ABSTRACT
Excessive fear generalisation is a feature characteristic of clinical anxiety and has been linked to its aetiology. Previous animal studies have shown that the mere passage of time increases fear generalisation and that brief exposure to training cues prior to long-term testing reverses this effect. The current study examined these phenomena in humans. Healthy participants learned the relationship between the presentation of a picture of a neutral male face and the delivery of a mild shock. One group was immediately tested with a novel picture of a somewhat different male face (generalisation test). Another group was tested one week later. A third group was also tested one week later and was additionally exposed to the training picture prior to testing. During picture presentations, shock-expectancy ratings were obtained as a measure of fear. Fear generalisation increased from the immediate test to the 1-week follow-up test. This result could not be attributed to level of neuroticism or a general increase in fear (incubation). Furthermore, the time-dependent increase in fear generalisation vanished following brief exposure to the training picture. Results indicate that human fear generalisation is a temporally dynamic process and that memory for stimulus details can be re-established following a reminder treatment.
Generalisation of threat expectancy increases with time
Generalisation of fear learning is an efficient way to deal with novel, potentially unsafe situations. Adaptive generalisation is essential for survival in a dynamic environment. Excessive fear generalisation, however, in which individuals respond fearfully to a broad range of innocuous stimuli, is characteristic of pathological fear (Dymond, Dunsmoor, Vervliet, Roche, & Hermans, Citation2015). For example, panic disorder (Lissek et al., Citation2010), generalised anxiety disorder (Lissek et al., Citation2014), and posttraumatic stress disorder (Lissek & Grillon, Citation2012) are associated with “overgeneralization”. Likewise, war veterans who demonstrate high levels of fear generalisation report more trauma re-experiencing symptoms (Kostek et al., Citation2014). It has further been theorised that fear generalisation is a key pathogenic mechanism in the aetiology of anxiety disorders (Kindt, Citation2014; Lenaert et al., Citation2014; Lissek et al., Citation2010; Lommen, Engelhard, & van den Hout, Citation2010). In support, Lenaert et al. (Citation2014) demonstrated in a large sample of first-year psychology students that level of fear generalisation is predictive of subclinical anxiety at 6 months follow-up. Given that fear generalisation is a risk factor for the development of pathological fear, mapping the factors that facilitate or reduce it is of theoretical and clinical importance. It is therefore surprising that little research has been devoted to the time-dependent nature of memory consolidation and memory performance, and its relation to fear generalisation (Jasnow, Lynch, Gilman, & Riccio, Citation2017). Although it has been well documented in the animal literature that fear generalisation intensifies over time (Jasnow, Cullen, & Riccio, Citation2012), it is unknown whether human fear generalisation is also a temporally dynamic rather than static process.
Conditioning theory postulates that (pathological) fear is a learned response that results from the interaction between humans and their environment (Mineka & Zinbarg, Citation2006). When an initially neutral stimulus coincides with an intrinsically aversive event (unconditioned stimulus; US), it (now coined conditioned stimulus; CS) comes to predict the US and will evoke preparatory fear responses (conditioned responses; CRs) when encountered on future occasions. Generalisation, then, refers to the triggering of CRs by novel stimuli (generalisation stimuli, GSs) that share some of the elements of the original CS or are conceptually related (for a review, see Dymond et al., Citation2015). As such, fear generalisation is considered a descriptive term for the behavioural alterations after fear conditioning, rather than a process or mechanism (cf. Lenaert et al., Citation2014; Lissek et al., Citation2010; Lommen et al., Citation2010). Experimental research has uncovered several conditions under which fear generalisation takes place, typically employing stimuli that share features across a perceptual dimension (Dunsmoor & Paz, Citation2015). For example, following repeated pairings of a neutral CS tone and electrocutaneous stimulation, exposure to similar tones with varying frequencies (GSs) results in skin conductance responses to these novel tones that diminish progressively with perceptual distance from the CS (Hovland, Citation1937). Such generalisation gradients – with peak responses to the CS and successively lower responses to stimuli that resemble the CS in decreasing steps of perceptual similarity – also arise with stimuli that share visual features, like circles of different sizes (e.g. Lissek et al., Citation2010).
