ABSTRACT
Several eye-tracking studies have revealed that spider phobic patients show a typical hypervigilance-avoidance pattern when confronted with images of spiders. The present experiment investigated if this pattern can be changed via placebo treatment. We conducted an eye-tracking experiment with 37 women with spider phobia. They looked at picture pairs (a spider paired with a neutral picture) for 7 s each in a retest design: once with and once without a placebo pill presented along with the verbal suggestion that it can reduce phobic symptoms. The placebo was labelled as Propranolol, a beta-blocker that has been successfully used to treat spider phobia. In the placebo condition, both the fixation count and the dwell time on the spider pictures increased, especially in the second half of the presentation time. This was associated with a slight decrease in self-reported symptom severity. In summary, we were able to show that a placebo was able to positively influence visual avoidance in spider phobia. This effect might help to overcome apprehension about engaging in exposure therapy, which is present in many phobic patients.
KEYWORDS:
1. Introduction
Spider phobia is a very common anxiety disorder, with a prevalence of 3.5%–6.1% in the general population. The disorder mainly affects women (APA, Citation2013). These people experience an overwhelming fear when faced with a spider, and this leads to avoidance behaviour (APA, Citation2013). The most effective treatment approach for spider phobia is cognitive behavioural therapy (CBT), during which patients learn to gain control over their dysfunctional cognitions, emotions, and behaviours via gradual exposure to the phobic object (Leutgeb, Schafer, & Schienle, Citation2009). In a meta-analysis by Wolitzky-Taylor, Horowitz, Powers, and Telch (Citation2008) on the psychological treatment of specific phobia, exposure therapy was clearly superior to other therapies without controlled confrontation; however, it was also found that placebo-related symptom reduction is possible. Placebos were significantly more effective than no treatment, suggesting that patients were moderately responsive to this type of intervention.
A placebo is defined as a substance or procedure … “that is objectively without specific activity for the condition being treated” (Moerman & Jonas, Citation2002, p. 471). The placebo effect is a powerful mechanism; it occurs due to processes such as expectancy or conditioning. In clinical trials, placebos are used to compare an inert drug or treatment with an active treatment, in order to determine the effectiveness of the new intervention. However, when interested in the placebo effect per se, it is necessary to compare the placebo condition to a condition in which no treatment is received. This was done in a series of studies on automatic emotion regulation, which compared placebo with no-placebo. Schienle, Ubel, and Scharmuller (Citation2014), Schienle, Ubel, Schongassner, Ille, and Scharmuller (Citation2014), Schienle, Übel, and Wabnegger (Citation2017) administered a disgust-reducing placebo (labelled as an herbal medicine) during functional magnetic resonance imaging: subjects received an inert pill with the verbal suggestion that it was able to reduce disgust symptoms, such as nausea. Participants were scanned in two sessions. They viewed disgusting images, once with and once without the placebo. The placebo reduced disgust feelings as well as insula activation and the activation of several regions in the visual cortex. The latter finding indicates that placebos are capable of influencing perceptual systems (Schienle et al., Citation2014). Clearly, when under the placebo, the emotional pictures were indeed viewed differently.
Following these results, Schienle, Gremsl, Ubel, and Korner (Citation2016) carried out an eye-tracking study to investigate if a disgust placebo would be able to provoke different eye movements while viewing disgust pictures. Once again, participants viewed images with disgusting content; this time, however, the disgust pictures were paired with neutral pictures. The placebo increased the dwell time (the overall time spent including all revisits) and enhanced the number of fixations for disgusting images compared with the no-placebo condition. Again, there was also a pronounced placebo-related reduction of disgust feelings. These effects were interpreted to reflect a greater willingness of the participants to view these stimuli, while they were on the placebo (Schienle et al., Citation2016).
Generally, affective pictures provoke specific visual scanning. Bradley, Houbova, Miccoli, Costa, and Lang (Citation2011) found that images with emotional content (e.g. animals, food, negative facial expressions), relative to neutral pictures (e.g. household objects), elicited broader scanning with more fixations. This exploration behaviour is typical of healthy, non-phobic individuals. In spider phobic patients, several eye-tracking studies have revealed a hypervigilance-avoidance pattern (Hermans, Vansteenwegen, & Eelen, Citation1999; Pflugshaupt et al., Citation2005; Rinck & Becker, Citation2006). An initial gaze direction towards the spider was followed by visual avoidance. Rinck and Becker (Citation2006) showed that after 500 ms, the gaze duration on the spider pictures already started to decrease. Hermans et al. (Citation1999) found that spider phobics started the avoidance after 1000–1500 ms. In the study by Pflugshaupt et al. (Citation2005), vigilance ended after 1700 ms.
