., Krypotos, A.-M., Boddez, Y., Effting, M., & Kindt, M. (2013). What’s wrong with fear conditioning? Biological Psychology, 92(1), 90–96. doi:10.1016/j.biopsycho.2011.12.015
- de Beurs, E., Van Dyck, R., Marquenie, L. A., Lange, A., & Blonk, R. W. B. (2001). De DASS: een vragenlijst voor het meten van depressie, angst en stress. Gedragstherapie, 34, 35–53. Retrieved from http://www2.psy.unsw.edu.au/dass/Dutch/DASS-manuscriptdeBeurs.pdf
- Blumenthal, T. D., Cuthbert, B. N., Filion, D. L., Hackley, S., Lipp, O. V., & van Boxtel, A. (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42(1), 1–15. doi:10.1111/j.1469-8986.2005.00271.x
- Clark, L. A., Watson, D., & Mineka, S. (1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103(1), 103–116. doi:10.1037/0021-843X.103.1.103
- De Houwer, J., Crombez, G., Baeyens, F., & Hermans, D. (2001). On the generality of the affective Simon effect. Cognition & Emotion, 15(2), 189–206. doi:10.1080/02699930125883
- Dunsmoor, J. E., Campese, V. D., Ceceli, A. O., LeDoux, J. E., & Phelps, E. A. (2015). Novelty-facilitated extinction: providing a novel outcome in place of an expected threat diminishes recovery of defensive responses. Biological psychiatry, 78(3), 203–209. doi: 10.1016/j.biopsych.2014.12.008
- Dunsmoor, J. E., White, A. J., & LaBar, K. S. (2011). Conceptual similarity promotes generalization of higher order fear learning. Learning & Memory, 18, 156–160. doi:10.1101/lm.2016411
- Dymond, S., Dunsmoor, J. E., Vervliet, B., Roche, B., & Hermans, D. (2015). Fear generalization in humans: Systematic review and implications for anxiety disorder research. Behavior Therapy, 46(5), 561–582. doi:10.1016/j.beth.2014.10.001
- Effting, M., & Kindt, M. (2007). Contextual control of human fear associations in a renewal paradigm. Behaviour Research and Therapy, 45(9), 2002–2018. doi:10.1016/j.brat.2007.02.011
- Eysenck, H. J., & Eysenck, S. B. G. (1975). Manual of the Eysenck Personality Questionnaire (junior and adult). Kent: Hodder and Stoughton.
- Gélat, T., & Chapus, C. F. (2015). Reaction time in gait initiation depends on the time available for affective processing. Neuroscience Letters, 609, 69–73. doi:10.1016/j.neulet.2015.10.003
- Haddad, A. D. M., Pritchett, D., Lissek, S., & Lau, J. Y. F. (2012). Trait anxiety and fear responses to safety cues: Stimulus generalization or sensitization? Journal of Psychopathology and Behavioral Assessment, 34(3), 323–331. doi:10.1007/s10862-012-9284-7
- Ishihara, S., & Ishihara, M. (1970). Ishihara’s design charts for color-blindness of unlettered persons. Tokyo: Isshinkai Foundation.
- Kaczkurkin, A. N., & Lissek, S. (2013). Generalization of conditioned fear and obsessive-compulsive traits. Journal of Psychology & Psychotherapy, 7(6), 487–2161. doi:10.4172/2161-0487.S7-003
- Klein, A. M., Becker, E. S., & Rinck, M. (2011). Approach and avoidance tendencies in spider fearful children: The approach-avoidance task. Journal of Child and Family Studies, 20(2), 224–231. doi:10.1007/s10826-010-9402-7
- Krieglmeyer, R., & Deutsch, R. (2010). Comparing measures of approach–avoidance behaviour: The manikin task vs. two versions of the joystick task. Cognition & Emotion, 24(5), 810–828. doi:10.1080/02699930903047298
- Krypotos, A.-M., Effting, M., Arnaudova, I., Kindt, M., & Beckers, T. (2014). Avoided by association: Acquisition, extinction, and renewal of avoidance tendencies toward conditioned fear stimuli. Clinical Psychological Science, 2(3), 336–343. doi:10.1177/2167702613503139
- Lissek, S., Biggs, A. L., Rabin, S. J., Cornwell, B. R., Alvarez, R. P., Pine, D. S., & Grillon, C. (2008). Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy, 46(5), 678–687. doi:10.1016/j.brat.2008.02.005
- Lissek, S., Kaczkurkin, A. N., Rabin, S., Geraci, M., Pine, D. S., & Grillon, C. (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75(11), 909–915. doi:10.1016/j.biopsych.2013.07.025
- Lissek, S., Pine, D. S., & Grillon, C. (2006). The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology, 72(3), 265–270. doi:10.1016/j.biopsycho.2005.11.004
- Lissek, S., Rabin, S., Heller, R. E., Lukenbaugh, D., Geraci, M., Pine, D. S., & Grillon, C. (2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry, 167(1), 47–55. doi:10.1176/appi.ajp.2009.09030410
- Lommen, M. J. J., Engelhard, I. M., & van den Hout, M. A. (2010). Neuroticism and avoidance of ambiguous stimuli: Better safe than sorry? Personality and Individual Differences, 49(8), 1001–1006. doi:10.1016/j.paid.2010.08.012