A key factor involved in the strength of fear generalisation thus entails the ability to detect (dis)similarities between novel stimuli and the original CS (Struyf, Zaman, Vervliet, & Van Diest, Citation2015). Such detection relies heavily on initial encoding and subsequent retrieval of the CS representation. For instance, the intensity of generalised responding depends on what specific stimulus features people pay attention to during learning (e.g. shape or colour), e.g. based on what the discrimination learning task requires them to focus on (Vervliet & Geens, Citation2014). Supposedly, poor memory of relevant stimulus details fuels fear generalisation. A crucial finding in this regard is that memory generally becomes less specific with time. For example, Bahrick, Clark, and Bahrick (Citation1967) required participants to identify a target stimulus (e.g. a drawing of a teacup) that was previously presented in isolation from an array of perceptually related stimuli, minutes, hours, or days after encoding. The number of “false positives” was shown to positively correlate not only with perceptual overlap between the target and test stimulus, but also with retention interval length. Likewise, Sekeres and colleagues (Citation2016) demonstrated that over the course of 7 days memory for peripheral (perceptual) details of naturalistic events (film clips) was progressively lost, and at a faster rate than memory for gist elements. Given that the broadness of generalisation depends heavily on recall performance, it can be hypothesised that the mere passage of time causes enhanced fear generalisation. Correspondingly, animal studies have repeatedly demonstrated that installation of a retention interval renders previously ineffective stimuli capable of evoking fear responses (for a review, see Jasnow et al., Citation2012). For example, mice trained with a footshock and tested 1, 14, 28, or 36 days later, show stable freezing responses over time in the training context, but in a novel environment freezing responses gradually increases over time, which reflects enhanced fear generalisation (Wiltgen & Silva, Citation2007). These effects have been attributed to a more rapid loss of memory for cues that were present in the training context compared to memory for the CR itself (Jasnow et al., Citation2017). From a neurobiological perspective, these findings are usually explained by the progressive reduction in hippocampal dependency (as memories are stored in the cortex), which results in recall of schematic information and consequently a broader range of stimuli being capable of eliciting the target response (Jasnow et al., Citation2017). Whether this time effect on fear generalisation is similar for humans is still unknown. A recently published human study on the effect of stress on fear generalisation found that remote fear memories (24 h old), but not newly formed fear memories (15 min old), were sensitive to the effects of stress (Dunsmoor, Otto, & Phelps, Citation2017). However, there was no time effect in the no-stress control conditions, meaning that there was no evidence that a 24-h interval affects fear generalisation. Possibly, the retention interval in this study was too short.
Promisingly, numerous animal studies have further revealed that brief exposure to the training context prior to testing eliminates fear generalisation to the novel context (Jasnow et al., Citation2012). Presumably, such a reminder treatment reactivates the initial memory, which re-enables differentiation between the training context and the test context. Similarly, it has recently been demonstrated in humans that cuing remote (non-trauma-related) episodic memory just prior to retrieval can restore some of the details that were initially forgotten over time (Sekeres et al., Citation2016).
In summary, the animal literature suggests that fear generalisation is a temporally dynamic process that, if left untreated, increases with time. Importantly, this may be of relevance to understanding anxiety-related disorders. Perhaps surprisingly, however, this topic has received little attention in the human fear conditioning literature. We therefore set out to test in humans the hypothesis that installation of a 1-week retention interval increases fear generalisation. To this end, one group was trained and tested on the same day, and another group was tested 7 days after training. We further predicted that a reminder treatment prior to testing reduces this effect. To examine this second hypothesis, a third group was tested 7 days after training and was exposed to the training stimulus just prior to the test phase.
Methods
Participants
Based on a power analysis (d = .80; cf. Leer et al., Citation2017), the sample size was set at 60 participants (n = 20 per group). Seventy-nine undergraduates participated in exchange for remuneration or course credits. Exclusion criteria were pregnancy, serious medical conditions, (past or present) psychiatric diagnoses, having an electronic implanted device (e.g. a pacemaker), pain or problems related to hands or wrists, and recent use of tranquilisers. Prior to data analysis, 19 participants were excluded because they did not show acquisition of differential US-expectancy (i.e. US-expectancy during the final CS trial of the acquisition phase ≤ US-expectancy during the final baseline trial of the acquisition phase; n = 10), did not show up at the follow-up test (n = 7), did not respond during the critical test trial (n = 1), or did not provide data on more than half of the trials and reported to not have understood the instructions (n = 1). The final sample comprised 60 participants (age: M = 21.88, SD = 2.63, range = 18–31; 37 females) that were allocated by order of appearance to one of three groups: “Day 1” (n = 21), “Day 8” (n = 19), or “Day 8 reminder” (n = 20).