The current eye-tracking experiment investigated whether this visual exploration behaviour by spider-phobic patients can be altered via placebo treatment. Women with spider phobia were asked to look at picture pairs (spider-neutral) in a retest design, once with and once without a placebo. The placebo was an inert pill presented along with the verbal suggestion that it can reduce phobic symptoms. The placebo was labelled as Propranolol, a beta-blocker that has been successfully used to treat spider phobia (Soeter & Kindt, Citation2015). We expected that under placebo, participants would spend more time on the spider images, compared to the no-placebo condition. We also investigated the time course of the placebo effect, in order to determine how the visual hypervigilance-avoidance pattern would be affected. In addition, we explored whether the placebo would influence self-reported symptom severity, and affective responses with regards to spiders.
2. Method
2.1. Participants
First, an online screening for spider phobia symptoms was conducted with the Spider Phobia Questionnaire (SPQ, Klorman, Weerts, Hastings, Melamed, & Lang, Citation1974). Forty-three women with elevated SPQ scores (cut off: 14) were invited to participate in the eye-tracking experiment. Prior to the experiment, a board-certified clinical psychologist assured the diagnosis of spider phobia according to DSM 5 (APA, Citation2013) by means of a clinical interview. Six patients were excluded from data analysis because of comorbidity (n = 5; depression, general anxiety disorder or obsessive-compulsive disorder), or because of drop out (one participant only completed one of the two experimental sessions). The final sample consisted of 37 women with a mean age of 22.7 years (SD = 4.8). All participants were free from somatic or mental disorders (except for spider phobia) and the intake of medication. The Ethics Committee of the University of Graz had approved the study (GZ. 39/74/63 ex 2015/16).
2.2. Material and design
2.2.1. Images
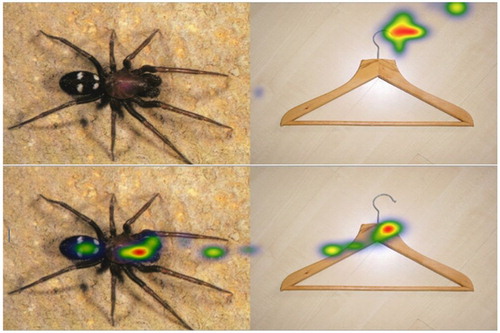
The participants viewed 48 picture pairs, while their eye movements were recorded. Sixteen pairs consisted of two neutral pictures, while the remaining 32 pairs contained one neutral image and one picture of a spider (see ). The location of the spider (left vs. right side) was balanced. The pairs were shown for 7000 ms each in random order. The images had been used in previous studies (Leutgeb et al., Citation2009; Schienle et al., Citation2014; Schienle et al., Citation2016) or were taken from the International Affective Picture System (IAPS, Lang, Bradley, & Cuthbert, Citation2001).
2.3. Procedure
The experiment consisted of two sessions (with and without placebo), which were separated by about one week. The sequence of the two sessions was counterbalanced.
During the placebo condition, participants received a placebo pill at the beginning of the session. The pill was introduced as Propranolol, a beta blocker, which is able to reduce spider-phobic symptoms. We told the participants that the medication needed about 10–15 min until the fear-reducing effect would occur. During this time, they read two manipulated scientific articles (inspired by the study of Soeter & Kindt, Citation2015), which described how patients were able to make contact with a tarantula after they had received this medication. The experimenters wore white coats during the testing to enhance the credibility of the cover story. After placebo administration, the eye tracking experiment was conducted. Subsequently, the participants were presented with two sheets of paper depicting all of the neutral or the spider pictures. We asked the participants to rate the intensity of experienced fear and disgust while watching the picture categories on 9-point Likert scales (1 = little; 9 = very intense). These two emotions were chosen since both are central in spider phobia (de Jong, Vorage, & van den Hout, Citation2000). In the placebo condition, participants completed the SPQ again after placebo administration.