- Lovibond, P. F., & Lovibond, S. H. (1995). The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behaviour Research and Therapy, 33(3), 335–343. doi:10.1016/0005-7967(94)00075-U
- van Meurs, B., Wiggert, N., Wicker, I., & Lissek, S. (2014). Maladaptive behavioral consequences of conditioned fear-generalization: A pronounced, yet sparsely studied, feature of anxiety pathology. Behaviour Research and Therapy, 57(1), 29–37. doi:10.1016/j.brat.2014.03.009
- Meyer, T. J., Miller, M. L., Metzger, R. L., & Borkovec, T. D. (1990). Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy, 28(6), 487–495. doi:10.1016/0005-7967(90)90135-6
- Milad, M. R., Goldstein, J. M., Orr, S. P., Wedig, M. M., Klibanski, A., Pitman, R. K., & Rauch, S. L. (2006). Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience, 120(6), 1196–1203. doi:10.1037/0735-7044.120.5.1196
- Ormel, J., Rosmalen, J., & Farmer, A. (2004). Neuroticism: A non-informative marker of vulnerability to psychopathology. Social Psychiatry and Psychiatric Epidemiology, 39(11), 906–912. doi:10.1007/s00127-004-0873-y
- Orr, S. P., Metzger, L. J., Lasko, N. B., Macklin, M. L., Peri, T., & Pitman, R. K. (2000). De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology, 109(2), 290–298. doi:10.1037/0021-843X.109.2.290
- Phaf, R. H., Mohr, S. E., Rotteveel, M., & Wicherts, J. M. (2014). Approach, avoidance, and affect: A meta-analysis of approach-avoidance tendencies in manual reaction time tasks. Frontiers in Psychology, 5, 378. doi:10.3389/fpsyg.2014.00378
- van Rijsoort, S., Emmelkamp, P., & Vervaeke, G. (1999). The Penn State Worry Questionnaire and the Worry Domains Questionnaire: Structure, reliability and validity. Clinical Psychology & Psychotherapy, 6(4), 297–307. doi:10.1002/(SICI)1099-0879(199910)6:4<297::AID-CPP206>3.0.CO;2-E
- Rinck, M., & Becker, E. S. (2007). Approach and avoidance in fear of spiders. Journal of Behavior Therapy and Experimental Psychiatry, 38(2), 105–120. doi:10.1016/j.jbtep.2006.10.001
- Sanderman, R., Arrindell, W. A., Ranchor, A. V., Eysenck, H. J., & Eysenck, S. B. G. (2012). Het meten van persoonlijkheidskenmerken met de Eysenck Personality Questionnaire (EPQ). Groningen, the Netherlands: UMCG/Rijksuniversiteit Groningen, Research institute SHARE.
- Soeter, M., & Kindt, M. (2012). Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology, 37(5), 1204–1215. doi:10.1038/npp.2011.307
- Strack, F., & Deutsch, R. (2004). Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review, 8(3), 220–247. doi:10.1207/s15327957pspr0803_1
- Tinoco-González, D., Fullana, M. A., Torrents-Rodas, D., Bonillo, A., Vervliet, B., Blasco, M. J., … Torrubia, R. (2015). Conditioned fear acquisition and generalization in generalized anxiety disorder. Behavior Therapy, 46(5), 627–639. doi:10.1016/j.beth.2014.12.004
- Torrents-Rodas, D., Fullana, M. A., Bonillo, A., Caseras, X., Andión, O., & Torrubia, R. (2013). No effect of trait anxiety on differential fear conditioning or fear generalization. Biological Psychology, 92(2), 185–190. doi:10.1016/j.biopsycho.2012.10.006
- Vansteenwegen, D., Vervliet, B., Hermans, D., Beckers, T., Baeyens, F., & Eelen, P. (2006). Stronger renewal in human fear conditioning when tested with an acquisition retrieval cue than with an extinction retrieval cue. Behaviour Research and Therapy, 44(12), 1717–1725. doi:10.1016/j.brat.2005.10.014
- Watson, D., & Clark, L. A. (1984). Negative affectivity: The disposition to experience aversive emotional states. Psychological Bulletin, 96(3), 465–490. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6393179 doi: 10.1037/0033-2909.96.3.465
- Watson, D., Gamez, W., & Simms, L. J. (2005). Basic dimensions of temperament and their relation to anxiety and depression: A symptom-based perspective. Journal of Research in Personality, 39(1), 46–66. doi:10.1016/j.jrp.2004.09.006
- Wiech, K., Ploner, M., & Tracey, I. (2008). Neurocognitive aspects of pain perception. Trends in Cognitive Sciences, 12(8), 306–313. doi:10.1016/j.tics.2008.05.005
- Wiers, R. W., Rinck, M., Dictus, M., & van den Wildenberg, E. (2009). Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes, Brain and Behavior, 8(1), 101–106. doi:10.1111/j.1601-183X.2008.00454.x
- Yonelinas, A. P., & Ritchey, M. (2015). The slow forgetting of emotional episodic memories: An emotional binding account. Trends in Cognitive Sciences, 19(5), 259–267. doi:10.1016/j.tics.2015.02.009