Apparatus and stimulus material
The conditioning task was programmed in E-Prime 2.0 (Psychology Software Tools) and presented via a 17-inch monitor (1024 × 768 pixels). shows the pictures of neutral male faces (511 × 768 pixels) that served as CS and GS (cf. Leer et al., Citation2017, exp. 2). The CS was obtained from the Radboud Faces Database (Langner et al., Citation2010). The GS was created by morphing the CS face with another neutral male face from the Radboud Face Database using Abrosoft Fantamorph software. Pictures of faces were used because initial formation of detailed memory was an important condition for testing our hypothesis. Arguably, for memory of abstract stimuli that are built up of only a few visual elements, e.g. circles, there is less room for a subsequent reduction in memory detail. The US was a 500-ms electrocutaneous stimulus generated by a Coulbourn Finger Stimulator. Shock electrodes were placed on the palmar side of the middle and index finger of the non-dominant hand.
Questionnaire
The neuroticism and extraversion scales of the Eysenck Personality Questionnaire were used (EPQ; Eysenck & Eysenck, Citation1975). Items of the two scales (21 for neuroticism and 19 for extraversion) were mixed and had a binary response format (0 = no, 1 = yes). Neuroticism was assessed to control for group differences that may affect (generalised) fearful responding (Lommen et al., Citation2010). Items of the extraversion scale were only included to minimise the impact of response tendencies.
Procedure
The experimental procedures were approved by the local ethics review board. Participants received written and oral information about the experimental procedures. After providing written informed consent and filling out the EPQ, they were subjected to a shock work-up procedure that aimed at selecting a level that was experienced as “highly uncomfortable, but not painful” (cf. Leer et al., Citation2017). Participants were given a series of different current flows, starting at 0.2 mA, gradually increasing, and maxing at 4.0 mA. They were asked to rate each shock (0 = I don’t feel anything; 1 = I feel something, but this is not annoying, it is just a sensation; 2 = it starts to feel annoying, but it is still very much tolerable; 10 = this is the maximum level I want to be exposed to) and they were urged to notify the experimenter when their maximum level was reached or when they wanted shock intensity to be turned down. Next, the experimental task commenced.
We used a non-differential fear acquisition and test protocol, i.e. there was no control stimulus signalling the absence of the US (cf. other human studies, e.g. Boddez, Bennett, van Esch, & Beckers, Citation2017; Leer et al., Citation2017, and most animal studies; Ghirlanda & Enquist, Citation2003). Inclusion of a control stimulus is known to affect fear generalisation, because responding is also determined by the perceived overlap between the test stimulus (GS) and the control stimulus (Ghirlanda & Enquist, Citation2003). Such an effect is undesirable given that our hypotheses specifically pertain to memory of the stimulus signalling the US. Furthermore, the nature of the control stimulus influences feature learning (i.e. prioritising the processing of stimulus features that uniquely predict the US) and thereby affects fear generalisation (Vervliet & Geens, Citation2014). Inclusion of a control stimulus may thus cause incomplete stimulus processing, which interferes with (testing) the hypothesised time-dependent changes in memory performance.
Practice phase. This phase served to help participants get familiar with the visual analogue scale (VAS) that was used throughout the experiment to assess online shock expectancy (0 = certainly no shock; 100 = certainly a shock). Participants were instructed that no shocks would be delivered and they were urged to fill out each VAS within 6 s, as it would disappear automatically. There were three practice trials, during which only the VAS was presented, with 3, 4, or 5 s in between.