2.4. Eye movement recording and analysis
Two-dimensional eye movements were recorded via an SMI RED250mobile with a sampling rate of 250 Hz. This remote eye tracker uses infrared cameras to monitor eye movements. A head movement compensation mechanism is included. To minimise head movements, we additionally used a chin rest. The eye-tracker was calibrated with a 9-point calibration procedure for both eyes. The picture pairs were presented on a 24-inch widescreen TFT monitor with a resolution of 1920 × 1080. Participants sat about 60 cm away from the monitor. Between the picture pairs a black fixation cross was shown in the middle of the screen. The eye-tracker was adjusted, so that it automatically switched to the next pair when participants looked at the fixation cross for 500 ms. The experiment was controlled with the SMI Experiment Suite. Data were exported with SMI Begaze and customised Python scripts.
We extracted the following eye-tracking variables: (1) fixation count: fixations were defined by the absence of a saccade. We counted the number of fixations during the 7000 ms viewing period of a picture pair. Fixation duration (2) is the mean time (in milliseconds) fixations lasted, and dwell time (3) is the overall time participants spent on one picture (in milliseconds).
3. Results
3.1. Eye-tracking
We first selected the pictures from the Spider-Neutral pairs and computed analyses of variance with the factors Picture Category (Spider, Neutral) and Treatment (Placebo, No-Placebo) for fixation count, fixation duration and dwell time. Previously, we made sure that there was no effect of sequence of placebo administration (placebo in the first session vs. placebo in the second session). For none of the studied eye-tracking parameters there were statistically significant sequence effects (all ps > .20).
For fixation count both main effects (Picture Category (F(1,36) = 1.46, p = .234, = .039, 95% CI [0.0, .210]); Treatment (F(1,36) = 1.17, p = .29,
= .032, 95% CI [0.0, .197]) were not significant, but the interaction was (F(1,36) = 10.21, p = .003,
= .221, 95% CI [.03, .422]). Post-hoc t-tests revealed that with placebo there were more fixations on the spiders than without the placebo (t(36) = 2.72, p = .01, d = .447, 95% CI [.106, .783]), and that with placebo there were more fixations on the spider pictures than on the neutral pictures (t(36) = 2.67, p = .01, d = .439, 95% CI [.099, .774]).
The ANOVA for the average fixation duration (ms) revealed a significant main effect Picture Category (F(1,36) = 6.62, p = .014, = .155, 95% CI [.006, .358]) with a shorter duration for spider pictures (M = 343.51, SD = 13.44) than for neutral pictures (M = 377.95, SD = 71.63). While the second main effect Treatment did not yield significance the interaction did (F(1,36) = 6.18, p = .018,
= .146, 95% CI [.004, .349]). Post hoc t-tests showed that the fixation duration was longer on the neutral pictures (M = 388.03, SD = 156.71) than on the spider pictures (M = 332.4, SD = 91.69) when no placebo was administered (t(36) = 3.11, p =.004, d = .511, 95% CI [.165, .851]).
The results for the dwell time revealed no significant main effect but a significant interaction Picture Category x Treatment (F(1,36) = 11.55, p = .002, = .243, 95% CI [.041, .442]). Post-hoc t-tests show that the dwell time on the spider picture was longer while under the influence of a placebo (t(36) = 3.06, p = .004, d = .503, 95% CI [.158, .842]; Mwith = 3418.45, SDwith = 647.3; Mwithout = 2964.6, SDwithout = 826.39). Means for the eye-tracking data are displayed in .
Table 1. Subjective data and eye-tracking parameters for spider – neutral pairs.