Acquisition phase. Participants were instructed that they would occasionally receive shocks and that it was their job to predict shock-occurrence and to indicate shock expectancy each time the VAS would appear on screen. They were then alternately exposed to 12 baseline trials and 12 CS trials, starting with a baseline trial. In between trials they looked at blank (white) screens for a random duration of 3, 4, or 5 s. Baseline trials involved a 6-s VAS presentation against a blank (white) background. CS trials comprised the simultaneous presentation of the CS and the VAS, for 6 s. At offset of nine randomly chosen CS trials the 500-ms shock US occurred immediately. Such partial reinforcement schedule (75%) was chosen for two reasons. First, it arguably reduces the impact of non-reinforced CS presentations during the test phase. Second, it allows for the examination of time-dependent increases in generalised fearful responding over and above time-dependent increases in conditioned fearful responding (i.e. incubation; Wiltgen & Silva, Citation2007), which is impossible when fear levels are maxing at pre-test.
Test phase. Only for participants in group “Day 1” the test phase was scheduled immediately after the acquisition phase (see ). For participants in group “Day 8” and group “Day 8 reminder” the test phase was scheduled 1 week later. Upon their return to the lab, the electrodes were reattached and participants were told that shock intensity was set at the level they had chosen in the previous session. Only participants in group “Day 8 reminder” were then exposed to the CS for 15 s and received the following instructions: “You will now be presented the picture that you saw in the first part of the experiment. Please encode as much detail as possible”. These instructions were added to maximise reinstatement of the CS representation. At the start of the test phase, all participants were told that they would be presented with the picture of the face they had seen in the first part of the experiment and other, somewhat different, faces. In fact, only one other face was presented, but giving this information may have resulted in reasoning-based rather than memory-based responding. They were further instructed that only the face that they had seen earlier would sometimes be followed by shock, that other faces would not, and that they should indicate their shock expectancy each time the VAS would appear on screen. Then, in a fixed order, participants were exposed to a baseline trial, a GS trial that involved the critical presentation of the GS (non-reinforced), another baseline trial, and a CS trial (non-reinforced). Note that some other fear generalisation test protocols involve random stimulus presentation, occasional reinforcement of the CS, and a set of different GSs (Lissek et al., Citation2010). However, occasional reinforcement and/or CS presentation preceding GS presentation would serve as a reminder treatment and thereby make it impossible to test the hypothesis. And, while using a set of different GSs enables investigation of the shape of the generalisation gradient, it comes with the limitation of CR extinction during the generalisation sequence (Lissek et al., Citation2010). Therefore, and in line with previous studies, we used only one GS (e.g. Barry, Vervliet, & Hermans, Citation2016; Vervliet, Vansteenwegen, & Eelen, Citation2004). During each trial, the VAS was presented for 6 s, which corresponded to the length of the CS and GS presentations. Inter-trial-intervals had a random duration of 3, 4, or 5 s.
Results
Randomisation check
There was no evidence for group differences in age, level of neuroticism, shock intensity, Fs < 1, or gender ratio, χ2(2, N = 60) = .04, p = .979, indicating successful randomisation (see ).
Table 1. Means (SD) for age, neuroticism, individually set shock intensity, and gender ratio.
Fear learning
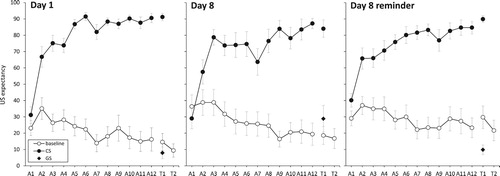
Mean US-expectancy ratings are shown in . To test whether acquisition of differential US-expectancy took place, a 2(Stimulus: CS vs. baseline) × 3(Group: Day 1 vs. Day 8 vs. Day 8 reminder) ANOVA was conducted on the last trial of the acquisition phase. There was a main effect of Stimulus, F(1, 57) = 236.93, p < .001, ηρ² = .806, with stronger shock expectancy during the CS (M = 87.55, SD = 14.69) than during baseline (M = 19.55, SD = 29.57). There were no other effects, Fs < 1, meaning that acquisition of differential US-expectancy was successful and unrelated to group.
Fear generalisation
First, we tested incubation, i.e. an increase in conditioned fearful responding over time, as this may interfere with our analysis of time-dependent fear generalisation (e.g. Wiltgen & Silva, Citation2007). A 2(Stimulus: CS vs. baseline) × 2(Group: Day 1 vs. Day 8) ANOVA on the CS and first baseline trial during the test phase only yielded a main effect of stimulus, F(1, 37) = 234.68, p < .001, ηρ² = .864, other Fs < 1, meaning that responding did not increase over time. Thus, any time-dependent changes in fear generalisation could not be accounted for by incubation.