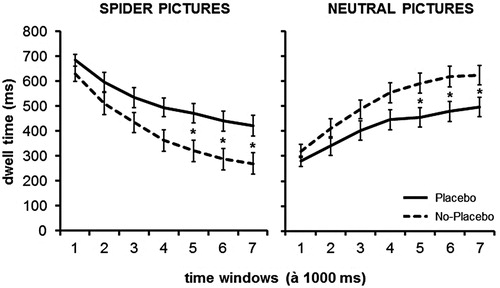
Time course of effects: We separated the total presentation duration of 7000 ms in seven equal time windows and calculated an ANOVA with the within-subject factors Picture Category (Spider, Neutral), Treatment (placebo, No-Placebo) and Time Window (seven windows à 1000 ms). The dependent variable was the dwell time. The main effect Picture category was not significant (F(1,36) = .003, p = .959), the main effect Treatment was not significant as well (F(1,36) = 1.61, p = .213). The main effect time window was significant (F(1,36) = 11.38, p < .001, = .240, 95% CI [.039, .44]). While the interactions treatment x picture category (F(1,36) = 9.51, p = .004,
= .209, 95% CI [.025, .411]) and picture category x time window (F(2.018,72.638) = 41.51, p < .001,
= .536, 95% CI [.366, .638]) were significant, the treatment x time window interaction was not (F(3.558, 128.072) = .643, p = .615). The three-way interaction yielded a significant result (F(2.379, 85.642) = 3.109, p = .041,
= .080, 95% CI [.0, .187]).
Post-hoc t-test with Bonferroni correction (Cut-off p = .007) showed that in the last three time windows participants spent more time on the spider picture when a placebo was administered compared to the no- placebo condition. Accordingly with placebo there was less gaze time on the neutral pictures than without placebo in the later phases (p’s < .003; compare ).
Figure 2. Mean dwell times and standard errors for spider and neutral pictures. Significant differences are labelled (*).
We also analyzed the picture pairs as a whole (we treated a pair as if it was one picture) to assure that the presence of a spider lead to specific gaze patterns compared to neutral pairs. Therefore, we compared Spider-Neutral pairs against Neutral-Neutral pairs. Analyses of variance for repeated measures with the factors Picture Pair (Spider – Neutral (SN), Neutral – Neutral (NN)), and Treatment (Placebo, No-Placebo) were computed for Fixation count and duration. Data showed hybrid interactions, where only one main effect is interpretable, that’s why we only report those: The main effect Picture Category was significant for fixation count (F(1,36) = 136.01, p < .001, = .791, 95% CI [.645, .855]) and fixation duration (F(1,36) = 28.6, p < .001,
= .443, 95% CI [.192, .605]). There were more and shorter fixations on the NN-pairs.
3.2. Self-report
Spider Phobia Questionnaire (SPQ): Participants reported lower SPQ scores (t(36) = 4.77, p < .001, d = .784, 95% CI [.411, 1.149]) while under the placebo (M = 17.22, SD = 4.83) compared to the No-Placebo condition (M = 20.89, SD = 3.64). The SPQ reduction occurred in both sequence groups that received the placebo in the first session (t(18) = 3.57, p = .002) or second session (t(17) = 3.55, p = .002).
Affective Ratings: Since the ratings for experienced fear and disgust for the neutral pictures were always “1” (except for one participant), we conducted paired t-tests for spider images only. For the fear ratings, the Bonferroni-corrected t-tests revealed that the ratings were lower with than without the placebo (t(36) = 2.86, p = .007, d = .470, 95% CI [.127, .807]). The disgust ratings for the spider pictures were significantly lowered by the placebo (t(36) = 3.70, p = .001, d = .608, 95% CI [.253, .956]). The means and standard deviation for the ratings are displayed in .
The test of sequence effects of placebo administration showed that only the group that received the placebo in the second session reported significant reduction of disgust and fear (ps < .01), while the other group did not (ps > .48).
We also calculated Pearson correlations to explore possible associations between SPQ scores, picture ratings and eye-tracking parameters. We first calculated difference scores: No-Placebo minus Placebo. We found significant correlations between dwell time on the spiders and placebo-induced disgust reduction (r(35) = .41, p = .01) and fear reduction (r(35) = .42, p = .01). The reduction of SPQ scores also correlated with the dwell time on spiders (r(35) = .47, p = .003).
4. Discussion
This eye-tracking study examined placebo effects in spider phobia. Participants viewed pictures of spiders paired with neutral images, once with a placebo (inert pill administered with the verbal suggestion of an active medication for the treatment of spider phobia) and once without a placebo. The treatment provoked specific changes in the chosen gaze parameters for phobic contents: more fixations and longer focussing (dwell time). These changes reflect reduced visual avoidance of spiders.
In order to further investigate the time course of the placebo effect, we separated the picture presentation in seven equal time windows of 1000 ms each. This enabled a more detailed analysis, and results revealed more gaze time spent on spider pictures at the beginning of the picture presentation. This indicated hypervigilance for the threatening stimulus, in both conditions.