Next, we tested whether there were differences between stimuli and groups in the test phase. A 3(Stimulus: CS vs. GS vs. first baseline trial) × 3(Group) ANOVA showed no main effect of Group, F(2, 55) = 1.34, p = .270. There was, however, a main effect of Stimulus, F(2, 110) = 192.63, p < .001, ηρ² = .778, which subsumed under a Stimulus × Group interaction, F(4, 110) = 2.82, p = .032, ηρ² = .093. To breakdown this interaction, separate ANOVAs were performed for each stimulus. Results showed group differences for US-expectancy ratings provided during the GS, F(2, 59) = 4.71, p = .013, ω = .332, but not for ratings provided during the CS, F(2, 59) = 1.22, p = .303, or the baseline trial, F(2, 57) = 1.80, p = .175 (see ).
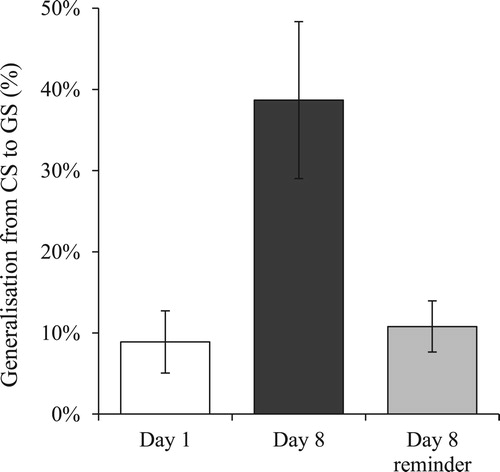
To test the hypotheses, we calculated generalisation indices (GIs) by taking the ratio of shock expectancy during the GS test trial to shock expectancy during the CS test trial (cf. Lenaert, van de Ven, Kaas, & Vlaeyen, Citation2016). As such, a GI can be interpreted as percentage generalisation from CS to GS, with 0% reflecting no generalisation and 100% reflecting full generalisation (see ). First, GIs of the three groups were compared by a one-way ANOVA. This analysis revealed a significant effect of Group, F(2, 59) = 7.38, p = .001, ω = .424. A first planned comparison showed a higher GI in group Day 8 (M = 0.39, SD = .42) than in group Day 1(M = 0.09, SD = .18), t(38) = 2.86, p = .009, d = .928 (variances were not equal; corrected p-value reported). This result indicates that installation of a 1-week retention interval led to increased fear generalisation, and supports the first hypothesis. A second planned comparison revealed a lower GI in group Day 8 reminder (M = 0.11, SD = .14) as compared to group Day 8, t(37) = 2.80, p = .012, d = .894 (variances were not equal; corrected p-value reported). This means that the reminder treatment at Day 8 resulted in weaker generalisation of US-expectancy, which is in support of the second hypothesis.
Discussion
The present experiment was designed to examine the time dependence of human fear generalisation. Results indicate that self-reported threat expectancy to a generalisation stimulus significantly increases following a 1-week retention interval and that brief exposure to the CS just prior to GS presentation strongly reduces this effect. The findings support the hypotheses and are in line with previous animal research demonstrating that rats and mice exhibit only low levels of fear expression when placed in a novel context shortly after training, exhibit progressively higher fear levels as the retention interval increases, and that these time effects can be eliminated when given a reminder treatment (Jasnow et al., Citation2017).
Of importance, we found no evidence that group differences in level of neuroticism or a general increase in fear over time (incubation) could account for the time-dependent increase in fear generalisation. More likely, alterations in memory performance, i.e. in discrimination acuity, were causally involved. Although no independent test of memory was performed, reinstatement of the CS representation strongly affected responding at the 1-week retention test. It may accordingly be inferred that reduced memory precision drove the effect of time on fear generalisation. Still, there might be another explanation of the findings. As an alternative to the result of recall performance, fear generalisation has been conceptualised as an active process driven by stimulus overlap (Struyf et al., Citation2015). This account holds that generalisation can occur even though the CS and GS are clearly perceptually discriminable, because the individual adopts a better safe than sorry strategy in the face of potential danger. It follows that changes in fear generalisation may be independent of changes in memory precision, but instead may have arisen because learning was emotionally relevant. Possibly, the passage of time feeds memory distrust, which in turn causes individuals to more readily apply a better safe than sorry approach, and thus show stronger fear generalisation. Furthermore, a reminder treatment would arguably restore confidence in memory and thereby decrease the tendency of acting in a precautionary way. Conclusions about the mechanisms underlying the current findings thus require further research, for example by including an independent test of memory precision or memory confidence, or by including confidence ratings of shock expectancy at test. Also, to disambiguate the role of emotion, future studies could test whether an aversive learning protocol is associated with stronger time-dependent increases in fear generalisation compared to a simple stimulus-stimulus protocol with a non-aversive outcome (e.g. a picture paired with another picture or sound).
Until the mechanisms of change are clear, clinical implications of the current findings may best be considered at a functional level. Time-dependent changes in fear generalisation may partly explain how normal fear turns into pathological fear. That is, following a traumatic learning experience, reductions in memory precision/confidence may increase the range of stimuli and situations that trigger the trauma memory and subsequent fear responses. Obviously, however, it would be excessive to state that the mere passage of time results in the development of anxiety disorders, because only a minority of individuals develops clinical problems following a traumatic event (Breslau et al., Citation1998). Alongside individual differences in other risk factors, there may be a tipping point in memory imprecision that should be reached in order for fear generalisation to go awry. This tipping point might be reached in some individuals, e.g. those low in autobiographical memory specificity, but not in others. At risk individuals or patients suffering from anxiety-related disorders might accordingly be encouraged to strengthen their memory for stimuli that were around at the time of the traumatic event. For example, retrieval practice – the act of calling information to mind – has been implicated in the long-term retention of information (a phenomenon referred to as the “testing effect”, Karpicke & Roediger, Citation2008). Occasionally recalling relevant cues that were present at the time of the traumatic event may thus prevent or attenuate excessive fear generalisation (Sekeres et al., Citation2016).
Several issues deserve further attention. First, the current findings not only warrant replication, but also cross-validation with other fear measures such as fear-potentiated startle or behavioural avoidance. Although US-expectancy is considered a valid measure of conditioned fear (Boddez et al., Citation2013), it is only weakly correlated with physiological and behavioural measures of fear (Mauss, Levenson, McCarter, Wilhelm, & Gross, Citation2005). It is therefore of interest to understand whether or not expression of time-dependent fear generalisation differentiates depending on the response system under investigation. Second, the present focus was on fear generalisation based on perceptual similarity, while many other factors have been identified that influence fear generalisation, such as the intensity of the CS and US, and conceptual or categorical associations (Dunsmoor & Paz, Citation2015). For instance, humans possess the ability to symbolically represent information and to use this to make predictions about the potential threat of novel stimuli, which enables fear generalisation based on non-perceptual information (Dymond et al., Citation2015). Because real-world fears typically involve multidimensional stimuli about which people likely have or will develop conceptual knowledge (e.g. in fear of public places), higher-order reasoning processes are likely to play a role in the development of clinical fears (Dunsmoor & Murphy, Citation2015). Whether the impact of non-perceptual factors on fear generalisation is also affected by the passage of time remains to be examined. Third, individuals show large differences in their ability to discriminate between faces and to remember faces (e.g. Wilmer et al., Citation2010) and it is unclear to what extent this affected the current results. Fourth, prior to the generalisation test phase we instructed participants that only the face that they had seen earlier would sometimes be followed by shock, which may have reduced levels of uncertainty and thereby dampened the strength of fear generalisation. Because the instructions were given in all groups, it seems unlikely that they mediated the observed group differences. Still, it may be interesting to test the current hypothesis using different instructions.
In summary, fear generalisation has been implicated in the pathogenesis of anxiety-related disorders, which underlines the importance of understanding what factors facilitate or reduce it. We provided the first evidence that the mere passage of time increases human fear generalisation, which may partly explain the transition from normal fear to pathological fear. We further demonstrated that a reminder treatment eliminates this effect. Future studies may be aimed at replicating and cross-validating the current findings, at uncovering underlying mechanisms, and at investigating the time-dependence of non-perceptual forms of fear generalisation.