The subsequent avoidance pattern, which followed this initial automatic attending to the threat, was reduced in the placebo condition; in the last three seconds of the picture presentation, phobics spent more time on the spiders when a placebo had been administered. Thus, it seems that the placebo was not able to change initial vigilance, but did facilitate controlled allocation of attention. This effect is in line with previous research on the psychophysiology of cognitive behavioural therapy (CBT) of spider phobia (e.g. Leutgeb et al., Citation2009; Leutgeb, Schafer, Kochel, & Schienle, Citation2012). The authors conducted an event-related potential study and found that after successful therapy, patients still showed initial attentiveness to spiders (reflected by early electrocortical potentials), but that they were able to control attention allocation to the threatening stimulus at later stages of processing (reflected by later electrocortical potentials).
After placebo administration in the current study, phobics reported a slight reduction in symptom severity (SPQ scores). In addition, the placebo treatment reduced fear and disgust experienced during the presentation of spider images, however only in those participants who received the placebo in the second session. Similar sequence effects on affective ratings were not present in previous investigations with “disgust placebos” (e.g. Schienle et al., Citation2014; Ubel, Leutgeb, & Schienle, Citation2015). These studies were characterised by more pronounced effects of the placebo that almost halved the intensity of reported disgust. In the current study the average placebo-related affective changes (disgust/fear ratings) were only modest and were assessed after the eye-tracking experiment. The participants were presented with an overview of the previously shown images (a sheet of paper with 32 spiders). This type of affective assessment might be considered a short-coming of the present study. However, a single rating of each picture would have prolonged the experiment considerably. Another type of limitation refers to the sample. We only studied women, because prevalence rates for spider phobia are higher in females than males. Therefore, we cannot generalise the results to men. In addition, it is important to note that we did not conduct a follow-up, so we cannot make assumptions about the temporal stability of the observed placebo effects. More information is needed on the specific expectations elicited by the placebo of the current study. We used the cover story of an active medication (the beta-blocker Propranolol) that triggered particular assumptions about the treatment. Other verbal suggestions or even open-label placebos (which do not involve deception) might be another option to be tested in future studies (e.g. Kam-Hansen et al., Citation2014).
Taken together, this eye-tracking study showed that a placebo was able to change visual avoidance of spiders; it seemed to increase the willingness for a controlled and prolonged confrontation. The overall time spent on the spider pictures positively correlated with placebo-related symptom reduction (decrease in experienced anxiety/ fear, reduction of SPQ scores). This effect has promising therapeutical implications. It is known that those suffering from specific phobias are often hesitant to seek treatment (Wolitzky-Taylor et al., Citation2008). In a study by Marks and O’Sullivan (Citation1988), 25% of phobic patients refused exposure-based treatment due to fear of facing the feared situation. More specifically, Öst (Citation1989) reported that 90% of the spider-phobic participants in his study would have refused the single-session exposure if they had been told in advance what the treatment entailed. Placebo interventions might help in overcoming apprehension to engage in exposure therapy. In addition, it is possible that placebos “boost” exposure therapy and reduce the time necessary for treatment. Therefore, administration of a placebo may be a valuable add-on to the well-established CBT for spider phobia. For future studies, it could be of interest to test other anxiety disorders like blood phobia, dental phobia or social anxiety disorder and explore the influence of a placebo with a similar design.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric.