Supplementary_Material
Download MS Excel (22.4 KB)Acknowledgements
We thank Alejandro Conduto, Arjen Muis and Dylan Neslo for their assistance with data collection, and we are grateful to Iris M. Engelhard for providing critical comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Miriam J. J. Lommen http://orcid.org/0000-0001-8845-4338
Additional information
Funding
References
- Bahrick, H. P., Clark, S., & Bahrick, P. (1967). Generalization gradients as indicants of learning and retention of a recognition task. Journal of Experimental Psychology, 75, 464–471. doi: 10.1037/h0025131
- Barry, T. J., Vervliet, B., & Hermans, D. (2016). Threat-related gaze fixation and its relationship with the speed and generalisability of extinction learning. Australian Journal of Psychology, 68, 200–208. doi: 10.1111/ajpy.12124
- Boddez, Y., Baeyens, F., Luyten, L., Vansteenwegen, D., Hermans, D., & Beckers, T. (2013). Rating data are underrated: Validity of US expectancy in human fear conditioning. Journal of Behavior Therapy and Experimental Psychiatry, 44, 201–206. doi: 10.1016/j.jbtep.2012.08.003
- Boddez, Y., Bennett, M. P., van Esch, S., & Beckers, T. (2017). Bending rules: The shape of the perceptual generalisation gradient is sensitive to inference rules. Cognition and Emotion, 4, 1444–1452. doi: 10.1080/02699931.2016.1230541
- Breslau, N., Kessler, R. C., Chilcoat, H. D., Schultz, L. R., Davis, G. C., & Andreski, P. (1998). Trauma and posttraumatic stress disorder in the community: The 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry, 55, 626–632. doi: 10.1001/archpsyc.55.7.626
- Dunsmoor, J. E., & Murphy, G. L. (2015). Categories, concepts, and conditioning: How humans generalize fear. Trends in Cognitive Sciences, 19, 73–77. doi: 10.1016/j.tics.2014.12.003
- Dunsmoor, J. E., Otto, A. R., & Phelps, E. A. (2017). Stress promotes generalization of older but not recent threat memories. Proceedings of the National Academy of Sciences of the United States of America, 114, 9218–9223. doi: 10.1073/pnas.1704428114
- Dunsmoor, J. E., & Paz, R. (2015). Fear generalization and anxiety: Behavioral and neural mechanisms. Biological Psychiatry, 78, 336–343. doi: 10.1016/j.biopsych.2015.04.010
- Dymond, S., Dunsmoor, J. E., Vervliet, B., Roche, B., & Hermans, D. (2015). Fear generalization in humans: Systematic review and implications for anxiety disorder research. Behavior Therapy, 46, 561–582. doi: 10.1016/j.beth.2014.10.001
- Eysenck, H. J., & Eysenck, S. B. G. (1975). Manual of the Eysenck Personality Questionnaire. New York: Hodder & Stoughton.
- Ghirlanda, S., & Enquist, M. (2003). A century of generalization. Animal Behaviour, 66, 15–36. doi: 10.1006/anbe.2003.2174
- Hovland, C. I. (1937). The generalization of conditioned responses. IV. The effects of varying amounts of reinforcement upon the degree of generalization of conditioned responses. Journal of Experimental Psychology, 21, 261–276. doi: 10.1037/h0061938
- Jasnow, A. M., Cullen, P. K., & Riccio, D. C. (2012). Remembering another aspect of forgetting. Frontiers in Psychology, 3, 175. doi: 10.3389/fpsyg.2012.00175
- Jasnow, A. M., Lynch, J. F., Gilman, T. L., & Riccio, D. C. (2017). Perspectives on fear generalization and its implications for emotional disorders. Journal of Neuroscience Research, 95, 821–835. doi: 10.1002/jnr.23837
- Karpicke, J. D., & Roediger, H. L. (2008). The critical importance of retrieval for learning. Science, 319, 966–968. doi: 10.1126/science.1152408
- Kindt, M. (2014). A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Behaviour Research and Therapy, 62, 24–36. doi: 10.1016/j.brat.2014.08.012
- Kostek, J. A., Beck, K. D., Gilbertson, M. W., Orr, S. P., Pang, K. C., Servatius, R. J., & Myers, C. E. (2014). Acquired equivalence in US veterans with symptoms of posttraumatic stress: Reexperiencing symptoms are associated with greater generalization. Journal of Traumatic Stress, 27, 717–720. doi: 10.1002/jts.21974
- Langner, O., Dotsch, R., Bijlstra, G., Wigboldus, D. H., Hawk, S. T., & Van Knippenberg, A. D. (2010). Presentation and validation of the Radboud Faces Database. Cognition and Emotion, 24, 1377–1388. doi: 10.1080/02699930903485076
- Leer, A., Engelhard, I. M., Lenaert, B., Struyf, D., Vervliet, B., & Hermans, D. (2017). Eye movement during recall reduces objective memory performance: An extended replication. Behaviour Research and Therapy, 92, 94–105. doi: 10.1016/j.brat.2017.03.002
- Lenaert, B., Boddez, Y., Griffith, J. W., Vervliet, B., Schruers, K., & Hermans, D. (2014). Aversive learning and generalization predict subclinical levels of anxiety: A six-month longitudinal study. Journal of Anxiety Disorders, 28, 747–753. doi: 10.1016/j.janxdis.2014.09.006
- Lenaert, B., van de Ven, V., Kaas, A. L., & Vlaeyen, J. W. (2016). Generalization on the basis of prior experience is predicted by individual differences in working memory. Behavior Therapy, 47(1), 130–140. doi: 10.1016/j.beth.2015.10.001
- Lissek, S., & Grillon, C. (2012). Learning models of PTSD. In The Oxford handbook of traumatic stress disorders (pp. 175–190). New York: Oxford University Press.
- Lissek, S., Kaczkurkin, A. N., Rabin, S., Geraci, M., Pine, D. S., & Grillon, C. (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75, 909–915. doi: 10.1016/j.biopsych.2013.07.025
- Lissek, S., Rabin, S., Heller, R. E., Lukenbaugh, D., Geraci, M., Pine, D. S., & Grillon, C. (2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry, 167, 47–55. doi: 10.1176/appi.ajp.2009.09030410
- Lommen, M. J., Engelhard, I. M., & van den Hout, M. A. (2010). Neuroticism and avoidance of ambiguous stimuli: Better safe than sorry? Personality and Individual Differences, 49, 1001–1006. doi: 10.1016/j.paid.2010.08.012
- Mauss, I. B., Levenson, R. W., McCarter, L., Wilhelm, F. H., & Gross, J. J. (2005). The tie that binds? Coherence among emotional experience, behavior, and autonomic physiology. Emotion, 5, 175–190. doi: 10.1037/1528-3542.5.2.175
- Mineka, S., & Zinbarg, R. (2006). A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist, 61, 10–26. doi: 10.1037/0003-066X.61.1.10
- Sekeres, M. J., Bonasia, K., St-Laurent, M., Pishdadian, S., Winocur, G., Grady, C., & Moscovitch, M. (2016). Recovering and preventing loss of detailed memory: Differential rates of forgetting for detail types in episodic memory. Learning & Memory, 23, 72–82. doi: 10.1101/lm.039057.115
- Struyf, D., Zaman, J., Vervliet, B., & Van Diest, I. (2015). Perceptual discrimination in fear generalization: Mechanistic and clinical implications. Neuroscience & Biobehavioral Reviews, 59, 201–207. doi: 10.1016/j.neubiorev.2015.11.004
- Vervliet, B., & Geens, M. (2014). Fear generalization in humans: Impact of feature learning on conditioning and extinction. Neurobiology of Learning and Memory, 113, 143–148. doi: 10.1016/j.nlm.2013.10.002
- Vervliet, B., Vansteenwegen, D., & Eelen, P. (2004). Generalization of extinguished skin conductance responding in human fear conditioning. Learning & Memory, 11, 555–558. doi: 10.1101/lm.77404
- Wilmer, J. B., Germine, L., Chabris, C. F., Chatterjee, G., Williams, M., Loken, E., … Duchaine, B. (2010). Human face recognition ability is specific and highly heritable. Proceedings of the National Academy of Sciences, 107, 5238–5241. doi: 10.1073/pnas.0913053107
- Wiltgen, B. J., & Silva, A. J. (2007). Memory for context becomes less specific with time. Learning & Memory, 14, 313–317. doi: 10.1101/lm.430907