- Bradley, M. M., Houbova, P., Miccoli, L., Costa, V. D., & Lang, P. J. (2011). Scan patterns when viewing natural scenes: Emotion, complexity, and repetition. Psychophysiology, 48(11), 1544–1553. doi: 10.1111/j.1469-8986.2011.01223.x
- de Jong, P. J., Vorage, I., & van den Hout, M. A. (2000). Counterconditioning in the treatment of spider phobia: Effects on disgust, fear and valence. Behaviour Research and Therapy, 38(11), 1055–1069. doi: 10.1016/S0005-7967(99)00135-7
- Hermans, D., Vansteenwegen, D., & Eelen, P. (1999). Eye movement registration as a continuous index of attention deployment: Data from a group of spider anxious students. Cognition & Emotion, 13(4), 419–434. doi: 10.1080/026999399379249
- Kam-Hansen, S., Jakubowski, M., Kelley, J. M., Kirsch, I., Hoaglin, D. C., Kaptchuk, T. J., & Burstein, R. (2014). Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Science Translational Medicine, 6(218), 218ra5–218ra5. doi: 10.1126/scitranslmed.3006175
- Klorman, R., Weerts, T. C., Hastings, J. E., Melamed, B. G., & Lang, P. J. (1974). Psychometric description of some specific-fear questionnaires. Behavior Therapy, 5(3), 401–409. doi: 10.1016/S0005-7894(74)80008-0
- Lang, P., Bradley, M., & Cuthbert, B. (2001). The international affective picture system. Gainesville, FL: University of Florida NIMH Center for the Study of Emotion and Attention (CSEA).
- Leutgeb, V., Schafer, A., Kochel, A., & Schienle, A. (2012). Exposure therapy leads to enhanced late frontal positivity in 8- to 13-year-old spider phobic girls. Biological Psychology, 90(1), 97–104. doi: 10.1016/j.biopsycho.2012.02.008
- Leutgeb, V., Schafer, A., & Schienle, A. (2009). An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology, 82(3), 293–300. doi: 10.1016/j.biopsycho.2009.09.003
- Marks, I., & O’Sullivan, G. (1988). Drugs and psychological treatments for agoraphobia/panic and obsessive- compulsive disorders: A review. The British Journal of Psychiatry, 153(5), 650–658. doi: 10.1192/bjp.153.5.650
- Moerman, D. E., & Jonas, W. B. (2002). Deconstructing the placebo effect and finding the meaning response. Annals of Internal Medicine, 136(6), 471–476. doi: 10.7326/0003-4819-136-6-200203190-00011
- Öst, L.-G. (1989). One-session treatment for specific phobias. Behaviour Research and Therapy, 27(1), 1–7. doi: 10.1016/0005-7967(89)90113-7
- Pflugshaupt, T., Mosimann, U. P., von Wartburg, R., Schmitt, W., Nyffeler, T., & Muri, R. M. (2005). Hypervigilance-avoidance pattern in spider phobia. Journal of Anxiety Disorders, 19(1), 105–116. doi: 10.1016/j.janxdis.2003.12.002
- Rinck, M., & Becker, E. S. (2006). Spider fearful individuals attend to threat, then quickly avoid it: Evidence from eye movements. Journal of Abnormal Psychology, 115(2), 231–238. doi:10.1037/0021-843X.115.2.231 doi: 10.1037/0021-843X.115.2.231
- Schienle, A., Gremsl, A., Ubel, S., & Korner, C. (2016). Testing the effects of a disgust placebo with eye tracking. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 101, 69–75. doi: 10.1016/j.ijpsycho.2016.01.001
- Schienle, A., Ubel, S., & Scharmuller, W. (2014). Placebo treatment can alter primary visual cortex activity and connectivity. Neuroscience, 263, 125–129. doi: 10.1016/j.neuroscience.2014.01.016
- Schienle, A., Ubel, S., Schongassner, F., Ille, R., & Scharmuller, W. (2014). Disgust regulation via placebo: An fMRI study. Social Cognitive and Affective Neuroscience, 9(7), 985–990. doi: 10.1093/scan/nst072
- Schienle, A., Übel, S., & Wabnegger, A. (2017). When opposites lead to the same: A direct comparison of explicit andimplicit disgust regulation via fMRI. Social Cognitive and Affective Neuroscience, 12(3), 445–451. https://doi.org/10.1093/scan/nsw144
- Soeter, M., & Kindt, M. (2015). An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biological Psychiatry, 78(12), 880–886. doi: 10.1016/j.biopsych.2015.04.006
- Ubel, S., Leutgeb, V., & Schienle, A. (2015). Electrocortical effects of a disgust placebo in children. Biological Psychology, 108, 78–84. doi: 10.1016/j.biopsycho.2015.03.015
- Wolitzky-Taylor, K. B., Horowitz, J. D., Powers, M. B., & Telch, M. J. (2008). Psychological approaches in the treatment of specific phobias: A meta-analysis. Clinical Psychology Review, 28(6), 1021–1037. doi: 10.1016/j.cpr.2008.02.007